Published online Jun 26, 2017. doi: 10.4330/wjc.v9.i6.531
Peer-review started: December 1, 2016
First decision: February 17, 2017
Revised: March 4, 2017
Accepted: April 18, 2017
Article in press: April 20, 2017
Published online: June 26, 2017
Processing time: 208 Days and 17.3 Hours
The implantable cardioverter-defibrillator (ICD) is effective to prevent sudden cardiac death (SCD) in selected patients with heart disease known to be at high risk for ventricular arrhythmia. Nevertheless, this invasive and definitive therapy is not indicated in patients with potentially transient or reversible causes of sudden death, or in patients with temporary contra-indication for ICD placement. The wearable cardioverter defibrillator (WCD) is increasingly used for SCD prevention both in patients awaiting ICD implantation or with an estimated high risk of ventricular arrhythmia though to be transient. We conducted a review of current clinical uses and benefits of the WCD, and described its technical aspects, limitations and perspectives.
Core tip: The wearable cardioverter defibrillator is increasingly used for sudden cardiac death prevention in patients thought to have a transient and/or reversible high risk for life-threatening ventricular arrhythmia. Evidences sustaining the use of this external device are growing. We provided an evidence base review in the light of new data.
- Citation: Barraud J, Cautela J, Orabona M, Pinto J, Missenard O, Laine M, Thuny F, Paganelli F, Bonello L, Peyrol M. Wearable cardioverter defibrillator: Bridge or alternative to implantation? World J Cardiol 2017; 9(6): 531-538
- URL: https://www.wjgnet.com/1949-8462/full/v9/i6/531.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i6.531
Sudden cardiac death (SCD) is an unpredictable event which leads to death in the absence of immediate resuscitation maneuvers and adequate therapies. Up to 23% of SCD are attributable to ventricular arrhythmias (VA)[1]. The implantable cardioverter-defibrillator (ICD) has proved to be highly effective for SCD secondary prevention. Otherwise, it has also been demonstrated to prevent SCD in selected patients with heart disease known to be at high risk for life-threatening VA[2-4]. However, long-term ICD-related complications, cost issues, social impact and quality of life force a rigorous evaluation of patients before ICD placement. Furthermore, some situations at high risk of VA-related SCD are known to be limited in time. For example, although SCD rate was 2.3% in patients with low left ventricular ejection fraction (LVEF) during the first month following myocardial infarction (MI), ICD implantation during the first 40 d post-MI failed to reduce total mortality. This result was essentially due to a large amount of non-arrhythmic death during this period[5]. In addition, up to 40% of patients with coronary artery disease and low LVEF do not meet the current criteria for ICD implantation after complete myocardial revascularization and/or optimization of medical therapy[6].
