Published online Mar 18, 2024. doi: 10.5312/wjo.v15.i3.266
Peer-review started: December 4, 2023
First decision: December 17, 2023
Revised: December 21, 2023
Accepted: January 23, 2024
Article in press: January 23, 2024
Published online: March 18, 2024
Processing time: 102 Days and 1.1 Hours
Multitudinous advancements have been made to the traditional microfracture (MFx) technique, which have involved delivery of various acellular 2nd generation MFx and cellular MFx-III components to the area of cartilage defect. The relative benefits and pitfalls of these diverse modifications of MFx technique are still not widely understood.
To comparatively analyze the functional, radiological, and histological outcomes, and complications of various generations of MFx available for the treatment of cartilage defects.
A systematic review was performed using PubMed, EMBASE, Web of Science, Cochrane, and Scopus. Patients of any age and sex with cartilage defects under
Forty-four RCTs were included in the analysis with patients of mean age of 39.40 (± 9.46) years. Upon comparing the results of the other generations with MFX-I as a constant comparator, we noted a trend towards better pain control and functional outcome (KOOS, IKDC, and Cincinnati scores) at the end of 1-, 2-, and 5-year time points with MFx-III, although the differences were not statistically significant (P > 0.05). We also noted statistically significant Magnetic resonance observation of cartilage repair tissue score in the higher generations of microfracture (weighted mean difference: 17.44, 95% confidence interval: 0.72, 34.16, P = 0.025; without significant heterogeneity) at 1 year. However, the difference was not maintained at 2 years. There was a trend towards better defect filling on MRI with the second and third generation MFx, although the difference was not statistically significant (P > 0.05).
The higher generations of traditional MFx technique utilizing acellular and cellular components to augment its potential in the management of cartilage defects has shown only marginal improvement in the clinical and radiological outcomes.
Core Tip: Chondral lesions have been reported in 60% of patients undergoing arthroscopic procedures of the knee; and such defects are described as one of the leading causes of chronic knee pain. As compared with the other cartilage restoration strategies, microfracture (MFx) is relatively cost-effective, simple, minimally-invasive and may also be performed in a single stage. Nevertheless, recent studies have demonstrated that modifications of the traditional MFx technique, such as the use of synthetic and autologous biological adjuvants may enhance the repair tissue quality, resilience, and overall efficacy of the procedure. Based on the current network meta-analysis we could conclude that the use of acellular and cellular adjuvants has shown only marginal improvement in the clinical (pain and functional scores) and radiological outcome in patients undergoing microfracture for cartilage defects of the knee. The safety and efficacy of the higher generation MFx procedures are also clearly evident from our review. However, there is a substantial potential for further improvement in the cellular components (chondrocytes over other cellular lineage), culture or processing methodology, delivery modalities (including appropriate scaffolds); as well as better surgical techniques to achieve demonstrable significant outcome improvement.
- Citation: Muthu S, Viswanathan VK, Sakthivel M, Thabrez M. Does progress in microfracture techniques necessarily translate into clinical effectiveness? World J Orthop 2024; 15(3): 266-284
- URL: https://www.wjgnet.com/2218-5836/full/v15/i3/266.htm
- DOI: https://dx.doi.org/10.5312/wjo.v15.i3.266
Lesions of the articular cartilage of the knee remain a challenging clinical entity in view of the limited capacity of the cartilaginous tissues to heal and potential progression to chronic degenerative arthritis[1]. The deficient endogenous cartilage repair mechanism has been attributed to the poor recruitment of regenerative cells into the area of cartilage defect[2]. Based upon the theory of marrow stimulation by subchondral drilling[1], Steadman et al[3] popularized the concept of microfracture (MFx) technique, whereby the migration of the growth factors and mesenchymal stem cells (MSCs) across the subchondral bone stimulates the development of the hyaline-like fibrocartilage. As compared with the other cartilage restoration strategies, MFx is relatively cost-effective, simple, minimally-invasive and may also be performed in a single stage[4]. Despite still being regarded as the gold-standard first-line treatment for cartilage deficiencies of the knee, there are concerns regarding their long-term outcomes and durability of the restored fibrocartilage[5,6]. In this context, alternate cartilage restoration procedures such as autologous chondrocyte implantation (ACI), osteoarticular transfer system and osteochondral allograft transplantation have been advocated as the better treatment strategies in the recent years. In fact, the United Kingdom National Institute for Health and Care Excellence, in a recent assessment, has recommended for the abandonment of MFx in favor of ACI in the management of articular knee defects[7-11].
Nevertheless, recent studies have demonstrated that modifications of the traditional MFx technique, such as the use of synthetic and autologous biological adjuvants may enhance the repair tissue quality, resilience and overall efficacy of the procedure[7,11]. Some researchers have purported that the suboptimal efficacy of the traditional marrow stimulating techniques may be attributed to the insufficient concentrations of MSCs and growth factors getting released from subchondral marrow. To circumvent this limitation, it has been proposed that supplementation of MFx with intra-articular adjuvants in the form of platelet-rich plasma (PRP) or hyaluronic acid (HA) can improve the outcome[12-18]. In addition, augmentation of defect with scaffolding matrix or cell-free polymer-based implant can provide a bioreactor-like structure, over which the marrow elements get trapped, concentrated and thereby, facilitate the restoration of an effective cartilage layer[19-21]. MFx has also been combined with diverse cellular additives like bone marrow aspiration concentrates (BMAC), MSCs, and peripheral blood stem cells (PBSCs). While individual studies on these biological augmentation [popularly described as “microfracture plus” (MFx+)] techniques have demonstrated encouraging histological and clinical outcomes, our understanding regarding these techniques has been limited by substantial heterogeneity among the study cohorts and paucity of high quality, prospective trials.
The purpose of our study was to consolidate the available evidence; compare the clinical, functional and radiological outcomes of three different generations of MFx techniques (traditional MFx, MFx + acellular additives, and MFx + cellular additives); and to provide the best recommendations on their relative efficacies, advantages, complications and pitfalls in the management of cartilaginous defects of the knee joint.
PROSPERO (International prospective register of systematic reviews) registration (CRD42022338329) was obtained for the study. Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) for Network Meta-analysis (NMA) guidelines[22] were followed for the conduction and reporting of the study.
PubMed, EMBASE, Medline, Cochrane, and Scopus electronic databases were used for literature search. The search was performed by three reviewers independently. The search strategy was built using the MeSH terms and corresponding keywords for knee cartilage defects and their different treatment methods with related complications, employing different boolean operators, as required. The model search strategy is described in Supplementary Table 1 following the PRESS guidelines[23].
The following PICOTS criteria were used for the inclusion of studies: (1) Population: Patients with cartilage defects; (2) Intervention: Treatment methods including various generations of MFx technique; (3) Comparator: Placebo or one of the alternate aforementioned treatment methods; (4) Outcome: Functional, radiological, histological outcome, or complications; (5) Time frame: Inception to 2022; and (6) Study type: Randomized controlled trials (RCTs).
Prospective non-randomized studies, retrospective studies, studies without comparator groups, and pre-clinical or animal model studies were excluded. Disagreements on decisions during the article selection were resolved through discussions among the authors. De-duplication of the articles screened from electronic databases was done using citation manager-Zotero. References of the articles included for the study were screened manually to identify the studies missed during the primary search.
Cochrane Consumers and Communication Group recommendations were followed for data extraction from the included studies. The following were extracted, and a master chart was prepared: (1) Study characteristics: Author name, country, publication year, number of patients in the study; (2) Baseline characteristics: Age for the individual treatment arms, gender proportions, cartilage defect size, interventions analyzed, and duration of follow-up; (3) Functional outcomes: Visual Analog Scale (VAS) score for pain, Western Ontario McMaster Universities Osteoarthritis Index score, Tegner score, Lysholm score, International Knee Documentation Committee (IKDC) score, Cincinnati score, and Knee Osteoarthritis Outcome Scale (KOOS) score; (4) Radiological outcomes: Magnetic resonance observation of cartilage repair tissue (MOCART) score, and successful magnetic resonance imaging (MRI)-based defect filling (≥ 2/3rd of the defect); and (5) Complications: Adverse events and failures (patient requiring revision surgeries).
Data extraction was performed independently by two reviewers. The different generations of MFx techniques, described in accordance with the ORG classification, include: First-generation MFx (MFX-I) representing the traditional MFx technique; second-generation MFx (MFX-II) involving MFX-I combined with acellular additives [such as PRP, HA, collagen, and procedures such as autologous matrix-induced chondrogenesis (AMIC)]; and third-generation MFx (MFX-III) involves combining MFX-I with cellular additives such as MSCs, BMAC, PBSCs, and stromal vascular fraction (SVF)[24].
We anticipated heterogeneity among the diverse studies in the duration of follow-up for the analysis of outcome measures. Therefore, we analyzed individual outcomes at short-term (1 years and 2 years), intermediate-term (5 years), and if available long-term (≥ 10 years), based on the available data at individual time points for the outcome concerned. The risk of bias of included studies was analyzed RoB2 tool from Cochrane group[25]. It was agreed upon that studies with a high risk of bias would be excluded from the study.
Relative effects of various treatment methods used in the management of cartilage defects were compared using NMA. Any bias in the outcome reporting of pairwise meta-analyses was reduced by employing multi-variate meta-analytic strategy[26]. Stata (16.1, Stata Corp LLC) was employed for the analysis. The outcomes, adjusted for the number of studies and number of subjects involved in the individual arms, were plotted into a network map. The difference between the direct effect (obtained by head-to-head comparisons) and the indirect effect estimates for the outcomes was used to assess the global inconsistency in the network. If a treatment belonged to a closed loop of evidence in the network (with both direct and indirect effects available), their difference was calculated along with their 95% confidence intervals (95%CI) and P values. The P values estimated the likelihood of conflict to be attributable to chance. A P ≤ 0.05 was considered to be suggestive of inconsistency; and the inconsistency model of NMA was utilized. The inconsistency was further explored with sensitivity analysis using the network side-split method[27]. If P > 0.05, a consistency model of NMA was employed.
Forest plot, using the pooled log odds ratio (OR) or weighted mean difference (WMD), was constructed for reporting the events and continuous outcomes (along with their 95%CI) for the individual arms in the network in order to demonstrate their effect on the outcome analysed (as compared to a constant comparator). We also described an individual pairwise comparison within the network. Random effects model of analysis using the common variance approach was employed in view of the heterogenicity in involved treatment arms[28]. Funnel plot for the outcomes in the included studies was employed for assessing the publication bias. CINeMA approach[29] using CINeMA app[30] was employed to analyse the confidence of the evidence generated.
