INTRODUCTION
Diabetes mellitus (DM) represents a persistent metabolic disorder characterized by elevated blood glucose levels, resulting from both genetic and environmental factors. Diabetic nephropathy, neuropathy, and retinopathy represent the predominant microvascular complications of diabetes while stroke and myocardial infarction are the principal macrovascular complications[1,2].
As of 2019, 7% of the United Kingdom population was living with diabetes[3], which has increased from 4.3% in 2005[4]. Diabetes and its associated complications account for £23.7 billion of public spending annually within the United Kingdom and contribute to 1.8% of the global gross domestic product[5,6]. The escalating prevalence of diabetes necessitates active surveillance and ongoing observation to prevent further economic impact.
Individuals with diabetes may manifest a range of orthopaedic issues due to sustained high blood sugar levels[7]. Among the spectrum of orthopaedic complications associated with diabetes, pathologies of the foot represent the most prevalent diabetes-related complication necessitating hospital admission in clinical practice[8]. In patients with diabetes, the convergence of two predominant pathologies, neuropathy and ischaemia, manifests distinctly in the feet. These result in the clinical presentations observed in the diabetic foot, notably neuropathic and ischaemic ulcerations, as well as Charcot neuroarthropathy. These conditions are frequently complicated by the onset of infection, potentially escalating to the necessity for limb amputation, whether minor or major. Furthermore, such complications in the diabetic foot are associated with an elevated risk of mortality.
Diabetic foot pathology frequently overlaps with other surgical specialities, notably vascular surgery and plastic surgery, and guidelines advocate for interdepartmental cooperation in management[9]. However, there is variation throughout the United Kingdom regarding models in delivering diabetic foot service and as vascular services increasingly adopt a 'hub and spoke' model, a consequent increase in orthopaedic involvement locally is anticipated[10].
The escalating financial strain and rising prevalence of diabetic foot pathology underscores the need for ongoing attention. This review aims to describe common diabetic orthopaedic presentations and evaluate the most recent advancements in multiple facets of orthopaedic surgery pertaining to the diabetic foot.
PATHOPHYSIOLOGY OF DIABETES
DM is a chronic metabolic disorder that affects glucose regulation in the body. It is broadly classified into two types, each with distinct pathophysiological features:
Type 1 DM - Primarily autoimmune. It involves the depletion of insulin production due to an autoimmune reaction. This process commences with insulitis, characterized by a T-lymphocyte-mediated autoimmune assault leading to the decimation of beta cells within the islets of Langerhans in the pancreas[11-14].
Type 2 DM - It is the most common form and comprises 90%–95% of all diabetes cases[15]. It has a polygenic nature and a multifaceted aetiology, manifesting through hyperinsulinemia and insulin resistance[16].
The National Institute for Health and Care Excellence (NICE) in the United Kingdom sets specific criteria for the diagnosis of diabetes, primarily focusing on Haemoglobin A1c (HbA1c) levels. Diabetes is diagnosed when HbA1c is 48 mmol/mol (6.5%) or higher, and levels between 42-47 mmol/mol (6.0%-6.4%) indicate a high risk of developing diabetes. Additionally, fasting plasma glucose levels of 7.0 mmol/L (126 mg/dL) or higher, or a 2-h plasma glucose level of 11.1 mmol/L (200 mg/dL) or higher after a 75 g oral glucose tolerance test, also confirm diabetes. In cases where there are typical symptoms of diabetes, such as excessive thirst or frequent urination, a random plasma glucose level of 11.1 mmol/L (200 mg/dL) or higher can lead to a diagnosis[17].
Diabetes induces neuropathic changes; high glucose levels damage peripheral nerves, leading to a loss of sensation in the feet[18]. This renders patients unaware of minor traumas or pressures on the foot. This patient group have been observed to have objective muscle weakness at the knee and leg with associated muscular atrophy manifesting in deformities such as cavus foot and claw toes[19-21].
Autonomic disturbances compromise the normal function of integumentary structures. Specifically, diabetes-induced neuropathy adversely affects the sweat and oil glands within the dermis, leading to a disruption in regular activity[22]. This manifests as xerosis, which becomes increasingly prone to fissuring and subsequent infection. Additionally, the autonomic nervous system is notably impaired, resulting in abnormal vasoconstrictive response during postural adjustments[23]. This culminates in increased risks of syncope, dizziness, and consequential falls, thereby elevating the propensity for fractures and associated morbidity[24].
Elevated blood glucose levels cause micro and macroangiopathy, impairing blood flow to the lower extremities[25]. This not only occurs via atherosclerotic disease but also various pathogeneses, such as vasculitis and fibromuscular dysplasia. Peripheral artery disease (PAD) can either manifest as subclinical or clinical. Detection of subclinical PAD can be beneficial as it can lead to earlier intervention and prevention of deterioration[26]. Severe clinical PAD can have severe risks such as an increased chance of infection and critical limb ischaemia[27].
The confluence of ischemia and neuropathy, often termed “diabetic foot”, predisposes individuals to foot ulcers, and infections, and, in severe cases, necessitates minor and major limb amputation[28]. The intersection of these pathologies, compounded by factors such as poor wound healing and immune response impairment, underscores the need for preventive care and stringent glucose control. These three pathologies can be categorised into diabetic foot ulceration, diabetic foot infection, and skeletal pathology.
DIABETIC FOOT ULCERS
Diabetic foot ulcers (DFUs) are a common manifestation of diabetic neuropathy. If left untreated these can progress into further infections of the bone and soft tissue. In patients with DFUs, there is a notable prevalence of concurrent lower extremity PAD, affecting approximately 50% of such individuals[29]. Evidence suggests that 50% of ulcers develop infection[18], with up to 20% of these infections necessitating amputation[30]. The long-term prognosis for patients with DFUs is poor, evidenced by a 24.6% mortality rate within five years of diagnosis[31].