The wearable cardioverter defibrillator (WCD) is increasingly used for SCD prevention both in patients awaiting ICD implantation or with an estimated high risk of VA though to be transient. This external device, which has been demonstrated to effectively terminate spontaneous and induced VA by automatic defibrillation shock delivery, requires no surgical intervention and is entirely removable. We conducted a review of current clinical uses and benefits of WCD, and described its technical aspects, limitations and perspectives.
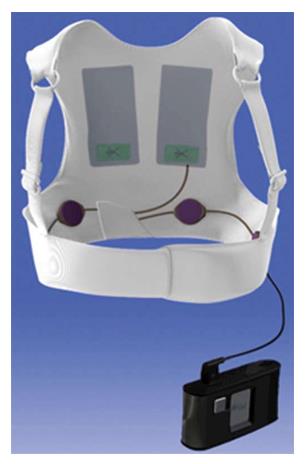
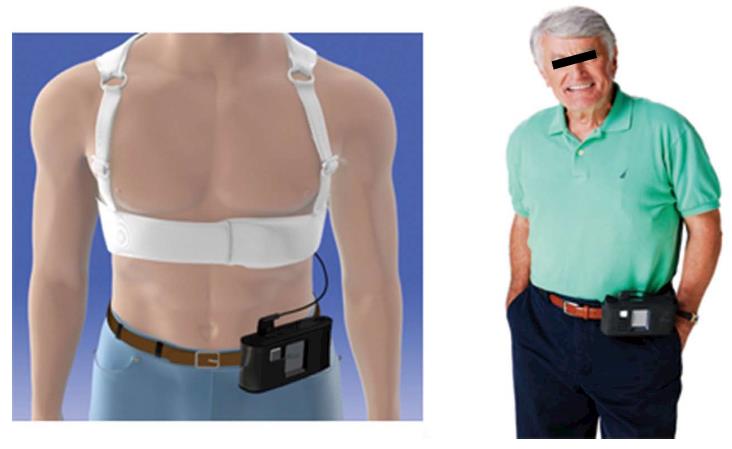
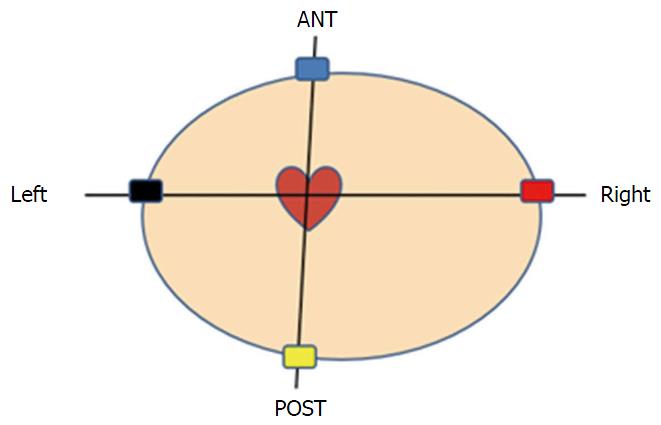
Currently available WCD is the Lifevest 4000® [ZOLL Lifecor Corporation (ZOLL), Pittsburgh, PA, United States]. With the LifeVest 4000®, the chest is surrounded by an elastic belt including an electrocardiographic (ECG) monitoring system with four dry, non-adhesive electrodes and the defibrillation system consisting in two posterior and one apical electrodes (Figure 1). The whole is maintained by shoulder straps forming a light washable vest and connected to a monitor unit including the battery, an LCD screen for message display and two “response buttons” for patient defibrillation shock withholding. The monitor unit is held in a holster or around the waist (Figure 2). Two batteries are delivered with the WCD; each one lasts for 24 h so that one is always in charge during the use of the other Total device weight is about 600 g. ECG electrodes provide two left-right and front-back bipolar ECG signals (Figure 3). The ECG is continuously recorded and analyzed. Following parameters can be programmed: (1) rate intervals for ventricular fibrillation (VF) zone: 120 to 250 bpm, default 200 bpm and ventricular tachycardia (VT) zone: 120 bpm to VF zone; (2) shock delay, i.e., time from arrhythmia detection to shock delivery: 60 to 180 s in VT zone and 25 to 55 s for VF zone. Further delay up to 30 s may be added at night; and (3) shock energy: 75 to 150 J.
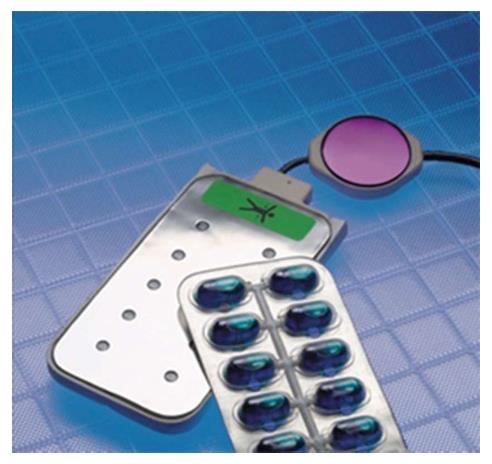
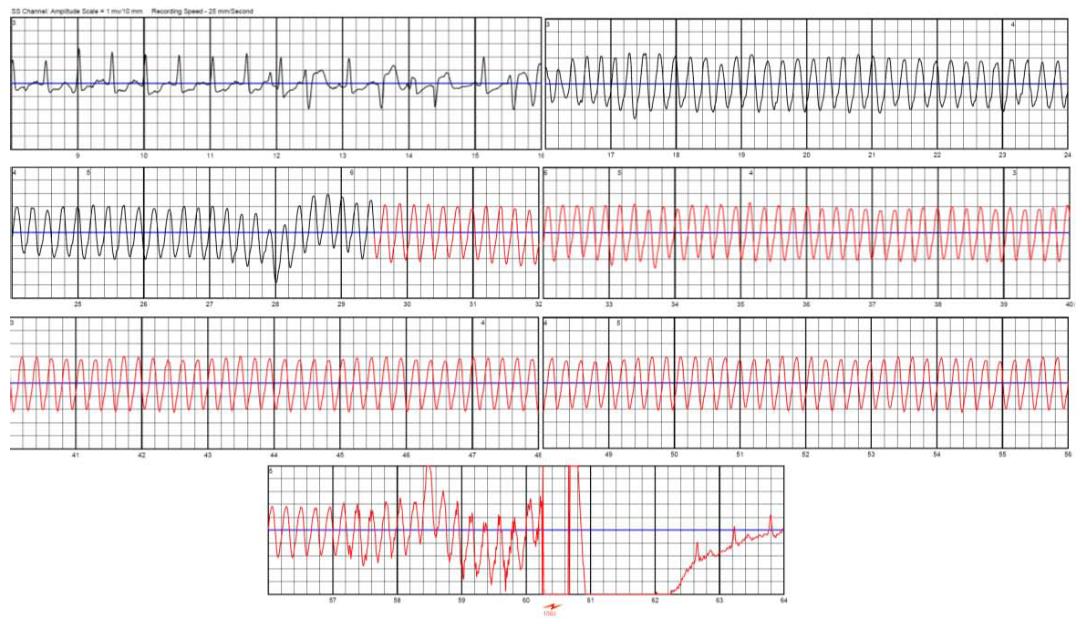
The WCD automatically delivers, i.e., without neither patient nor witness intervention, defibrillation shocks for termination of life-threatening VA. Arrhythmia detection and discrimination (for arrhythmia detected in the VT zone) occur within few seconds after the rhythm disorder onset. In case of VA detection within the programmed VT or VF zone, the device alerts the patient of the imminence of a shock starting by vibrations of the defibrillation electrodes during 5 s, followed by a low monotonal sound signal then high bitonal sound signal. Finally, a voice warning during the few lasts s precedes the shock delivery. During this period, the patient, if still conscious, can withhold shock delivery by pressing the two response buttons on the monitor unit. Without this well-done intervention, defibrillation shock is delivered, synchronized to the R-wave signal in case of monomorphic VT. In order to improve shock impedance, and to prevent skin burns, the defibrillation electrodes release a conductive gel contained in small capsules before shock delivery (Figure 4). Up to five shocks can be delivered for the same episode. ECG signal is continuously recorded and reviewable 30 s prior to the detection of arrhythmia to 15 s after the alarms stop (Figure 5). Total duration from the onset of the arrhythmia to shock delivery, (including time of fulfilling detection criteria, confirmation, alarms and capacitor charging) is about 50 s. Daily remote monitoring advices medical staff about VA occurrence and therapies, daily ECGs, as well as patient compliance (assessed by the daily wear time).