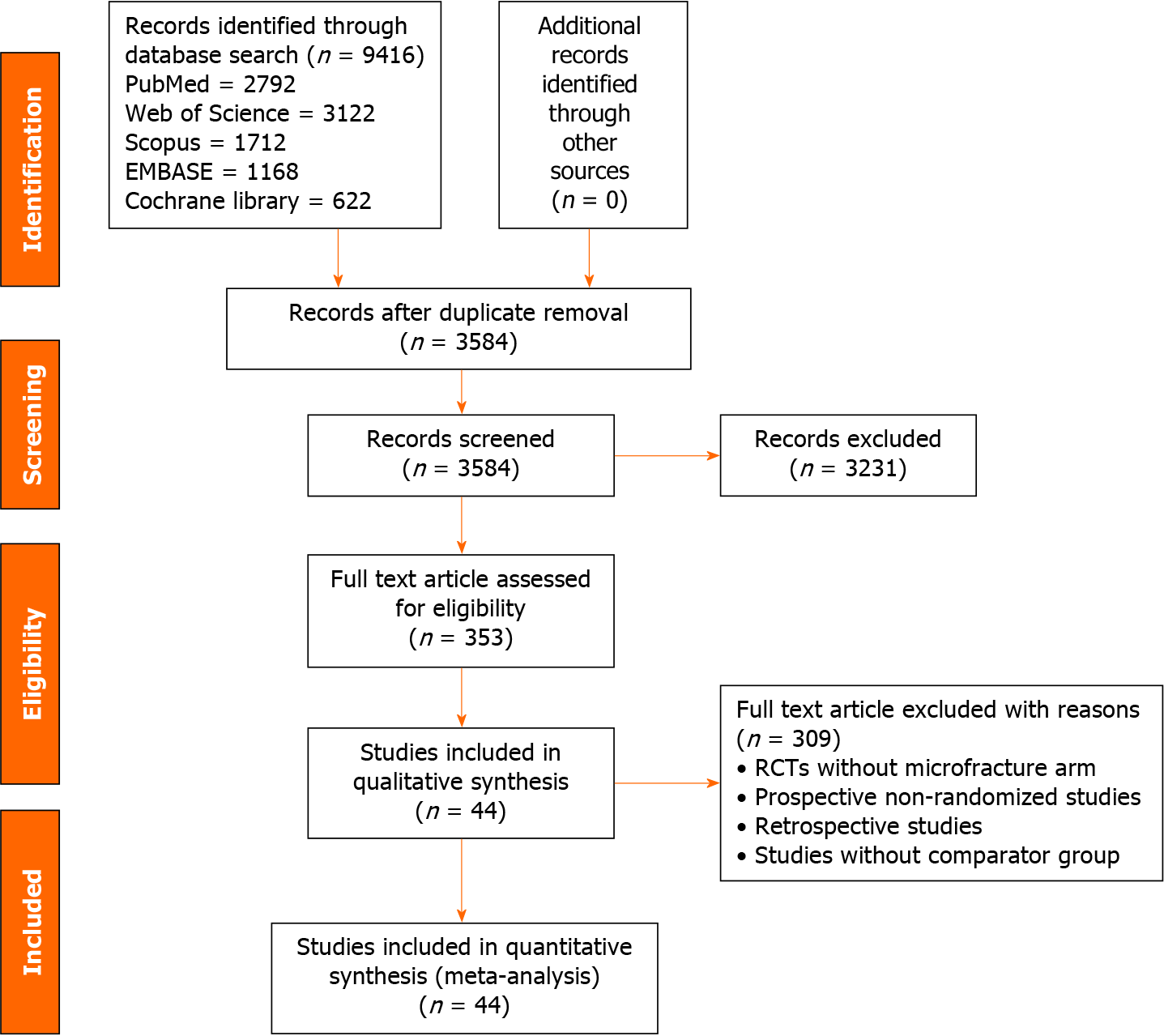
Overall, 9416 articles were shortlisted for initial screening. De-duplication resulted in 3584 articles. Title and abstract screening excluded 3231 articles. Among them, 353 articles qualified for full-text review; and 44 eligible RCTs[4,9,13,15,19,20,31-68] with 2629 included patients qualified for inclusion in the study. PRISMA flow diagram for the inclusion of studies is shown in Figure 1.
The included studies reported at least one of the generations of MFx employed in cartilage defect management. The baseline characteristics of the studies included in the network are presented in Table 1. Norway (n = 6), Germany (n = 5), and United States (n = 5) were the leading countries reporting the highest number of RCTs in the field. The network plot has been presented in Figure 1. The network had 36 possible pair-wise comparisons, among which, 14 had direct evidence data. The network had 42 two-armed studies and 2 multi-armed studies. We did not find significant variability among the characteristics of the included patients in the network concerning age and gender proportions. The mean age of the patients included in the trials was 39.40 (± 9.46) years. The mean follow-up in the included trials ranged between 1 and 15 years.
| Ref. | Country | Study design | Sample size | Treatment | Mean age | Female | Mean defect size | Follow-up (months) | |||||
| Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | ||||
| Volz et al[31], 2017 | Germany | RCT | 34 | 13 | AMIC | Microfracture | 40.0 | 36.5 | 7 | 3 | 3.9 | 2.9 | 60 |
| Niemeyer et al[32], 2019 | Germany | RCT | 52 | 50 | MACI | Microfracture | 36.0 | 37.0 | 19 | 22 | 2.7 | 2.4 | 24 |
| Fossum et al[33], 2019 | Norway | RCT | 21 | 20 | ACI-C | AMIC | 37.2 | 38.3 | 7 | 12 | 4.9 | 5.2 | 24 |
| Ulstein et al[34], 2014 | Norway | RCT | 11 | 14 | Microfracture | AOT | 31.7 | 32.7 | 11 | 9 | 2.6 | 3.0 | 120 |
| Visna et al[35], 2004 | Czech Republic | RCT | 25 | 25 | Autologous chondrograft transplantation | Microfracture | 29.4 | 32.2 | 7 | 9 | 4 | 3.3 | 12 |
| Assche et al[36], 2010 | Belgium | RCT | 33 | 34 | ACI-P | Microfracture | 34.0 | 34.0 | 11 | 10 | 2.5 | 2.3 | 24 |
| Saw et al[37], 2013 | United States | RCT | 24 | 25 | Microfracture with HA | Microfracture with PBSC | 42.0 | 38.0 | 17 | 15 | NA | NA | 18 |
| Anders et al[38], 2013 | Germany | RCT | 22 | 8 | AMIC | Microfracture | 41.0 | 38.0 | 17 | 15 | 3.7 | 3.5 | 24 |
| Lee et al[15], 2013 | Republic of Korea | RCT | 25 | 24 | Microfracture | Microfracture with PRP | 46.0 | 46.0 | 10 | 10 | 3.0 | 3.0 | 24 |
| Brittberg et al[39], 2018 | Sweden | RCT | 65 | 63 | MACI | Microfracture | 38.0 | 34.0 | 23 | 20 | 5.1 | 4.9 | 60 |
| Lim et al[40], 2012 | South Korea | RCT | 30 | 22 | Microfracture | AOT | 32.9 | 30.4 | 12 | 10 | 2.7 | 2.7 | 60 |
| 18 | ACI-P | 25.1 | 8 | 2.8 | 60 | ||||||||
| Knutsen et al[41], 2007 | Norway | RCT | 40 | 40 | ACI-P | Microfracture | 33.3 | 31.1 | 5.0 | 5.0 | 60 | ||
| Knutsen et al[42], 2016 | Norway | RCT | 40 | 40 | ACI-P | Microfracture | 33.3 | 31.1 | 5.0 | 5.0 | 180 | ||
| Liu et al[43], 2021 | Taiwan | RCT | 10 | 5 | Kartigen | Microfracture | 54.8 | 67.8 | 5 | 3 | 2.9 | 1.0 | 24 |
| Yoon et al[44], 2020 | Republic of Korea | RCT | 20 | 10 | ACI-CCP | Microfracture | 41.5 | 47.2 | 6 | 7 | 3.5 | 2.5 | 12 |
| Kon et al[45], 2018 | Italy | RCT | 51 | 49 | Collagen HA | Microfracture | 34.0 | 35.2 | 15 | 18 | 3.4 | 3.4 | 24 |
| Vanlauwe et al[46], 2011 | Belgium | RCT | 51 | 61 | ACI-P | Microfracture | 33.9 | 33.9 | 22 | 20 | 2.6 | 2.4 | 60 |
| Stanish et al[20], 2013 | Canada | RCT | 41 | 39 | Microfracture with BST-CarGel | Microfracture | 35.1 | 37.2 | 18 | 14 | NA | NA | 12 |
| Basad et al[47], 2010 | Germany | RCT | 40 | 20 | MACI | Microfracture | 33.0 | 37.5 | 15 | 3 | 7.0 | 7.0 | 24 |
| Solheim et al[48], 2018 | Norway | RCT | 20 | 20 | Microfracture | Mosaicplasty | 35.0 | 31.0 | 6 | 6 | 4.0 | 4.0 | 180 |
| Bisicchia et al[49], 2020 | Italy | RCT | 20 | 20 | Microfracture with SVF | Microfracture | 49.8 | 46.1 | 8 | 7 | 3.2 | 3.1 | 12 |
| Saris et al[50], 2014 | Netherlands | RCT | 72 | 72 | MACI | Microfracture | 34.8 | 32.9 | 27 | 24 | 4.9 | 4.7 | 24 |
| Saris et al[51], 2008 | Netherlands | RCT | 57 | 61 | ACI-P | Microfracture | 33.9 | 33.9 | 22 | 20 | 2.6 | 2.4 | 12 |
| Saris et al[9], 2009 | Netherlands | RCT | 57 | 61 | ACI-P | Microfracture | 33.9 | 33.9 | 22 | 20 | 2.6 | 2.4 | 36 |
| Qiao et al[52], 2020 | China | RCT | 10 | 10 | Microfracture | Microfracture with HA | 62.3 | 59.7 | 7 | 5 | 4.0 | 4.0 | 12 |
