Published online Oct 18, 2024. doi: 10.5312/wjo.v15.i10.908
Revised: August 31, 2024
Accepted: September 11, 2024
Published online: October 18, 2024
Processing time: 87 Days and 3.5 Hours
The field of orthopedic and regenerative medicine is rapidly evolving with the increasing utilization of orthobiologic. These biologically derived therapies, inc
Core Tip: Orthobiologics, including platelet-rich plasma, mesenchymal stem cells, bone marrow aspirate concentrate, stromal vascular fraction, and autologous chondrocyte implantation, show significant potential in enhancing musculoskeletal healing and reducing the need for invasive surgeries. Despite their growing popularity, inconsistencies in treatment protocols and evidence levels highlight the need for standardized, high-quality research. Future advancements in delivery systems, personalized medicine, and novel cell sources may further optimize their efficacy and safety.
- Citation: Jeyaraman M, Jeyaraman N, Ramasubramanian S, Balaji S, Muthu S. Evidence-based orthobiologic practice: Current evidence review and future directions. World J Orthop 2024; 15(10): 908-917
- URL: https://www.wjgnet.com/2218-5836/full/v15/i10/908.htm
- DOI: https://dx.doi.org/10.5312/wjo.v15.i10.908
The field of orthopedic and regenerative medicine is witnessing a surge in interest and application of orthobiologics. These biologically derived therapies, such as platelet-rich plasma (PRP), Adipose tissue-derived Mesenchymal Stem Cells (AD-MSCs), and Bone Marrow Aspirate Concentrate (BMAC), are being recognized for their potential to significantly improve the treatment outcomes of musculoskeletal injuries and degenerative conditions. The promise of these therapies lies in their ability to enhance the body's natural healing processes, offering a less invasive alternative to traditional surgical methods.
Orthobiologics is a dynamic and rapidly advancing field dedicated to the application of biologically derived substances and techniques to enhance healing and regeneration in musculoskeletal tissues. This domain encompasses a diverse range of therapies, including cell-based treatments such as MSCs and cultured chondrocytes, blood-derived products like PRP, tissue grafts, growth factors, hormones, and extracellular matrix components such as hyaluronic acid. While the focus of this paper is primarily on specific cell-based therapies and blood-derived products, it is crucial to recognize the broader and continually evolving nature of the orthobiologics landscape.
Orthobiologics involves using biological substances to promote the repair and regeneration of musculoskeletal tissues. These therapies are increasingly employed to address a wide array of conditions, including osteoarthritis, tendon and ligament injuries, cartilage defects, muscle injuries, and bone fractures[1-4]. To provide a comprehensive understanding of the field, it is essential to outline the major categories of orthobiologics currently in use or under investigation:
Utilized in the form of PRP, platelet-rich fibrin, platelet lysate, autologous conditioned serum, autologous protein solution, autologous conditioned plasma, hyperacute serum, growth factor concentrates, plasma rich in growth factors, and gold-indued cytokines.
These multipotent cells can be harvested from several sources namely: (1) Autologous (bone marrow, adipose tissue, umbilical cord, amniotic fluid, dental pulp, hair follicle, periosteum, menstrual blood, peripheral blood, and synovial fluid); and (2) allogenic.
Utilized in the form of BMA and BMAC.
Utilized in the form of adipose-derived stem cells, stromal vascular fraction (SVF), micro-fat, nano-fat, microvascular fragments, and exosomes.
The patient's chondrocytes are cultured and reimplanted.
Expanded under laboratory conditions to increase cell numbers.
Including bone morphogenetic proteins (BMPs), which are utilized to enhance bone healing.
Autologous or allogeneic grafts used for tissue repair and regeneration.
The clinical use of these orthobiologic techniques varies across the globe, influenced by differing regulatory frame
Despite the growing popularity and enthusiasm surrounding these therapies, the scientific validation of their efficacy and safety remains inconsistent and often controversial. Recent studies have shown promising results for the use of orthobiologics. For instance, PRP therapy has demonstrated significant potential in managing early knee osteoarthritis[5] and enhancing the healing process following rotator cuff repairs[6]. Similarly, stem cell therapies are being explored for their ability to regenerate cartilage in osteoarthritic joints[7] and improve recovery from tendon injuries[8]. However, the field faces challenges such as variability in treatment protocols, inconsistent outcomes, and regulatory concerns. The clinical application of orthobiologics also extends to chronic wound healing, where therapies like PRP have shown effectiveness in promoting tissue regeneration and reducing healing time[9]. Moreover, advancements in molecular biology and drug delivery systems are paving the way for more targeted and controlled release of bioactive molecules, enhancing the therapeutic potential of orthobiologics. This editorial aim to provide a concise overview of the current evidence levels in the field of orthobiologics.