Patient education by specialized healthcare givers on how to properly wear the device, change the battery and disable shock delivery is a crucial step. In our experience, 10% to 15% patients eligible for this therapy are not able to understand instructions to withhold therapies or change battery and therefore are not treated with the WCD. In order to improve patient knowledge and handle of the WCD, we systematically schedule an additional patient education session 10 to 15 d after hospital discharge.
Similarly, understanding and knowledge of his cardiac disease and potentials benefits associated with the use of the WCD is a critical part of patient care, aiming high device compliance which is the prerequisite of effective SCD protection. Lack of compliance might have dramatics consequences. Indeed, in various studies, the majority of SCD observed during follow-up were observed in patients not or not-correctly wearing the WCD[7,8]. Weight and footprint of the device were the main reasons for low compliance. On the other hand, as high as 22.5% of patients discontinued the use of WCD due to comfort or lifestyle issues in study from Feldman et al[8]. A 40% reduction of size and weight of the device was associated with a significant decrease in the rate of WCD therapy interruption (14.2%) in a more recent report[9].
Overall, national registries showed good compliance with the actual WCD[6,10]. In United States’ experience, of 3569 patients wearing WCD, > 50% of patients achieved a 90% wear time compliance[9]. In the German registry, this number grows to 72%[11]. In both studies, long period of therapy was associated with higher time of wearing. Otherwise, remote monitoring allows measurement of daily WCD wear time and medical staff is alerted in case of low patient compliance so that prompt corrective measures can be taken.
Auricchio et al[12] were the firsts to report the efficacy of the first generation WCD (WCD™ device, LIFECOR, Pittsburgh, Pennsylvania) for termination of life-threatening VA. This device reliably stopped induced VT or VF by automatically delivering a 230 J defibrillation shock in 15 SCD survivors. The firsts prospective multicenter studies demonstrating clinical benefit of the WCD were the Wearable Defibrillator Investigate Trial (WEARIT) and Bridge to ICD in Patients at Risk Of Arrhythmic Death (BIROAT) studies[8]. Inclusion criteria for the WEARIT study was symptomatic NYHA III or IV ambulatory heart failure and LVEF < 0.30. Differently, the BIROAD study enrolled: (1) patients after a recent MI or coronary artery bypass grafting (CABG) and having complications such as VA, syncope or low LVEF < 0.30, but not receiving an ICD for up to 4 mo; and (2) patients who met criteria for an ICD but refused therapy or had to wait for at least 4 mo before implantation. A total of 289 patients were enrolled in both studies, united into one at the request of the Federal Drug Administration, and followed during a total of 901 mo of patient use. During the follow-up, 6 of 8 defibrillation attempts were successful. No patient died while correctly wearing the WCD.