| 10 | Microfracture with MSC | 62.0 | 7 | 4.0 | 12 | ||||||||
| Nguyen et al[53], 2017 | Vietnam | RCT | 15 | 15 | Microfracture with SVF | Microfracture | 58.6 | 58.2 | 12 | 12 | NA | NA | 18 |
| Lim et al[54], 2021 | Republic of Korea | RCT | 43 | 46 | Microfracture with MSC | Microfracture | 55.3 | 54.4 | 28 | 30 | 4.9 | 4.0 | 60 |
| Venosa et al[55], 2022 | Italy | RCT | 19 | 19 | Microfracture with PRP | Microfracture with MSC | 56.4 | 55.8 | 7 | 10 | 1.0 | 1.0 | 12 |
| Shive et al[19], 2015 | Canada | RCT | 34 | 26 | Microfracture with BST-CarGel | Microfracture | 34.3 | 40.1 | 12 | 12 | 2.4 | 2.0 | 60 |
| Koh et al[13], 2016 | Republic of Korea | RCT | 40 | 40 | Microfracture with MSC | Microfracture | 39.1 | 38.4 | 24 | 26 | 4.8 | 4.6 | 24 |
| Knutsen et al[4], 2004 | Norway | RCT | 40 | 40 | ACI-P | Microfracture | 33.0 | 31.1 | 16 | 16 | 5.1 | 4.5 | 24 |
| Kim et al[56], 2017 | South Korea | RCT | 14 | 14 | Microfracture | Microfracture with Collagen | 55.7 | 55.4 | 0 | 1 | 2.9 | 3.6 | 12 |
| Kim et al[57], 2020 | South Korea | RCT | 48 | 52 | Microfracture | Microfracture with Collagen | 51.7 | 48.9 | 9 | 12 | 4.6 | 3.9 | 24 |
| Kane et al[58], 2018 | United States | RCT | 21 | 9 | Neocart | Microfracture | 41.4 | 38.8 | 2 | 3 | 2.2 | 1.7 | 60 |
| Ibarra et al[59], 2021 | United States | RCT | 24 | 24 | MACI | Microfracture | 33.7 | 35.8 | 7 | 10 | 1.9 | 1.7 | 72 |
| Hashimoto et al[60], 2019 | Japan | RCT | 7 | 4 | Microfracture with MSC | Microfracture | 42.6 | 46.3 | 4 | 0 | 3.0 | 4.4 | 12 |
| Gudas et al[61], 2006 | Lithuania | RCT | 28 | 29 | AOT | Microfracture | 24.6 | 24.3 | 10 | 12 | 2.8 | 2.7 | 36 |
| Gudas et al[62], 2013 | Lithuania | RCT | 28 | 29 | AOT | Microfracture | 24.6 | 24.3 | 10 | 12 | 2.7 | 2.8 | 120 |
| Gudas et al[63], 2005 | Lithuania | RCT | 29 | 28 | Microfracture | AOT | 24.3 | 24.6 | 12 | 10 | 2.8 | 2.7 | 36 |
| Glasbrenner et al[64], 2020 | Germany | RCT | 12 | 12 | Microfracture | Microfracture with BMAC | 36.7 | 47.9 | 3 | 6 | 1.7 | 1.7 | 12 |
| Dasar et al[65], 2016 | Turkey | RCT | 20 | 20 | Microfracture | Carbon fibre rod | 36.4 | 38.5 | 15 | 15 | 3.5 | 4.0. | 24 |
| Crawford et al[66], 2012 | United States | RCT | 21 | 9 | NeoCart | Microfracture | 41.0 | 39.0 | 2 | 3 | 2.8 | 2.5 | 24 |
| Cole et al[67], 2011 | United States | RCT | 9 | 20 | Microfracture | MACI | 33.0 | 32.7 | 4 | 6 | 3.4 | 2.7 | 24 |
| Chung et al[68], 2014 | South Korea | RCT | 24 | 12 | Microfracture with BMAC | Microfracture | 47.4 | 44.3 | 10 | 10 | 1.3 | 1.5 | 24 |
None of the included studies demonstrated high risk of bias to warrant exclusion from the study. The risk of bias in the pairwise comparisons is presented in Supplementary Figure 1. We did not find any significant publication bias using the funnel plot for most of the outcome measures analyzed. When publication bias was noted, we adjusted using the “trim and fill” method to identify the missing studies and their effects on the overall estimate. We did not find any significant impact of the missing studies on the overall outcomes, as shown in Supplementary Figure 2.
We performed a pooled NMA using a frequentist approach to every outcome of interest. Among all the treatment arms in the network, MFX-I had high data strength as compared with all the other comparators (as shown in the network plots in Supplementary Figure 3). Therefore, MFX-I is taken as the constant comparator and all the outcomes have been reported in comparison to the performance of MFX-I. The outcomes have been analyzed in terms of pain, functional outcomes, radiological outcomes, adverse effects, and failures.
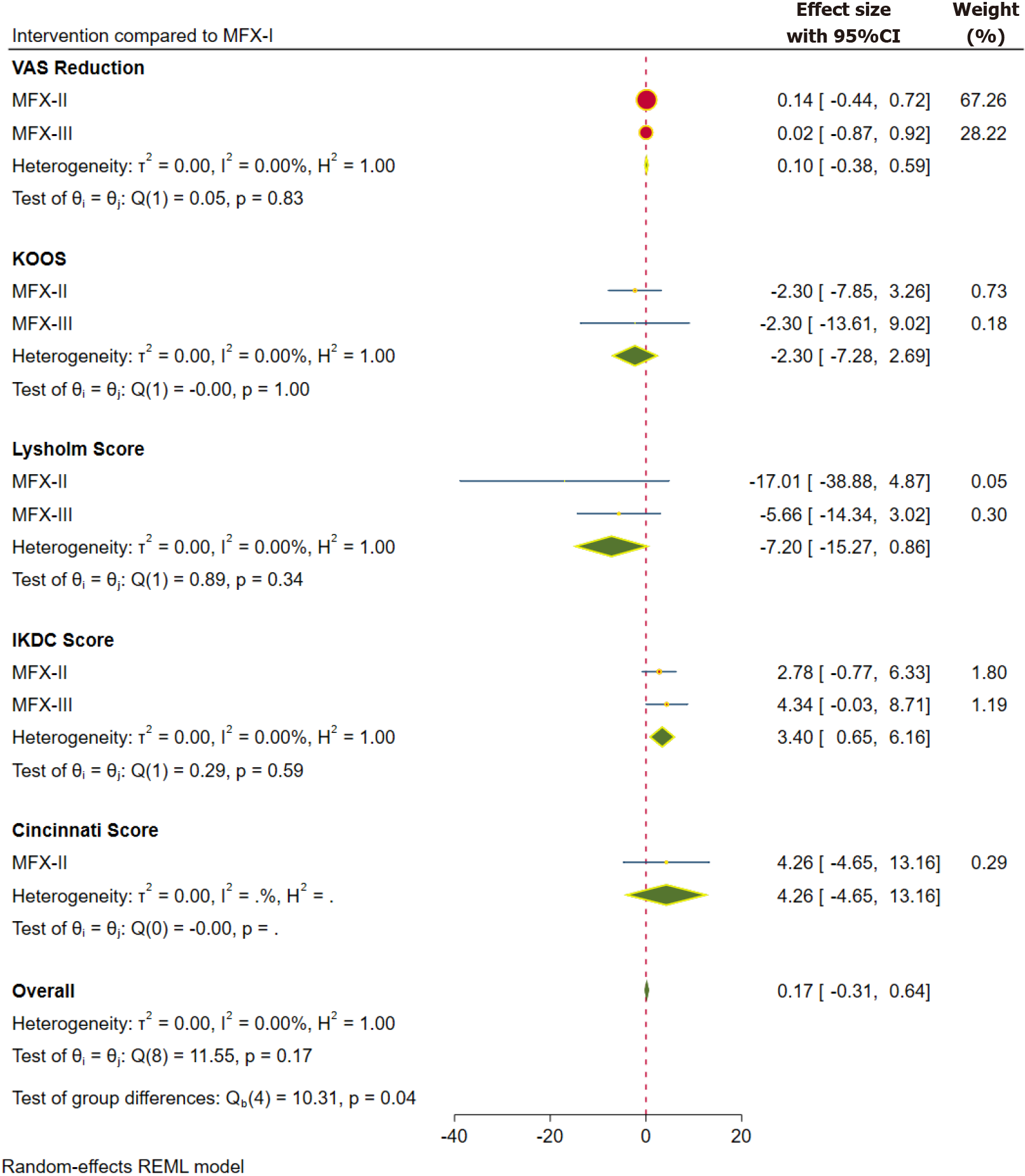
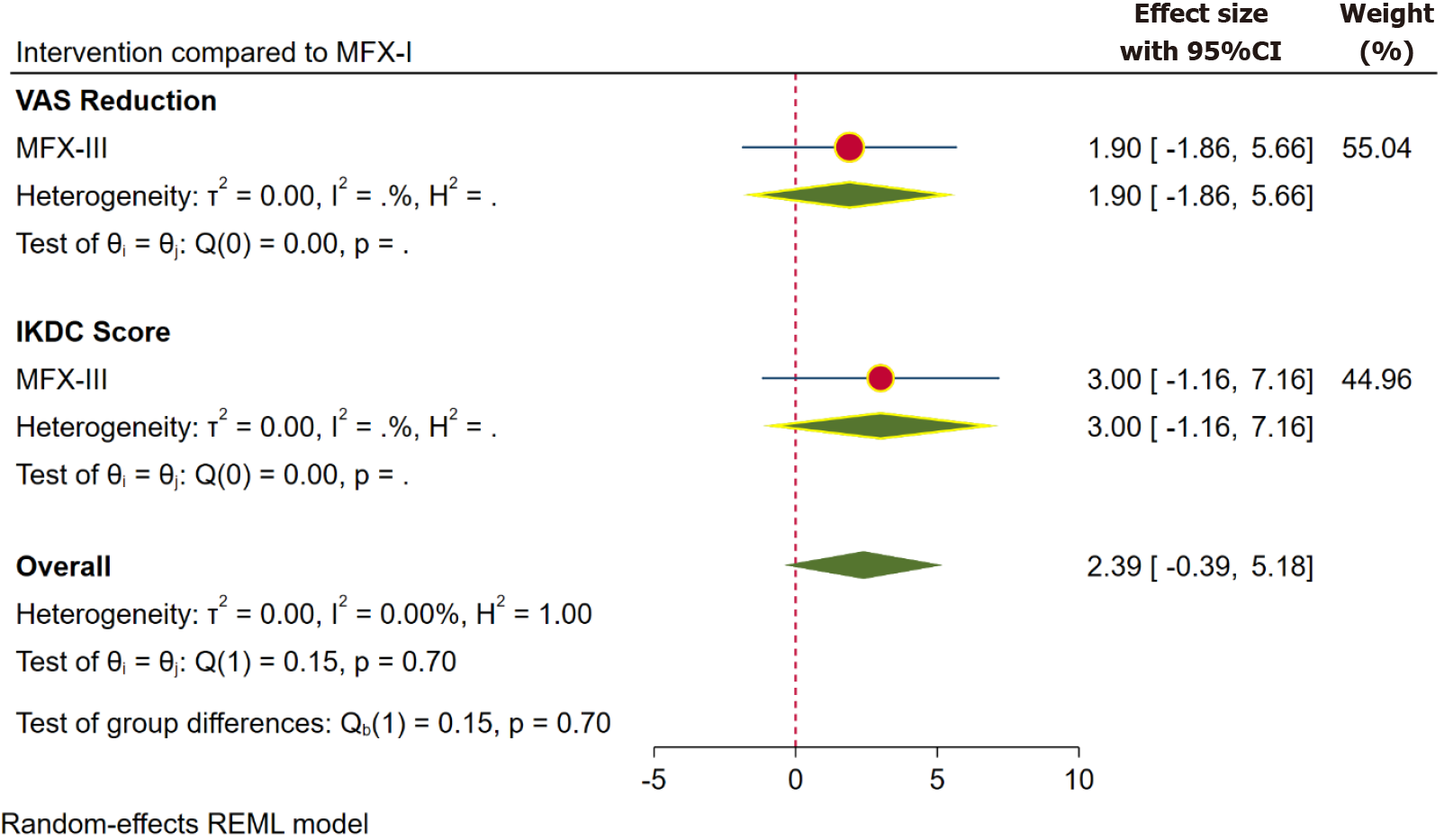
Pain: Inference from the VAS score is taken into consideration for pain outcomes. VAS score was reported at one year in 13 studies[4,15,33,38,41,44,45,49,53,55-58] involving 676 patients, at two years in 10 studies[4,15,33,38,41,45,50,53,57,68] involving 690 patients and at 5 years in 3 studies[39,41,54] involving 297 patients. The pooled forest plot of the VAS score outcome based on the aforementioned follow-up time points is presented in Figures 2, 4, and 5 respectively. Although we did not note a statistically significant improvement in the pain reduction with the advancements to the traditional MFx, the SUCRA ranking of the interventions were consistent in favouring the higher generations in the following order MFX-III > MFX-II > MFX-I as shown in Table 2.
