Published online Apr 26, 2017. doi: 10.4330/wjc.v9.i4.304
Peer-review started: September 23, 2016
First decision: November 2, 2016
Revised: January 5, 2017
Accepted: February 8, 2017
Article in press: February 13, 2017
Published online: April 26, 2017
Processing time: 220 Days and 2.6 Hours
Incidental diagnosis of left ventricular systolic dysfunction (LVD) is common in clinical practice. The prevalence of asymptomatic LVD (Ejection Fraction, EF < 50%) is 6.0% in men and 0.8% in women and is twice as common as symptomatic LVD. The timely and definitive exclusion of an ischemic etiology is central to optimizing care and reducing mortality in LVD. Advances in cardiovascular imaging provide many options for imaging of patients with left ventricular dysfunction. Clinician experience, patient endurance, imaging modality characteristics, cost and safety determine the choice of testing. In this review, we have compared the diagnostic utility of established tests - nuclear and echocardiographic stress testing with newer techniques like coronary computerized tomography and cardiac magnetic resonance imaging and highlight their inherent limitations in patients with underlying left ventricular dysfunction.
Core tip: Left ventricular systolic dysfunction is common in clinical practice and may be detected in asymptomatic patients. The timely and definitive exclusion of an ischemic etiology is central to optimizing care and reducing mortality. Clinician experience, imaging modality characteristics, cost and safety determine the choice of testing. We compare the diagnostic utility of established tests like nuclear and echocardiographic stress testing with newer techniques like coronary computerized tomography and cardiac magnetic resonance imaging. Due to limitations inherent to each non-invasive modality, oftentimes cardiac catheterization remains the definitive method to exclude coronary artery disease in patients with underlying left ventricular dysfunction.
- Citation: Bomb R, Kumar S, Chockalingam A. Coronary artery disease detection - limitations of stress testing in left ventricular dysfunction. World J Cardiol 2017; 9(4): 304-311
- URL: https://www.wjgnet.com/1949-8462/full/v9/i4/304.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i4.304
Incidental diagnosis of left ventricular systolic dysfunction (LVD) is common in clinical practice. The prevalence of asymptomatic LVD (ejection fraction, EF < 50%) is 6.0% in men and 0.8% in women[1]. Asymptomatic LVD is at least twice as common as symptomatic LVD[2]. The diagnosis of LVD is usually made by demonstration of reduced systolic contractility and low EF by echocardiography. To determine whether LVD is due to coronary artery disease (CAD) is critical in the management of these patients as coronary revascularization has been shown to substantially reduce mortality in ischemic LVD. Modalities available for CAD diagnosis are either invasive coronary angiography (CA) or various non-invasive techniques such as dobutamine stress echocardiography (DSE), myocardial perfusion imaging (MPI) or single photon emission computerized tomography (SPECT), positron emission tomography (PET), cardiac magnetic resonance imaging (CMR) and coronary computerized tomography (CCT). Clinician experience, patient endurance, imaging modality characteristics, cost, safety and local availability determine the choice of testing.
ACC/AHA in 2005 recommended CA for patients with heart failure who have angina or significant ischemia; CA was felt to be reasonable in patients with chest pain that may or may not be cardiac in origin in whom coronary anatomy is not known, those with known or suspected CAD as well as patients with myocardial viability on noninvasive tests[3]. The 2013 revised guidelines finds it reasonable (class IIa) to pursue either non-invasive imaging or CA in revascularization eligible patients[4]. CA is an invasive procedure with potentially serious complications such as atheroembolism, bleeding, renal failure, myocardial infarction, ventricular tachyarrhythmias, stroke and death. The low yield of CA in the setting of LVD further highlights the unfavorable risk benefit ratio. Therefore, a noninvasive method that can identify ischemic myocardial scaring or coronary luminal narrowing would be ideal in this setting. This would reduce the number of unwanted CA in patients with a truly non-ischemic cardiomyopathy. On the other hand, importantly, an ischemic etiology for cardiomyopathy can be missed when relying solely on non-invasive tests. In patients undergoing cardiac transplantation, severe CAD was found in all patients with a pretransplant diagnosis of ischemic cardiomyopathy (57 percent of a total 112 patients); unexpectedly at the time of transplant, severe CAD was also found in 9 of 38 patients previously thought to have idiopathic dilated cardiomyopathy (DCM) and 3 of 4 with presumptive alcoholic cardiomyopathy[5]!
DSE and MPI are commonly used modalities for evaluation of CAD. Both have proven to be clinically useful in large series, are widely available and provide prognostic information as well. Despite the high sensitivity and specificity reported with these tests over the last 2 decades, clinicians still have to deal with ambiguous test results when evaluating systolic heart failure patients. Available literature suggests that the sensitivity and specificity of non-invasive methods ranges between 80%-95%; this implies that the etiology of cardiomyopathy may be misdiagnosed in approximately 1 out of every 10 patients. Myocardial perfusion using newer imaging modalities like CMR and PET provide physiological data similar to DSE and SPECT while CCT predominantly provides anatomical information along the lines of invasive angiography. In this review, we will focus specifically on CAD detection in patients with LVD; role of imaging in LVD associated with myocarditis or specific cardiomyopathies like tachycardia induced cardiomyopathy, peripartum cardiomyopathy and stress cardiomyopathy are beyond the scope of this review.
