INTRODUCTION
Spinal cord injury (SCI) is the transient or permanent loss of usual spinal motor, sensory and autonomic function; it can be a devastating injury for several reasons. SCI often happens unexpectedly, for example, the young motorcyclist who develops paraplegia following a road traffic accident, the gymnast who breaks their neck and becomes a wheelchair user for the rest of their lives, or the rugby player who injures their cervical spine during a scrum. These are all people in their prime, looking forward to life and severely disabled in seconds — a lifechanging event with years of good quality life lost. Another reason is the consequences of SCI are enormous for both the Individual and society as patients are left with significant psychological sequelae and physical dependence; thus managing these patients requires extensive medical, rehabilitative and social care. These resources are costly; in the United States it is estimated each patient costs $375000-$1149000 in the first year of injury and the lifetime cost of a 25-year-old tetraplegic patient is around $5100000[1].
In the United Kingdom, major trauma is the most significant cause of mortality in the under-50s[2], and survivors can be left with permanent disabilities. There are an estimated 1200 traumatic spinal cord injuries in the United Kingdom annually[3], and around 40000 people currently live with long term disabilities following SCI[4] although certain charities believe this may be higher[5]. Approximately 14% of spinal fractures damage the spinal cord, of which 50% are incomplete[6]. If managed correctly, half of these will walk on discharge. Appropriate initial management is thus essential and should target the preservation of cord function whilst avoiding further complications. This article aims to explore the initial management of patients with SCI, when surgery should be considered, and options for non-surgical management.
ACUTE MANAGEMENT
Trauma management begins before reaching the hospital. Up to 25% of traumatic spinal cord injuries occur after the primary insult during extraction, transport, or handling[7]. In patients with a mechanism of injury compatible with spinal trauma, spinal immobilisation is a priority and leads to better outcomes[8]. Gold standard spinal protection consists of three-point immobilisation of the cervical spine using blocks, collar and tape, and a rigid spinal board to maintain alignment of the rest of the spine[4,9]. Although these protective measures are essential to prevent further spinal injury, they have their associated complications including pressure sores[10], aspiration[11], increased intracranial pressure[12] and may hinder initial assessment. As soon as safety allows, immobilising devices should be removed.
Up to 57% of patients with SCI have other injuries or even concomitant spinal fractures[13-15], and 25% to 50% of acute SCI patients have concomitant head injury[16]. Associated injuries in the context of SCI is pertinent for several reasons. Attempting to treat other life-threatening injuries in the face of suspected SCI can be challenging when establishing a secure airway or treating hypotension compounded by neurogenic shock. Other injuries can also precipitate hypoxia and hypotension, which in turn inflict secondary damage to the spinal cord. Consequently, all trauma patients must be assessed and treated according to an Advanced Trauma and Life Support (ATLS) protocol to ensure life/Limb-threatening injuries and other spinal cord injuries are not missed. Life-threatening injuries should take priority; however, spinal alignment must always be maintained.
Patients with spinal cord injuries can present with systemic effects. Cervical lesions in the C3-C5 region can affect diaphragmatic innervation leading to respiratory difficulties in patients who may require ventilation[17]. These patients are also at risk at regurgitation and pulmonary aspiration secondary to paralytic ileus and reduced gastroesophageal sphincter tone[18]; this can be addressed by decompressing the stomach with a nasogastric tube. Another phenomenon commonly seen in up to 20%[19] of patients with high spinal cord injuries is neurogenic shock which can cause decreased systemic vascular resistance, profound hypotension, bradycardia and unopposed vagal tone[20]. This can often be mistaken for haemorrhagic shock secondary to internal bleeding in the context of major trauma. However, hypotension in neurogenic shock is often fluid-resistant and inotropic/vasopressor support may be more appropriate. In the absence of pelvic fractures, urinary catheterisation can be useful when assessing fluid balance, especially in patients with a denervated bladder and at risk of urinary retention.