DFUs exert a negative impact on individuals’ well-being and quality of life. A crucial aspect affecting patients' experiences with DFU is the loss of mobility, resulting from the requirement to avoid weight-bearing during treatment[32]. Evidence demonstrates a substantial improvement in patient’s well-being and health status following the healing of DFUs, while a decline is observed when DFUs persist or reoccur[33].
These ulcers frequently originate from minor, repetitive trauma or inflammation during weight-bearing activities, resulting in sub-callus haemorrhages. Upon callus removal, these haemorrhages are often revealed as full-thickness ulcers, extending beyond the epidermis and dermis into subcutaneous layers[34]. Additionally, DFUs can develop from continuous low-pressure sources, such as ill-fitting footwear leading to tissue necrosis, or from acute high-pressure trauma like puncture wounds[8].
In the landscape of DFU classification systems, the Wagner system[35], initially prevalent, is now advised against by NICE[36], likely due to its omission of critical factors like neuropathy and ulcer size, key predictors of healing duration. The WIfI system has been identified for expert evaluation and ongoing assessment of peripheral tissue blood flow[37]. Conversely, in auditing clinical outcomes, the SINBAD system outperforms others[38]. Its broad validation across various research contexts and consistent results, underscored by the extensive number of participants involved, affirm its reliability[39].
DIABETIC FOOT INFECTION
Diabetic foot osteomyelitis
Diabetic Foot Osteomyelitis (DFO) refers to the infection of bone and bone marrow. It occurs in 10%-15% of moderate and up to 50% of severe ulcer infections[40]. Clinically diagnosing these infections is critical, with microbiological analyses playing a pivotal role in identifying the causative bacteria and guiding targeted antibiotic therapy. The most common causative bacteria S.Aureus followed by pseudomonas and E.Coli[41]. Magnetic resonance imaging remains the gold standard in imaging in cases of suspected osteomyelitis[42]. According to the latest International Working Group on the Diabetic Foot (IWGDF) guidelines an intraosseous abscess and ‘bone oedema with other associated signs of osteomyelitis’ is associated with > 90% and 51%-90% probability of diagnosing osteomyelitis respectively[43].
Prognosis varies based on its location; forefoot infections have better outcomes. Hindfoot infections are associated with a 50% risk of amputations proximal to the ankle compared to 18.5% and 0.33% for the midfoot and forefoot respectively[44].
Diabetic foot attack
A diabetic foot attack (DFA) represents a serious and rapidly worsening complication in diabetic foot disease, often leading to limb-threatening conditions. It manifests primarily in two forms: Typical DFAs and atypical DFAs such as ischemic and Charcot neuroarthropathy attacks[45].
A typical DFA presents a significant clinical challenge, often precipitated by a severe infection. This pathologic cascade typically initiates with a minor local infection, which can be attributed to compromised skin integrity. Elevated blood glucose levels create an environment conducive to bacterial proliferation, further exacerbating the risk of infection[45]. As the infection progresses, it may culminate in the formation of an abscess, a localised collection of pus within the tissue, signifying a deep-seated infection. Concurrently, the infection may invade muscular tissues leading to pyomyositis[46].
In its most severe form, this infectious process can escalate to a rapidly spreading necrosis. Two critical manifestations of this are necrotizing fasciitis and myonecrosis. Necrotizing fasciitis is a rapidly progressive and life-threatening infection, that involves the fascial planes and subcutaneous tissues, leading to widespread tissue death[47]. Myonecrosis specifically denotes muscular tissue necrosis, often a consequence of the unchecked spread of bacterial infection[48].
The two forms of atypical DFA vary from the typical form of DFA. The ischemic variant includes severe critical limb ischemia, with or without tissue loss. Unlike the typical form, infection is not the main cause; rather, severe ischemia drives the condition and immediate intervention is crucial to prevent the progression to limb-threatening ischemia[45]. The other, Charcot Neuroarthropathy Attack, presents typically as a hot, swollen foot without ulceration[45].
The most inclusive guidelines identified for Diabetic foot infection are published by the IWGDF[49]. NICE employs urgency on the acute debridement and washout of DFAs, however, there is a lack of guidelines regarding the recommended technique of surgical intervention[50]. This perspective is further corroborated by the joint colleges through their publication, "Operational Delivery of the Multi-Disciplinary Care Pathway for Diabetic Foot," wherein they underscore the imperative nature of immediate infection control in such clinical scenarios[9].
CHARCOT ARTHROPATHY
Charcot arthropathy is a progressive, destructive joint disease affecting the extremities of diabetic patients. It affects 0.1%-0.3% of patients with diabetes and up to 7.5% of patients with ‘diabetes and neuropathy’[51,52]. Historically rare, its prevalence is rising alongside escalating obesity and diabetes.
Charcot arthropathy is an accumulation of two distinct aetiologies, neurotraumatic and neurovascular.
Neurotraumatic: Primarily arises due to repetitive trauma to a joint that is not sensed properly due to underlying neuropathy. Lack of sensation results in minor injuries or stresses to the foot that are not recognised, leading to progressive damage. Clinically, neurotraumatic Charcot arthropathy is characterized by joint dislocation, fractures, and deformities. The body's normal repair processes are unable to properly heal these repeated traumas, resulting in chronic inflammation and further joint destruction[53].
Neurovascular: Involves an abnormal autonomic nervous system response, leading to increased blood flow to the affected area. The increased blood flow can result in bone resorption. This weakens the bone structure and, combined with neuropathy, increases the risk of fractures and joint dislocations[53].
Charcot arthropathy progresses clinically and radiographically through four distinct stages as outlined by Eichenholtz[54]:
Stage 0, the initial stage with localized swelling, redness, increased temperature, and joint instability. Diagnosis relies on clinical findings and is supported by advanced imaging (i.e., bone scans), though radiographs are normal.
Stage I, the acute or developmental phase, features swelling, redness, warmth, and bony fragmentation, often mistaken for soft tissue infections, thus delaying treatment. Elevation of the extremity often reduces the redness, a significant clinical finding.
Stage II, the coalescence phase, sees a decrease in swelling and redness and consolidation of fractured fragments.