Thereafter, some large studies validated the clinical benefit of this therapy and evaluated the occurrence of VA during the period of the WCD use in patients with low LVEF in the setting of ischemic heart disease. Rate of patients receiving appropriate shock within the 3 mo following percutaneous coronary intervention (PCI) or CABG varied from 1.3% to 1.7%[9,10]. Prolonging WCD wearing period to 15 mo resulted in increasing rate of appropriate WCD shock to 4.1%[13]. In the United States’ experience, first shock success was of 99% for all VT/VF events, and survival after VT/VF events was 89.5%[9]. Importantly, no death could be attributed to WCD technical failure since its introduction. For note, at the end of the WCD period use, about 60% of patients were not eligible anymore for ICD implantation, mainly because of left ventricular ejection fraction improvement[6].
From 0.4% to 3% of patients experienced inappropriate WCD shock[6,9,10,14]. The WCD is an external device, which is dramatically exposed to noise detection. Inappropriate shocks are mainly related to noise artifacts or T wave oversensing[15]. Compared to conventional transvenous ICD, the rate of inappropriate shock with the WCD is low. This fact is explained by the possibility for the patient to withhold shock delivery while pressing the response buttons. Incidence of false alarms attributable to artifacts is unknown.
According to current guidelines for management of patients presenting with VA and the prevention of SCD, “the WCD may be considered for adult patients with poor LV systolic function who are at risk of sudden arrhythmic death for a limited period, but are not candidates for an implantable defibrillator (e.g., bridge to transplant, bridge to transvenous implant, peripartum cardiomyopathy, active myocarditis and arrhythmias in the early post-myocardial infarction phase). In patients presenting with high risk of SCD, but non-indicated for an ICD implantation because of temporary contra-indication, in expectation of a diagnosis, or if the arrhythmic risk may evolve”[16]. For the Heart Rhythm Society, the use of WCD is reasonable in patients with a clear indication of ICD placement but with temporary contra indication to the procedure (infection for example) or as a bridge therapy to heart transplantation. Otherwise, the use of the WCD should be considered in additional clinical situations: In patients with high risk for SCD due to LV dysfunction that may resolve over time (following myocardial revascularization, myocarditis…) or with a potentially treatable cause (arrhythmia-induced or chemotherapy-induced LV dysfunction)[17].
After acute myocardial infarction: Sudden cardiac death occurred in 2.3% of patients with severely depressed LVEF during the first month post-MI[18]. However, the risk of life-threatening VA significantly decreases with LVEF recovery after acute event[19]. Furthermore, in primary prevention studies, ICD benefit occurred years after implantation[3,20]. Former studies showed no impact of early implantation of ICD after AMI on overall mortality[5,21]. The DINAMIT was an open-label trial including 674 patients 6 to 40 d after an AMI, with LVEF > 0.35 and impaired cardiac autonomic function. Patients were randomized in a 1/1 fashion for medical treatment or medical treatment and ICD placement. This study did not found statistical difference in overall mortality between the 2 groups. Indeed, a smaller proportion of SCD observed in the ICD group was offset by an increase in the rate of non-arrhythmic deaths among these patients. These results are consistent with findings from the IRIS study[21]. The United States’ experience with the WCD was derived from a national database and included 8453 patients with ejection fraction < 0.35 early after acute MI[10]. One point four percent of patients were correctly treated by WCD, whose 75% in the first month of use. The median time to first WCD therapy was 9 d. The resuscitation survival rate was of 91%. The VEST Prevention of Early Sudden Death Trial and VEST Registry (VEST) is a randomized simple blind trial currently enrolling patients with LVEF < 0.35 following AMI. This study aims to demonstrate a reduction of SCD within the first three mo following AMI. Enrollment exceeded 1700 patients in 2015, results are awaited[22].
After revascularization procedures: Life-threatening VA are a frequent cause of SCD after elective revascularization CABG or PCI[23]. ICD implantation is mandated in patients with reduced LVEF < 0.35 evaluated at least 3 mo after revascularization because of possible LV systolic function recovery. In the setting of LV dysfunction <0.35 after CABG or PCI, Zishiri et al[24] found a significant reduction in early mortality hazard in patients treated with the WCD (3% vs 7%, P < 0.05). In this subset of patients, appropriate therapy rate was 1.3%.
Terminal cardiomyopathy listed for heart transplantation: The risk of SCD in patients awaiting heart transplantation is about 10% at one year[25]. Although ICD is largely used in this population of patients, complications, such as infection, are frequent, particularly in LV assist devices receivers[26]. The WCD was found to be a safe and efficient transitory solution to protect this population as a bridge to transplantation[27].