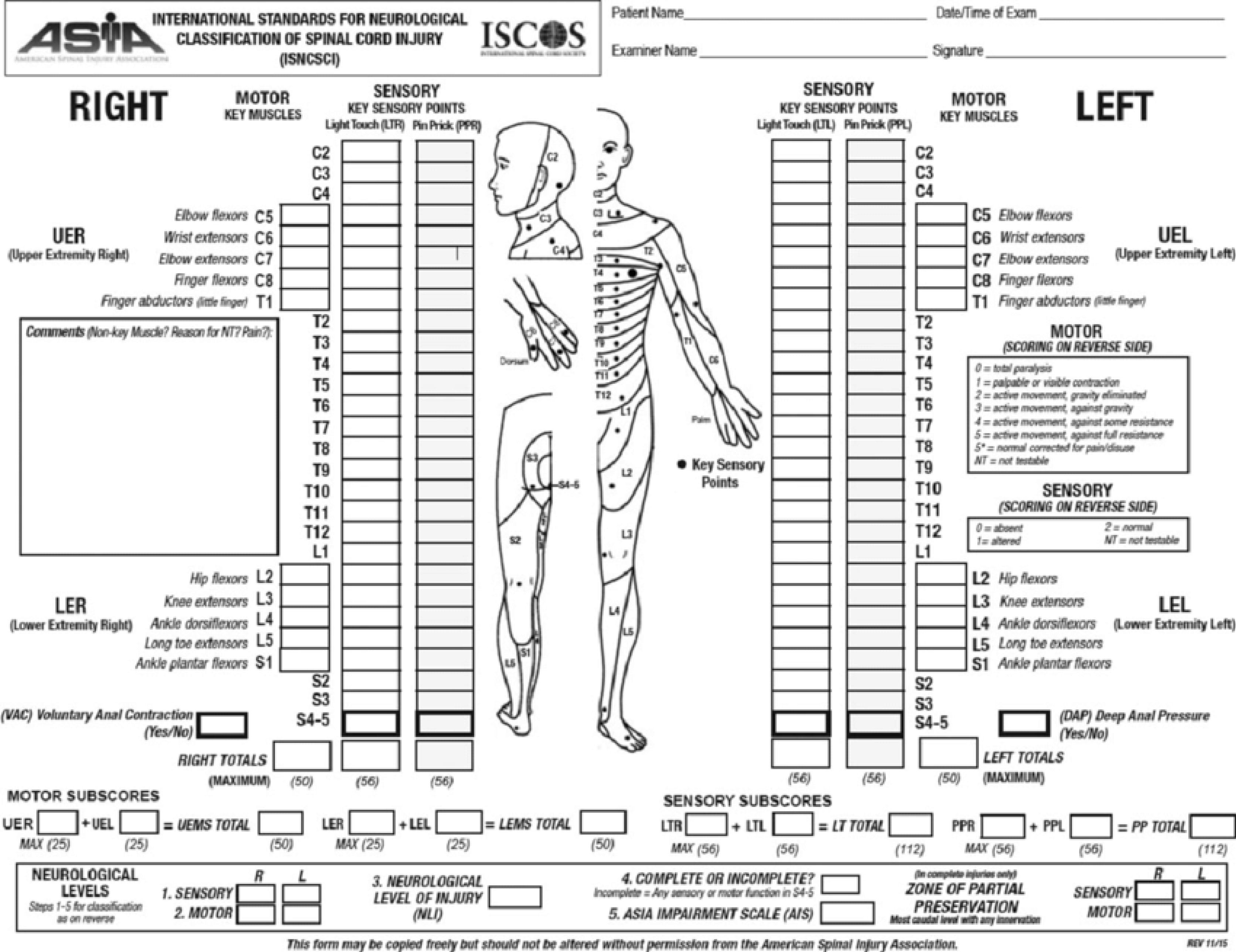
Once life and limb-threatening injuries have been addressed whilst maintaining spinal immobilisation, the focus can be turned to assessing the spine. The patient needs to be evaluated for spinal injuries and appropriately imaged. A neurological examination, including anal tone, should be performed and documented on an American Spinal Injury Association (ASIA) chart (Figure 1). Acute SCI patients often present with spinal shock, which is an acute state of diminished spinal function characterised by loss of all sensorimotor functions caudal to the site of injury. Spinal shock should not be confused with neurogenic shock; the former describes depressed spinal cord function following acute insult to the cord whilst the latter refers to circulatory collapse as previously described. Spinal shock is transient; thus, the initial neurological examination may be misleading, repeat examination within the days following the initial injury is vital to determine the true extent of cord injury.