Orthobiologics are increasingly being utilized in various clinical scenarios to promote the healing and regeneration of musculoskeletal tissues. They are particularly prominent in the treatment of sports injuries, osteoarthritis, and spinal disorders. PRP, in particular, has demonstrated effectiveness in treating tendinopathies like tennis elbow and patellar tendinitis. It achieves this by delivering growth factors that promote healing and reduce inflammation. Similarly, stem cell therapies, especially those involving MSCs, are being explored for their potential to repair and regenerate damaged ligaments and tendons. These therapies show promise in accelerating recovery and reducing the necessity for surgical in
The popularity and market growth of orthobiologics are driven by several factors, including the increasing prevalence of musculoskeletal conditions, advancements in biological therapies, and a growing preference for minimally invasive treatments. The global orthobiologics market is projected to surpass USD 11.4 billion by 2032. This expansion is fueled by the increasing prevalence of musculoskeletal conditions and sports injuries, advancements in biologic therapies, a gro
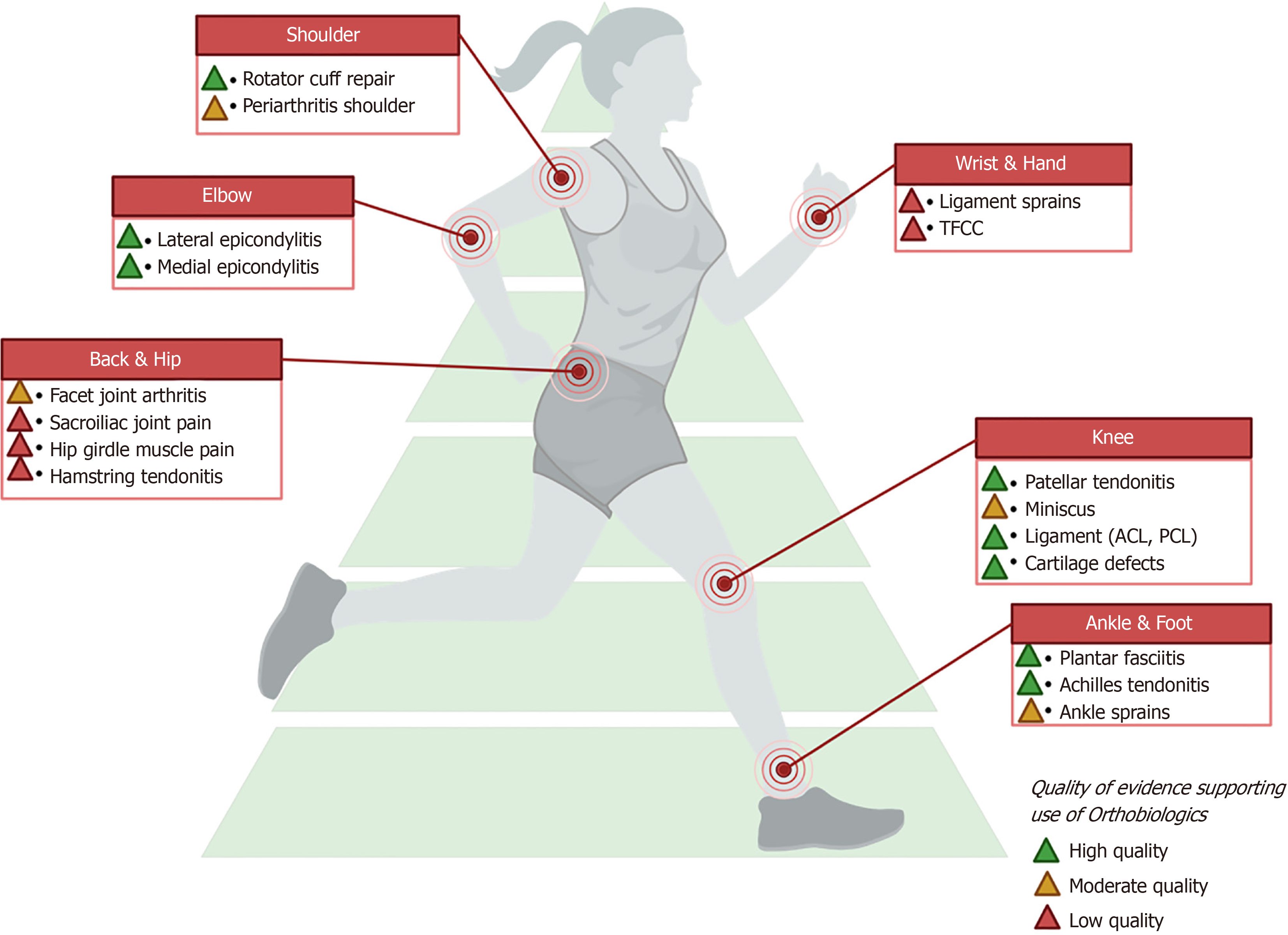
Table 1 summarizes the indications for orthobiologic products and the level of evidence supporting their usage across various musculoskeletal conditions. We used the Level of Evidence table to ascertain the level of evidence of the studies available for a given orthobiologic[15]. Further, we graded the studies for their quality, based on the bias in their study design into high, moderate, and low[16,17]. For example: Despite the availability of randomized controlled trials (RCTs) for a given orthobiologic if the quality parameters are not satisfied they are downgraded by one level. Orthobiologics such as PRP, BMAC/Microfragmented Adipose Tissue/(SVF/MFAT/AD-MSC), allogeneic MSC, and cultured cho
| Indication | Orthobiologic product | Level of evidence | Ref. |
| Knee osteoarthritis | PRP | 1 | [18-20] |
| BMAC | 1 | [21,22] | |
| SVF/MFAT/AD-MSC | 1 | [23-25] | |
| Allogeneic MSC | 1 | [26-28] | |
| Cultured chondrocytes (ACI/MACI) | 1 | [29] | |
| Avascular necrosis of femoral head | BMAC | 1 | [30-32] |
| SVF/MFAT/AD-MSC | 4 | [33,34] | |
| Cultured osteoblasts | 4 | [35-37] | |
| Lateral epicondylitis | PRP | 1 | [1,38] |
| BMAC | 1 | [39] | |
| Achilles tendinopathy | PRP | 1 | [40,41] |
| BMAC | 1 | [39] | |
| Patellar tendinopathy | PRP | 1 | [42,43] |
| BMAC | 1 | [39] | |
| Adhesive capsulitis | PRP | 1 | [44,45] |
| Plantar fasciitis | PRP | 1 | [46,47] |
| SVF | 4 | [48] | |
| Degenerative disc disease | PRP | 1 | [49,50] |