ICD infections, before re-implantation: Cardiac implanted electronic devices infections require complete system removal, associated with antibiotic therapy for 2 to 6 wk. Period before re-implantation is long, so that patients could beneficiate from WCD protection without deleting hospital discharge, as the risk of SCD remains unchanged[7] during this period. Highest incidence rate of appropriate therapies remains to patients after ICD explantation for infection in expectation of reimplantation compared with other indications[14]. Therefore, the AHA guidelines sustain its use in this clinical setting with a Class IIA recommendation (level of evidence C)[17].
Nonischemic cardiomyopathy: Benefice of ICD in prevention of SCA in non-ischemic cardiomyopathy (NICM) patients is still a matter of debate. Low LVEF < 0.35 remains the only criterion validated to stratify the risk of SCD among these patients[4,28,29]. Plurality of etiologies, absence of criteria that define the likelihood of reversibility and potential for recovery after optimal medical therapy[30] make difficult the assessment of the long term risk of VA in this population of patients. Early ICD implantation, within the firsts mo after diagnosis failed to improve long term survival[28,31]. Therefore, LVEF assessment for SCD risk stratification is recommended at least 3 mo after optimal medical treatment[16], and some studies tend to delay ICD placement to 9 mo[32-34]. Furthermore, in a recent large randomized study, prophylactic ICD implantation in patients with symptomatic NICM showed no impact on mortality[35]. Indeed, the DANISH study included 556 patients with symptomatic systolic NICM and LVEF ≤ 0.35 who were assigned to receive an ICD, and 560 patients assigned to receive medical care, both group receiving CRT if indicated. Primary evaluation criteria was death from any cause. No difference was observed between the two groups after a median follow-up period of 67 mo. Only patients younger than 68 years of age showed a lower rate of death after ICD implantation, independent of CRT status.
Small cohorts aimed to evaluate the benefit of WCD in patients with NICM. Incidence of appropriate therapies varied from 0% to 5.5%[6,8,9,15,36]. Prospective studies are lacking in this heterogeneous population to specify real benefit.
Tako-tsubo cardiomyopathy: Tako-tsubo cardiomyopathy is a heterogeneous provider of SCD, and life-threatening VA occur during the first wk after disease onset. Prevalence of VA varies between 8% and 13.5%[37,38]. Patients with QT prolongation after stress cardiomyopathy demonstrated a higher risk of VA. This subset of patients might have substantial benefit of the WCD use[39,40].
Peripartum cardiomyopathy: Peripartum cardiomyopathy patients with severely reduced LVEF have an elevated risk of VA[41,42]. Up to 38% of deaths in this population are sudden and most of them (87%) occur within the 6 mo following the diagnosis[43]. The WCD was found to correctly treat these VA during the first mo after diagnosis, until ICD implantation or systolic function recovery[44].
Prediction of cardiomyopathy and evaluation of SCD risk after acute myocarditis is difficult. Assessment of the LVEF appears to be an insufficient criterion[45,46]. Similarly to Tako-tsubo cardiomyopathy or peripartum cardiomyopathy, myocarditis has a potentially high likelihood of cardiac recovery so that the WCD may be limited to patients in secondary prevention or with particularly high-risk features[17].
Pharmacology-induced cardiomyopathies (alcohol, methamphetamine, trastuzumab) are associated with a great potential of recovery of LV systolic function after withdrawal of the putative agent and optimal medical therapy.
In all these various clinical settings known to result in both potentially transient LV dysfunction and high SCD risk, the WCD might be a valuable tool in both for SCD prevention and to provide additional information for subsequent SCD risk stratification.
Unexplained syncope: The diagnostic of syncope encompass various causes. First, it can be the precursor event of SCD. Then it is a major step in the rhythmic risk in patients presenting with inherited arrhythmia syndromes or structural heart diseases such as hypertrophic cardiomyopathy. During this time of evaluation, no rhythmic protection can be offered by classical monitoring approaches, such as implantable cardiac monitors. The WCD may bridge this vulnerable period until diagnostic has been established. The Ambulatory Post-Syncope Arrhythmia Protection Feasibility Study currently enrolling patients, aims to assess utility of WCD in patients with high rhythmic risk after unexplained syncope[47].
End-stage renal disease: Hemodialysed (HD) patients are known to be at high risk of SCD[48]. In a retrospective study, 75 hemodialysed patients presenting with SCD while wearing a WCD were included[49]. Seventy-eight point six percent of SCD were linked to VT/VF episodes. One-year survival after SCA was 31.4%. In comparison with historical data, the WCD therapy was associated with an improved survival ref. The ICD was associated with better survival in HD patients yet[50], but is more exposed to complications such as device infections[51].