| Follow-up | Outcome | Intervention | Coeffecient | Standard error | SUCRA ranking |
| 1 yr | VAS | mFX-II | 0.139 | 0.296 | MFX-III > MFX-II > MFX-I1 |
| mFX-III | 0.023 | 0.457 | |||
| KOOS | mFX-II | -2.296 | 2.835 | MFX-III > MFX-II > MFX-I1 | |
| mFX-III | -2.296 | 5.775 | |||
| Lysholm score | mFX-II | -17.008 | 11.160 | MFX-I > MFX-III > MFX-II3 | |
| mFX-III | -5.660 | 4.427 | |||
| IKDC score | mFX-II | 2.782 | 1.811 | MFX-III > MFX-II > MFX-I1 | |
| mFX-III | 4.339 | 2.228 | |||
| Cincinnati score | mFX-II | 4.257 | 4.543 | MFX-II > MFX-I1 | |
| MRI filling | mFX-II | 0.383 | 0.312 | MFX-III > MFX-II > MFX-I1 | |
| mFX-III | 1.860 | 1.770 | |||
| MOCART score | mFX-II | 11.950 | 7.419 | MFX-III > MFX-II > MFX-I1 | |
| mFX-III | 30.700 | 14.168 | |||
| Adverse events | mFX-II | -0.529 | 0.373 | MFX-III > MFX-II > MFX-I1 | |
| mFX-III | -0.138 | 0.546 | |||
| Failure events | mFX-II | -0.520 | 0.777 | MFX-II > MFX-I1 | |
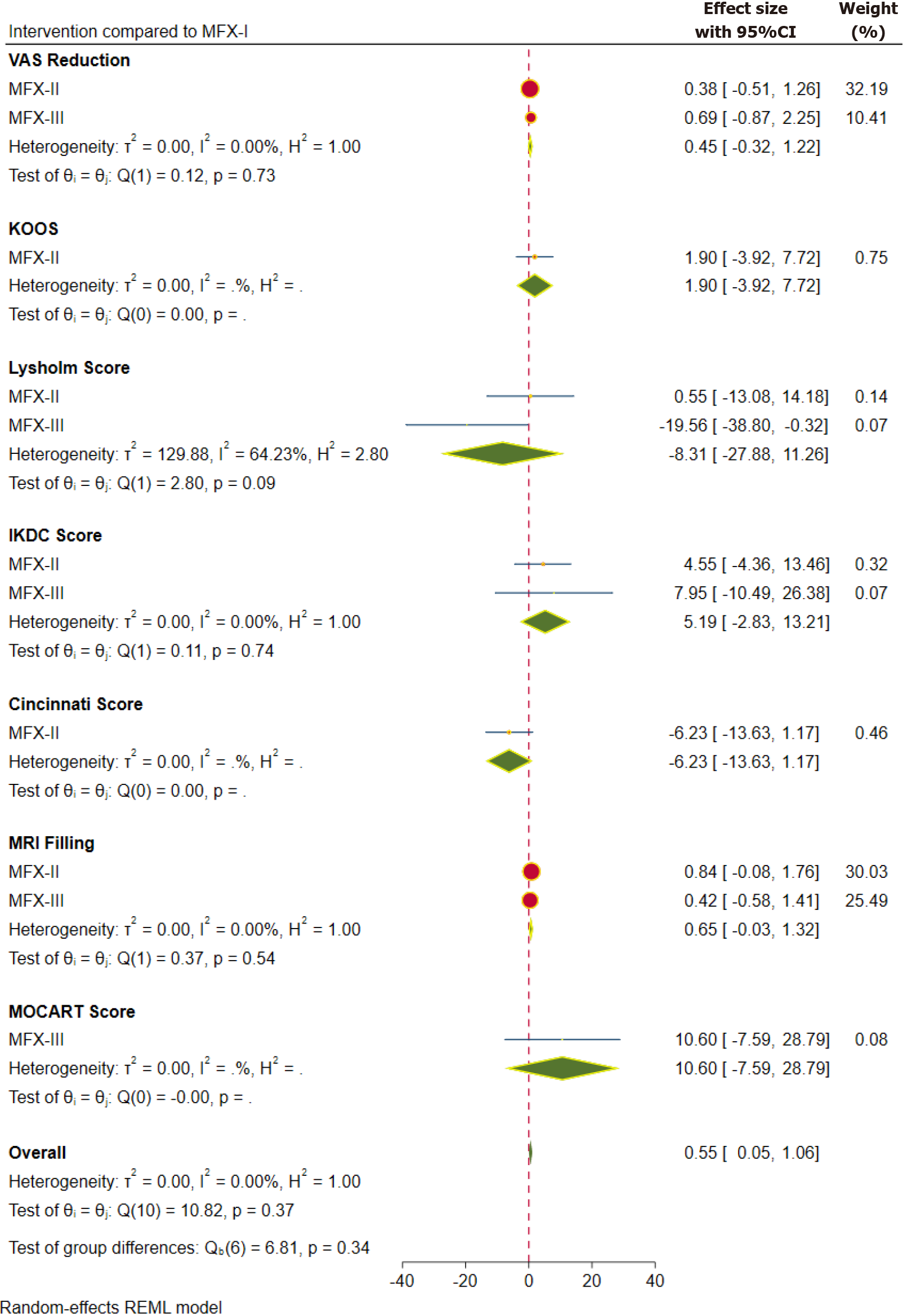
| 2 yr | VAS | mFX-II | 0.377 | 0.452 | MFX-III > MFX-II > MFX-I1 |
| mFX-III | 0.690 | 0.795 | |||
| KOOS | mFX-II | 1.899 | 2.971 | MFX-II > MFX-I1 | |
| Lysholm score | mFX-II | 0.550 | 6.952 | MFX-II > MFX-I > MFX-III3 | |
| mFX-III | -19.560 | 9.814 | |||
| IKDC score | mFX-II | 4.548 | 4.545 | MFX-III > MFX-II > MFX-I1 | |
| mFX-III | 7.947 | 9.405 | |||
| Cincinnati score | mFX-II | -6.227 | 3.775 | MFX-II = MFX-I2 | |
| MRI filling | mFX-II | 0.840 | 0.468 | MFX-II > MFX-III > MFX-I3 | |
| mFX-III | 0.418 | 0.508 | |||
| MOCART | mFX-III | 10.600 | 9.281 | MFX-III > MFX-I1 | |
| 5 yr | VAS | mFX-III | 1.900 | 1.917 | MFX-III > MFX-I1 |
| IKDC score | mFX-III | 3.000 | 2.121 | MFX-III > MFX-I1 |
Functional outcomes: The functional outcomes were reported using KOOS, Lysholm score, IKDC score, and Cincinnati score. Figure 2 shows the pooled forest plot of various scores. KOOS score was reported at one year in 8 studies[32,33,44,46,51,55-57] involving 569 patients, and at 2 years in 4 studies[32,33,51,57] involving 361 patients. Lysholm score was reported at 1 year in 10 studies[4,33,35,41,44,47,48,53,59,65] involving 499 patients, and at 2 years in 8 studies[4,15,33,39,41,47,53,59] involving 516 patients. IKDC score was reported at 1 year in 15 studies[15,35,37,43-45,56-60,64,66,67] involving 631 patients, at 2 years in 13 studies[15,37,39,43,45,50,57-59,64,66-68] involving 782 patients, and at 5 years in 4 studies[39,54,58,59] involving 295 patients. Cincinnati score was reported at 1 year in 3 studies[31,38,65] involving 117 patients, and at 2 years in 4 studies[31,38,39,50] involving 349 patients.
The functional outcomes reported at 1, 2, and 5-year time points using the aforementioned scores were clubbed together for the sake of understanding (despite the limitation of such an approach), in view of the heterogenicity in the reporting of functional outcomes among the reviewed studies.
One-year functional outcomes: The pooled forest plot of the functional outcomes, sub-grouped based on the individual scores at 1 year, is presented in Figure 2. We observed statistically significant outcome in the higher generations of MFx evaluated with IDKC score (WMD = 3.40; 95%CI: 0.65, 6.16; P = 0.045; without significant heterogeneity). However, the difference was not clinically relevant; and less than the minimum clinical difference for the outcome concerned. Although we did not note a statistically significant improvement in most of the functional outcomes with the advancements to the traditional MFx; we observed that (with the exception of Lysholm score) the SUCRA ranking of the interventions consistently favoured the higher generations in the following order: MFX-III > MFX-II > MFX-I (Table 2).
Two-year functional outcome: The pooled forest plot of the functional outcomes, sub-grouped based on the individual scores at 2 years, is presented in Figure 4. We did not note statistically significant difference with the higher generations of MFx with regard to the functional scores such as KOOS, Lysholm score, IDKC score, and Cincinnati score. Nevertheless, similar to the functional outcome at 1-year time point; SUCRA rankings of interventions were consistent in favouring the higher generations in the following order MFX-III > MFX-II > MFX-I (for all outcome measures except the Lysholm score (Table 2).
Five-year functional outcomes: We did not have sufficient data points to evaluate mid-term and long-term functional outcomes. However, based on the available data, there was no significant change in the functional outcome with the higher generations of MFx, as compared to the traditional technique (based on IKDC score; Figure 5). Nevertheless, as with the earlier time points, the SUCRA ranking of interventions favoured the higher generations (in the order MFX-III > MFX-I; Table 2).