We performed a search of MEDLINE, PUBMED, SCOPUS, Clinical trials.gov and The Cochrane Library from January 1975 through Dec 2015. We set no geographic or language restrictions. To increase yield, we also searched the references of all the retrieved manuscripts and review article. MeSH terms used were Coronary Artery Disease; Ventricular Dysfunction, Left; Magnetic Resonance ImagingCine; Computerized Tomogram, Echocardiography, Stress; Dobutamine; Positron-Emission Tomography; Tomography, X-Ray Computed; and Tomography, Emission-Computed, Single-Photon.
After extensive review it was noted that though modalities like DSE, SPECT, PET and magnetic resonance imaging (MRI) have been extensively studied and written about as regards to assessment of viability in patients with cardiomyopathy, recent published literature is scant specifically with diagnosis of CAD in these patients. A few recent reviews extensively discuss specific technical aspects[6,7]. We present our review highlighting the limited literature specifically pertaining to diagnostic accuracy of various cardiac imaging modalities in left ventricular (LV) systolic dysfunction.
SPECT allows direct assessment of myocardial perfusion. Parameters that factor into SPECT reporting are myocardial perfusion, wall motion abnormalities and LV ejection fraction. An inducible perfusion abnormality indicates impaired perfusion reserve which in turn corresponds to epicardial coronary obstruction.
Various studies have evaluated the utility of SPECT in detection of CAD. Bulkley et al[8] in 1977 reported that SPECT could reliably differentiate between ischemic and idiopathic cardiomyopathy obviating the need for cardiac catheterization. A similar conclusion was made by Tauberg et al[9] in 1993; based on the size of perfusion deficit, they showed that large defects have 97% predictive value for ischemic cardiomyopathy and 94% predictive value for idiopathic cardiomyopathy, and could reliably differentiate the two entities[9]. In contrast, Dunn et al[10] in 1982 reported lower accuracy (80%) for SPECT in differentiating between the two entities. Moreover, only complete perfusion defects indicated CAD in this study; partial perfusion defects as well as reversible defects were seen both in CAD as well as DCM. A study by Wu et al[11] in 2003 reported that SPECT was only of modest value to distinguish between ischemic and idiopathic cardiomyopathy and concluded that SPECT cannot be relied upon in an individual patient to differentiate the two entities. Overall, the existing literature points to a high sensitivity for SPECT in CAD detection (nearly 100% in some published studies) while specificity on average is only about 40%-50% in LVD patients[12-15].
Although individual studies report high diagnostic accuracy for detecting CAD, SPECT has limitations specific to prior LVD that impact reliability. Regional wall motion abnormalities may point to CAD if located in particular coronary distributions. However, in a study of 50 DCM patients, 64% had regional wall motion abnormalities[16]; other studies report the presence of regional wall motion abnormalities in 30%-50% of patients with DCM[17-19]. Thus, regional wall motion abnormalities do not automatically imply an ischemic etiology for the cardiomyopathy.
Reversible myocardial perfusion defects are traditionally considered specific for ischemia. However reversible perfusion defects can occur in dilated cardiomyopathy as well. In one study, complete perfusion defects in thallium redistribution studies appeared to imply an ischemic etiology but was seen in only a few patients[11]; the key finding in this study was that partially reversible defects occur in both DCM and ischemic cardiomyopathy when the LVD is severe (EF in the 25% range). In most instances, there is overlap of perfusion abnormalities between DCM and ischemic cardiomyopathy (even when large or reversible perfusion defects are present), thereby limiting the role of SPECT in the setting of LVD.
There are several possible reasons for the presence of perfusion defects in DCM. Structural changes in the myocardium of DCM patients like fibrosis and scarring could account for fixed perfusion defects[19]. Dilatation of ventricle and abnormal cell membrane permeability could lead to variable radioactive tracer uptake and redistribution. Stress testing induced LV geometry changes are also known to cause reversible defects in DCM[20]. Exercise induced coronary spasm[21], mitral valve prolapse[22], and aortic stenosis[23] have been associated with SPECT abnormalities. Clinicians should consider the role of these confounders while interpreting SPECT results.
In a given patient with LVD, DCM is likely if SPECT shows normal perfusion and global (i.e., non-regional) systolic dysfunction. However, if reversible defects are detected, especially in coronary territories, possibility of CAD remains high[24]. Another important concern in patients with severe LVD is balanced ischemia. Severe left main or triple vessel disease could cause equal reduction in tracer uptake in all major segments. As SPECT does not involve absolute quantification of tracer uptake, this matched perfusion defect in multiple territories appears as “normal” in this qualitative comparison of segments relative to each other[25]. In patients with balanced ischemia, diffuse ST depression during stress, subtle perfusion defects and transient cavity dilatation (TID) may be the only clues for underlying severe CAD.