Although the WCD is able to automatically terminate VA, daily maintenance is necessary. A non-negligible proportion of patients are unable to correctly use and handle the device, change battery or respond to device alarms. This issue might be kept in mind before patient selection. The WCD cannot deliver antitachycardia and/or anti-bradycardia pacing. In patients with cardiac pacemakers, bipolar pacing mode should be programmed in order to avoid oversensing of pacing artifacts during VF leading to termination of the treatment algorithm[52]. In contrast, time to shock delivery, which is substantially longer compared to ICD, doesn’t seem to be a limitation. As shown in the MADIT-RIT trial[53], prolonged delays in therapy delivery were associated with reductions in inappropriate therapies and overall mortality. Finally, cost impact of this device has to be underlined. Few studies evaluated the cost-effectiveness of the WCD. After ICD removal for infection, WCD seemed to be cost-effective for SCD prevention compared to in-hospital monitoring or discharge to a skilled nursing facility before reimplantation[54].
The WCD is a life-saving therapy as it has been demonstrated to promptly detect and terminate VT/VF by automatically delivering defibrillation shock. This device represents a safe, easy to handle, non-invasive and reversible way to prevent SCD in patients with SCD risk though to be high for a limited period or having a transient contraindication to permanent ICD implantation. Data sustaining the use of the WCD therapy in patients with low LVEF following myocardial revascularization are strong. Similarly, current guidelines sustain the use of the WCD in patients with ICD infection requiring device removal. Further prospective and randomized studies are awaited to better guide its indications and its benefit in other clinical settings.
We thank Dr. Kent Volosin and Bertrand Colombo, ZOLL Medical Corporation, for their technical support.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aggarwal A, Carbucicchio C, De Maria E, Kettering K, Ozaydin M S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2843] [Cited by in RCA: 3868] [Article Influence: 351.6] [Reference Citation Analysis (0)] |
| 2. | Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2967] [Cited by in RCA: 2785] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 3. | Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5067] [Cited by in RCA: 4890] [Article Influence: 203.8] [Reference Citation Analysis (0)] |
| 4. | Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4998] [Cited by in RCA: 4843] [Article Influence: 230.6] [Reference Citation Analysis (0)] |
| 5. | Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, Fain E, Gent M, Connolly SJ. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1094] [Cited by in RCA: 1044] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 6. | Kutyifa V, Moss AJ, Klein H, Biton Y, McNitt S, MacKecknie B, Zareba W, Goldenberg I. Use of the wearable cardioverter defibrillator in high-risk cardiac patients: data from the Prospective Registry of Patients Using the Wearable Cardioverter Defibrillator (WEARIT-II Registry). Circulation. 2015;132:1613-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 7. | Tanawuttiwat T, Garisto JD, Salow A, Glad JM, Szymkiewicz S, Saltzman HE, Kutalek SP, Carrillo RG. Protection from outpatient sudden cardiac death following ICD removal using a wearable cardioverter defibrillator. Pacing Clin Electrophysiol. 2014;37:562-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Feldman AM, Klein H, Tchou P, Murali S, Hall WJ, Mancini D, Boehmer J, Harvey M, Heilman MS, Szymkiewicz SJ. Use of a wearable defibrillator in terminating tachyarrhythmias in patients at high risk for sudden death: results of the WEARIT/BIROAD. Pacing Clin Electrophysiol. 2004;27:4-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Chung MK, Szymkiewicz SJ, Shao M, Zishiri E, Niebauer MJ, Lindsay BD, Tchou PJ. Aggregate national experience with the wearable cardioverter-defibrillator: event rates, compliance, and survival. J Am Coll Cardiol. 2010;56:194-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 239] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 10. | Epstein AE, Abraham WT, Bianco NR, Kern KB, Mirro M, Rao SV, Rhee EK, Solomon SD, Szymkiewicz SJ. Wearable cardioverter-defibrillator use in patients perceived to be at high risk early post-myocardial infarction. J Am Coll Cardiol. 2013;62:2000-2007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Klein HU, Meltendorf U, Reek S, Smid J, Kuss S, Cygankiewicz I, Jons C, Szymkiewicz S, Buhtz F, Wollbrueck A. Bridging a temporary high risk of sudden arrhythmic death. Experience with the wearable cardioverter defibrillator (WCD). Pacing Clin Electrophysiol. 2010;33:353-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Auricchio A, Klein H, Geller CJ, Reek S, Heilman MS, Szymkiewicz SJ. Clinical efficacy of the wearable cardioverter-defibrillator in acutely terminating episodes of ventricular fibrillation. Am J Cardiol. 