Published online May 26, 2017. doi: 10.4330/wjc.v9.i5.429
Peer-review started: November 14, 2016
First decision: February 15, 2017
Revised: February 24, 2017
Accepted: March 12, 2017
Article in press: March 13, 2017
Published online: May 26, 2017
Processing time: 187 Days and 7.8 Hours
Implantable cardioverter defibrillator (ICD) programming involves several parameters. In recent years antitachycardia pacing (ATP) has gained an increasing importance in the treatment of ventricular arrhythmias, whether slow or fast. It reduces the number of unnecessary and inappropriate shocks and improves both patient’s quality of life and device longevity. There is no clear indication regarding the type of ATP to be used, except for the treatment of fast ventricular tachycardias (188 bpm-250 bpm) where it has been shown a greater efficacy and safety of burst compared to ramp; 8 impulses in each sequence of ATP appears to be the best programming option in this setting. Beyond ATP use, excellent clinical results were obtained with programming standardization following these principles: extended detection time in ventricular fibrillation (VF) zone; supraventricular discrimination criteria up to 200 bpm; first shock in VF zone at the maximum energy in order to reduce the risk of multiple shocks. The MADIT-RIT trial and some observational registries have also recently demonstrated that programming with a widespread use of ATP, higher cut-off rates or delayed intervention reduces the number of inappropriate and unnecessary therapies and improves the survival of patients during mid-term follow-up.
Core tip: Antitachycardia pacing (ATP) has a great importance in the treatment of ventricular arrhythmias, whether slow or fast. It reduces the number of unnecessary shocks and it improves both patient’s quality of life and device longevity. Beyond ATP use, excellent clinical results were obtained with programming standardization following these principles: Extended detection in ventricular fibrillation (VF) zone; supraventricular discrimination criteria up to 200 bpm; first shock in VF zone at the maximum energy in order to reduce the risk of multiple shocks. The MADIT-RIT trial and some registries have also recently demonstrated that programming with a widespread use of ATP, higher cut-off rates or delayed intervention reduces the number of inappropriate therapies and improves the survival of patients during medium term follow-up.
- Citation: De Maria E, Giacopelli D, Borghi A, Modonesi L, Cappelli S. Antitachycardia pacing programming in implantable cardioverter defibrillator: A systematic review. World J Cardiol 2017; 9(5): 429-436
- URL: https://www.wjgnet.com/1949-8462/full/v9/i5/429.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i5.429
The efficacy of implantable cardioverter-defibrillator (ICD) in reducing sudden death and total mortality is well documented[1], initially in secondary prevention[2-4], more recently also in primary prevention[5,6]. In particular, two big trials, MADIT II[5] and SCD-HeFT[6], helped to define high risk patients after a myocardial infarction (MI) or with heart failure (HF) associated with reduced left ventricle ejection fraction.
These studies showed that the overall survival rate was higher in patients with ICD compared with those who received conventional medical therapy[5,6].
In the last decade, ICD implants have grown exponentially[7], leading manufacturers to heavily invest in this field to improve therapies and develop sophisticated algorithms with high sensitivity and specificity for arrhythmias discrimination. Once detected, the ICD can treat ventricular arrhythmias with high-energy shocks or antitachycardia pacing (ATP).
Although ICDs are usually well accepted by most patients, there are several clinical reports of anxiety and depression after implantation[8,9]. Quality of life can, in fact, be negatively influenced when receiving painful shocks, especially if multiple[10]. The main benefit of ATP therapy, from the patient’s point of view, is to avoid painful shocks; actually, ATP is rarely noticed by patients and therefore well tolerated. Moreover, battery life of the device is extended if the high-energy shock therapy is avoided. Even more important, it has also been demonstrated that shock therapy is associated with a higher risk of mortality compared to ATP sequences only[11].
With the increase in ICD indications, the choice of an optimal device programming, both for discrimination and therapy, is becoming increasingly important. A critical analysis of the most important clinical studies in this field is crucial in order to achieve an effective and safe therapy that improves patient’s outcome without adversely affecting quality of life.
ATP consists of one or more trains of pacing stimuli (usually 8 impulses for each train) conventionally expressed as a percentage of the tachycardia cycle length for a given RR interval, from the onset of the preceding R wave. Pace stimulation delivered at very short coupling intervals (i.e., < 84%) is more likely to enter a reentrant circuit but also accelerate the arrhythmia. Unlike shock therapy, the locations of the ICD generator and shocking coils do not affect ATP efficacy. ATP is usually delivered from the right ventricular apex (RVA), but efficacy is similar also when pacing from outflow tract. ATP from RVA is less effective in terminating ventricular tachycardia (VT) with a basal exit site[1].