Figure 1 American Spinal Injury Association Impairment Scale.
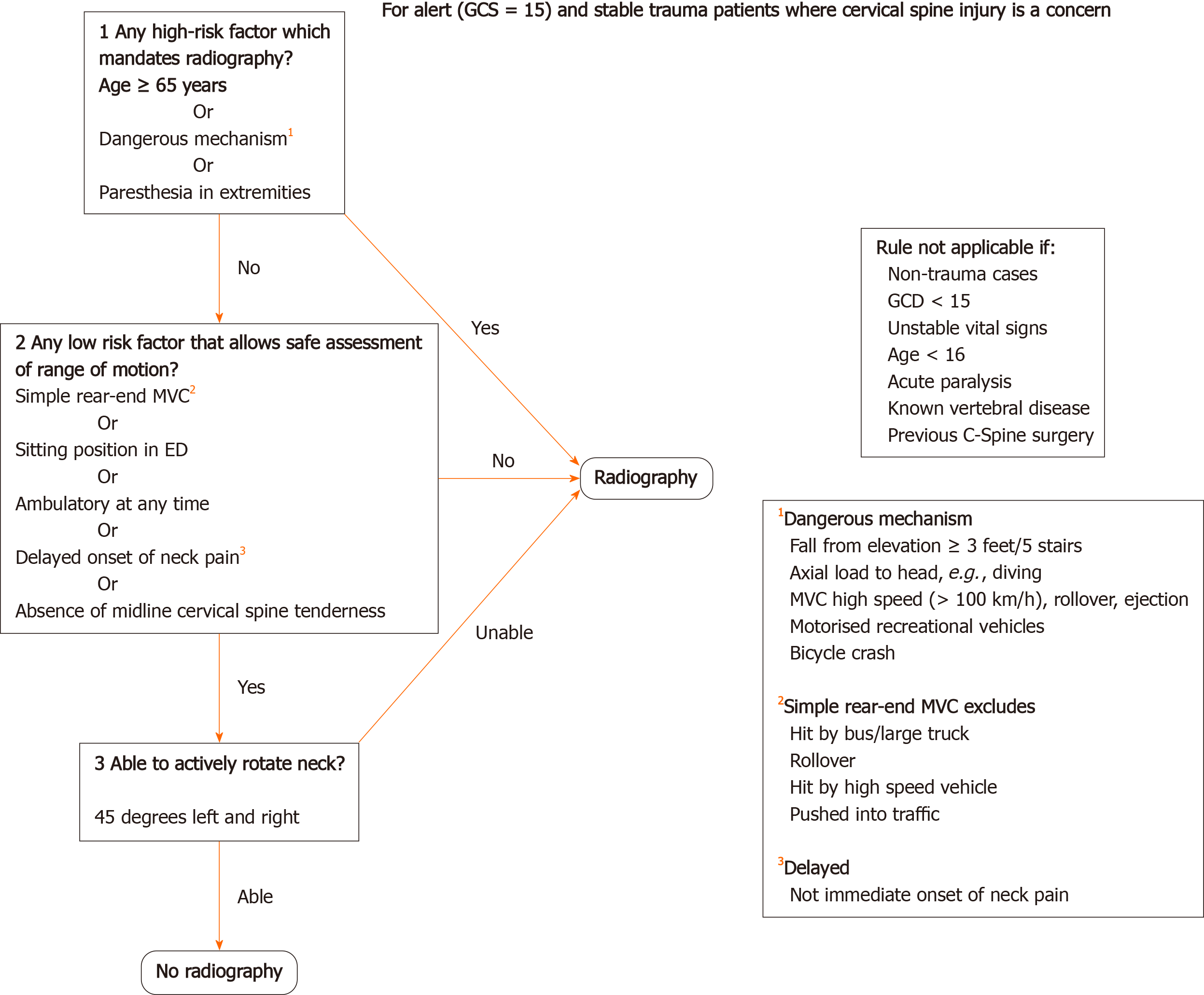
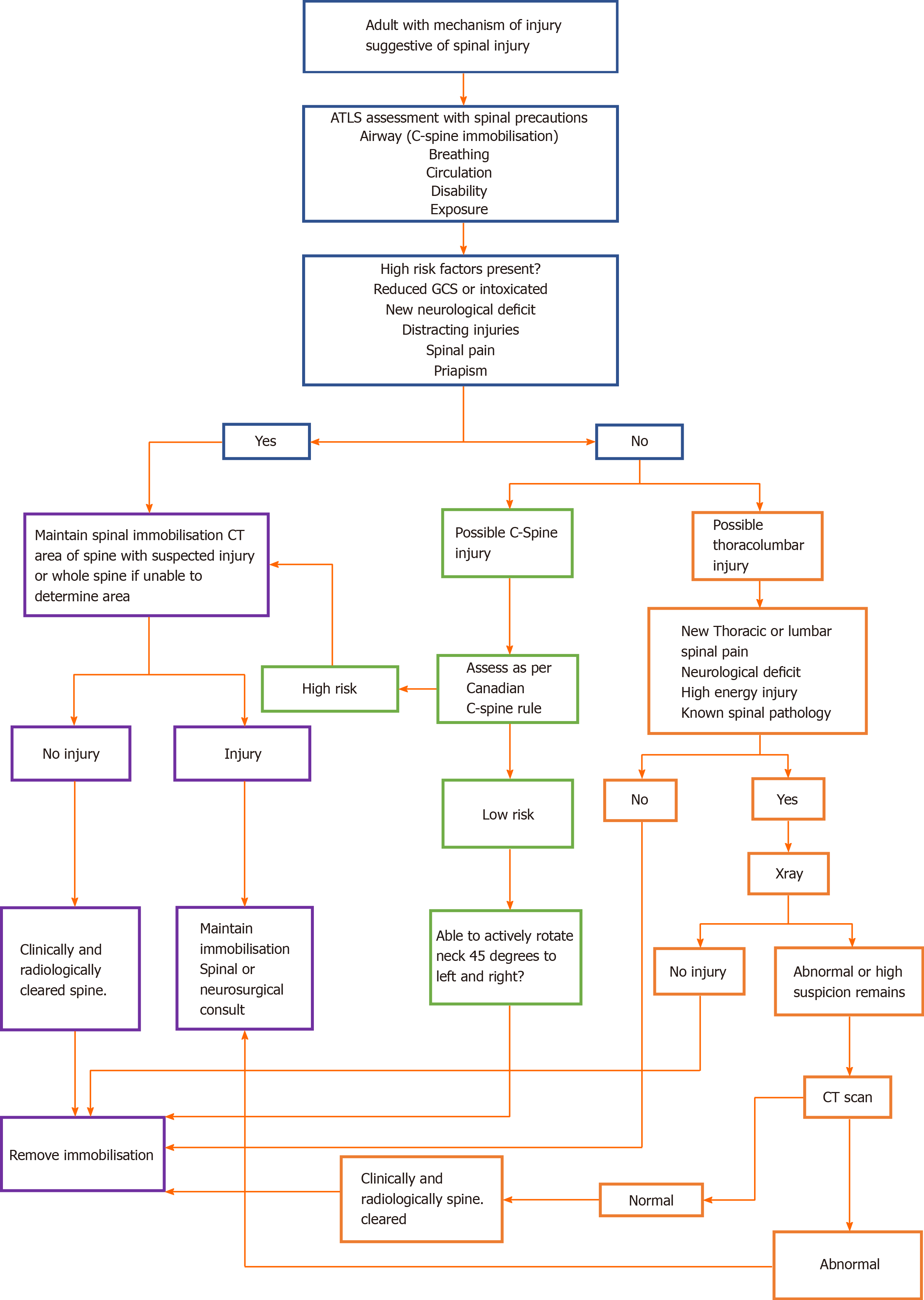
Guidelines form the UK National Institute for Health and Care Excellence[4] summarise the immobilisation, assessment, and imaging of patients with suspected spinal injuries. Adult patients with distracting injuries, spinal pain, limb weakness/paraesthesia, priapism, confusion or altered consciousness require a computed tomography (CT) of the cervical spine or X-ray of the thoracolumbar spine depending on the suspected injury location. If X-rays are inconclusive, then further CT of the thoracolumbar spine is required. Adult patients without the above risk factors but still suspected of having C spine injuries should be assessed using the Canadian C-spine rule (Figure 2). Patients who have a high-risk factor, such as paraesthesia in the lower/upper limbs, a dangerous mechanism of injury, or are above 65 years of age require a CT scan of the C-spine. If only low-risk factors are present and they can rotate their neck 45 degrees to the left and right no further immobilisation or imaging are warranted. Patients with no radiological abnormality on X-ray/CT and clinically have no spinal pain or neurological deficit can have their spines 'cleared' and immobilisation devices can be removed. In patients under the age of 16, the imaging modality of choice is an magnetic resonance imaging (MRI) scan if there is a strong suspicion of C spine injury based on the Canadian C-spine rule and clinical assessment. CT and MRI scans should be reported by a senior radiologist prior to clearing the spine.