1998;81:1253-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Lamichhane M, Gardiner JC, Bianco NR, Szymkiewicz SJ, Thakur RK. National experience with long-term use of the wearable cardioverter defibrillator in patients with cardiomyopathy. J Interv Card Electrophysiol. 2017;48:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Wäßnig NK, Günther M, Quick S, Pfluecke C, Rottstädt F, Szymkiewicz SJ, Ringquist S, Strasser RH, Speiser U. Experience With the Wearable Cardioverter-Defibrillator in Patients at High Risk for Sudden Cardiac Death. Circulation. 2016;134:635-643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Salehi N, Nasiri M, Bianco NR, Opreanu M, Singh V, Satija V, Jhand AS, Karapetyan L, Safadi AR, Surapaneni P. The Wearable Cardioverter Defibrillator in Nonischemic Cardiomyopathy: A US National Database Analysis. Can J Cardiol. 2016;32:1247.e1-1247.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace. 2015;17:1601-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 216] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 17. | Piccini JP, Allen LA, Kudenchuk PJ, Page RL, Patel MR, Turakhia MP. Wearable Cardioverter-Defibrillator Therapy for the Prevention of Sudden Cardiac Death: A Science Advisory From the American Heart Association. Circulation. 2016;133:1715-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 18. | Solomon SD, Zelenkofske S, McMurray JJ, Finn PV, Velazquez E, Ertl G, Harsanyi A, Rouleau JL, Maggioni A, Kober L. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352:2581-2588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 612] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 19. | Exner DV, Kavanagh KM, Slawnych MP, Mitchell LB, Ramadan D, Aggarwal SG, Noullett C, Van Schaik A, Mitchell RT, Shibata MA. Noninvasive risk assessment early after a myocardial infarction the REFINE study. J Am Coll Cardiol. 2007;50:2275-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 258] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 20. | Wilber DJ, Zareba W, Hall WJ, Brown MW, Lin AC, Andrews ML, Burke M, Moss AJ. Time dependence of mortality risk and defibrillator benefit after myocardial infarction. Circulation. 2004;109:1082-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Steinbeck G, Andresen D, Seidl K, Brachmann J, Hoffmann E, Wojciechowski D, Kornacewicz-Jach Z, Sredniawa B, Lupkovics G, Hofgärtner F. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009;361:1427-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 536] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 22. | Olgin JE. Vest Prevention of Early Sudden Death Trial and VEST Registry. [accessed 2015 Feb 18]. Available from: https://clinicaltrials.gov/ct2/show/NCT01446965. |
| 23. | Vaughan-Sarrazin MS, Hannan EL, Gormley CJ, Rosenthal GE. Mortality in Medicare beneficiaries following coronary artery bypass graft surgery in states with and without certificate of need regulation. JAMA. 2002;288:1859-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Zishiri ET, Williams S, Cronin EM, Blackstone EH, Ellis SG, Roselli EE, Smedira NG, Gillinov AM, Glad JA, Tchou PJ. Early risk of mortality after coronary artery revascularization in patients with left ventricular dysfunction and potential role of the wearable cardioverter defibrillator. Circ Arrhythm Electrophysiol. 2013;6:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 25. | Fieguth HG, Wahlers T, Trappe HJ, Borst HG. Arrhythmogenic mortality in heart-transplant candidates. Transpl Int. 1996;9 Suppl 1:S219-S220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Pettit SJ, Petrie MC, Connelly DT, Japp AG, Payne JR, Haj-Yahia S, Gardner RS. Use of implantable cardioverter defibrillators in patients with left ventricular assist devices. Eur J Heart Fail. 2012;14:696-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Opreanu M, Wan C, Singh V, Salehi N, Ahmad J, Szymkiewicz SJ, Thakur RK. Wearable cardioverter-defibrillator as a bridge to cardiac transplantation: A national database analysis. J Heart Lung Transplant. 2015;34:1305-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1548] [Cited by in RCA: 1473] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 29. | Iacoviello M, Monitillo F. Non-invasive evaluation of arrhythmic risk in dilated cardiomyopathy: From imaging to electrocardiographic measures. World J Cardiol. 2014;6:562-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2275] [Cited by in RCA: 2160] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 31. | Bänsch D, Antz M, Boczor S, Volkmer M, Tebbenjohanns J, Seidl K, Block M, Gietzen F, Berger J, Kuck KH. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the Cardiomyopathy Trial (CAT). Circulation. 2002;105:1453-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 411] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 32. | Marchlinski FE, Jessup M. Timing the implantation of implantable cardioverter-defibrillators in patients with nonischemic cardiomyopathy. J Am Coll Cardiol. 2006;47:2483-2485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Kadish A, Schaechter A, Subacius H, Thattassery E, Sanders W, Anderson KP, Dyer A, Goldberger J, Levine J. Patients with recently diagnosed nonischemic cardiomyopathy benefit from implantable cardioverter-defibrillators. J Am Coll Cardiol. 2006;47:2477-2482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | McClellan MB, Tunis SR. Medicare coverage of ICDs. N Engl J Med. 2005;352:222-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N Engl J Med. 2016;375:1221-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1118] [Cited by in RCA: 1348] [Article Influence: 134.8] [Reference Citation Analysis (0)] |