The rationale for ATP efficacy relies of the fact that monomorphic VT can be interrupted with appropriately timed pacing stimuli delivered into the excitable gap of a reentrant circuit. The chance of arrhythmia interruption depends on several factors: The conduction time from pacing stimulus site to the reentrant circuit; the duration of the excitable gap; the presence of anatomic/functional barriers; the state of the sympathetic nervous system. For example, beta-blockers drugs increase the duration of excitable gap, thus increasing ATP efficacy[10].
VT with spontaneous RR interval variability are more likely be ATP responsive, while those with greater variation in QRS morphology are less responsive. This is the reason why polymorphic VT and ventricular fibrillation (VF), usually lacking an organized reentry, are rarely interrupted by pacing.
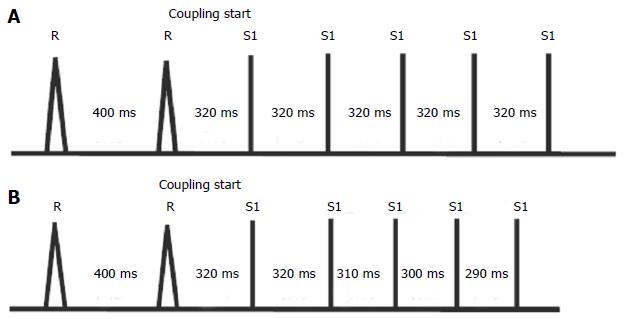
ICDs allow delivering different ATP therapy types, in particular the two most important are: (1) burst with impulse trains at constant coupling in a programmable number; and (2) ramp with autodecremental coupling (Figure 1).
Schaumann et al[12], in 1998, evaluated whether ATP could be safely used even in those patients in whom ATP testing on induced VT was not performed. All devices were programmed with the same ATP scheme in the VT zone (< 200 bpm): 3 ramps from 8 to 10 impulses, with 8 ms decrease and 81% coupling. The study enrolled 200 patients divided in two groups: The first included subjects in whom efficacy of ATP was demonstrated on sustained VT induced in the electrophysiology laboratory (Tested group); in the second group ATP was programmed empirically (Empirical group). During the follow-up period (20 ± 11 mo) ATP therapy proved to be highly effective in both groups. In particular, success rate was 95% in the T group and 90% of the E group. Moreover, this study provided a strong response to one of the most frequent criticism to pacing therapy, the risk of tachycardia acceleration. Acceleration after ATP occurred only in 2% in group T and 5% in group E. The conclusions of the study were, therefore, that the success rate of ATP therapy was not dependent on efficacy testing and ATP was recommended for VT treatment in any patient with ICD.
During the 90s other studies were published about the efficacy and the safety of the ATP therapy. These studies reported that ATP sequences successfully interrupted 78%-94% of slow VT (< 188 bpm), with an acceleration rate between 2% and 4%[13-15]. Based on these results the ATP was conventionally programmed only for slower TV, presumably hemodynamically well tolerated.
Fast VTs (from 188 to 250 bpm) were, instead, still treated like VF, with high energy shocks. Faster VTs have a shorter excitable gap that is more difficult to be penetrated and interrupted by a pacing stimulus.
Nevertheless, in 2001, Wathen et al[16] with the PainFREE Rx I trial showed, for the first time, that ATP therapy was also effective for fast VT (FVT). This trial, however, had many limitations: Only patients with coronary artery disease were included; it was nonrandomized; ICDs were programmed with a short detection interval (12 of 16 beats), which could imply that many treated arrhythmias were non-sustained VTs, destined to run out on their own.