Figure 2 Canadian C-Spine rule.
GCS: Glasgow Coma Scale.
TIMING OF SURGERY
It is crucial to appreciate that damage to the spinal cord in SCI is not limited to the initial insult but also includes a secondary injury in the early acute phase (minutes to hours) and subacute phase (days to weeks). During the acute period, vasogenic oedema, microvessel vasospasm, inflammation, failure of sodium ion pumps and free radicals are the main pathophysiologic changes accounting for secondary injury to the spinal cord. In the subacute phase, microglial stimulation, apoptosis and macrophage activity are the principal mechanisms of cellular damage[21,22].
The literature remains divisive over the optimal timing for spinal decompression and stabilisation following surgery. Some pre-clinical evidence in animals suggests a secondary injury due to the persistent compression of the spinal cord after the initial injury may be reversible if addressed quickly. This can improve outcomes by reducing neural tissue damage. Fehlings et al[23] performed a review of several experimental studies to evaluate the effect of early decompression on outcomes in dogs and rats. They concluded that early decompression improved evoked potentials and neurological recovery. Delamarter et al[24] analysed the effect of spinal cord compression on dogs and showed that decompression at one hour produced significantly greater neurological recovery that at six hours.
Human data, unfortunately, is more complicated. Liu et al[25] conducted a retrospective review in the timing of surgical decompression for traumatic cervical spinal cord injury. They concluded that early surgical intervention leads to higher rates of mortality and neurological deterioration compared with later surgery but had significantly shorter hospital stay. Early was defined as surgery in the first 72 h. Vaccaro et al[26] performed a prospective randomised trial on cervical SCI and concluded that early surgery, within 72 h, did not provide any significant neurologic benefit compared to later intervention. They also showed that there was no significant difference in length of stay or time for rehabilitation. On the other hand, Cengiz et al[27] performed a prospective randomised controlled study on the timing of thoracolumbar stabilisation following trauma. Their definition of 'Early' was different to the previous studies and defined as surgery within 8 h and late as 3-15 d following injury. They concluded that patients treated early had significantly better neurologic improvement on the ASIA score, shorter overall hospital and intensive therapy unit (ITU) stay, and less systemic complications such as pneumonia. Similarly, the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS) showed that decompression before 24 h conferred a significant improved neurologic outcome of at least a grade 2 ASIA Improvement Score (AIS) in adults with a cervical SCI[28].
Early surgical intervention may have beneficial outcomes for other measures. Bellabarba et al[29] performed a systematic review identifying seven studies which reported on hospital length of stay (LOS), intensive care unit LOS, and respiratory complications in patients with thoracolumbar spinal trauma. Overall, there was a significant reduction in hospital LOS and respiratory morbidity in favour of early surgery (< 72 h following injury). ITU LOS also showed a significant decrease but the effect was limited to patients with thoracic spinal injury. Other studies have also found similar results favouring early surgical intervention in hospital LOS, ITU LOS and morbidity[30]. With regards to mortality, Schinkel et al[31] reported a statistically significant decrease in mortality with early fixation in patients with thoracic fracture. In contrast, other studies have shown no difference in mortality with regards to the timing of surgery for thoracolumbar and lumbar fractures[32]. Other studies have also shown a significant cost saving with early stabilisation in thoracic spinal injuries ranging from $40000 to $80000 in hospital-related charges. Interestingly these same studies did not show similar findings for lumbar fractures[32,33].
Although the Spinal Trauma Study group[28] has identified the initial 24 h following SCI as the optimum period for decompression, we have seen that different timescales have been used to define 'early' and 'late' surgical decompression. This inconsistency could have hindered the development of firm conclusions regarding the optimal timing of surgery. To date, no surgical guidelines explore the merits of early vs late surgical decompression and stabilisation in SCI with regards to the 24-h threshold. Other guidelines on surgical timing have suggested class 2 evidence supporting (1) early surgery (< 72 h) in SCI patients who have been haemodynamically optimised (2) expeditious reduction of bilateral locked facets in SCI patients with tetraplegia and (3) urgent decompression in the presence of neurologic deterioration[34].
It seems that the evidence supports early surgical intervention to reduce morbidity, length of stay, healthcare costs and, if performed early enough, can even improve neurological recovery. Disappointingly, in one survey involving 2000 neurosurgeons, 80% were in favour of early surgery, but fewer than 50% found that operating within 24 h was feasible (30). The main obstacles were the lack of resources for overnight or weekend operating. Of note, Macis et al[35] found a 33% reduction in long term paralysis if patients were treated at a trauma centre.
NON-SURGICAL MANAGEMENT
The decision to operate or not in spinal trauma is multifactorial. Patient factors include fitness for surgery and the presence of other life or limb-threatening injuries whilst having the right resources and skill are apparent considerations too. Non-operative management has a limited role in established cases of neurological deficit and instability but may have a role in a stable spine with no neurology. Prolonged immobility, however, can increase the risk of pressure sores, deep vein thrombosis and pneumonia[36].
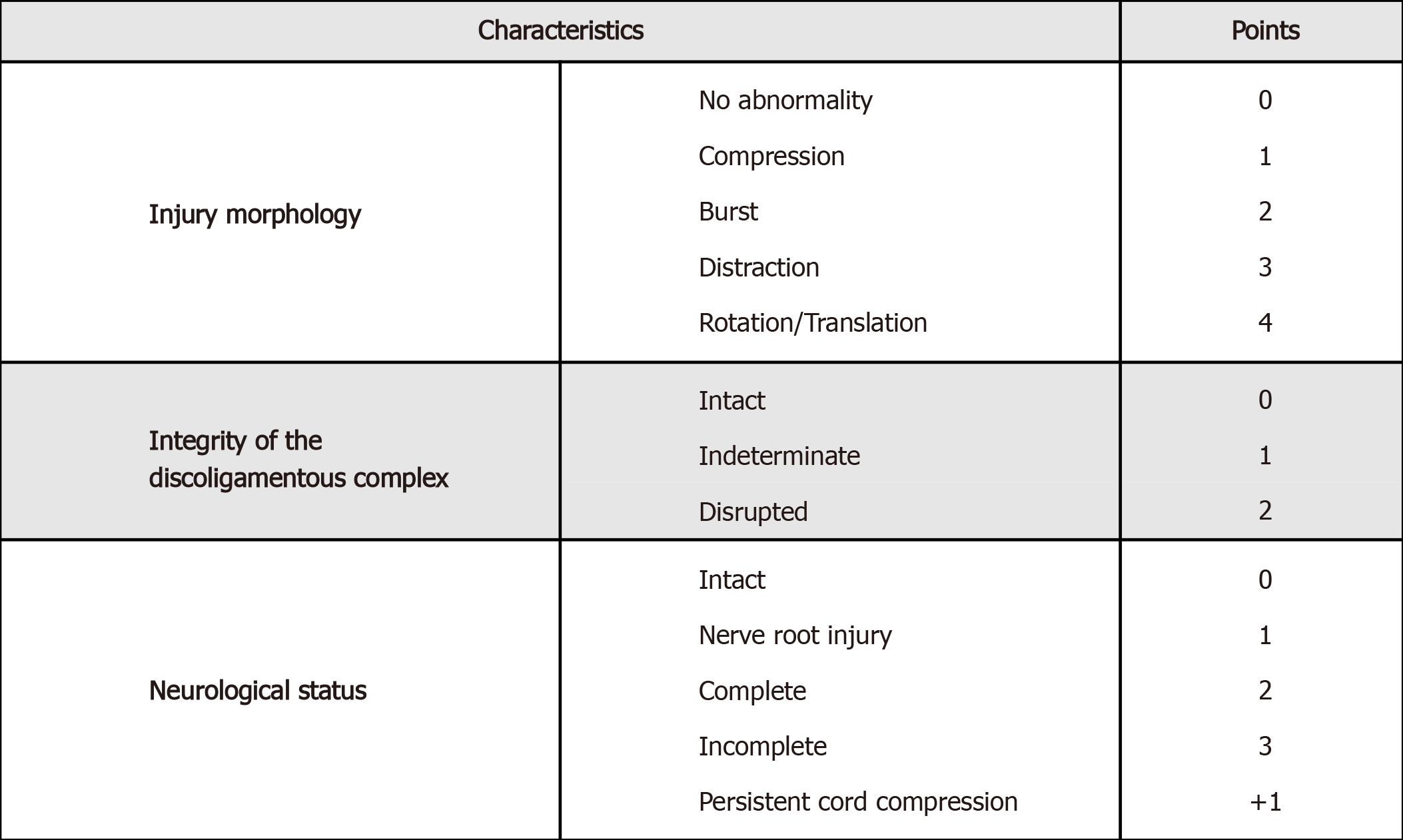
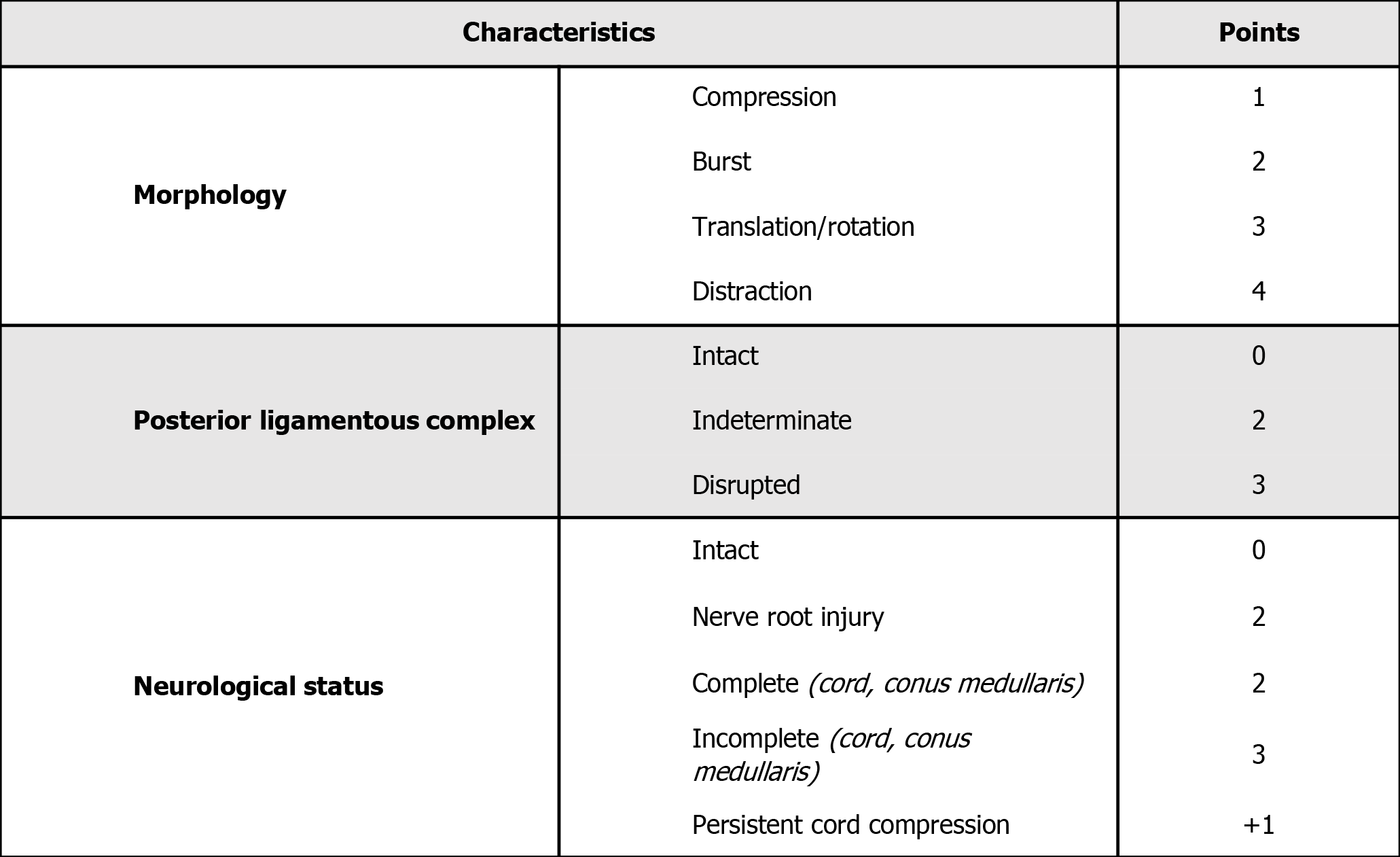
The Thoracolumbar Injury Classification and Severity Score (TLICS)[37] (Figure 3) and the sub-axial Injury Classification and Severity Scale (SLIC)[38] (Figure 4) are classification systems used to score spinal fracture patterns in the thoracolumbar and sub-axial cervical regions respectively. These scoring systems are based on the fracture morphology, the integrity of the disco-ligamentous complex (DLC)/posterior ligamentous complex (PLC) and neurological status. Generally, scores <4 can typically be managed conservatively, a score of > 4 would favour surgical intervention, and a score of 4 is generally based on the surgeon's individual preference[37]. These scoring systems have shown good inter and intraobserver reliability and are reliable when predicting which patients can be managed non-operatively[39].
Figure 3 Subaxial Cervical Spine Injury Classification System[38].
Figure 4 Thoracolumbar injury classification score[37].
In scores of less than 4 spinal orthoses can be used to facilitate early ambulation and improve acute pain. Standing X-rays should be obtained before discharge to check for stability, alignment, and occult ligamentous injury. In the thoracolumbar region, non-operative management is predominantly used for compression or bust fracture in the absence of neurological deficit and PLC injury[40], transverse and spinous process fractures do generally not warrant surgery either[41]. Cervical injuries can be divided into C1, C2 and sub-axial (C3-C7) injuries. Simple C1 injuries with an intact transverse alar ligament and C2 dens fractures type 1 and 3 can be managed with a rigid collar; however, individual patient factors need to be considered for risk of non-union with C2 dens fractures[42]. Management of sub-axial injuries should consider the SLIC scoring system when deciding between operative and non-operative management.
The role of corticosteroids in acute SCI is controversial. The National Acute Spinal Cord Injury Study (NASCIS) II and III were initially pivotal studies recommending the use of steroids in acute SCI[43,44]. However, other studies have shown significantly higher rates of morbidity and mortality in patients treated with corticosteroids, particularly for pneumonia, sepsis, gastrointestinal haemorrhage, acute respiratory distress syndrome and death[7]. Consequently, steroids are not recommended by the British Association of Spinal Cord Injury Specialists and not routinely used for acute SCI in the United Kingdom or United States[4,45].
CONCLUSION
Patients presenting with acute spinal cord injury can be challenging. They may present with other injuries and multisystem problems. Nonetheless, the management, assessment, classification, and medical treatment of patients with acute SCI is becoming increasingly standardised. Figure 5 summarises the treatment algorithm for trauma patients presenting to the emergency department with suspected traumatic spinal cord injury. The main role of surgery is to prevent further neurological deterioration through decompression whilst stabilisation reduces complications related to immobility and facilitates earlier rehabilitation which remains critical in optimising outcomes. Ultimately, we need a better understanding of SCI pathophysiology to develop novel treatments with the aim of restoring neurological function to preinjury levels.
Figure 5 Spinal cord injury evaluation.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang X S-Editor: Gao CC L-Editor: A P-Editor: Li JH