| BMAC | 1 | [51-53] | |
| Fracture | PRP | 1 | [54-56] |
| BMAC | 1 | [57-59] | |
| ACL augment | PRP | 1 | [60,61] |
| BMAC | 1 | [62] | |
| Meniscus repair | PRP | 1 | [63-65] |
| BMAC | 4 | [66,67] | |
| MFAT | 1 | [68] | |
| Rotator cuff repair augment | PRP | 1 | [69,70] |
| BMAC | 1 | [39,71,72] | |
| Ankle sprain | PRP | 1 | [73] |
| Acute muscle injuries | PRP | 1 | [74] |
| Ankle osteoarthritis | PRP | 1 | [75,76] |
| Carpal tunnel syndrome | PRP | 1 | [77,78] |
For knee osteoarthritis, multiple orthobiologics such as PRP, BMAC, SVF/MFAT/AD-MSC, allogeneic MSC, and cultured chondrocytes demonstrate a high level of evidence (Level 1). Similarly, PRP and BMAC show strong support (Level 1) in the treatment of conditions like lateral epicondylitis, Achilles tendinopathy, patellar tendinopathy, adhesive capsulitis, plantar fasciitis, degenerative disc disease, fractures, ACL augmentation, and meniscus repair (with MFAT also showing Level 1 evidence). In contrast, avascular necrosis of the femoral head shows high-level evidence (Level 1) for BMAC, but a lower level of evidence (Level 4) for SVF/MFAT/AD-MSC and cultured osteoblasts. Other conditions like plantar fasciitis treated with SVF and meniscus repair with BMAC exhibit Level 4 evidence, suggesting the need for further research to validate their efficacy. The data suggest that PRP and BMAC are extensively supported by high-qua
The development and application of orthobiologics face significant regulatory challenges. Regulatory bodies like the European Medicines Agency and the United States Food and Drug Administration require extensive clinical data to ensure safety and efficacy, but the rapid advancement of these therapies often outpaces regulatory frameworks. This mismatch can lead to delays in approval and commercialization[79-81]. Ethically, the use of stem cells and other bio
Despite the promising potential of orthobiologics, several research gaps need to be addressed. One of the key gaps is the lack of standardized protocols for the preparation and application of these therapies. The variability in methods for isolating and concentrating biological materials, such as PRP and stem cells, leads to inconsistent results across studies, making it challenging to draw definitive conclusions about their efficacy. Moreover, long-term safety and efficacy data are scarce. Most studies focus on short-term outcomes, and there is a need for longitudinal studies that follow patients over several years to understand the durability of the benefits and any potential long-term adverse effects. There is also a need for more high-quality RCTs to provide robust evidence that can guide clinical practice.
The future of orthobiologics is promising, driven by advancements in biotechnology and personalized medicine. One exciting prospect is the development of more sophisticated delivery systems that can target biological materials precisely to the site of injury or disease. For instance, advances in nanotechnology and drug delivery systems could enhance the efficacy of growth factors and stem cells by ensuring sustained and controlled release. Personalized medicine also holds great potential for orthobiologics. By leveraging genomic and proteomic data, clinicians can tailor biologic therapies to the individual patient’s biological profile, improving outcomes and reducing the risk of adverse reactions. This approach aligns with the broader trend in medicine towards more personalized and precision-based treatments.
iPSCs represent a promising frontier for future orthobiologic therapies, offering several key advantages that could revolutionize the field. Derived from adult somatic cells, iPSCs circumvent the ethical concerns associated with em
The field of orthobiologics shows immense potential in enhancing musculoskeletal healing and regeneration. Despite pro
| 1. | Muthu S, Patel S, Gobbur A, Patil SC, Ks KH, Yadav V, Jeyaraman M. Platelet-rich plasma therapy ensures pain reduction in the management of lateral epicondylitis - a PRISMA-compliant network meta-analysis of randomized controlled trials. Expert Opin Biol Ther. 2022;22:535-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Ranjan R, Kumar R, Jeyaraman M, Arora A, Kumar S, Nallakumarasamy A. Autologous platelet-rich plasma in the delayed union of long bone fractures - A quasi experimental study. J Orthop. 2023;36:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Jeyaraman M, Jeyaraman N, Jayakumar T, Ramasubramanian S, Ranjan R, Jha SK, Gupta A. Efficacy of stromal vascular fraction for knee osteoarthritis: A prospective, single-centre, non-randomized study with 2 years follow-up. World J Orthop. 2024;15:457-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Muthu S, Patel S, Selvaraj P, Jeyaraman M. Comparative analysis of leucocyte poor vs leucocyte rich platelet-rich plasma in the management of lateral epicondylitis: Systematic review & meta-analysis of randomised controlled trials. J Clin Orthop Trauma. 2021;19:96-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Zhao K, Liu YS, Nie LY, Qian LN, Nie NF, Leptihn S, Bunpetch V, Xu JQ, Zou XH, Ouyang H. The influence of sample size and gender composition on the meta-analysis conclusion of platelet-rich plasma treatment for osteoarthritis. J Orthop Translat. 2020;22:34-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Poff G, Spencer E, Scott B, Sleadd R, Broyles J. Comparison of Clinical Outcomes after Platelet-Rich Plasma and Rotator Cuff Repair in High-Grade Intrasubstance Partial Rotator Cuff Tears. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Pabinger C, Lothaller H, Kobinia GS. Intra-articular injection of bone marrow aspirate concentrate (mesenchymal stem cells) in KL grade III and IV knee osteoarthritis: 4 year results of 37 knees. Sci Rep. 2024;14:2665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 8. | Cho WS, Chung SG, Kim W, Jo CH, Lee SU, Lee SY. Mesenchymal Stem Cells Use in the Treatment of Tendon Disorders: A Systematic Review and Meta-Analysis of Prospective Clinical Studies. Ann Rehabil Med. 2021;45:274-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Qu W, Wang Z, Hunt C, Morrow AS, Urtecho M, Amin M, Shah S, Hasan B, Abd-Rabu R, Ashmore Z, Kubrova E, Prokop LJ, Murad MH. The Effectiveness and Safety of Platelet-Rich Plasma for Chronic Wounds: A Systematic Review and Meta-analysis. Mayo Clin Proc. 2021;96:2407-2417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Cavallo C, Roffi A, Grigolo B, Mariani E, Pratelli L, Merli G, Kon E, Marcacci M, Filardo G. Platelet-Rich Plasma: The Choice of Activation Method Affects the Release of Bioactive Molecules. Biomed Res Int. 2016;2016:6591717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 189] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 11. | Han Y, Huang H, Pan J, Lin J, Zeng L, Liang G, Yang W, Liu J. Meta-analysis Comparing Platelet-Rich Plasma vs Hyaluronic Acid Injection in Patients with Knee Osteoarthritis. Pain Med. 2019;20:1418-1429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Jeyaraman M, Karthik KS, Choudary D, Jeyaraman N, Nallakumarasamy A, Ramasubramian S. Autologous Bone Marrow Aspiration Concentrate (BMAC) Therapy for Primary Knee Osteoarthritis-An Observational and Dose Escalation Study. Indian J Orthop. 2024;58:1016-1026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Jeyaraman M, Jeyaraman N, Ramasubramanian S, Ranjan R, Jha SK, Gupta A. Bone Marrow Aspirate Concentrate for Treatment of Primary Knee Osteoarthritis: A Prospective, Single-Center, Non-randomized Study with 2-Year Follow-Up. Indian J Orthop. 2024;58:894-904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Rodeo SA. Orthobiologics: Current Status in 2023 and Future Outlook. J Am Acad Orthop Surg. 2023;31:604-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 15. | Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128:305-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1559] [Cited by in RCA: 1506] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 16. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 18678] [Article Influence: 2668.3] [Reference Citation Analysis (0)] |
| 17. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 12507] [Article Influence: 1250.7] [Reference Citation Analysis (2)] |
| 18. | Simental-Mendía M, Ortega-Mata D, Tamez-Mata Y, Olivo CAA, Vilchez-Cavazos F. Comparison of the clinical effectiveness of activated and non-activated platelet-rich plasma in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Clin Rheumatol. 2023;42:1397-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 19. | Xiong Y, Gong C, Peng X, Liu X, Su X, Tao X, Li Y, Wen Y, Li W. Efficacy and safety of platelet-rich plasma injections for the treatment of osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Front Med (Lausanne). 2023;10:1204144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 20. | Nie LY, Zhao K, Ruan J, Xue J. Effectiveness of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Clinical Trials. Orthop J Sports Med. 2021;9:2325967120973284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 21. | Keeling LE, Belk JW, Kraeutler MJ, Kallner AC, Lindsay A, McCarty EC, Postma WF. Bone Marrow Aspirate Concentrate for the Treatment of Knee Osteoarthritis: A Systematic Review. Am J Sports Med. 2022;50:2315-2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 22. | Belk JW, Lim JJ, Keeter C, McCulloch PC, Houck DA, McCarty EC, Frank RM, Kraeutler MJ. Patients With Knee Osteoarthritis Who Receive Platelet-Rich Plasma or Bone Marrow Aspirate Concentrate Injections Have Better Outcomes Than Patients Who Receive Hyaluronic Acid: Systematic Review and Meta-analysis. Arthroscopy. 2023;39:1714-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 23. | Anil U, Markus DH, Hurley ET, Manjunath AK, Alaia MJ, Campbell KA, Jazrawi LM, Strauss EJ. The efficacy of intra-articular injections in the treatment of knee osteoarthritis: A network meta-analysis of randomized controlled trials. Knee. 2021;32:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 24. | Jeyaraman M, Muthu S, Ganie PA. Does the Source of Mesenchymal Stem Cell Have an Effect in the Management of Osteoarthritis of the Knee? Meta-Analysis of Randomized Controlled Trials. Cartilage. 2021;13:1532S-1547S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | Bolia IK, Bougioukli S, Hill WJ, Trasolini NA, Petrigliano FA, Lieberman JR, Weber AE. Clinical Efficacy of Bone Marrow Aspirate Concentrate Versus Stromal Vascular Fraction Injection in Patients With Knee Osteoarthritis: A Systematic Review and Meta-analysis. Am J Sports Med. 2022;50:1451-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Han X, Yang B, Zou F, Sun J. Clinical therapeutic efficacy of mesenchymal stem cells derived from adipose or bone marrow for knee osteoarthritis: a meta-analysis of randomized controlled trials. J Comp Eff Res. 2020;9:361-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Jeyaraman M, Muthu S, Nischith DS, Jeyaraman N, Nallakumarasamy A, Khanna M. PRISMA-Compliant Meta-Analysis of Randomized Controlled Trials on Osteoarthritis of Knee Managed with Allogeneic vs Autologous MSCs: Efficacy and Safety Analysis. Indian J Orthop. 2022;56:2042-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Iijima H, Isho T, Kuroki H, Takahashi M, Aoyama T. Effectiveness of mesenchymal stem cells for treating patients with knee osteoarthritis: a meta-analysis toward the establishment of effective regenerative rehabilitation. NPJ Regen Med. 2018;3:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 29. | Dhillon J, Decilveo AP, Kraeutler MJ, Belk JW, McCulloch PC, Scillia AJ. Third-Generation Autologous Chondrocyte Implantation (Cells Cultured Within Collagen Membrane) Is Superior to Microfracture for Focal Chondral Defects of the Knee Joint: Systematic Review and Meta-analysis. Arthroscopy. 2022;38:2579-2586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 30. | Jindal K, Aggarwal S, Kumar P, Rathod P. Core decompression with bone marrow aspirate concentrate in post collapse avascular necrosis of hip: A systematic review and meta-analysis. J Clin Orthop Trauma. 2021;17:78-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Ulusoy İ, Yılmaz M, Kıvrak A. Efficacy of autologous stem cell therapy in femoral head avascular necrosis: a comparative study. J Orthop Surg Res. 2023;18:799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Jeyaraman M, Muthu S, Jain R, Khanna M. Autologous bone marrow derived mesenchymal stem cell therapy for osteonecrosis of femoral head: A systematic overview of overlapping meta-analyses. J Clin Orthop Trauma. 2021;13:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Tantuway V, Jeyaraman M, Nallakumarasamy A, Prikh MB, Sharma AK, Sharma R. Functional Outcome Analysis of Autologous Stromal Vascular Fraction (SVF) (Sahaj Therapy(®)) Using Direct Sonication in Osteonecrosis of the Femoral Head (ONFH): A 6-Year Follow-Up Study. Indian J Orthop. 2024;58:68-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 34. | Zhang W, Zheng C, Yu T, Zhang H, Huang J, Chen L, Tong P, Zhen G. The therapeutic effect of adipose-derived lipoaspirate cells in femoral head necrosis by improving angiogenesis. Front Cell Dev Biol. 2022;10:1014789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 35. | Patro BP, Jeyaraman N, Jayakumar T, Das G, Nallakumarasamy A, Jeyaraman M. Efficacy of Autologous Adult Live-Cultured Osteoblast (AALCO) Implantation in Avascular Necrosis of the Femoral Head: A Mid-Term Outcome Analysis. Indian J Orthop. 2024;58:1053-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 36. | Sadat-Ali M, Al-Omran AS, AlTabash K, Acharya S, Hegazi TM, Al Muhaish MI. The clinical and radiological effectiveness of autologous bone marrow derived osteoblasts (ABMDO) in the management of avascular necrosis of femoral head (ANFH) in sickle cell disease (SCD). J Exp Orthop. 2022;9:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 37. | Palekar G, Bhalodiya HP, Archik S, Trivedi K. Retrospective Study on Implantation of Autologous-Cultured Osteoblasts for the Treatment of Patients with Avascular Necrosis of the Femoral Head. Orthop Res Rev. 2021;13:15-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 38. | Chen XT, Fang W, Jones IA, Heckmann ND, Park C, Vangsness CT Jr. The Efficacy of Platelet-Rich Plasma for Improving Pain and Function in Lateral Epicondylitis: A Systematic Review and Meta-analysis with Risk-of-Bias Assessment. Arthroscopy. 2021;37:2937-2952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Imam MA, Holton J, Horriat S, Negida AS, Grubhofer F, Gupta R, Narvani A, Snow M. A systematic review of the concept and clinical applications of bone marrow aspirate concentrate in tendon pathology. SICOT J. 2017;3:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 40. | Nauwelaers AK, Van Oost L, Peers K. Evidence for the use of PRP in chronic midsubstance Achilles tendinopathy: A systematic review with meta-analysis. Foot Ankle Surg. 2021;27:486-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 41. | Arthur Vithran DT, Xie W, Opoku M, Essien AE, He M, Li Y. The Efficacy of Platelet-Rich Plasma Injection Therapy in the Treatment of Patients with Achilles Tendinopathy: A Systematic Review and Meta-Analysis. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 42. | Dupley L, Charalambous CP. Platelet-Rich Plasma Injections as a Treatment for Refractory Patellar Tendinosis: A Meta-Analysis of Randomised Trials. Knee Surg Relat Res. 2017;29:165-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Barman A, Sinha MK, Sahoo J, Jena D, Patel V, Patel S, Bhattacharjee S, Baral D. Platelet-rich plasma injection in the treatment of patellar tendinopathy: a systematic review and meta-analysis. Knee Surg Relat Res. 2022;34:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 44. | Yu S, Hu R, Feng H, Huang D. Efficacy of platelet-rich plasma injection in the treatment of frozen shoulder: A systematic review and meta-analysis. J Back Musculoskelet Rehabil. 2023;36:551-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Lin HW, Tam KW, Liou TH, Rau CL, Huang SW, Hsu TH. Efficacy of Platelet-Rich Plasma Injection on Range of Motion, Pain, and Disability in Patients With Adhesive Capsulitis: A Systematic Review and Meta-analysis. Arch Phys Med Rehabil. 2023;104:2109-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 46. | Hohmann E, Tetsworth K, Glatt V. Platelet-Rich Plasma Versus Corticosteroids for the Treatment of Plantar Fasciitis: A Systematic Review and Meta-analysis. Am J Sports Med. 2021;49:1381-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 47. | Fei X, Lang L, Lingjiao H, Wei C, Zhou X. Platelet-rich plasma has better mid-term clinical results than traditional steroid injection for plantar fasciitis: A systematic review and meta-analysis. Orthop Traumatol Surg Res. 2021;107:103007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Wu B, Xiao S, Yang S, Wei Z, Deng C. A New Minimally Invasive Procedure for Treating Plantar Heel Pain: Stromal Vascular Fraction Gel Grafting. Ann Plast Surg. 2023;91:609-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 49. | Schneider BJ, Hunt C, Conger A, Qu W, Maus TP, Vorobeychik Y, Cheng J, Duszynski B, McCormick ZL. The effectiveness of intradiscal biologic treatments for discogenic low back pain: a systematic review. Spine J. 2022;22:226-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 50. | Chang MC, Park D. The Effect of Intradiscal Platelet-Rich Plasma Injection for Management of Discogenic Lower Back Pain: A Meta-Analysis. J Pain Res. 2021;14:505-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Her YF, Kubrova E, Martinez Alvarez GA, D'Souza RS. The Analgesic Efficacy of Intradiscal Injection of Bone Marrow Aspirate Concentrate and Culture-Expanded Bone Marrow Mesenchymal Stromal Cells in Discogenic Pain: A Systematic Review. J Pain Res. 2022;15:3299-3318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 52. | Law L, Hunt CL, van Wijnen AJ, Nassr A, Larson AN, Eldrige JS, Mauck WD, Pingree MJ, Yang J, Muir CW, Erwin PJ, Bydon M, Qu W. Office-Based Mesenchymal Stem Cell Therapy for the Treatment of Musculoskeletal Disease: A Systematic Review of Recent Human Studies. Pain Med. 2019;20:1570-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Yim RL, Lee JT, Bow CH, Meij B, Leung V, Cheung KM, Vavken P, Samartzis D. A systematic review of the safety and efficacy of mesenchymal stem cells for disc degeneration: insights and future directions for regenerative therapeutics. Stem Cells Dev. 2014;23:2553-2567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 54. | Jamal MS, Hurley ET, Asad H, Asad A, Taneja T. The role of Platelet Rich Plasma and other orthobiologics in bone healing and fracture management: A systematic review. J Clin Orthop Trauma. 2022;25:101759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 55. | Li S, Xing F, Luo R, Liu M. Clinical Effectiveness of Platelet-Rich Plasma for Long-Bone Delayed Union and Nonunion: A Systematic Review and Meta-Analysis. Front Med (Lausanne). 2021;8:771252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 56. | Zhang Y, Xing F, Luo R, Duan X. Platelet-Rich Plasma for Bone Fracture Treatment: A Systematic Review of Current Evidence in Preclinical and Clinical Studies. Front Med (Lausanne). 2021;8:676033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 57. | Palombella S, Lopa S, Gianola S, Zagra L, Moretti M, Lovati AB. Bone Marrow-Derived Cell Therapies to Heal Long-Bone Nonunions: A Systematic Review and Meta-Analysis-Which Is the Best Available Treatment? Stem Cells Int. 2019;2019:3715964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Attia AK, Robertson GAJ, McKinley J, d'Hooghe PP, Maffulli N. Surgical Management of Jones Fractures in Athletes: Orthobiologic Augmentation: A Systematic Review and Meta-analysis of 718 Fractures. Am J Sports Med. 2023;51:2216-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 59. | Imam MA, Holton J, Ernstbrunner L, Pepke W, Grubhofer F, Narvani A, Snow M. A systematic review of the clinical applications and complications of bone marrow aspirate concentrate in management of bone defects and nonunions. Int Orthop. 2017;41:2213-2220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 60. | Zhu T, Zhou J, Hwang J, Xu X. Effects of Platelet-Rich Plasma on Clinical Outcomes After Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis. Orthop J Sports Med. 2022;10:23259671211061535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 61. | Lv ZT, Zhang JM, Pang ZY, Wang Z, Huang JM, Zhu WT. The efficacy of platelet rich plasma on anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Platelets. 2022;33:229-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 62. | Cao Y, Zhang Z, Song G, Ni Q, Zheng T, Li Y. Biological enhancement methods may be a viable option for ACL arthroscopic primary repair - A systematic review. Orthop Traumatol Surg Res. 2022;108:103227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 63. | Li Z, Weng X. Platelet-rich plasma use in meniscus repair treatment: a systematic review and meta-analysis of clinical studies. J Orthop Surg Res. 2022;17:446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 64. | Zaffagnini S, Poggi A, Reale D, Andriolo L, Flanigan DC, Filardo G. Biologic Augmentation Reduces the Failure Rate of Meniscal Repair: A Systematic Review and Meta-analysis. Orthop J Sports Med. 2021;9:2325967120981627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 65. | Xie Y, Xing Q, Wang S, Yang Z, Hu A, Wu Q. Can platelet-rich plasma enhance the effect of meniscus repair? A meta-analysis of randomized controlled trials Platelet-rich plasma and meniscus repair. J Orthop Surg (Hong Kong). 2022;30:10225536221131483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Massey PA, Sampognaro G, Starnes E, Lowery MT, Duncan M, Sherman WF, Zhang AS. Improved Outcomes After Reinforced Radial Meniscus Repair Augmented With Bone Marrow Aspirate Concentrate. Arthrosc Sports Med Rehabil. 2023;5:e843-e851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 67. | Dancy ME, Marigi EM, Krych AJ, Werner BC, Camp CL. Impact of Biologic Augmentation on Revision Surgery Rates After Meniscus Repair: A Matched-Cohort Analysis of 3420 Patients. Orthop J Sports Med. 2023;11:23259671231186990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 68. | Conte P, Anzillotti G, Di Matteo B, Gallese A, Vitale U, Marcacci M, Kon E. Orthobiologic injections for treating degenerative meniscus lesions: a matter of facts? Ten years of clinical experience in a systematic review. J Cartilage Joint Preser. 2023;3:100104. [DOI] [Full Text] |
| 69. | Zhao JG, Zhao L, Jiang YX, Wang ZL, Wang J, Zhang P. Platelet-rich plasma in arthroscopic rotator cuff repair: a meta-analysis of randomized controlled trials. Arthroscopy. 2015;31:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 70. | Hurley ET, Lim Fat D, Moran CJ, Mullett H. The Efficacy of Platelet-Rich Plasma and Platelet-Rich Fibrin in Arthroscopic Rotator Cuff Repair: A Meta-analysis of Randomized Controlled Trials. Am J Sports Med. 2019;47:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 71. | Muthu S, Mogulesh C, Viswanathan VK, Jeyaraman N, Pai SN, Jeyaraman M, Khanna M. Is cellular therapy beneficial in management of rotator cuff tears? Meta-analysis of comparative clinical studies. World J Meta-Anal. 2022;10:162-176. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 72. | Liu F, Meng Q, Yin H, Yan Z. Stem Cells in Rotator Cuff Injuries and Reconstructions: A Systematic Review and Meta-Analysis. Curr Stem Cell Res Ther. 2019;14:683-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 73. | Milo AMM, Braganza CL. Platelet-Rich Plasma on Ankle Sprains - Efficacy on Pain Reduction and Shorter Return to Play: A Systematic Review of Available Randomized Control Trials. J Med University St Tomas. 2023;7:1153-1160. [DOI] [Full Text] |
| 74. | Grassi A, Napoli F, Romandini I, Samuelsson K, Zaffagnini S, Candrian C, Filardo G. Is Platelet-Rich Plasma (PRP) Effective in the Treatment of Acute Muscle Injuries? A Systematic Review and Meta-Analysis. Sports Med. 2018;48:971-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 75. | Laohajaroensombat S, Prusmetikul S, Rattanasiri S, Thakkinstian A, Woratanarat P. Platelet-rich plasma injection for the treatment of ankle osteoarthritis: a systematic review and meta-analysis. J Orthop Surg Res. 2023;18:373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 76. | Ding SL, Ji LF, Zhang MZ, Xiong W, Sun CY, Han ZY, Wang C. Safety and efficacy of intra-articular injection of platelet-rich plasma for the treatment of ankle osteoarthritis: a systematic review and meta-analysis. Int Orthop. 2023;47:1963-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 77. | Catapano M, Catapano J, Borschel G, Alavinia SM, Robinson LR, Mittal N. Effectiveness of Platelet-Rich Plasma Injections for Nonsurgical Management of Carpal Tunnel Syndrome: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Arch Phys Med Rehabil. 2020;101:897-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 78. | Dong C, Sun Y, Qi Y, Zhu Y, Wei H, Wu D, Li C. Effect of Platelet-Rich Plasma Injection on Mild or Moderate Carpal Tunnel Syndrome: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biomed Res Int. 2020;2020:5089378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 79. | Master Z, Matthews KRW, Abou-El-Enein M. Unproven stem cell interventions: A global public health problem requiring global deliberation. Stem Cell Reports. 2021;16:1435-1445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 80. | Guleria I, de Los Angeles Muñiz M, Wilgo M, Bapat A, Cui W, Hsu YS, Jeyaraman M, Muthu S, Rodriguez F, Fesnak A, Celluzzi C, Sesok-Pizzini D, Reich-Slotky R, Spitzer T. How do I: Evaluate the safety and legitimacy of unproven cellular therapies? Transfusion. 2022;62:518-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | George B. Regulations and guidelines governing stem cell based products: Clinical considerations. Perspect Clin Res. 2011;2:94-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 82. | Vaish A, Vaishya R. Stem cells in orthopaedics and sports injuries: A comprehensive review and future research directions. J Orthop Rep. 2024;3:100344. [DOI] [Full Text] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/