In 2004, the same authors published a prospective, single-blinded trial, the PainFREE Rx II[17], which exceeded the limits of the first study. This trial enrolled 637 ICD patients randomized to ATP (n = 315) or shock therapy (n = 322) to evaluate episode duration between the two arms (primary endpoint). Baseline clinical variables were similar between the 2 groups, in particular age (average, 67 years), ejection fraction (average, 32%), sex (male 77%), coronary artery disease (85%), syncope (35%). Both groups received similar pharmacological therapies. Primary prevention indication for ICD involved 44% of patients. Fast VT was defined as a zone between 240 and 320 ms (188-250 bpm); faster rates were considered as VF. In the first group the initial therapy in the FVT zone was ATP (8 impulses burst at 88% of arrhythmia cycle length), while in the second group shock was directly delivered at the defibrillation threshold (DFT) + 10 J. The detection for FVT, as well as for VF, was 18 of 24 beats. The first important result was that the FVTs represented 76% of all ventricular arrhythmias. In the ATP group the bursts resulted effective in 77% of the FVTs; when it failed, shocks were effectively delivered to interrupt the arrhythmia. There was no significant difference between percentage (and number) of patients who had fast VT in either the ATP or shock arms (15% vs 16% respectively). In addition, after accounting for 2 patients in the ATP arm who together had 131 episodes, numbers of fast VT in the ATP and shock arms were similar (151 vs 144, respectively). Moreover, there was no significant difference in episode duration between the shock and ATP arms (10 s vs 9.7 s, respectively). Not all patients with fast VT episodes received shock therapy in the shock arm (only 67% of episodes being shocked) and 30% of fast VT episodes self-terminated after detection. In comparison, only 20% of patients in the ATP arm received shocks. The acceleration was rare, with 2% incidence in the ATP arm and 1% the shock arm. Syncopal events were also low and comparable between the two groups. It is interesting to note that the success rate of the first shock (92%) was identical in both groups, even if delivered after an ineffective ATP. In conclusion, this study showed that ATP therapy was safe and effective compared to shocks also for the treatment of FVT, with a 70% relative reduction of shocks in the ATP group.
After these studies, scientific community started to consider ICDs as stimulation devices with a defibrillation backup, only as a security option, with a consequent improvement in patient’s quality of life. It is noteworthy that these trials used bursts coupled to 88% of arrhythmia cycle: This was a “little aggressive” therapy, so the risk of arrhythmias acceleration was low[18].
In order to avoid significant delays in the delivery of shock therapy (in case of ATP failure) algorithms of ATP sequences during or before capacitor charging were soon implemented in the VF zone for most manufacturers; this strategy has been subsequently clinically validated as safe and effective[11].
The importance of the ATP therapy in the context of ICD programming is well documented, but we should understand if there is a specific pacing pattern to prefer, especially in relation to the type of ventricular arrhythmia.
Several studies comparing the efficacy of two different types of ATP pattern (burst and ramp) on induced VTs did not show significant differences in the percentage of success: Gills et al[19] reported 76% efficacy for burst and 68% for ramp in a study with 21 patients enrolled; Calkins et al[20] reported a success rate of 70% for burst and 72% for ramp (44 patients); Kantoch et al[21] reported a 69% success for burst and 72% for ramp in 31 patients. The rate of acceleration was low and did not change significantly between the two patterns.
Burst vs Ramp were evaluated also in the setting of spontaneous VTs: Gills at al[19] found, in this case, a success rate of 96% for burst and 93% for ramp; Ardashev et al[22] reported 61% efficacy for burst and 76% for ramp with 54 patients enrolled. The latter study, therefore, was the only indicating a significant difference in efficacy between the two techniques (P < 0.01), in favor of the ramp.
Overall, from the analysis of several studies, there was not a clear difference in the efficacy of burst and ramp for treatment of non-FVT, in ischemic and non-ischemic cardiomyopathies. The choice of the pattern was, therefore, left to the clinician’s experience and to an empirical case-by-case approach[18]. An important exception is represented by patients with arrhythmogenic right ventricular dysplasia: The success rate of ramp fell down to 25%, with an acceleration rate of 24%, while the burst resulted in a better outcome[22].
Different considerations have to be made for FVT in which burst seems to be better. The PITAGORA ICD[23] trial was a randomized Italian study that aimed to compare two ATP strategies (burst and ramp) in terms of efficacy, arrhythmia acceleration and syncope on FVT episodes. Two hundred and six patients were randomized into two groups, with two different therapies: 88% coupling-8 impulses burst vs 91% coupling-8 impulses ramp with 10 ms decrement. The FVT zone was programmed between 188 and 250 bpm, with a detection of 18 of 24 beats. The study demonstrated that burst was significantly more effective than ramp (75% vs 54%; P = 0.015) to interrupt FVT episodes. Regarding safety, burst was associated with a lower percentage of acceleration compared to ramp (2% vs 7%), although this difference was not statistically significant. The overall incidence of syncope was 1%. The adopted strategy, with ATP as the first therapy and prolonged detections (18 of 24 compared to 12 of 16), allowed the end of the arrhythmic episode before shock delivery in 81% of the cases[23].
In 2010, the results of the trial ADVANCED-D[24] were published. This study aimed to compare 8 impulses burst with 15 impulses burst on FVT. Nine hundred and twenty-five patients were enrolled and randomized into two groups treated with the two different sequences of ATP. The window of FVT remained between 188 and 250 bpm and detection 18 of 24 beats. No significant difference was detected between the two sequences, 8 pulses burst terminated 64% of episodes compared to 70% of 15 pulses burst. Moreover, there were not significant differences also regarding syncope or rate of acceleration. The sequence of 15 pulses was more effective only in patients with no history of HF (P = 0.014) and with left ventricular ejection fraction > 40% (P = 0.016). The conclusion of the study was that an ATP sequence of 15 pulses can be considered effective, but also safe, in FVT comparable with a sequence of 8 pulses.
Thanks to the coronary sinus lead which stimulates the left ventricle, cardiac resynchronization therapy devices equipped with a cardioverter defibrillator (CRTD) offer the potential for alternative sites for ATP. The possibility to deliver therapy from either the left ventricular lead (LV-ATP) or the left and the right ones simultaneously (BiV-ATP) may theoretically increase the rate of success compared to right ventricular stimulation (RV-ATP). Several studies reported an increased efficacy of BiV-ATP configuration compared to the RV-ATP for termination of VT events both slow and fast[25]. However, the ADVANCED CRT-D[26] trial demonstrated a significant superiority of BiV-ATP only in ischemic patients with FVTs. Moreover, few papers compared LV-ATP with the other configurations. In this context, a study by Haghjoo et al[27] compared efficacy and safety of the three ATP therapy sites (RV, LV and BiV) for VT treatment in patients with a CRTD device. The study enrolled 89 patients (with ischemic and non-ischemic etiologies) divided into 3 groups with 3 different pacing sites during ATP. The mean follow-up was 38 mo with 259 detected VT episodes in 46 patients. The results showed: (1) greater efficacy of BiV-ATP compared to both LV-ATP and RV-ATP for the treatment of FVT (188-250 bpm); (2) higher success rate and lower acceleration rate of both LV-ATP and BiV-ATP compared to RV-ATP for slower VTs (< 188 bpm)[27]. Therefore, left ventricular lead allows further possibilities to increase the success of ATP; in patients with CRTD it is recommended to set either biventricular or left ventricular ATP therapy for the slowest therapy zone (< 188 bpm), while biventricular ATP should be programmed for faster arrhythmias.
The therapeutic programming of an ICD involves several parameters. It is worthwhile to understand if a strategic standardized choice can be as effective and safe as a patient-tailored programming, which is inevitably time-consuming for the physician. Indeed, the customization of the ICD setting is useful only if it provides improvements in clinical outcomes or in patient’s quality of life; otherwise both the simplification and the standardization of the therapy can be convenient and minimize the risk of random errors.
In this framework, EMPIRIC trial[10] randomized 900 patients with ICDs (48% implanted for primary prevention, 52% for secondary prevention) to a standardized (EMPIRIC group) or a physician-tailored (TAILORED group) VT/VF programming and followed them for 1 year. The EMPIRIC programming (Table 1) was created by taking into account some key strategies to safely reduce the number of shocks for VT/VF and supraventricular tachycardias (SVTs) and to avoid untreated slow VT: (1) three attempts of ATP for VT < 200 bpm. In particular, 2 burst of 8 intervals coupled at 88% with 20 ms decrement and 1 ramp of 8 intervals coupled at the 81% with 10 ms decrement; (2) a sequence of ATP for FVT between 200 bpm and 250 bpm. In particular, 1 burst of 8 intervals coupled at the 88% (as in the PainFREE Rx II); (3) long detection time (18 out of 24) in VF and FVT (as in the PainFREE Rx II, PITAGORA ICD e ADVANCED-D trials); (4) first shock at the maximum energy in VF and FVT zones to reduce the risk of multiple shocks; and (5) discrimination criteria for SVT in the VF zone.
| Detection | Threshold (bpm) | Beats to detect | Therapies |
| VF | 250 | 18 out of 24 | 30 J × 6 |
| FVT | 200 | 18 out of 24 | 1 × burst, 30 J × 5 |
| VT | 150 | 16 | 2 × burst, 1 × ramp, 30 J × 3 |
The results of the study reported no significant differences in the number of deaths, syncope events and arrhythmias acceleration between the two groups of patients. Moreover, the rate of hospitalization was significantly lower (P = 0.001) in the EMPIRIC group[10]. The overall ATP efficacy was 92%; consequently, a significant reduction of VT shocks was reported in the EMPIRIC group compared to the TAILORED group (P < 0.001). It is interesting to observe that the EMPIRIC group had a threshold for the VT zone of 150 bpm, value which is lower than the average in the TAILORED group (171 bpm). Nevertheless, the study did not show an increase of the SVTs inappropriately treated, thanks to the discrimination algorithms. To conclude, EMPIRIC study entails that a simplified and standardized programming is possible, without reducing efficacy and safety of the therapy.
The PREPARE study[1] analyzed a different standardized setting with the aim of reducing shocks occurrence, syncopes and untreated symptomatic VT in patients with ICDs for primary prevention[28]. The PREPARE programming was developed on the basis of some key strategies: (1) to detect only fast tachycardias (> 182 bpm); (2) to discriminate only sustained tachycardias (discrimination set to 30 of 40 in the FVT and FV zones); (3) to deliver ATP as the first therapy for FVT; (4) to always deliver the high-energy shock (at least 30 J); and (5) to use discrimination criteria for SVT up to 200 bpm.
The PREPARE programming (Table 2) provided a lower mortality (P = 0.01) compared to a control cohort of patients form the EMPIRIC[10] the MIRACLE ICD (Multicenter InSync Implantable Cardioverter Defibrillators Trial) studies. The extension of the detection duration (30 out of 40 beats), fast rate cutoffs for the therapy (182 bpm), the use of SVT discrimination criteria reduced the number of shocked episodes. Moreover, at 12 mo follow up the incidence of syncopal events was 1.6% and mortality 4.9%. This study demonstrated how a strategically chosen tachycardia detection and conservative therapy options, can make the device more acceptable by the patient without negatively affecting its efficacy and safety.
| Detection | Threshold (bpm) | Beats to detect | Therapies |
| VF | 250 | 18 out of 24 | 30 J/35 J × 6 |
| FVT | 182 | 18 out of 24 | 1 × Burst, 30 J/35 J × 6 |
| VT | 167 | 32 | Off |
In 2012 a large randomized multicenter study, Multicenter Automatic Defibrillator Implantation Trial-Reduce Inappropriate Therapy (MADIT-RIT)[29], was published in the New England Journal of Medicine. The aim of this study was to test two ICD programming strategies in patients with an ICD implanted for primary prevention. In particular, the first strategy was characterized by therapies only for high heart rates (> 200 bpm) while the second was to increase of the detection delay duration before the initiation of therapies, with values variable in relation to the heart rate (60 s delay for rates between 170 bpm and 199 bpm, 12 s delay for rates between 200 bpm and 249 bpm, 2.5 s delays for higher rates). As explained by the authors, the MADIT-RIT study was based on the hypothesis that these two strategies would have reduced the number of patients receiving appropriate and inappropriate shocks and ATPs, compared to a conventional programming, without increasing mortality and morbidity. The study involved 98 centers in the United States, Canada, Europe, Israel and Japan, enrolling a total of 1500 patients with either ischemic or non-ischemic heart disease and indicated for implantation of an ICD or a CRTD in primary prevention. Patients with atrial fibrillation were excluded. The first episode of inappropriate therapy represented the primary endpoint of the study: This outcome was evaluated by comparing each treatment group with the control group. The rates of both syncope and mortality (for any cause) were secondary endpoints. A significant reduction in the risk of any inappropriate therapy was observed in the two groups with “unconventional” programming in a follow-up of 1.4 years: The results showed a 79% relative risk reduction for patients with “High-Rate Therapy” and a 76% risk reduction for those with “Delayed Therapy” (HR of “High-Rate therapy” vs conventional therapy: 0.21, 95%CI, P < 0.001; HR of “Delayed Therapy” vs conventional therapy: 0.24, 95%CI, P < 0.001). Another significant result of the MADIT-RIT regarded one of the secondary endpoints of the study. The “High-Rate Therapy” programming reduced the risk of death from any cause (HR = 0.45, P = 0.01) by a factor of 55% compared to conventional therapy. The group with “Delayed Therapy” showed a 44% reduction of the mortality risk, but it did not reach statistical significance compared to the conventional therapy group (HR = 0.56, P = 0.06). Concerning syncopal episodes, no difference among the three groups was observed.
Recently, the OBSERVational registry on long-term outcome of ICD patients[30] confirmed, in a “real world setting”, the results of MADIT-RIT trial. OBSERVO-ICD was a multicenter, retrospective, registry enrolling (from 2010 to 2012) all consecutive patients undergoing ICD implantation in 5 Italian centers. The aim of the study was to test whether a too aggressive ICD programming could be associated with electrical storms (ES). A total of 1319 patients were enrolled, both primary and secondary prevention. During follow up (median 39 mo) 4.7% of patients experienced at least 1 ES episode. Patients with ES presented with significantly lower VF detection zone (P = 0.002), more frequently had ATP therapies during capacitor charging programmed off (P = 0.001), and less frequently had delayed therapies for VT zone (P = 0.042) and VF zone (P = 0.036). Patients with ES had a significantly higher incidence of death and HF–related death compared to patients with no VTs and patients with unclustered VTs/VFs (P = 0.025 and P = 0.001, respectively). In conclusion, patients with less aggressive ICD programming (higher VF detection rates, higher detection times, ATP therapies during capacitor charging turned on) had a decreased likelihood of ES and lower risk of death.
ATP is a safe, effective and painless therapy for VTs with large clinical evidence supporting its routine use in primary and secondary ICD patients[31,32].
ATP therapy is effective in interrupting VTs, both slow and fast, with a consequent reduction of unnecessary shocks and an improvement of clinical outcome, patients’ quality of life and device longevity[12,16,17,31].
In a recent expert consensus document on ICD programming, from the most important world leading arryhthmological societies[32], it was stated that “in all patients with structural heart disease... that ATP therapy be active for all ventricular tachyarrhythmia detection zones to include arrhythmias up to 230 bpm, to reduce total shocks except when ATP is documented to be ineffective or proarrhythmic”.
In patient with inherited cardiac channelopathies (Brugada syndrome, Long and Short QT syndrome, catecholaminergic polymorphic VT, early repolarization syndromes) the index clinical arrhythmia is polymorphic VT or VF: These arrhythmias usually lack an organized reentry and are rarely interrupted by pacing, so ATP should not be routinely programmed[31,32].
As concerns the type of ATP to be used, clear conclusions cannot be drawn, except for the treatment of fast TV (188 bpm-250 bpm) for which greater efficacy and safety of burst was showed compared to ramp[23,31,32]. So, as a first choice, it is indicated to program burst in preference to ramp. Ramp should be reserved for patients in whom burst fails and ramp is proven to be effective. A “little aggressive” burst programming (several studies used impulses coupled at the 88%) seems to be related to lower rates of arrhythmia acceleration[18]. Moreover, the optimal number of impulses in each sequence of ATP has been proved to be minimum 8[24,32].
In patients with biventricular devices the lead placed in the coronary sinus offers further opportunities for a successful ATP therapy, due to biventricular pacing (ATP-BiV) or left ventricular only pacing (LV-ATP)[25-27].
In the last years, a great effort has been devoted to standardize the ICD programming, particularly in the primary prevention. Two studies provided excellent results in this field: EMPIRIC[10] and PREPARE[1]. The fundamental principles of these programming strategies were: (1) prolonged detection for the VF zone (18 out of 24 and 30 out of 40); (2) delayed detection time in any window; (3) SVT discrimination criteria up to 200 bpm; (4) ATP as first therapy for FVT; and (5) first shock at maximum energy in the VF zone to reduce the risk of multiple shocks.
The MADIT-RIT[29] trial and the OBSERVO-ICD registry[30] have recently confirmed this programming philosophy, showing that higher cut-off rates, more prolonged detections and ATP during capacitor charging reduce the number of inappropriate and unnecessary therapies compared to a more “conventional” programming. This reduction translates in a better clinical outcome in terms of morbidity and even mortality. The results of these studies add new chapters in the development of the ICD therapy, especially in primary prevention patients.
More studies are needed, instead, in a secondary prevention setting to design effective ATP strategies. Secondary prevention patients can represent an opportunity to a more “tailored” approach compared to primary prevention, on the basis of the knowledge of arrhythmia history (ECG morphology, cycle length, arrhythmia mechanism, patient’s tolerance, hemodynamic impact)[31]. In patients with prior known VTs the device must be programmed to cover all clinical arrhythmias; slower monomorphic and better tolerated VTs should be treated with at least 2-3 sequences of ATP and at least 8 impulses. A second burst of ATP increases efficacy from 64% to 83% in FVT range, although programming > 2 bursts usually does not add further benefit[31,32].
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cheng TH, Kettering K S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Wilkoff BL, Williamson BD, Stern RS, Moore SL, Lu F, Lee SW, Birgersdotter-Green UM, Wathen MS, Van Gelder IC, Heubner BM. Strategic programming of detection and therapy parameters in implantable cardioverter-defibrillators reduces shocks in primary prevention patients: results from the PREPARE (Primary Prevention Parameters Evaluation) study. J Am Coll Cardiol. 2008;52:541-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 417] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 2. | A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med. 1997;337:1576-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2438] [Cited by in RCA: 2217] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 3. | Connolly SJ, Gent M, Roberts RS, Dorian P, Roy D, Sheldon RS, Mitchell LB, Green MS, Klein GJ, O’Brien B. Canadian implantable defibrillator study (CIDS) : a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000;101:1297-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1078] [Cited by in RCA: 981] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 4. | Kuck KH, Cappato R, Siebels J, Rüppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH). Circulation. 2000;102:748-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 967] [Cited by in RCA: 898] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 5. | Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5067] [Cited by in RCA: 4892] [Article Influence: 203.8] [Reference Citation Analysis (0)] |
| 6. | Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4998] [Cited by in RCA: 4845] [Article Influence: 230.7] [Reference Citation Analysis (0)] |
| 7. | Gadler F, Valzania C, Linde C. Current use of implantable electrical devices in Sweden: data from the Swedish pacemaker and implantable cardioverter-defibrillator registry. Europace. 2015;17:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Heller SS, Ormont MA, Lidagoster L, Sciacca RR, Steinberg S. Psychosocial outcome after ICD implantation: a current perspective. Pacing Clin Electrophysiol. 1998;21:1207-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Herrmann C, von zur Mühen F, Schaumann A, Buss U, Kemper S, Wantzen C, Gonska BD. Standardized assessment of psychological well-being and quality-of-life in patients with implanted defibrillators. Pacing Clin Electrophysiol. 1997;20:95-103. [PubMed] |
| 10. | Wilkoff BL, Ousdigian KT, Sterns LD, Wang ZJ, Wilson RD, Morgan JM. A comparison of empiric to physician-tailored programming of implantable cardioverter-defibrillators: results from the prospective randomized multicenter EMPIRIC trial. J Am Coll Cardiol. 2006;48:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 173] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Sweeney MO, Sherfesee L, DeGroot PJ, Wathen MS, Wilkoff BL. Differences in effects of electrical therapy type for ventricular arrhythmias on mortality in implantable cardioverter-defibrillator patients. Heart Rhythm. 2010;7:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 260] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 12. | Schaumann A, von zur Mühlen F, Herse B, Gonska BD, Kreuzer H. Empirical versus tested antitachycardia pacing in implantable cardioverter defibrillators: a prospective study including 200 patients. Circulation. 1998;97:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 96] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Yee R, Klein GJ, Guiraudon GM, Jones DL, Sharma AD, Norris C. Initial clinical experience with the pacemaker-cardioverter-defibrillator. Can J Cardiol. 1990;6:147-156. [PubMed] |
| 14. | Luceri RM, Habal SM, David IB, Puchferran RL, Muratore C, Rabinovich R. Changing trends in therapy delivery with a third generation noncommitted implantable defibrillator: results of a large single center clinical trial. Pacing Clin Electrophysiol. 1993;16:159-164. [PubMed] |
| 15. | Trappe HJ, Klein H, Fieguth HG, Kielblock B, Wenzlaff P, Lichtlen PR. Clinical efficacy and safety of the new cardioverter defibrillator systems. Pacing Clin Electrophysiol. 1993;16:153-158. [PubMed] |
| 16. | Wathen MS, Sweeney MO, DeGroot PJ, Stark AJ, Koehler JL, Chisner MB, Machado C, Adkisson WO. Shock reduction using antitachycardia pacing for spontaneous rapid ventricular tachycardia in patients with coronary artery disease. Circulation. 2001;104:796-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 222] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 17. | Wathen MS, DeGroot PJ, Sweeney MO, Stark AJ, Otterness MF, Adkisson WO, Canby RC, Khalighi K, Machado C, Rubenstein DS. Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators: Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (PainFREE Rx II) trial results. Circulation. 2004;110:2591-2596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Peinado R, Almendral J, Rius T, Moya A, Merino JL, Martínez-Alday J, Pérez-Villacastín J, Arenal A, Ormaetxe J, Tercedor L. Randomized, prospective comparison of four burst pacing algorithms for spontaneous ventricular tachycardia. Am J Cardiol. 1998;82:1422-1445, 1422-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Gillis AM, Leitch JW, Sheldon RS, Morillo CA, Wyse DG, Yee R, Klein GJ, Mitchell LB. A prospective randomized comparison of autodecremental pacing to burst pacing in device therapy for chronic ventricular tachycardia secondary to coronary artery disease. Am J Cardiol. 1993;72:1146-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Calkins H, el-Atassi R, Kalbfleisch S, Langberg J, Morady F. Comparison of fixed burst versus decremental burst pacing for termination of ventricular tachycardia. Pacing Clin Electrophysiol. 1993;16:26-32. [PubMed] |
| 21. | Kantoch MJ, Green MS, Tang AS. Randomized cross-over evaluation of two adaptive pacing algorithms for the termination of ventricular tachycardia. Pacing Clin Electrophysiol. 1993;16:1664-1672. [PubMed] |
| 22. | Ardashev AV, Dzhandzhgava AO, Zheliakov EG. [Antitachycardia pacing in patients with implanted cardioverter defibrillators]. Kardiologiia. 2011;51:65-73. [PubMed] |
| 23. | Gulizia MM, Piraino L, Scherillo M, Puntrello C, Vasco C, Scianaro MC, Mascia F, Pensabene O, Giglia S, Chiarandà G. A randomized study to compare ramp versus burst antitachycardia pacing therapies to treat fast ventricular tachyarrhythmias in patients with implantable cardioverter defibrillators: the PITAGORA ICD trial. Circ Arrhythm Electrophysiol. 2009;2:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Santini M, Lunati M, Defaye P. Prospective multicenter randomized trial of fast ventricular tachycardia termination by prolonged versus conventional anti-tachyarrhythmia burst pacing in implantable cardioverter-defibrillator patients-Atp DeliVery for pAiNless ICD therapy (ADVANCED-D) Trial results. J Interv Card Electrophysiol. 2010;27:127-135. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Byrd IA, Rogers JM, Smith WM, Pollard AE. Comparison of conventional and biventricular antitachycardia pacing in a geometrically realistic model of the rabbit ventricle. J Cardiovasc Electrophysiol. 2004;15:1066-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Gasparini M, Anselme F, Clementy J, Santini M, Martínez-Ferrer J, De Santo T, Santi E, Schwab JO; ADVANCE CRT-D Investigators. BIVentricular versus right ventricular antitachycardia pacing to terminate ventricular tachyarrhythmias in patients receiving cardiac resynchronization therapy: the ADVANCE CRT-D Trial. Am Heart J. 2010;159:1116-1123.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Haghjoo M, Hajahmadi M, Fazelifar AF, Sadr-Ameli MA. Efficacy and safety of different antitachycardia pacing sites in the termination of ventricular tachycardia in patients with biventricular implantable cardioverter-defibrillator. Europace. 2011;13:509-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Morgan JM, Sterns LD, Hanson JL, Ousdigian KT, Otterness MF, Wilkoff BL. A trial design for evaluation of empiric programming of implantable cardioverter defibrillators to improve patient management. Curr Control Trials Cardiovasc Med. 2004;5:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, Estes NA, Greenberg H, Hall WJ, Huang DT. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275-2283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1032] [Cited by in RCA: 1086] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 30. | Guerra F, Palmisano P, Dell’Era G, Ziacchi M, Ammendola E, Bonelli P, Patani F, Cupido C, Devecchi C, Accogli M, Occhetta E, Santangelo L, Biffi M, Boriani G, Capucci AItalian Association of Arrhythmology and Cardiac Pacing (AIAC). Implantable cardioverter-defibrillator programming and electrical storm: Results of the OBSERVational registry On long-term outcome of ICD patients (OBSERVO-ICD). Heart Rhythm. 2016;13:1987-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 31. | Cantillon DJ, Wilkoff BL. Antitachycardia pacing for reduction of implantable cardioverter-defibrillator shocks. Heart Rhythm. 2015;12:1370-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Wilkoff BL, Fauchier L, Stiles MK, Morillo CA, Al-Khatib SM, Almendral J, Aguinaga L, Berger RD, Cuesta A, Daubert JP. 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Heart Rhythm. 2016;13:e50-e86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 208] [Article Influence: 18.9] [Reference Citation Analysis (0)] |