| 36. | Singh M, Wang NC, Jain S, Voigt AH, Saba S, Adelstein EC. Utility of the Wearable Cardioverter-Defibrillator in Patients With Newly Diagnosed Cardiomyopathy: A Decade-Long Single-Center Experience. J Am Coll Cardiol. 2015;66:2607-2613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Stiermaier T, Rommel KP, Eitel C, Möller C, Graf T, Desch S, Thiele H, Eitel I. Management of arrhythmias in patients with Takotsubo cardiomyopathy: Is the implantation of permanent devices necessary? Heart Rhythm. 2016;13:1979-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 38. | Stiermaier T, Eitel C, Denef S, Desch S, Schuler G, Thiele H, Eitel I. Prevalence and Clinical Significance of Life-Threatening Arrhythmias in Takotsubo Cardiomyopathy. J Am Coll Cardiol. 2015;65:2148-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 39. | Nascimento FO, Krishna RK, Hrachian H, Santana O. Wearable cardioverter defibrillator in stress cardiomyopathy and cardiac arrest. BMJ Case Rep. 2013;2013:pii: bcr2013009789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Peters S, Klein HU. WCD LifeVest: risk stratification in a case of Tako-Tsubo cardiomyopathy with QT interval prolongation. Herz. 2012;37:219-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Elkayam U. Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Coll Cardiol. 2011;58:659-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 232] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 42. | Honigberg MC, Givertz MM. Arrhythmias in peripartum cardiomyopathy. Card Electrophysiol Clin. 2015;7:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Whitehead SJ, Berg CJ, Chang J. Pregnancy-related mortality due to cardiomyopathy: United States, 1991-1997. Obstet Gynecol. 2003;102:1326-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 44. | Duncker D, Haghikia A, König T, Hohmann S, Gutleben KJ, Westenfeld R, Oswald H, Klein H, Bauersachs J, Hilfiker-Kleiner D. Risk for ventricular fibrillation in peripartum cardiomyopathy with severely reduced left ventricular function-value of the wearable cardioverter/defibrillator. Eur J Heart Fail. 2014;16:1331-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 45. | Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 1999;99:1091-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 368] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 46. | Kindermann I, Kindermann M, Kandolf R, Klingel K, Bültmann B, Müller T, Lindinger A, Böhm M. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118:639-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 463] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 47. | Szymkiewicz S. Ambulatory Post-Syncope Arrhythmia Protection Feasibility Study (ASAP). [accessed 2015 Feb 18]. Available from: https://clinicaltrials.gov/ct2/show/NCT02188147. |
| 48. | Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W. ‘United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & amp; end-stage renal disease in the United States. Am J Kidney Dis. 2012;59:A7, e1-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 520] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 49. | Wan C, Herzog CA, Zareba W, Szymkiewicz SJ. Sudden cardiac arrest in hemodialysis patients with wearable cardioverter defibrillator. Ann Noninvasive Electrocardiol. 2014;19:247-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Herzog CA, Li S, Weinhandl ED, Strief JW, Collins AJ, Gilbertson DT. Survival of dialysis patients after cardiac arrest and the impact of implantable cardioverter defibrillators. Kidney Int. 2005;68:818-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Guha A, Maddox WR, Colombo R, Nahman NS, Kintziger KW, Waller JL, Diamond M, Murphy M, Kheda M, Litwin SE. Cardiac implantable electronic device infection in patients with end-stage renal disease. Heart Rhythm. 2015;12:2395-2401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 52. | LaPage MJ, Canter CE, Rhee EK. A fatal device-device interaction between a wearable automated defibrillator and a unipolar ventricular pacemaker. Pacing Clin Electrophysiol. 2008;31:912-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, Estes NA, Greenberg H, Hall WJ, Huang DT. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275-2283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1032] [Cited by in RCA: 1085] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 54. | Healy CA, Carrillo RG. Wearable cardioverter-defibrillator for prevention of sudden cardiac death after infected implantable cardioverter-defibrillator removal: A cost-effectiveness evaluation. Heart Rhythm. 2015;12:1565-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |