Published online Jan 18, 2024. doi: 10.5312/wjo.v15.i1.45
Peer-review started: August 20, 2023
First decision: November 2, 2023
Revised: November 13, 2023
Accepted: December 13, 2023
Article in press: December 13, 2023
Published online: January 18, 2024
Processing time: 148 Days and 20 Hours
Previous studies investigating the association between loss of estrogen at menopause and skeletal muscle mass came to contradictory conclusions.
To evaluate the association between serum estradiol level and appendicular lean mass index in middle-aged postmenopausal women using population-based data.
This study included 673 postmenopausal women, aged 40-59 years, from the National Health and Nutrition Examination Survey between 2013 and 2016. Weighted multivariable linear regression models were used to evaluate the association between serum E2 Level and appendicular lean mass index (ALMI). When non-linear associations were found by using weighted generalized additive model and smooth curve fitting, two-piecewise linear regression models were further applied to examine the threshold effects.
There was a positive association between serum E2 level and ALMI. Compared to individuals in quartile 1 group, those in other quartiles had higher ALMI levels. An inverted U-shaped curve relationship between serum E2 Level and ALMI was found on performing weighted generalized additive model and smooth curve fitting, and the inflection point was identified as a serum E2 level of 85 pg/mL.
Our results demonstrated an inverted U-shaped curve relationship between serum E2 levels and ALMI in middle-aged postmenopausal women, suggesting that low serum E2 levels play an important in the loss of muscle mass in middle-aged postmenopausal women.
Core Tip: This paper evaluated the association between serum E2 level and appendicular lean mass index in middle-aged postmenopausal women from the National Health and Nutrition Examination Survey between 2013 and 2016, and found an inverted U-shaped curve relationship between them, with the point of inflection at a serum E2 level of 85 pg/mL.
- Citation: Jin F, Wang YF, Zhu ZX. Association between serum estradiol level and appendicular lean mass index in middle-aged postmenopausal women. World J Orthop 2024; 15(1): 45-51
- URL: https://www.wjgnet.com/2218-5836/full/v15/i1/45.htm
- DOI: https://dx.doi.org/10.5312/wjo.v15.i1.45
Most women experience menopausal transition in middle age, when aging-related hormonal changes accelerate[1]. The onset of sarcopenia, a multifactorial condition related to the loss of muscle mass and quality, has been intimately linked to menopause[2,3].
Compared with the anabolic effects of androgens on the skeletal muscle mass in men[4,5], the effects of estrogens on the skeletal muscle mass in women are less clearly understood[6]. Moreover, previous studies on the association between the loss of estrogen at menopause and skeletal muscle mass or function came to contradictory conclusions[7]. As the most potent estrogen hormone, estradiol (E2) is responsible for the maintenance of sexual characteristics and muscle health[8]. Thus, we aimed to evaluate the association between serum E2 level and appendicular lean mass index (ALMI) in middle-aged postmenopausal women using population-based data.
The National Health and Nutrition Examination Survey (NHANES) is a large, ongoing cross-sectional survey conducted annually in a nationally representative sample of the non-institutionalized United States population. Data for this study were pooled from the NHANES between 2013 and 2016. The study population was restricted to postmenopausal women aged 40-59 years. Individuals with a regular period in the past 12 mo (n = 840), or with an unrecorded menopausal status (n = 287), as well as those with missing serum E2 Levels (n = 69) or ALMI data (n = 171) were excluded. Finally, 673 women were included in the analysis.
Written informed consent was obtained from all participants and the Institutional Review Board of the National Center for Health Statistics (NCHS) approved the survey protocols (Protocol #2011-17).
The exposure variable was the serum E2 level, which was measured based on the reference method of the National Institute for Standards and Technology, using isotope dilution liquid chromatography tandem mass spectrometry. The outcome variable was ALMI, which was measured by dual-energy X-ray absorptiometry whole-body scans and calculated as the appendicular lean mass (kg) divided by height squared (m2). The covariates included in this study were age, race, educational level, body mass index (BMI), ratio of family income to poverty, moderate activities, total protein, blood urea nitrogen, and serum uric acid and calcium levels. Detailed information on these variables can be found on the NHANES website (https://www.cdc.gov/nchs/nhanes/).
All estimates were applied with weights, in accordance with the guidelines edited by the NCHS[9], to account for the NHANES sampling method. All analyses were performed using EmpowerStats software (http://www.empowerstats.com) and R software (version 3.4.3). The statistical significance was set at P < 0.05. Weighted multivariable linear regression models were used to evaluate the association between serum E2 level and ALMI. Following the Strengthening the Reporting of Observational Studies in Epidemiology statement[10], we constructed three models: Model 1, no covariates were adjusted; Model 2, age and race were adjusted; and Model 3, all covariates presented in Table 1 were adjusted. When non-linear associations were found by using weighted generalized additive model and smooth curve fitting, two-piecewise linear regression models were further applied to examine the threshold effects.
| Serum estradiol level (pg/mL) | Q1 (≤ 3.80) | Q2 (3.88-7.42) | Q3 (7.45-17.50) | Q4 (≥ 17.60) | P value |
| Age (yr) | 54.4 ± 4.1 | 53.6 ± 4.0 | 52.9 ± 4.8 | 49.6 ± 4.9 | < 0.001 |
| Race/Ethnicity (%) | 0.584 | ||||
| Non-Hispanic White | 70.9 | 68.3 | 70.1 | 73.6 | |
| Non-Hispanic Black | 7.8 | 14.1 | 10.9 | 10.4 | |
| Mexican American | 6.1 | 8.3 | 8.3 | 6.3 | |
| Other race/ethnicity | 15.2 | 9.3 | 10.7 | 9.7 | |
| Education level (%) | 0.520 | ||||
| Less than high school | 13.3 | 14.1 | 12.7 | 10.1 | |
| High school | 24.5 | 19.2 | 24.9 | 19.0 | |
| More than high school | 62.2 | 66.6 | 62.3 | 71.0 | |
| Body mass index (kg/m2) | 25.6 ± 4.7 | 28.8 ± 4.8 | 32.2 ± 5.9 | 32.0 ± 8.3 | < 0.001 |
| Income to poverty ratio | 3.0 ± 1.8 | 3.3 ± 1.7 | 3.1 ± 1.5 | 3.4 ± 1.6 | 0.143 |
| Moderate activities (%) | 0.965 | ||||
| Yes | 49.1 | 47.2 | 49.7 | 49.8 | |
| No | 50.9 | 52.8 | 50.3 | 50.2 | |
| Total protein (g/L) | 69.9 ± 4.6 | 70.5 ± 4.1 | 71.1 ± 4.0 | 70.0 ± 3.4 | 0.022 |
| Blood urea nitrogen (mg/dL) | 5.0 ± 1.6 | 4.8 ± 1.6 | 4.8 ± 1.8 | 4.6 ± 1.2 | 0.076 |
| Serum uric acid (umol/L) | 263.8 ± 57.0 | 287.1 ± 69.7 | 302.9 ± 68.2 | 286.6 ± 67.6 | < 0.001 |
| Serum calcium (mg/dL) | 2.4 ± 0.1 | 2.4 ± 0.1 | 2.4 ± 0.1 | 2.3 ± 0.1 | 0.092 |
| Appendicular lean mass index (kg/m2) | 6.1 ± 1.0 | 6.8 ± 1.0 | 7.3 ± 1.1 | 7.5 ± 1.4 | < 0.001 |
Demographic characteristics of the participants subclassified based on the serum E2 level quartiles (Q1: ≤ 3.80 pg/mL; Q2: 3.88-7.42 pg/mL; Q3: 7.45-17.50 pg/mL; and Q4: ≥ 17.60 pg/mL) are shown in Table 1. Compared with the Q1 group, individuals in other groups were younger, and had lower levels of blood urea nitrogen, and higher levels of income to poverty ratio, BMI, total protein, serum uric acid, and ALMI.
The association between serum E2 level and ALMI was positive in each model, with a significant P for trend among the different serum E2 level quartile groups (Table 2). In the subgroup analysis stratified by BMI and race, this positive association was significant in the group with BMI < 25 kg/m2 (Table 3).
| Model 1 β (95%CI) | Model 2 β (95%CI) | Model 3 β (95%CI) | |
| Serum estradiol level | 0.004 (0.002, 0.007)a | 0.003 (0.001, 0.005)a | 0.001 (0.000, 0.002)b |
| Serum estradiol level categories | |||
| Q1 | Reference | Reference | Reference |
| Q2 | 0.665 (0.406, 0.924) | 0.607 (0.356, 0.859) | 0.090 (-0.036, 0.216) |
| Q3 | 1.222 (0.969, 1.475) | 1.199 (0.953, 1.445) | 0.128 (-0.002, 0.258) |
| Q4 | 1.369 (1.126, 1.612) | 1.385 (1.133, 1.637) | 0.268 (0.133, 0.402) |
| P value | < 0.001 | < 0.001 | < 0.001 |
| Model 1 β (95%CI) | Model 2 β (95%CI) | Model 3 β (95%CI) | |
| Stratified by BMI | |||
| BMI (< 25 kg/m2) | 0.002 (-0.000, 0.004) | 0.001 (-0.001, 0.004) | 0.002 (0.000, 0.003)a |
| BMI (25-29.9 kg/m2) | 0.003 (0.001, 0.005)b | 0.002 (0.000, 0.004)a | 0.001 (-0.001, 0.003) |
| BMI (≥ 30 kg/m2) | 0.006 (0.004, 0.009)c | 0.005 (0.002, 0.008)a | 0.001 (-0.001, 0.003) |
| Stratified by race | |||
| Non-Hispanic White | 0.003 (-0.000, 0.007) | 0.002 (-0.002, 0.006) | 0.002 (-0.000, 0.004) |
| Non-Hispanic Black | 0.004 (0.000, 0.007)a | 0.004 (-0.000, 0.007) | 0.001 (-0.000, 0.003) |
| Mexican American | 0.003 (-0.002, 0.008) | 0.003 (-0.002, 0.008) | -0.003 (-0.005, 0.000) |
| Other race | 0.015 (0.009, 0.022)c | 0.013 (0.007, 0.020)c | 0.002 (-0.001, 0.006) |
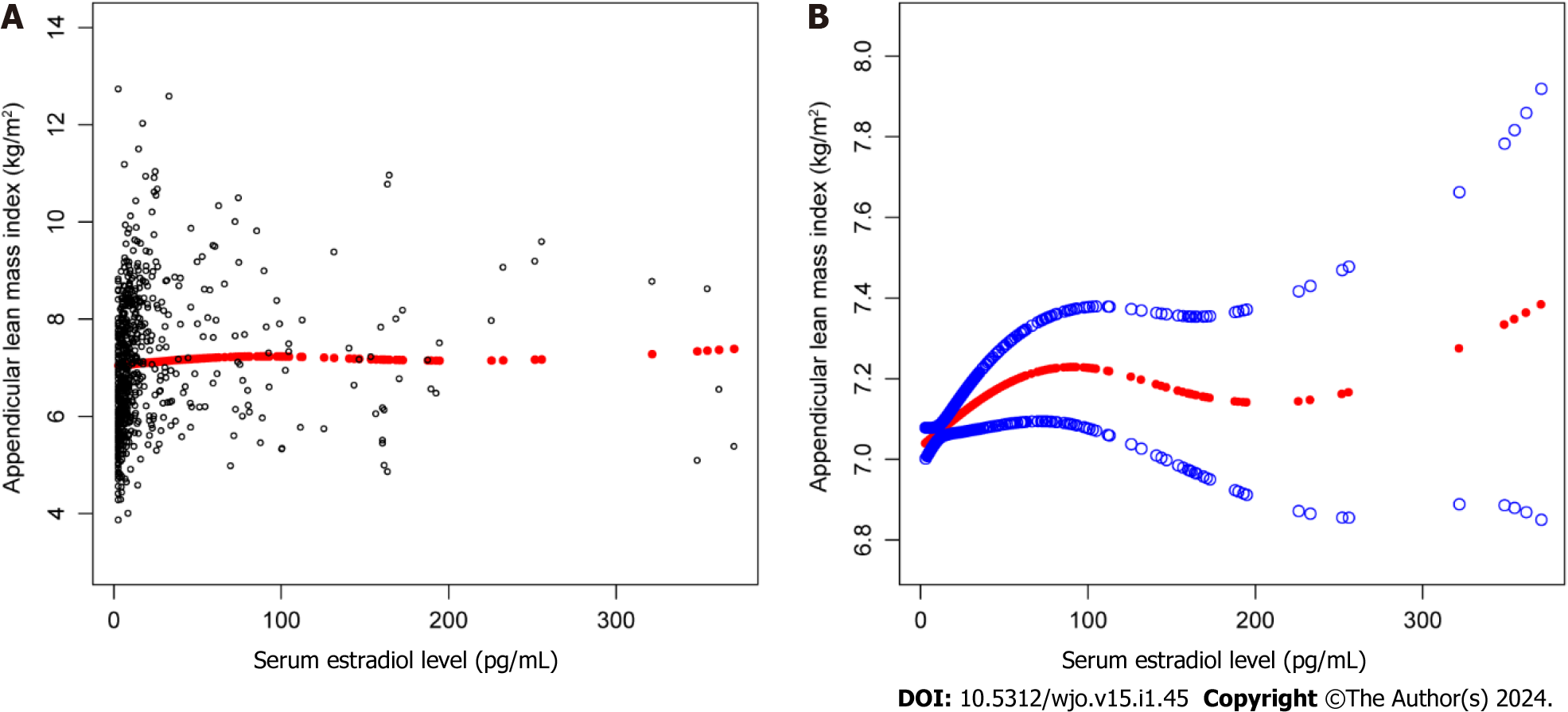
An inverted U-shaped curve relationship between serum E2 level and ALMI was found, as shown in Figure 1, and the inflection point was identified at a serum E2 level of 85 pg/mL (Table 4).
| Appendicular lean mass index | Adjusted β (95%CI), P value |
| Serum estradiol level | |
| Fitting by standard linear model | 0.001 (0.000, 0.002), 0.006 |
| Fitting by two-piecewise linear model | |
| Inflection point | 85 (pg/mL) |
| Serum estradiol level < 85 (pg/mL) | 0.004 (0.002, 0.007), < 0.001 |
| Serum estradiol level > 85 (pg/mL) | -0.001 (-0.003, 0.001), 0.280 |
| Log likelihood ratio | 0.003 |
This study evaluated the association between serum E2 level and ALMI in middle-aged postmenopausal women, and found an inverted U-shaped curve relationship between them, with the point of inflection at a serum E2 level of 85 pg/mL.
Estrogens, especially E2, are known to play an important role in the preservation of muscle health. Several studies have investigated the effects of hormone replacement therapy (HRT) and found that it has a positive and measurable impact on muscle function[11,12]. Conversely, other studies found that HRT does not protect against muscle loss[13,14]. Moreover, it was reported that menopausal HRT was associated with an increased risk of adverse events, such as dementia[15], stroke[16], and breast cancer[17]. Therefore, it is important to balance the potential benefits against risks. Our results revealed an inverted U-shaped curve relationship between serum E2 level and ALMI, suggesting that adequate E2 supplementation may be a useful adjunct therapy for individuals with a low serum E2 level.
The exact mechanism underlying the effects of E2 on skeletal muscle remains unclear. A possible explanation for the potentially beneficial effect is that E2 can stimulate the proliferative activity of the muscle satellite cells (stem cells) that are responsible for muscle tissue maintenance[18,19]. Another possible explanation is that estrogen deficiency results in the loss of muscle mass through apoptotic mechanisms[20,21]. Despite these possibilities, the molecular mechanism of the impact of E2 on muscle function needs to be further explored.
Data from the NHANES surveys were acquired following standard protocols, which ensured that the data were accurate and consistent. However, the limitations of this study should also be noted. First, a causal relationship between serum E2 level and ALMI in middle-aged postmenopausal women could not be determined due to the cross-sectional design of the NHANES surveys. Second, biases caused by unmeasured confounding factors cannot be excluded. Third, the conclusion cannot be generalized to older women because the population of this study was restricted to middle-aged postmenopausal women.
Overall, this study showed an inverted U-shaped curve relationship between serum E2 levels and ALMI in middle-aged postmenopausal women, suggesting that low serum E2 levels play a crucial role in the loss of muscle mass in middle-aged postmenopausal women.
The onset of sarcopenia, a multifactorial condition related to the loss of muscle mass and quality, has been intimately linked to menopause.
Compared with the anabolic effects of androgens on the skeletal muscle mass in men, the effects of estrogens on the skeletal muscle mass in women are less clearly understood. Moreover, previous studies on the association between the loss of estrogen at menopause and skeletal muscle mass or function came to contradictory conclusions.
We aimed to evaluate the association between serum E2 level and appendicular lean mass index (ALMI) in middle-aged postmenopausal women using population-based data.
This study included 673 postmenopausal women, aged 40-59 years, from the National Health and Nutrition Examination Survey between 2013 and 2016. Weighted multivariable linear regression models were used and when non-linear associations were found by using weighted generalized additive model and smooth curve fitting, two-piecewise linear regression models were further applied to examine the threshold effects.
There was a positive association between serum E2 level and ALMI. Compared to individuals in quartile 1 group, those in other quartiles had higher ALMI levels. An inverted U-shaped curve relationship between serum E2 level and ALMI was found on performing weighted generalized additive model and smooth curve fitting, and the inflection point was identified as a serum E2 Level of 85 pg/mL.
Our results demonstrated an inverted U-shaped curve relationship between serum E2 levels and ALMI in middle-aged postmenopausal women, suggesting that low serum E2 Levels play an important in the loss of muscle mass in middle-aged postmenopausal women.
The molecular mechanism of the impact of E2 on muscle function needs to be further explored.
| 1. | Nelson HD. Menopause. Lancet. 2008;371:760-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 553] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 2. | Mellen RH, Girotto OS, Marques EB, Laurindo LF, Grippa PC, Mendes CG, Garcia LNH, Bechara MD, Barbalho SM, Sinatora RV, Haber JFDS, Flato UAP, Bueno PCDS, Detregiachi CRP, Quesada K. Insights into Pathogenesis, Nutritional and Drug Approach in Sarcopenia: A Systematic Review. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 3. | Yang L, Smith L, Hamer M. Gender-specific risk factors for incident sarcopenia: 8-year follow-up of the English longitudinal study of ageing. J Epidemiol Community Health. 2019;73:86-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | De Spiegeleer A, Beckwée D, Bautmans I, Petrovic M; Sarcopenia Guidelines Development group of the Belgian Society of Gerontology and Geriatrics (BSGG). Pharmacological Interventions to Improve Muscle Mass, Muscle Strength and Physical Performance in Older People: An Umbrella Review of Systematic Reviews and Meta-analyses. Drugs Aging. 2018;35:719-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Ottenbacher KJ, Ottenbacher ME, Ottenbacher AJ, Acha AA, Ostir GV. Androgen treatment and muscle strength in elderly men: A meta-analysis. J Am Geriatr Soc. 2006;54:1666-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Anderson LJ, Liu H, Garcia JM. Sex Differences in Muscle Wasting. Adv Exp Med Biol. 2017;1043:153-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 156] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 7. | Priego T, Martín AI, González-Hedström D, Granado M, López-Calderón A. Role of hormones in sarcopenia. Vitam Horm. 2021;115:535-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 8. | Geraci A, Calvani R, Ferri E, Marzetti E, Arosio B, Cesari M. Sarcopenia and Menopause: The Role of Estradiol. Front Endocrinol (Lausanne). 2021;12:682012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 9. | Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, Curtin LR. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat 2. 2013;1-24. [PubMed] |
| 10. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6805] [Cited by in RCA: 13562] [Article Influence: 713.8] [Reference Citation Analysis (3)] |
| 11. | Taaffe DR, Newman AB, Haggerty CL, Colbert LH, de Rekeneire N, Visser M, Goodpaster BH, Nevitt MC, Tylavsky FA, Harris TB. Estrogen replacement, muscle composition, and physical function: The Health ABC Study. Med Sci Sports Exerc. 2005;37:1741-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Sørensen MB, Rosenfalck AM, Højgaard L, Ottesen B. Obesity and sarcopenia after menopause are reversed by sex hormone replacement therapy. Obes Res. 2001;9:622-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 150] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Kenny AM, Dawson L, Kleppinger A, Iannuzzi-Sucich M, Judge JO. Prevalence of sarcopenia and predictors of skeletal muscle mass in nonobese women who are long-term users of estrogen-replacement therapy. J Gerontol A Biol Sci Med Sci. 2003;58:M436-M440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Javed AA, Mayhew AJ, Shea AK, Raina P. Association Between Hormone Therapy and Muscle Mass in Postmenopausal Women: A Systematic Review and Meta-analysis. JAMA Netw Open. 2019;2:e1910154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 15. | Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH; Women's Health Initiative Memory Study. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947-2958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 925] [Cited by in RCA: 925] [Article Influence: 42.0] [Reference Citation Analysis (1)] |
| 16. | Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ, Wactawski-Wende J, Jackson RD, Limacher M, Margolis KL, Wassertheil-Smoller S, Beresford SA, Cauley JA, Eaton CB, Gass M, Hsia J, Johnson KC, Kooperberg C, Kuller LH, Lewis CE, Liu S, Martin LW, Ockene JK, O'Sullivan MJ, Powell LH, Simon MS, Van Horn L, Vitolins MZ, Wallace RB. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310:1353-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 956] [Cited by in RCA: 1115] [Article Influence: 85.8] [Reference Citation Analysis (4)] |
| 17. | Chlebowski RT, Anderson GL, Aragaki AK, Manson JE, Stefanick ML, Pan K, Barrington W, Kuller LH, Simon MS, Lane D, Johnson KC, Rohan TE, Gass MLS, Cauley JA, Paskett ED, Sattari M, Prentice RL. Association of Menopausal Hormone Therapy With Breast Cancer Incidence and Mortality During Long-term Follow-up of the Women's Health Initiative Randomized Clinical Trials. JAMA. 2020;324:369-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 267] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 18. | La Colla A, Pronsato L, Milanesi L, Vasconsuelo A. 17β-Estradiol and testosterone in sarcopenia: Role of satellite cells. Ageing Res Rev. 2015;24:166-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Forcina L, Miano C, Pelosi L, Musarò A. An Overview about the Biology of Skeletal Muscle Satellite Cells. Curr Genomics. 2019;20:24-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 20. | Kerksick C, Taylor L 4th, Harvey A, Willoughby D. Gender-related differences in muscle injury, oxidative stress, and apoptosis. Med Sci Sports Exerc. 2008;40:1772-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Laakkonen EK, Soliymani R, Karvinen S, Kaprio J, Kujala UM, Baumann M, Sipilä S, Kovanen V, Lalowski M. Estrogenic regulation of skeletal muscle proteome: a study of premenopausal women and postmenopausal MZ cotwins discordant for hormonal therapy. Aging Cell. 2017;16:1276-1287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mostafavinia A, Iran S-Editor: Liu JH L-Editor: A P-Editor: Yuan YY