Stage III, the reconstruction phase, involves joint ankylosis and bone hypertrophy, leading to deformity, instability, and joint dysfunction[54]. Infections, further complicate Charcot arthropathy diagnosis and management, exacerbated by hypertrophic exostoses and ulcer formation[55].
Charcot neuropathy can be classified into its anatomical position. Sanders and Frykberg identified five distinct categories of this destruction[52].
Type I, affecting the forefoot, accounts for 15% of cases.
Type II, involves the tarsometatarsal joints and represents 40% of cases.
Type III, impacting the naviculocuneiform, talonavicular, and calcaneocuboid joints, constitutes 30%.
Type IV, affects the ankle and/or subtalar joint and is seen in 10% of cases.
Type V, involving the calcaneus, is observed in 5% of cases.
Furthermore, Brodsky's classification highlights the prevalence of disease location[56], with the midfoot being the most common site (60%), followed by the hindfoot (30%-35%), the ankle (9%), and the calcaneus (2%).
GUIDELINES
IWGDF/Infectious Disease Society of America
The "IWGDF/Infectious Disease Society of America (IDSA) Guidelines on the Diagnosis and Treatment of Diabetes-related Foot Infections (IWGDF/IDSA 2023)" offer a comprehensive set of recommendations for healthcare professionals managing foot infections in individuals with diabetes[49]. These guidelines are developed through the Grade of Recommendations Assessment, Development and Evaluation framework[57]. They cover various aspects of diagnosis and treatment, including the classification of infection severity, microbiological sample collection, and antibiotic therapy choices. The guidelines also address the diagnosis of osteomyelitis, recommend diagnostic methodologies, and provide specific treatment recommendations for different infection severities and conditions.
eIWGDF – 2023
The 2023 IWGDF Guidelines provide comprehensive recommendations for healthcare professionals[43]. The IWGDF 2023 guidelines are divided into 7 sections, prevention, classification, management of foot infection in diabetic ulcer patients, management of PAD in diabetic ulcer patients, offloading foot ulcers, interventions to enhance healing in diabetic patients and acute Charcot neuro-osteoarthropathy (CNO) guidance.
NICE
NICE Guidelines recommend regular foot assessments and risk stratification for patients with diabetes, provide guidance on ulcer management, debridement and dressings, and highlight the importance of patient education and self-management[50]. NICE Guidelines recommend tailored local pathways to coordinate multidisciplinary team care, suggest an appropriate duration of referral to foot protection services based on risk, and guide appropriate research of alternative interventions.
ULCER AND OSTEOMYELITIS MANAGEMENT
Ulcer management
The primary goal while managing a DFU is to achieve wound closure. In addition to patient education and attaining diabetic control, proper offloading, otherwise termed “pressure modulation” is critical in managing neuropathic ulcers[58]. Common techniques include podiatry offloading, which includes felted foam/padding, casting techniques such as Total Contact Cast (TCC) or Air cast boot and surgical offloading. Based on IWGDF guidelines, the preferred off-loading treatment is a non-removable knee-high offloading device, such as total contact casting. Several surgical procedures exist which are adjuncts to cast offloading which can aid in ulcer healing. These include Achilles Tendon lengthening, distal metatarsal osteotomy[43], metatarsal head excision or MTPJ excision arthroplasty.
PAD of the lower limb has a significant impact on the healing potential of a foot ulcer. If peripheral pulses are weak or absent, the vascular team should be involved with further investigations including arterial doppler, measurement of ankle and toe pressures and calculating the Ankle Brachial Index (ABI) and Toe Brachial Index (TBI). In the presence of triphasic or biphasic pedal doppler waveforms, ABI 0.9-1.3 and TBI ≥ 0.70, PAD is less likely. However, in case of ankle pressure < 50 mmHg, toe pressure < 30 mmHg, ABI < 0.4 or TcpO2 (transcutaneous pressure of oxygen) < 25 mmHg, urgent vascular imaging, and revascularisation to restore in-line flow may be considered. Despite optimal treatment, if an ulcer does not show signs of healing in 4-6 wk, consider angiography and revascularisation[59].
DFO management
Management of DFO should be a multifaceted approach with medical management guided by microbiology and surgical intervention. Conservative treatment alone shows success rates in the region of 68%, while in combination with surgical intervention the reported success rates vary from 76%-86%[60,61]. However, it is important to employ a patient-centred approach offering surgical intervention to candidates based on individual comorbidity profiles and functional status.
A paucity of evidence exists delineating between oral and intravenous antibiotic administration[62,63]. Current clinical guidelines advocate for a total of 6 wk in conservatively managed cases[64]. In cases where osteomyelitis is surgically excised, a postoperative course of antibiotics, for five days, is recommended. Conversely, if residual osteomyelitis persists post-excision, an extended antibiotic regimen spanning three weeks is indicated[49].
The use of antibiotic-loaded calcium sulphate hydroxyapatite composites is gaining prominence in the surgical management of DFO. This biomaterial is notable for its capacity to integrate seamlessly into the host tissue and be substituted by newly formed bone. Additionally, its structure facilitates ongoing osteointegration. This not only delivers effective antimicrobial activity but also aids in the obliteration of dead spaces, a critical aspect in the treatment of such infections[65].
Surgical management encompasses a range of interventions, from conservative surgery to minor and major amputations. There has been a significant shift in standard diabetic foot surgical practices, evolving from predominantly amputation-focused approaches to more conservative strategies. This change reflects an evolving understanding of the complexities of DFO management and a commitment to preserving patient quality of life.
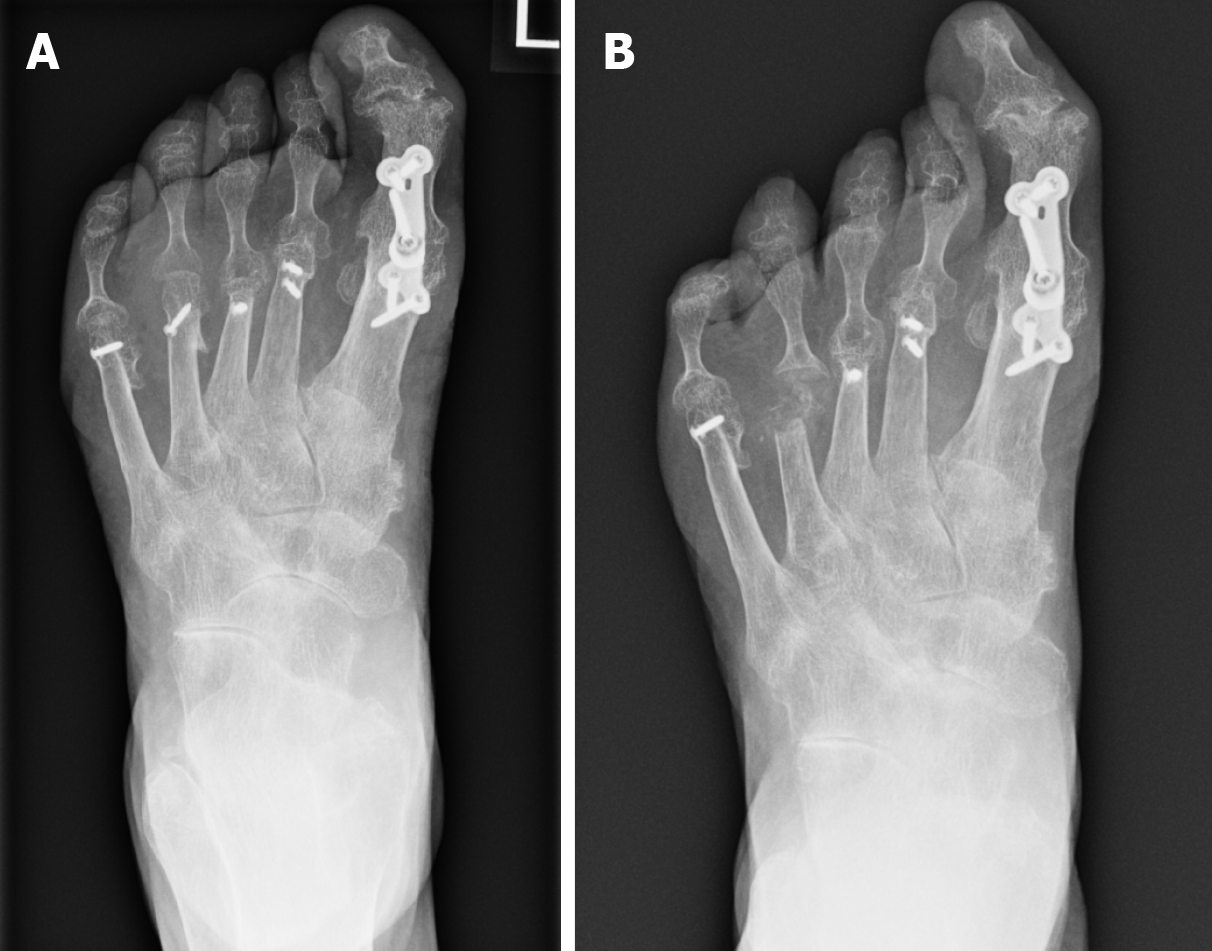
Conservative surgery focuses on the removal of only the infected bone without necessitating amputation (Figure 1). It has been associated with numerous benefits which include reduced rates of both minor and major amputations and a decrease in the frequency of urgent surgical interventions[66]. However, at present there is a paucity of evidence comparing patient focussed outcomes with minor amputation, vs conservative management of osteomyelitis[60,67].
Figure 1 Radiograph demonstrating osteomyelitis affecting the 4th distal metatarsal and base of proximal phalanx.
A: Before conservative surgery; B: Post conservative surgery.
Location of DFO necessitates different conservative surgical techniques. Lesser toe osteomyelitis can be managed by Distal phalangectomy with acceptable outcomes[68]. Distal Hallux ulcer with associated osteomyelitis typically necessitates either partial or complete phalangectomy, or the implementation of the Distal Syme Hallux Amputation technique and partial hallux amputations are predominantly executed via distal phalangectomy[69,70]. Arthroplasty of the interphalangeal joint (IPJ), preferably without fixation, has been identified as a viable intervention for IPJ osteomyelitis[71].
Keller Arthroplasty has emerged as a beneficial intervention for elderly individuals suffering from degenerative hallux valgus litmus with persistent diabetic ulcers[72,73]. This surgical procedure entails excising the first metatarsal joint. Its efficacy is underscored by a high long-term success rate of 95% and a low postoperative complication rate of 4.7%. However, the removal of the metatarsal joint can result in a shorter and less rigid toe[73].
In DFUs located on the plantar surface of the first metatarsal head, sesamoid bones are often implicated. Sesamoidectomy, the surgical excision of one or both sesamoid bones, is contingent upon the extent of the infection. This procedure may be complemented by arthroplasty of the first metatarsal head in cases where the metatarsal head is affected but does not necessitate resection[74]. Recent evidence emphasises the necessity of tailoring the surgical approach to the specific characteristics of the ulcer, aiming to mitigate both immediate and long-term postoperative complications[75]. The resection of the metatarsal head is associated with a re-ulceration rate of 24.7% however the risk of complications is 7%[76].
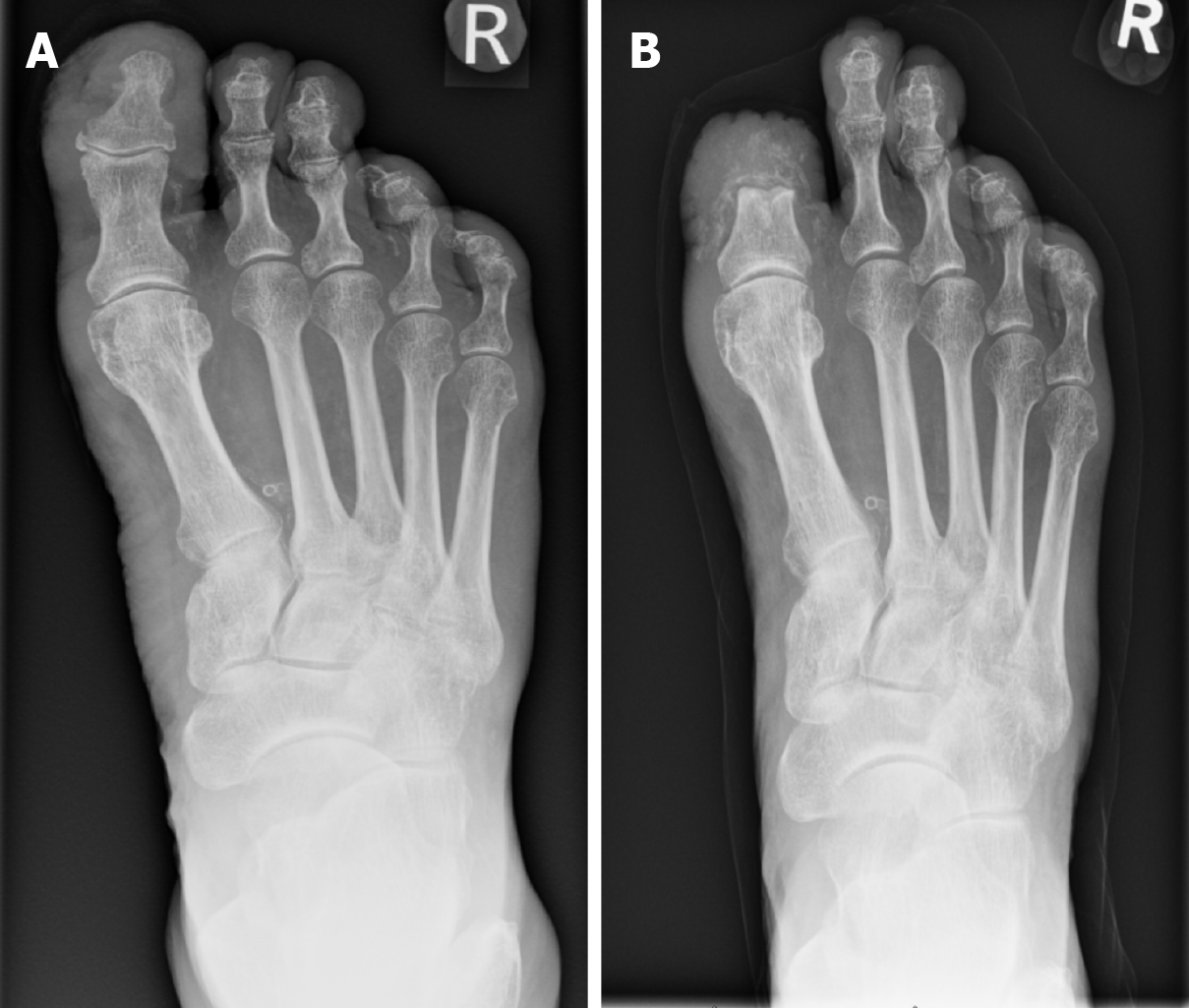
In the case of rapidly progressing high-risk DFO infection, amputation may be necessary to avoid worse patient outcomes. High-risk indications include peripheral arterial occlusion, refractive ulcers, extensive soft tissue infections and gangrene[77]. Notably, PAD plays a pivotal role, with 84.1% of amputations attributed to DFO being a direct consequence of this condition[78]. In defining major amputation, it is widely accepted as amputations occurring above the Syme level. Debate exists within the literature as to the nomenclature surrounding “minor amputation”. It varies between being seen as any amputation from the ankle to forefoot, to the more conservative approach of resecting infected bone while sparing healthy tissue (Figure 2)[79].
Figure 2 Radiograph demonstrating osteomyelitis affecting the right hallux distal phalanx and proximal phalanx.
A: Before minor amputation; B: Post minor amputation.
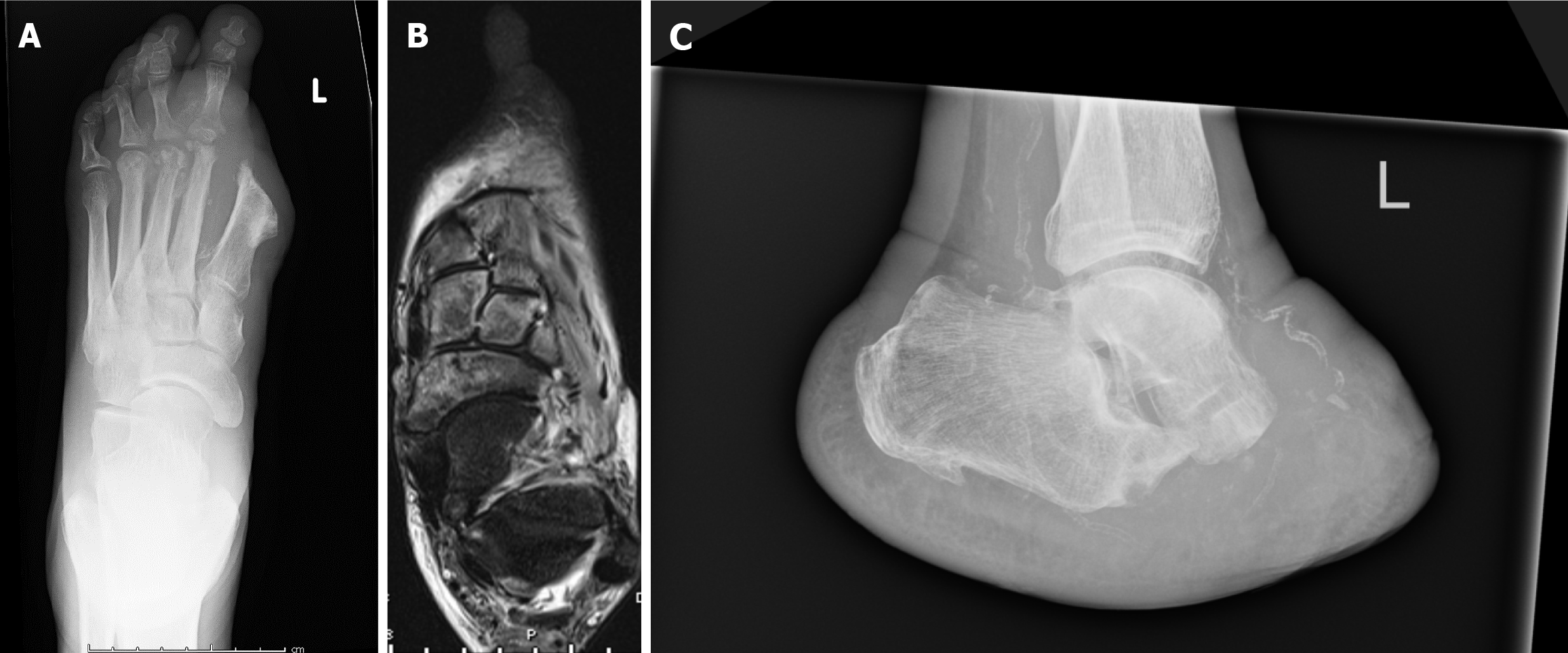
Decisions regarding level of amputation required have two facets for consideration; the aim to perform amputation as distally as possible, and the other, there is the aim to avoid further amputations as this can lead to a decline in the patient's health status, functional abilities, and psychological well-being. Readmission rates following minor amputation for further debridement or amputation within 6 months can be as high 54.3% (Figure 3). Furthermore, minor amputations have a higher failure rate and result in readmission for a major amputation in 4.5% of cases[78].
Figure 3 Imaging demonstrating recurrent osteomyelitis post-minor amputation (left hallux amputation).
A: Radiograph demonstrating the involvement of the 2nd and 3rd metatarsals; B: An MRI Scan showing involvement to just before the talo-navicular and calcaneo-cuboid joints; C: A radiograph demonstrating result of a Choparts's Amputation.
Evidence increasingly supports conservative surgical osteomyelitis management in diabetic feet, yet comparative studies on surgical approaches and long-term outcomes remain scarce. Minor interventions preserve health-related quality of life, contrasting major amputations' detrimental impact[80].
DIABETIC FOOT ATTACK MANAGEMENT
In patients experiencing a typical diabetic foot episode, the pervasive issue is a severe infection. Consequently, initial management should prioritise rapid infection control. This encompasses immediate surgical intervention, thorough debridement of all infected and necrotic tissues, and the exploration of any tracking paths, concomitant with the initial administration of broad-spectrum antibiotics. Following this, antibiotic therapy should be refined based on culture results. Even in the presence of severe ischemia, infection control procedures, including surgery, should not be postponed for further vascular assessments. Concurrent management should include metabolic and glucose regulation, as well as the treatment of any co-occurring medical issues, such as acute kidney injury, with specialist assistance. Once the initial infection is under control, it is essential to evaluate vascular status, if not already done. In cases of compromised vascular health, prompt revascularization is imperative. Continued necrosis may necessitate additional exploratory surgeries. Post-infection control, the focus shifts to wound stabilization, achievable through advanced wound care techniques like negative pressure wound therapy, alongside ongoing adjustments to antibiotic therapy based on culture findings. Importantly, daily, consistent multidisciplinary involvement at the patient's bedside is critical for optimal care[81].
Vas et al[45] produced guidelines for typical DFAs which were divided into three categories phases[45].
Phase 1 – Hospital admission with immediate surgical consultation. Follow local Sepsis protocols and arrange appropriate imaging. Arterial Duplex if possible, however, this should not delay progression to phase 2.
Phase 2 – Identify the proximal aspect of infection spread and radical debridement of all infected tissue down the healthy tissue. Pro-active planning to be conducted, such as a surgical re-look within 48 h.
Phase 3 - Avoid weight bearing on the affected limb and daily bedside assessments. Revascularization assessment and management as a priority. Targeted antibiotics and medical optimisation. Skin grafting when appropriate.
Following these three steps has been shown to equate to better patient outcomes and effective management.
The ‘Red-Amber-Green’ (RAG) model is a structured approach for debriding osteomyelitis and diabetic foot infections[82]. This model identifies the core of the infected ulcer, containing necrotic tissue, as the 'red zone'. Encircling this area is the 'amber zone', comprising relatively avascular and fibrous tissues, often harbouring infection. Beyond this lies the 'green zone', representing normal, healthy tissue. Complete excision of tissues in the red and amber zones, extending to the unaffected green zone, is critical. Implementing this protocol can decrease hospital stay and reduce the need for further surgical debridements[82].
In managing typical diabetic foot episodes, prioritizing infection control through immediate surgical intervention, comprehensive debridement, and broad-spectrum antibiotics is crucial for effective outcomes. The integration of a phased approach and the RAG model further refines this process, emphasizing early, aggressive management and multidisciplinary care to enhance patient recovery and reduce complications.
CHARCOT MANAGEMENT
Diagnosing Charcot foot involves clinical history, physical and radiological examinations, and identifying fractures or dislocations without bone infection. The goals of treatment are; to maintain/obtain a ‘shoeable’, plantigrade and non-ulcerated foot come the end of treatment, irrespective of surgical or conservative management[83,84].
Principles of conservative management
Initially, evidence directs the use of knee-high, non-removable immobilisation in any diabetic patient as soon as CNO is suspected and while investigation is ongoing[85,86].
Off-loading and immobilisation, until the deformity settles (for 4-6 months or longer) with vigilant monitoring are recommended. The IWGDF guidelines highlight the importance of multiple factors in evaluating remission, noting that oedema alone is not a reliable indicator. Studies suggest that ending immobilization based on foot temperature may lower recurrence rates, though radiological assessments indicate longer resolution times[87]. Prefabricated knee-high walkers provide effective off-loading, similar to TCC and non-removable walkers, by accommodating foot shape, redistributing pressures, and minimizing mechanical stress, thus preventing further inflammatory disease progression and deformities. Removable devices are preferred when frequent skin inspections are necessary.
Principles of surgical management
Acute reconstruction in active CNO has been traditionally discouraged due to the pro-inflammatory environment and complex patient comorbidities, leading to higher complication rates. Conservative management aiming for a stable, non-ulcerated foot is preferred[88]. However, surgery may be required in cases of severe deformity, instability, persistent pain, or when conservative measures fail to immobilize the foot[89,90]. Early surgical intervention, before ulceration and structural collapse, tends to yield better outcomes[84,91]. Surgery focuses on off-loading the foot internally, correcting deformities, preserving foot length, and stabilizing the foot through various procedures like exostectomy, tendon lengthening, osteotomy, and arthrodesis, tailored to the deformity and instability level. Dealing with proximal hindfoot and ankle deformities presents additional challenges due to the difficulty in off-loading these areas with conventional devices.
Tendoachilles lengthening
Equinus deformity has a prevalence of 10.3%-37% among diabetics[92,93]. This deformity produces higher plantar pressures, altering forefoot loading patterns and potentially resulting in ulceration[92]. Hindfoot equinus produces moments that directly oppose those of the midfoot during loading, and this incongruency of biomechanics contributes to midfoot (medial column) break and in turn a rocker-bottom deformity[94,95].
Correcting equinus deformity, often involving percutaneous Tendoachilles lengthening (TAL) and TCC, has been shown to reduce pressure, aid ulcer repair, and slow the Charcot process[96]. Lower ulcer recurrence rates have been associated with the TAL group[97-100].
Exostectomy
Exostectomy relieves the pressure on the skin to avert the development of ulcers. Healing rates for this procedure are better for medial sided exostoses, with healing rates between 75% and 94.4% when addressing exostosis-related ulcers located on the medial plantar midfoot, compared to approximately 33% for exostosis-related ulcers on the lateral plantar midfoot[101-103]. It is a limited procedure, particularly effective for patients with severe co-morbidities and poor soft tissue envelope. When associated with an equinus ankle contracture, this should be combined with an Achilles tendon lengthening procedure. One of the main risk of exostectomy is progression of deformity or iatrogenic destabilisation.
Charcot foot reconstruction
Charcot foot reconstruction represents a diverse array of procedures and their combinations, involving the mid and hindfoot and the ankle. Evidence suggests surgical reconstruction of Charcot foot is becoming more frequently used and is superior in terms of outcomes[104]. In a comparative analysis of Charcot reconstruction modalities, the complication rates were reported at 36% for reconstruction and 26% for external fixation. The resumption of weight-bearing activities was achieved by 91% of subjects within an average duration of 16.5 wk. Fusion rates were notably high at an aggregate of 86%, with external fixation achieving 94%, internal fixation at 81%, and a combined internal-external technique culminating at a 95% success rate[104].
Super construct techniques
‘Super construct’ refers to a long fixation that extends beyond the area affected by active CNO or damage[105]. As a substantial part of the interface between device and bone is in non-CNO areas, this improves fixation and is recommended in CNO surgery. Based on current guidelines[105], a super construct should extend fusion beyond the injury to encompass healthy joints, shorten the limb with bone resection to correct deformity and decrease soft tissue strain, select the most robust device the soft tissue can support, and position hardware to enhance mechanical efficiency.
Midfoot techniques
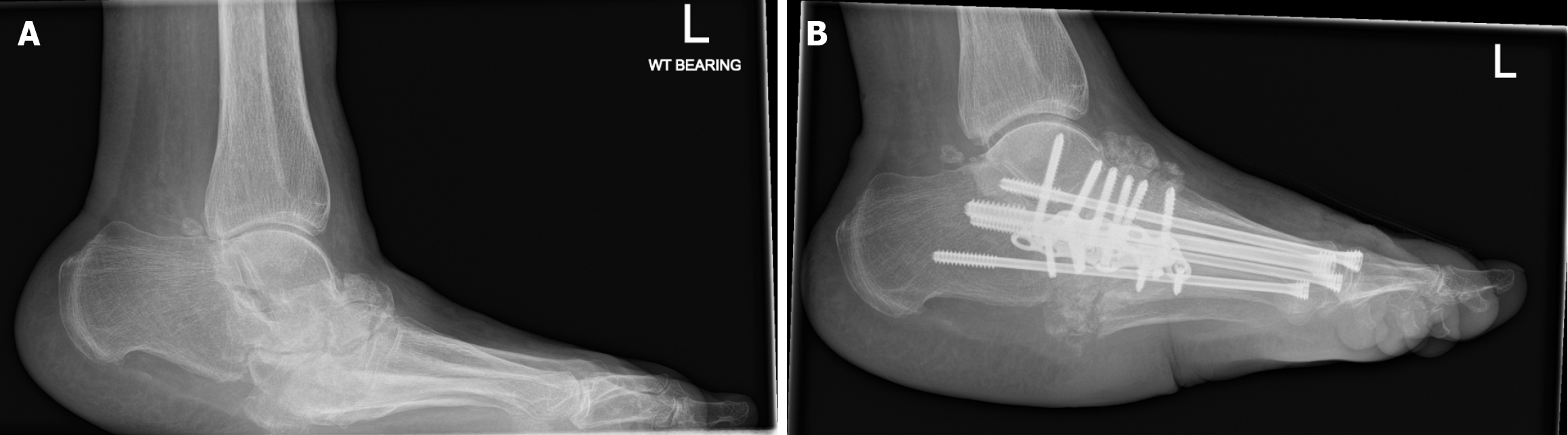
The most common area for surgical reconstruction in CNO is the midfoot, specifically the tarsometatarsal region (27% to 60%) (Figure 4)[106].
Figure 4 A radiograph demonstrating left midfoot charcot arthropathy.
A: Before Charcot reconstruction surgery; B: Post Charcot reconstruction surgery.
A "rocker-bottom" deformity, characterized by a collapsed plantar arch and potential ulceration, can result from midfoot break and collapse[107]. Restoring the plantar arch involves either internal fixation, external fixation, or a combination of both. Internal fixation is typically executed through intramedullary beaming or plantar plating, targeting arthrodesis. Beaming employs large-diameter screws inserted axially into the medullary canal of the affected bones, extending into the midtarsal region. The most common approach is medial column beaming, where a screw extends from the first metatarsal head through adjacent bones to create a supportive structure. Lateral column beaming involves the fourth and fifth metatarsal bases, cuboid, and calcaneus. Evidence suggests that using both columns for beaming can reduce instability and prevent premature loosening that might occur with only medial column fixation[108,109].
The alternative is plantar or dorsal-medial plating. Beam and plate techniques of medial column reconstruction have been associated with comparable construct stiffness and cycles to failure. However, beamed constructs could withstand a significantly higher load before failure[106]. Beaming offers load-sharing in the midfoot, diminishing stress at the union site, compressing segments for fusion, enabling minimally invasive fusion, and mitigating axial deformity and cantilever bending risks[110].
A concern with beaming is reduced correction and control in the coronal plane. Plastic deformation of the implant, particularly at the shaft-screw thread junction[111], is the most common reason for intramedullary beam failure, particularly at the points where screws traversed the choparts and talonavicular joints[106]. In the context of midfoot fixation, the evidence fails to denote the superiority of beams, plates, or external methods. Intramedullary beaming and plating is favourable for CNO with no infection and healthy soft tissues, while external fixation suits cases with infection or requiring soft tissue surveillance[112].
An advantage of external fixation is that it offers triplane stability and deformity control at multiple levels[113]. Modern Hexapod systems can be used to plan and execute deformity correction, and post-correction residuals can be used to increase the accuracy of correction. However, pin site infection is a problem and is associated with complications in 18% of patients[113]. The other disadvantages include lengthy treatment regiments, and a steep surgical learning curve requiring significant expertise[109]. There are therefore advocates for a combined or staged minimally invasive technique of initial deformity correction with an external fixator, followed by arthrodesis with percutaneous internal fixation[114].
Ankle techniques
Charcot neuroarthropathy at the hindfoot and ankle level is associated with more challenging complications compared to the midfoot. Rates of infection are higher and the rate of successful correction and fusion decrease when correcting proximal to the talonavicular joint[115,116]. It is accepted that hindfoot and ankle Charcot is less amenable to bracing[117].
In the domain of Charcot reconstruction pertaining to the hindfoot, arthrodesis stands as the cornerstone technique and most commonly involves tibiotalocalcaneal or tibiocalcaneal fusion[118]. Intramedullary nail (IMN), internal fixation or external fixation can be employed in isolation or combination. Biomechanical advantages of IMN include load sharing to allow earlier mobilisation and better stability and load distribution in axial compression and torsion than external fixation[119]. This in turn can aid fusion[119,120].
IMN fixation results in solid fusion in 83.1% of cases and stable fibrous unions in 7.1%[121]. However, there is a moderate infection rate of 45.85%. When external fixation is used for reconstruction, anatomical reduction is achieved in 71.64% of patients, and fibrous union occurs in 14.92% of cases[121]. However, this method comes with challenges such as long treatment durations, a 17.84% pin-tract infection rate, and significant surgical expertise[121,122].
Ankle arthrodesis is associated with a 53% solid fusion rate, with 3 screws being more effective than 2[123]. Stable fibrous unions is seen in 29.4% of cases and a 23.5% rate of superficial wound infections. Crossed cannulated or solid screw techniques were associated with a 53% solid fusion rate, with 18% developing unstable pseudoarthrosis, leading to amputation[123]. Blade plate techniques have been associated with a 100% limb salvage but higher infection rates of 60%[124,125].
Charcot foot management, emphasises early diagnosis through clinical, radiological, and physical assessments, aims for a stabilised, plantigrade, and ulcer-free foot. Conservative strategies prioritize immobilization with knee-high, non-removable devices to off-load and protect the foot, with remission being guided by thermographic and radiological evaluations. Surgical interventions, reserved for severe cases, focus on deformity correction, internal off-loading, and maintaining foot structure through various reconstructive techniques, considering patient-specific complexities. The multifaceted approach, integrating conservative and surgical methods, underscores the necessity of personalised treatment plans to optimise patient outcomes and minimise recurrence.
CONCLUSION
The surgical management of the diabetic foot requires a multifaceted approach that integrates timely diagnosis, comprehensive patient education, and individualized treatment strategies to address the complex interplay of neuropathy, ischemia, and infection inherent in diabetic foot pathology. The evidence underscores the importance of an interdisciplinary team comprising endocrinologists, podiatrists, vascular surgeons, orthopaedic surgeons, and infectious disease specialists to optimize outcomes. Advances in surgical techniques, from conservative bone resections to complex reconstructions, offer hope for limb salvage and improved quality of life for patients with diabetic foot complications. However, the paramount importance of preventive care, rigorous glycemic control, and patient education cannot be overstated, as these measures can significantly reduce the incidence and severity of diabetic foot complications. Future research should focus on refining surgical techniques, improving wound care strategies, and developing innovative technologies for early diagnosis and treatment to further enhance patient outcomes in the management of diabetic foot. The collaborative efforts of the multidisciplinary team, combined with ongoing advancements in medical science, hold the key to reducing the morbidity and mortality associated with diabetic foot complications, ultimately leading to a significant improvement in the quality of life for individuals affected by this challenging condition.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: Https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country of origin: United Kingdom
Peer-review report’s classification
Scientific Quality: Grade A, Grade B
Novelty: Grade A, Grade B
Creativity or Innovation: Grade A, Grade B
Scientific Significance: Grade A, Grade B
P-Reviewer: M Amin KF, Iraq; Papotto G, Italy S-Editor: Li L L-Editor: A P-Editor: Zhao YQ