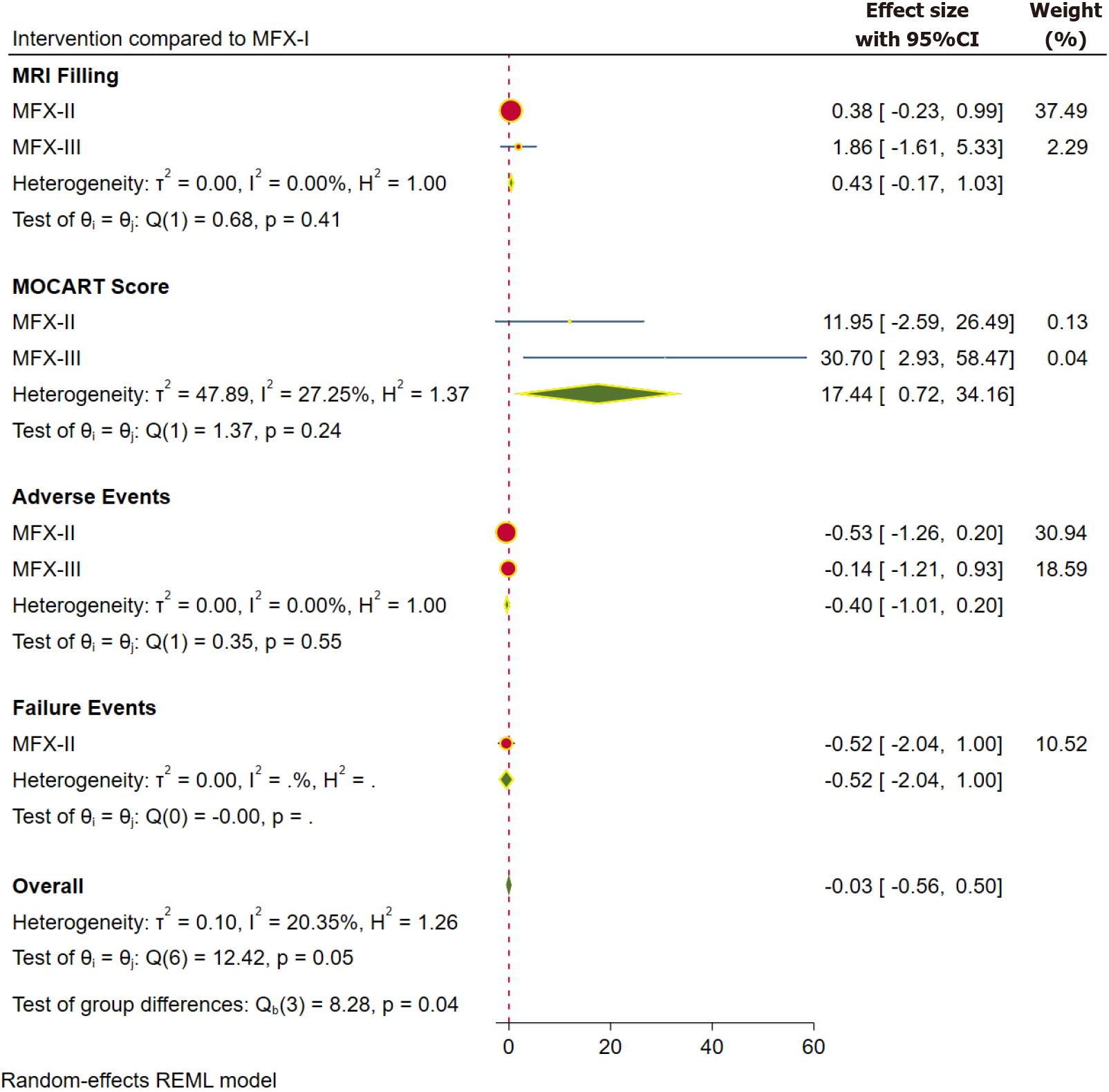
The MOCART (magnetic resonance observation of cartilage repair tissue) Score and MRI defect filling (> 2/3rd) have been used to report the radiological outcomes in the included studies. The MOCART score was reported at 1 year in 8 studies[4,32,44,56,57,59,60,65] involving 439 patients, and at 2 years in 3 studies[13,32,59] involving 230 patients. The MRI-based defect filling was reported at 1 year in 17 studies[19,20,31,37,38,40,43-45,47,56,57,60,62-64,67] involving 847 patients, and at 2 years in 10 studies[13,19,31,38,45,47,50,64,67,68] involving 610 patients.
The pooled forest plots of the radiological outcomes, sub-grouped based on the individual scores at 1- and 2-year time points, are presented in Figures 3 and 4, respectively. We observed statistically better MOCART score in the higher generations of MFx (WMD = 17.44; 95%CI: 0.72, 34.16; P = 0.025; without significant heterogeneity) at 1 year. However, the difference was not maintained at 2 years. Although we did not note a statistically significant improvement in the MRI-filling with the advancements to the traditional MFx, the SUCRA ranking of the interventions were consistent in favouring the higher generations in the following order MFX-III > MFX-II > MFX-I (Table 2).
Adverse events: The adverse events following the compared interventions were reported in 32 studies[9,19,20,31-33,37-39,43,44,46-48,50-55,57,58,60-63,65-67,69-75] involving 1752 patients. Figure 3 shows the pooled forest plot of the reported complications for the analyzed interventions. In comparison with MFX-I, there was no statistically significant difference in the reported rates of adverse events in the higher generations. On the other hand, the SUCRA ranking of the interventions favoured the higher generations in the following order MFX-III > MFX-II > MFX-I (Table 2); thereby, highlighting the safety of the higher generations in comparison with the traditional technique.
Failures: The need for subsequent procedures following the interventions was considered as treatment failure, and the same was reported in 31 studies[4,31,33,34,38-42,46,48,57,59,61,63-65,69,72,73,76,77] involving 1059 patients. Figure 3 shows the pooled forest plot of the failure events for the reported interventions. In comparison with MFX-I, there was no statistically significant difference in the failure events among the higher generations of MFx techniques. Moreover, the SUCRA ranking of the interventions favoured the higher generations in the following order MFX-III > MFX-II > MFX-I (Table 2); thus, highlighting the reliability of the higher generations in comparison to the traditional technique.
We did not observe significant heterogeneity across various outcomes analyzed in the network (based upon the heterogeneity values in the corresponding individual forest plots of pairwise comparisons of interventions). We sub-grouped and analyzed the studies based on the outcome measures and follow-up time point in order to avoid any heterogeneity in the pooled results.
We did not observe any significant evidence of global inconsistency, which could have affected the transivity of the network results. The consistency analysis was performed for the individual outcomes; and the chi-square values in the corresponding pair-wise comparison forest plots were presented. We noted the indirect pooled estimates to have wider CI compared to direct estimates in some of the paired networks analysed (although without any evidence of systematic differences concerning the potential effect modifiers). We considered these apparent inconsistencies to be the effect of true differences between the direct and indirect estimates. The indirect estimates were considered to reflect a more precise estimate, since they were from a network involving a larger number of studies.
Upon grading the paired comparisons in the network using the CINeMA approach, a “high” confidence was noted across a majority of the paired comparisons (Table 3). However, some of the comparison pairs demonstrated “moderate” confidence. The lack of precision was the most common reason, which downgraded the quality of evidence in the indirect estimates, in view of wider CIs extending on either side of the axes. We also observed some concerns due to certain “within-study bias”, following selective reporting of some of the outcome measures.
| Comparison | Number of studies | Within-study bias | Reporting bias | Indirectness | Imprecision | Heterogeneity | Incoherence | Confidence rating | Reasons for downgrading |
| MFx-I: MFx-II | 7 | Some concerns | Some concerns | No concerns | Major concerns | Some concerns | No concerns | Moderate | Imprecision in results |
| MFx-I: MFx -III | 1 | Some concerns | Some concerns | No concerns | Major concerns | Some concerns | No concerns | Moderate | Imprecision in results |
| MFx-II: MFx -III | 1 | Some concerns | Some concerns | No concerns | Major concerns | Some concerns | No concerns | Moderate | Imprecision in results |
Chondral lesions have been reported in 60% of patients undergoing arthroscopic procedures of the knee; and such defects are described as one of the leading causes of chronic pain[78-81]. These defects may result from acute trauma, repetitive microtrauma, osteochondritis dessicans or early osteoarthritis; and can produce symptoms like pain, swelling, catching, stiffness and locking[33]. Hunter et al[82,83] described the challenge of cartilaginous injury by stating that, “once the cartilage is destroyed, it never recovers”. These observations still hold true; and the avascular as well as aneural nature of cartilage substantially limits its ability to self-regenerate[84]. If left untreated, a transgressed cartilage gradually results in severe osteoarthritis of the joint and ensuing long-standing disability[85].
Superficial cartilage deficiencies do not induce a local inflammatory response; therefore, despite proliferation of matrix molecules and chondrocytes, the surface is not adequately restored[86]. When the cartilage defect penetrates the subchondral plate, the vascularized bone marrow can enable the formation of clot rich in chondroprogenitor cells, fibrin and bioactive molecules; which in turn, facilitates the formation of type I collagen and fibrocartilage[87]. This is the rationale underlying the MFx technique, which has traditionally remained the first-line treatment for small to medium-sized defects[88]. The purported benefits of the procedure include low cost, easy technique and proven improvement in short-term outcome[87,88]. Nevertheless, 47% to 80% of patients have been reported to demonstrate substantial functional deterioration at 18 to 36 months post-surgically[10], which may be attributed to the poor viscoelastic properties of the restored fibrocartilage[89]. Since the initial description of MFx technique, multitudinous attempts have been made in the fields of tissue engineering and cartilage repair in an attempt to find the “holy grail”, which enables the restoration of hyaline cartilage that can consistently integrate into the deficiency[42].
In the traditional MFx technique described by Steadman et al[3], the debridement of the unstable cartilaginous tissues is initially performed arthroscopically; and a well-shouldered vertical wall is created around the periphery of the lesion. Following this, layers of calcified cartilage are removed using a curette. An arthroscopic awl is then utilized in a direction perpendicular to the bone in order to create holes in the subchondral plate around 3-4 mm apart (ascertaining that the interposed subchondral bone between the MFx perforations is maintained intact). Alternately, microdrilling using a 1.5 mm drill may be performed to perforate the subchondral plate to a depth of 1 cm.
While lesions smaller than 2 cm2 in low-demand individuals are amenable to treatment with traditional MFx technique; lesions larger than 4 cm2 have been purported to require additional adjuvant modalities too[90]. Diverse acellular biomaterials such as alginate, collagen, tri-copolymer and poly-lactic-glycolic acid have been utilized for engineering of cartilaginous tissues[91]. These tissues serve as carriers for delivery of cells and growth factors; as well as provide an appropriate milieu for tissue regeneration[92].
The cell therapy for cartilage repair was initially proposed in the 1980s using the technology of tissue engineering[93]; and cellular therapeutic innovation was eventually realized in 1994, when Brittberg et al[94] described the ACI technique. Further on, scaffold-based ACI (matrix-induced ACI-MACI: FDA-approved in 2016) technique has also been described as a modification of the traditional MFx. The discovery of adult stem cells resulted in a paradigm shift in the field of regenerative medicine[95]. A variety of stem cell-based therapies involving multipotent MSCs implantation (like bone marrow, adipose tissue, synovium, periosteum, peripheral blood, etc.) have been employed for cartilage repair. The chondrogenesis and development of neo-cartilaginous tissues from such undifferentiated MSCs can be guided using growth factors, and other biophysical or biomechanical stimuli[96,97].
As an alternative form of cell-based therapy, Gobbi et al[10] described the technique of implanting the bone marrow aspirate concentrate delivered via HA-based scaffold (HA-BMAC) over the micro-fractured area. Such an approach relies on the presence of MSCs and growth factors at the deficient zone so as to steer chondrogenesis. They concluded that such an approach yielded successful medium-term clinical outcome with restoration of durable cartilage, irrespective of the size and age of the lesion.
Despite such extensive publications, there has been a substantial dearth of large-scale, high-quality RCTs on this subject. In a recent systematic review; among 540 reviewed manuscripts, only 10 studies were found to be methodologically sufficient to be included for final analysis. The current evidence on this subject is therefore, still largely unclear[98]. The purpose of the current NMA was to comprehensively analyse the existing literature on chondral injuries of the knee; and comparatively evaluate the histological, radiological and clinical outcome following 3 different generations of MFx, namely traditional MFx (MFx-I), modified MFx technique using acellular adjuvant (MFx-II); and modified MFx technique using cellular adjuvant (MFX-III).
Clinical and functional outcome: Overall, in our meta-analysis, we compared the pain scores and functional outcome measures (KOOS, Lysholm score, IKDC score, and Cincinnati scores) among the three generations of MFx. We could clearly observe a trend of improved pain scores and functional outcome scores (KOOS, IKDC and Cincinnati scores) with the use of cellular adjuvants (MFx-III-MSC, BMAC, PBSC, and SVF). Although the difference in the pain and functional scores improved with the use of acellular adjuvants (such as PRP, HA, collagen, and AMIC) too in comparison with traditional MFx, the differences were not as substantial as for cellular adjuvants.
This observation is in concurrence with a majority of the studies, which have demonstrated overall improved clinical outcome with acellular (MFx-II) adjuvants. In a prospective, multicenter clinical trial[31], AMIC with biodegradable type I/III collagen membrane showed significantly improved longer-term radiological (MRI defect filling) and functional outcome (as assessed by Cincinnati and modified ICRS scores) at the 5-year time point, in comparison with MFx-I. In another recent RCT, Shive et al[19] concluded that the use of BST-CarGel (soluble polymer scaffold containing polysaccharide chitosan dispersed in uncoagulated blood) following MFx leads to improved cartilage resurfacing and wound healing. On a similar note, various prospective studies have also reported meliorated outcome (clinical and radiological) following the use of diverse cellular components after MFx (MFx-III). Some such cellular components, which have been successfully tried in cartilage defects, include single-stage cell-based therapy using autologous cartilage fragments (cartilage autograft implantation system-CAIS)[67], collagen-covered ACI (ACI-C), AMIC[33], micro-fragmented stromal-vascular fraction (rich in adipose-derived MSCs-ADMSC)[49], and tri-layered collagen hydroxyapatite biomimetic osteochondral scaffold (CHAS) seeded intra-operatively with autologous chondrocytes (AC) or filtered bone marrow stem/stromal cells (fBMSC)[99]. In a prospective series by Liu et al[43], it was demonstrated that the application of Kartigen (matrix with autologous bone marrow MSC-derived chondrocyte precursors embedded in atelocollagen) enabled the restoration of columnar surface of articular cartilage, collagen type 2 and glycosaminoglycan in similar composition to native hyaline cartilage (on histology).
Radiological outcome: A majority of the studies reported on MOCART score and MRI filling defect during the follow-up. There was a statistically significant improvement in the MOCART score at the end of 1 year in patients following the use of cellular adjuvants after MFx, indicating a substantially improved cartilage tissue quality and integration. Although the radiological outcome scores at the subsequent follow-up time points were not statistically different; similar to the clinical outcome, there was a definitive trend towards better outcome after the use of cellular and acellular adjuvants following MFx (cellular > acellular).
In a prospective randomized study by Ibarra et al[59], it was concluded that structural outcome (as assessed by MRI-T2 mapping and MOCART score) and significantly improved clinical outcome (as evaluated by KOOS subscale and Tegner scale) at 1 to 6 years and 4 to 6 years, respectively in patients undergoing matrix-assisted autologous chondrocyte transplantation, as compared with traditional MFx. Patients undergoing adjuvant cell therapy also demonstrated higher response and lower failure rates in this series. Similar prospective cohort studies have demonstrated improved cartilage fill on T2WI MRI and mean MOCART score following surgical treatment with PRP-loaded scaffold (MFx-II)[100], scaffold augmentation using BMAC (MFx-III)[100] and transplantation of autologous BMSCs (BMSC-MFx-III)[60].
Complications and adverse events: Based on our network analysis, we could also clearly identify mitigated complication and failure rates with the higher generations of MFx (although the differences were not statistically significant. In a prospective series by Martinčič et al[99], tri-layered CHAS seeded intra-operatively with AC or fBMSC demonstrated significantly improved outcome, in comparison with MFx. In this study, blood soaking of the scaffold prior to cell seeding substantially reduced early post-operative complications like synovitis and arthrofibrosis.
Limitations: Though our study is one of the most comprehensively-performed reviews of the existing literature on this subject, there are certain limitations. The long-term data on histological and radiological outcomes following recent generations of MFx are limited. There is substantial paucity as well as heterogeneity in the reporting on the diverse functional outcome measures, which prevented uniform comparison of events.
Current status and future directions: Based on our comprehensive review and NMA, we could conclude that the use of acellular and cellular adjuvants (2nd and 3rd generation) marginally improves the overall clinical status (pain and functional scores) and radiological outcome (MOCART score and MRI-filling) in patients undergoing MFx for cartilage defects of the knee. The safety and efficacy of the higher generation MFx procedures are also clearly evident from our review. However, there is a substantial potential for further improvement in the cellular components (chondrocytes over other cellular lineage), culture or processing methodology, delivery modalities (including appropriate scaffolds); as well as better surgical techniques[6].
The use of acellular and cellular adjuvants (2nd and 3rd generation) has shown only marginal improvement in the clinical (pain and functional scores) and radiological outcome (MOCART score and MRI-filling) in patients undergoing MFx for cartilage defects of the knee.
We have noted improvements in the traditional microfracture (MFx) techniques over the decades of its routine use in the management of cartilage defects. The recent generations include the addition of acellular components and cellular components to the cartilage defect. However, the effectiveness of these modifications is not explored further.
To explore the clinical effectiveness of the various generations of the MFx technique to understand their clinical effect in the management of cartilage defects.
To comparatively explore the clinical, radiological and histological outcomes along with the complications reported in the various generations of MFx in the context of the management of cartilage defects.
We made a systematic review by utilizing the databases such as PubMed, EMBASE, Web of Science, Cochrane, and Scopus to identify the randomized controlled trials (RCTs) reporting the outcomes of utilization of various generations of MFx in the management of cartilage defects. Network meta-analysis was performed among the three generations for the outcomes analysed using Stata.
Forty-four RCTs were included in the analysis with patients of mean age of 39.40 (± 9.46) years. Upon comparing the results of the other generations with MFX-I as a constant comparator, we noted a trend towards better pain control and functional outcome (KOOS, IKDC and Cincinnati scores) at the end of 1-, 2-, and 5-year time points with MFx-III, although the differences were not statistically significant (P > 0.05). We also noted statistically significant MOCART score in the higher generations of MFx (WMD = 17.44; 95%CI: 0.72, 34.16; P = 0.025; without significant heterogeneity) at 1 year. However, the difference was not maintained at 2 years. There was a trend towards better defect filling on MRI with the second and third generation MFx, although the difference was not statistically significant (P > 0.05).
The higher generations of traditional MFx technique utilizing acellular and cellular components to augment its potential in the management of cartilage defects has shown only marginal improvement in the clinical and radiological outcomes.
Future work could focus on the improvement in the cellular components (chondrocytes over other cellular lineage), culture or processing methodology, delivery modalities (including appropriate scaffolds); as well as better surgical techniques to make the clinical impact with their further advancements.
| 1. | Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 516] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 2. | Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469:2696-2705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 3. | Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;S362-S369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 834] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 4. | Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grøntvedt T, Solheim E, Strand T, Roberts S, Isaksen V, Johansen O. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 995] [Cited by in RCA: 864] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 5. | Hoemann CD, Tran-Khanh N, Chevrier A, Chen G, Lascau-Coman V, Mathieu C, Changoor A, Yaroshinsky A, McCormack RG, Stanish WD, Buschmann MD. Chondroinduction Is the Main Cartilage Repair Response to Microfracture and Microfracture With BST-CarGel: Results as Shown by ICRS-II Histological Scoring and a Novel Zonal Collagen Type Scoring Method of Human Clinical Biopsy Specimens. Am J Sports Med. 2015;43:2469-2480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Muthu S, Korpershoek JV, Novais EJ, Tawy GF, Hollander AP, Martin I. Failure of cartilage regeneration: emerging hypotheses and related therapeutic strategies. Nat Rev Rheumatol. 2023;19:403-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 159] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 7. | Bedi A, Feeley BT, Williams RJ 3rd. Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:994-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 316] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 8. | Krych AJ, Harnly HW, Rodeo SA, Williams RJ 3rd. Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee: a retrospective comparative study. J Bone Joint Surg Am. 2012;94:971-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Saris DB, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, Luyten FP; TIG/ACT/01/2000&EXT Study Group. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37 Suppl 1:10S-19S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 355] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 10. | Gobbi A, Karnatzikos G, Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22:1986-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 223] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 11. | Solheim E, Hegna J, Inderhaug E, Øyen J, Harlem T, Strand T. Results at 10-14 years after microfracture treatment of articular cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24:1587-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Case JM, Scopp JM. Treatment of Articular Cartilage Defects of the Knee With Microfracture and Enhanced Microfracture Techniques. Sports Med Arthrosc Rev. 2016;24:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Koh YG, Kwon OR, Kim YS, Choi YJ, Tak DH. Adipose-Derived Mesenchymal Stem Cells With Microfracture Versus Microfracture Alone: 2-Year Follow-up of a Prospective Randomized Trial. Arthroscopy. 2016;32:97-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 14. | Lee KB, Wang VT, Chan YH, Hui JH. A novel, minimally-invasive technique of cartilage repair in the human knee using arthroscopic microfracture and injections of mesenchymal stem cells and hyaluronic acid--a prospective comparative study on safety and short-term efficacy. Ann Acad Med Singap. 2012;41:511-517. [PubMed] |
| 15. | Lee GW, Son JH, Kim JD, Jung GH. Is platelet-rich plasma able to enhance the results of arthroscopic microfracture in early osteoarthritis and cartilage lesion over 40 years of age? Eur J Orthop Surg Traumatol. 2013;23:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Siclari A, Mascaro G, Gentili C, Kaps C, Cancedda R, Boux E. Cartilage repair in the knee with subchondral drilling augmented with a platelet-rich plasma-immersed polymer-based implant. Knee Surg Sports Traumatol Arthrosc. 2014;22:1225-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Saw KY, Hussin P, Loke SC, Azam M, Chen HC, Tay YG, Low S, Wallin KL, Ragavanaidu K. Articular cartilage regeneration with autologous marrow aspirate and hyaluronic Acid: an experimental study in a goat model. Arthroscopy. 2009;25:1391-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Strauss E, Schachter A, Frenkel S, Rosen J. The efficacy of intra-articular hyaluronan injection after the microfracture technique for the treatment of articular cartilage lesions. Am J Sports Med. 2009;37:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Shive MS, Stanish WD, McCormack R, Forriol F, Mohtadi N, Pelet S, Desnoyers J, Méthot S, Vehik K, Restrepo A. BST-CarGel® Treatment Maintains Cartilage Repair Superiority over Microfracture at 5 Years in a Multicenter Randomized Controlled Trial. Cartilage. 2015;6:62-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Stanish WD, McCormack R, Forriol F, Mohtadi N, Pelet S, Desnoyers J, Restrepo A, Shive MS. Novel scaffold-based BST-CarGel treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J Bone Joint Surg Am. 2013;95:1640-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 178] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 21. | Strauss EJ, Barker JU, Kercher JS, Cole BJ, Mithoefer K. Augmentation Strategies following the Microfracture Technique for Repair of Focal Chondral Defects. Cartilage. 2010;1:145-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4165] [Cited by in RCA: 5893] [Article Influence: 535.7] [Reference Citation Analysis (1)] |
| 23. | McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol. 2016;75:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1398] [Cited by in RCA: 3433] [Article Influence: 343.3] [Reference Citation Analysis (1)] |
| 24. | Orthopaedic Research Group. ORG Cartilage Treatment Classifier Tool. [cited 12 December 2023]. Available from: https://orthopaedicresearchgroup.com/contact.php. |
| 25. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 18837] [Article Influence: 2691.0] [Reference Citation Analysis (0)] |
| 26. | Hwang H, DeSantis SM. Multivariate network meta-analysis to mitigate the effects of outcome reporting bias. Stat Med. 2018;37:3254-3266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Lu G, Ades A. Modeling between-trial variance structure in mixed treatment comparisons. Biostatistics. 2009;10:792-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 29. | Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, Salanti G. CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17:e1003082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1002] [Cited by in RCA: 1162] [Article Influence: 193.7] [Reference Citation Analysis (0)] |
| 30. | Papakonstantinou T, Nikolakopoulou A, Higgins JPT, Egger M, Salanti G. CINeMA: Software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst Rev. 2020;16:e1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 345] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 31. | Volz M, Schaumburger J, Frick H, Grifka J, Anders S. A randomized controlled trial demonstrating sustained benefit of Autologous Matrix-Induced Chondrogenesis over microfracture at five years. Int Orthop. 2017;41:797-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 32. | Niemeyer P, Laute V, Zinser W, Becher C, Kolombe T, Fay J, Pietsch S, Kuźma T, Widuchowski W, Fickert S. A Prospective, Randomized, Open-Label, Multicenter, Phase III Noninferiority Trial to Compare the Clinical Efficacy of Matrix-Associated Autologous Chondrocyte Implantation With Spheroid Technology Versus Arthroscopic Microfracture for Cartilage Defects of the Knee. Orthop J Sports Med. 2019;7:2325967119854442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 33. | Fossum V, Hansen AK, Wilsgaard T, Knutsen G. Collagen-Covered Autologous Chondrocyte Implantation Versus Autologous Matrix-Induced Chondrogenesis: A Randomized Trial Comparing 2 Methods for Repair of Cartilage Defects of the Knee. Orthop J Sports Med. 2019;7:2325967119868212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Ulstein S, Årøen A, Røtterud JH, Løken S, Engebretsen L, Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2014;22:1207-1215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 35. | Visna P, Pasa L, Cizmár I, Hart R, Hoch J. Treatment of deep cartilage defects of the knee using autologous chondrograft transplantation and by abrasive techniques--a randomized controlled study. Acta Chir Belg. 2004;104:709-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Van Assche D, Staes F, Van Caspel D, Vanlauwe J, Bellemans J, Saris DB, Luyten FP. Autologous chondrocyte implantation versus microfracture for knee cartilage injury: a prospective randomized trial, with 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2010;18:486-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Saw KY, Anz A, Siew-Yoke Jee C, Merican S, Ching-Soong Ng R, Roohi SA, Ragavanaidu K. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy. 2013;29:684-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 187] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 38. | Anders S, Volz M, Frick H, Gellissen J. A Randomized, Controlled Trial Comparing Autologous Matrix-Induced Chondrogenesis (AMIC®) to Microfracture: Analysis of 1- and 2-Year Follow-Up Data of 2 Centers. Open Orthop J. 2013;7:133-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 39. | Brittberg M, Recker D, Ilgenfritz J, Saris DBF; SUMMIT Extension Study Group. Matrix-Applied Characterized Autologous Cultured Chondrocytes Versus Microfracture: Five-Year Follow-up of a Prospective Randomized Trial. Am J Sports Med. 2018;46:1343-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 194] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 40. | Lim HC, Bae JH, Song SH, Park YE, Kim SJ. Current treatments of isolated articular cartilage lesions of the knee achieve similar outcomes. Clin Orthop Relat Res. 2012;470:2261-2267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 41. | Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89:2105-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 427] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 42. | Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Ludvigsen TC, Løken S, Solheim E, Strand T, Johansen O. A Randomized Multicenter Trial Comparing Autologous Chondrocyte Implantation with Microfracture: Long-Term Follow-up at 14 to 15 Years. J Bone Joint Surg Am. 2016;98:1332-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 43. | Liu YL, Yen CC, Liu TT, Chang CH, Shih TT, Wang JH, Yang MC, Lin FH, Liu HC. Safety and Efficacy of Kartigen(®) in Treating Cartilage Defects: A Randomized, Controlled, Phase I Trial. Polymers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Yoon KH, Park JY, Lee JY, Lee E, Lee J, Kim SG. Costal Chondrocyte-Derived Pellet-Type Autologous Chondrocyte Implantation for Treatment of Articular Cartilage Defect. Am J Sports Med. 2020;48:1236-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 45. | Kon E, Filardo G, Brittberg M, Busacca M, Condello V, Engebretsen L, Marlovits S, Niemeyer P, Platzer P, Posthumus M, Verdonk P, Verdonk R, Victor J, van der Merwe W, Widuchowski W, Zorzi C, Marcacci M. A multilayer biomaterial for osteochondral regeneration shows superiority vs microfractures for the treatment of osteochondral lesions in a multicentre randomized trial at 2 years. Knee Surg Sports Traumatol Arthrosc. 2018;26:2704-2715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 46. | Vanlauwe J, Saris DB, Victor J, Almqvist KF, Bellemans J, Luyten FP; TIG/ACT/01/2000&EXT Study Group. Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med. 2011;39:2566-2574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 253] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 47. | Basad E, Ishaque B, Bachmann G, Stürz H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010;18:519-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 303] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 48. | Solheim E, Hegna J, Strand T, Harlem T, Inderhaug E. Randomized Study of Long-term (15-17 Years) Outcome After Microfracture Versus Mosaicplasty in Knee Articular Cartilage Defects. Am J Sports Med. 2018;46:826-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 49. | Bisicchia S, Bernardi G, Pagnotta SM, Tudisco C. Micro-fragmented stromal-vascular fraction plus microfractures provides better clinical results than microfractures alone in symptomatic focal chondral lesions of the knee. Knee Surg Sports Traumatol Arthrosc. 2020;28:1876-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Saris D, Price A, Widuchowski W, Bertrand-Marchand M, Caron J, Drogset JO, Emans P, Podskubka A, Tsuchida A, Kili S, Levine D, Brittberg M; SUMMIT study group. Matrix-Applied Characterized Autologous Cultured Chondrocytes Versus Microfracture: Two-Year Follow-up of a Prospective Randomized Trial. Am J Sports Med. 2014;42:1384-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 51. | Saris DB, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, Vandekerckhove B, Almqvist KF, Claes T, Handelberg F, Lagae K, van der Bauwhede J, Vandenneucker H, Yang KG, Jelic M, Verdonk R, Veulemans N, Bellemans J, Luyten FP. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 449] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 52. | Qiao Z, Tang J, Yue B, Wang J, Zhang J, Xuan L, Dai C, Li S, Li M, Xu C, Dai K, Wang Y. Human adipose-derived mesenchymal progenitor cells plus microfracture and hyaluronic acid for cartilage repair: a Phase IIa trial. Regen Med. 2020;15:1193-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 53. | Nguyen PD, Tran TD, Nguyen HT, Vu HT, Le PT, Phan NL, Vu NB, Phan NK, Van Pham P. Comparative Clinical Observation of Arthroscopic Microfracture in the Presence and Absence of a Stromal Vascular Fraction Injection for Osteoarthritis. Stem Cells Transl Med. 2017;6:187-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 54. | Lim HC, Park YB, Ha CW, Cole BJ, Lee BK, Jeong HJ, Kim MK, Bin SI, Choi CH, Yoo JD; Cartistem Research Group, Yoon JR, Chung JY. Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cell Implantation Versus Microfracture for Large, Full-Thickness Cartilage Defects in Older Patients: A Multicenter Randomized Clinical Trial and Extended 5-Year Clinical Follow-up. Orthop J Sports Med. 2021;9:2325967120973052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 55. | Venosa M, Calafiore F, Mazzoleni M, Romanini E, Cerciello S, Calvisi V. Platelet-Rich Plasma and Adipose-Derived Mesenchymal Stem Cells in Association with Arthroscopic Microfracture of Knee Articular Cartilage Defects: A Pilot Randomized Controlled Trial. Adv Orthop. 2022;2022:6048477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 56. | Kim MS, Koh IJ, Choi YJ, Pak KH, In Y. Collagen Augmentation Improves the Quality of Cartilage Repair After Microfracture in Patients Undergoing High Tibial Osteotomy: A Randomized Controlled Trial. Am J Sports Med. 2017;45:1845-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 57. | Kim MS, Chun CH, Wang JH, Kim JG, Kang SB, Yoo JD, Chon JG, Kim MK, Moon CW, Chang CB, Song IS, Ha JK, Choi NY, In Y. Microfractures Versus a Porcine-Derived Collagen-Augmented Chondrogenesis Technique for Treating Knee Cartilage Defects: A Multicenter Randomized Controlled Trial. Arthroscopy. 2020;36:1612-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Kane MS, Williams RJ III, DeBerardino TM, Taylor D, Ma CB, Anderson DE, Crawford DC. Review of an exploratory phase II FDA regulated clinical trial of a novel surgical innovation: completion of a prospective, randomized, controlled trial to compare NeoCart with the standard-of-care, microfracture, for articular cartilage repair. Annals of Joint. 2018;3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 59. | Ibarra C, Villalobos E, Madrazo-Ibarra A, Velasquillo C, Martinez-Lopez V, Izaguirre A, Olivos-Meza A, Cortes-Gonzalez S, Perez-Jimenez FJ, Vargas-Ramirez A, Franco-Sanchez G, Ibarra-Ibarra LG, Sierra-Suarez L, Almazan A, Ortega-Sanchez C, Trueba C, Martin FB, Arredondo-Valdes R, Chavez-Arias D. Arthroscopic Matrix-Assisted Autologous Chondrocyte Transplantation Versus Microfracture: A 6-Year Follow-up of a Prospective Randomized Trial. Am J Sports Med. 2021;49:2165-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 60. | Hashimoto Y, Nishida Y, Takahashi S, Nakamura H, Mera H, Kashiwa K, Yoshiya S, Inagaki Y, Uematsu K, Tanaka Y, Asada S, Akagi M, Fukuda K, Hosokawa Y, Myoui A, Kamei N, Ishikawa M, Adachi N, Ochi M, Wakitani S. Transplantation of autologous bone marrow-derived mesenchymal stem cells under arthroscopic surgery with microfracture versus microfracture alone for articular cartilage lesions in the knee: A multicenter prospective randomized control clinical trial. Regen Ther. 2019;11:106-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 61. | Gudas R, Stankevicius E, Monastyreckiene E, Pranys D, Kalesinskas RJ. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 62. | Gudas R, Gudaitė A, Mickevičius T, Masiulis N, Simonaitytė R, Cekanauskas E, Skurvydas A. Comparison of osteochondral autologous transplantation, microfracture, or debridement techniques in articular cartilage lesions associated with anterior cruciate ligament injury: a prospective study with a 3-year follow-up. Arthroscopy. 2013;29:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 63. | Gudas R, Kalesinskas RJ, Kimtys V, Stankevicius E, Toliusis V, Bernotavicius G, Smailys A. A prospective randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy. 2005;21:1066-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 391] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 64. | Glasbrenner J, Petersen W, Raschke MJ, Steiger M, Verdonk R, Castelli CC, Zappalà G, Fritschy D, Herbort M. Matrix-Augmented Bone Marrow Stimulation With a Polyglycolic Acid Membrane With Hyaluronan vs Microfracture in Local Cartilage Defects of the Femoral Condyles: A Multicenter Randomized Controlled Trial. Orthop J Sports Med. 2020;8:2325967120922938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 65. | Dasar U, Gursoy S, Akkaya M, Algin O, Isik C, Bozkurt M. Microfracture technique versus carbon fibre rod implantation for treatment of knee articular cartilage lesions. J Orthop Surg (Hong Kong). 2016;24:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Crawford DC, DeBerardino TM, Williams RJ 3rd. NeoCart, an autologous cartilage tissue implant, compared with microfracture for treatment of distal femoral cartilage lesions: an FDA phase-II prospective, randomized clinical trial after two years. J Bone Joint Surg Am. 2012;94:979-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 67. | Cole BJ, Farr J, Winalski CS, Hosea T, Richmond J, Mandelbaum B, De Deyne PG. Outcomes after a single-stage procedure for cell-based cartilage repair: a prospective clinical safety trial with 2-year follow-up. Am J Sports Med. 2011;39:1170-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 179] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 68. | Chung JY, Lee DH, Kim TH, Kwack KS, Yoon KH, Min BH. Cartilage extra-cellular matrix biomembrane for the enhancement of microfractured defects. Knee Surg Sports Traumatol Arthrosc. 2014;22:1249-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 69. | Barié A, Kruck P, Sorbi R, Rehnitz C, Oberle D, Walker T, Zeifang F, Moradi B. Prospective Long-term Follow-up of Autologous Chondrocyte Implantation With Periosteum Versus Matrix-Associated Autologous Chondrocyte Implantation: A Randomized Clinical Trial. Am J Sports Med. 2020;48:2230-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 70. | Clavé A, Potel JF, Servien E, Neyret P, Dubrana F, Stindel E. Third-generation autologous chondrocyte implantation versus mosaicplasty for knee cartilage injury: 2-year randomized trial. J Orthop Res. 2016;34:658-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 71. | de Queiroz AAB, Debieux P, Amaro J, Ferretti M, Cohen M. Hydrogel implant is as effective as osteochondral autologous transplantation for treating focal cartilage knee injury in 24 months. Knee Surg Sports Traumatol Arthrosc. 2018;26:2934-2941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 72. | Gooding CR, Bartlett W, Bentley G, Skinner JA, Carrington R, Flanagan A. A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: Periosteum covered versus type I/III collagen covered. Knee. 2006;13:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 73. | Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, Bentley G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87:640-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 510] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 74. | Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, Skinner JA, Pringle J. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 734] [Cited by in RCA: 615] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 75. | Zeifang F, Oberle D, Nierhoff C, Richter W, Moradi B, Schmitt H. Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am J Sports Med. 2010;38:924-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 76. | Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. J Bone Joint Surg Br. 2012;94:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 187] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 77. | Zaffagnini S, Boffa A, Andriolo L, Reale D, Busacca M, Di Martino A, Filardo G. Mosaicplasty vs Matrix-Assisted Autologous Chondrocyte Transplantation for Knee Cartilage Defects: A Long-Term Clinical and Imaging Evaluation. App Sci-Basel. 2020;10:4615. [DOI] [Full Text] |
| 78. | GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789-1858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9354] [Cited by in RCA: 8390] [Article Influence: 1048.8] [Reference Citation Analysis (4)] |
| 79. | Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13:456-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1032] [Cited by in RCA: 924] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 80. | Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18:730-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 639] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 81. | Arøen A, Løken S, Heir S, Alvik E, Ekeland A, Granlund OG, Engebretsen L. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 375] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 82. | Hunter W. Of the structure and disease of articulating cartilages. 1743. Clin Orthop Relat Res. 1995;3-6. [PubMed] |
| 83. | William H. Of the structure and diseases of articulating cartilagesPhil. Trans R Soc. 1743;42:514-521. [RCA] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 240] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 84. | Buckwalter JA. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther. 1998;28:192-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 323] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 85. | Muthu S. Osteoarthritis, an old wine in a new bottle! World J Orthop. 2023;14:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 86. | Kim HK, Moran ME, Salter RB. The potential for regeneration of articular cartilage in defects created by chondral shaving and subchondral abrasion. An experimental investigation in rabbits. J Bone Joint Surg Am. 1991;73:1301-1315. [PubMed] |
| 87. | Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75:532-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 850] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 88. | Everhart JS, Campbell AB, Abouljoud MM, Kirven JC, Flanigan DC. Cost-efficacy of Knee Cartilage Defect Treatments in the United States. Am J Sports Med. 2020;48:242-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 89. | Hunziker EB, Lippuner K, Keel MJ, Shintani N. An educational review of cartilage repair: precepts & practice--myths & misconceptions--progress & prospects. Osteoarthritis Cartilage. 2015;23:334-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 90. | Niemeyer P, Becher C, Brucker PU, Buhs M, Fickert S, Gelse K, Günther D, Kaelin R, Kreuz P, Lützner J, Nehrer S, Madry H, Marlovits S, Mehl J, Ott H, Pietschmann M, Spahn G, Tischer T, Volz M, Walther M, Welsch G, Zellner J, Zinser W, Angele P. Significance of Matrix-augmented Bone Marrow Stimulation for Treatment of Cartilage Defects of the Knee: A Consensus Statement of the DGOU Working Group on Tissue Regeneration. Z Orthop Unfall. 2018;156:513-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 91. | Zheng MH, Willers C, Kirilak L, Yates P, Xu J, Wood D, Shimmin A. Matrix-induced autologous chondrocyte implantation (MACI): biological and histological assessment. Tissue Eng. 2007;13:737-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 92. | Wang CC, Yang KC, Lin KH, Liu YL, Liu HC, Lin FH. Cartilage regeneration in SCID mice using a highly organized three-dimensional alginate scaffold. Biomaterials. 2012;33:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 93. | Vacanti JP. Beyond transplantation. Third annual Samuel Jason Mixter lecture. Arch Surg. 1988;123:545-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 115] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 94. | Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4207] [Cited by in RCA: 3675] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 95. | Teo AQA, Wong KL, Shen L, Lim JY, Toh WS, Lee EH, Hui JHP. Equivalent 10-Year Outcomes After Implantation of Autologous Bone Marrow-Derived Mesenchymal Stem Cells Versus Autologous Chondrocyte Implantation for Chondral Defects of the Knee. Am J Sports Med. 2019;47:2881-2887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 96. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15369] [Article Influence: 569.2] [Reference Citation Analysis (2)] |
| 97. | Prajwal GS, Jeyaraman N, Kanth V K, Jeyaraman M, Muthu S, Rajendran SNS, Rajendran RL, Khanna M, Oh EJ, Choi KY, Chung HY, Ahn BC, Gangadaran P. Lineage Differentiation Potential of Different Sources of Mesenchymal Stem Cells for Osteoarthritis Knee. Pharmaceuticals (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 98. | Devitt BM, Bell SW, Webster KE, Feller JA, Whitehead TS. Surgical treatments of cartilage defects of the knee: Systematic review of randomised controlled trials. Knee. 2017;24:508-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 99. | Martinčič D, Leban J, Filardo G, Busacca M, Barlič A, Veber M, Drobnič M. Autologous chondrocytes versus filtered bone marrow mesenchymal stem/stromal cells for knee cartilage repair-a prospective study. Int Orthop. 2021;45:931-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 100. | Krych AJ, Nawabi DH, Farshad-Amacker NA, Jones KJ, Maak TG, Potter HG, Williams RJ 3rd. Bone Marrow Concentrate Improves Early Cartilage Phase Maturation of a Scaffold Plug in the Knee: A Comparative Magnetic Resonance Imaging Analysis to Platelet-Rich Plasma and Control. Am J Sports Med. 2016;44:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li J, China S-Editor: Chen YL L-Editor: A P-Editor: Yuan YY