In contemporary clinical practice, DSE and exercise stress echocardiography play a major role is detection of CAD and risk stratification. A graded dobutamine infusion starting at 5 mg/kg per minute and increasing at 3-min intervals to 10, 20, 30 and 40 mg/kg per minute is the standard for dobutamine stress testing[26]. If LVD is known to be of ischemic etiology, presence of myocardial viability and the probability of recovery after revascularization can be reliably predicted based on demonstrating contractile reserve with low dose dobutamine stress testing. There is a paucity of literature for exercise stress echocardiography in LVD.
Geleijnse et al[27] reported the sensitivity, specificity and accuracy of DSE for detection of CAD to be 80%, 84% and 81% respectively. In a study by Marcovitz et al[28], DSE had 96% sensitivity and 66% specificity for detection of CAD based on resting or inducible wall motion abnormalities. Similarly, in a study evaluating chest pain, Hennesse et al[29] showed that the overall sensitivity and specificity of DSE were 82% and 65% respectively: Positive and negative predictive values were 89% and 51% respectively. In a recent meta-analysis of 32 studies, DSE had a higher sensitivity (94% vs 75%, P < 0.001) and lower negative likelihood ratio (0.21 vs 0.47, P < 0.001) compared to SPECT for detection of left main or triple vessel disease[30]. These studies were performed predominantly in patients with preserved cardiac function.
Few DSE studies have been specifically performed in patients with LVD. One study by Jong et al[31] showed that most of the patients with DCM were found to have an abnormal regional myocardial contractile response to dobutamine. Cohen et al[32] reported in a study that although dobutamine had a reasonable specificity and positive predictive value, it lacked sensitivity in diagnosis of CAD in DCM patients. Sharp et al[33] reported that using the change in global wall motion score index from low to peak dose, DSE had a sensitivity of 83% and a specificity of 71% for detection of CAD. In one study, changes in left ventricular geometry as seen in patients with DCM can lead to false positive and false negative results in approximately 22% patients, hence reducing the accuracy of DSE[34]. Vigna et al[35] showed that although DSE has a specificity of 96% it has a lower sensitivity of 80% in diagnosis of CAD in patients with DCM.
Similar to SPECT, the reliability of DSE for CAD detection remains a challenge in the setting of LVD. Coronary territory specific hypokinesia, characteristic thinning and scar related hyperechoic signals would help confirm an ischemic etiology. When baseline LVD is significant with predominantly global hypokinesia, lack of response to dobutamine could mean a poor contractile reserve and not necessarily ischemia. On the other hand, some regional variability in improved contractility may be due to endothelial dysfunction, microvascular abnormalities or focal fibrosis. These factors together with increased wall stress may lead to a reduced myocardial perfusion reserve and wall motion abnormalities in the absence of CAD. Segmental wall motion abnormalities and abnormal contractile response that could be interpreted as ischemia or hibernation are common in patients with DCM despite the absence of CAD[36]. Finally, DSE is an observer and patient dependent procedure, the accuracy of which depends on the experience of the interpreter as well the acoustic windows available during stress testing.
Not much data is available comparing the accuracy of SPECT and DSE in detection of ischemia in patients with prior LVD. There are multiple studies comparing these modalities in patients with preserved cardiac function and a pooled analysis concluded that MPI was more sensitive compared to DSE (84% vs 80%) but DSE was more specific (86% vs 77%)[37]. The accuracy for both modalities is likely to be significantly lower in patients with LVD and dilated hearts.
Many new techniques are clinically useful in LVD providing information about etiology, ischemia and prognosis. Prominent among them are coronary computed tomography (CT) and cardiac magnetic resonance imaging (MRI). Currently 64-slice CT is considered the minimum standard for evaluation of coronary stenosis. In a study using 64-slice CT, the accuracy, sensitivity, specificity, positive and negative predictive value were found to be 95%, 90%, 97%, 93% and 95% respectively for identifying ischemic cardiomyopathy[38]. Another new study also using 64-slice CT showed sensitivity, specificity, positive and negative predictive values of 96%, 99%, 94%, and 100% respectively for detection of > 70% coronary stenosis in patients with cardiomyopathy of unknown etiology[39]. Compared to CA, CT technology has advanced rapidly with 256- and 320-slice CT becoming available in many centers; it is likely that these newer scanners will provide better results. Thus, CCT is a non-invasive alternative to CA in patients with LVD for detection of CAD; however, CCT in its current state cannot overcome the inherent limitation of luminography, i.e., ability to provide only coronary anatomic information. Detection of atherosclerosis or stenotic lesions may not prove causality in LVD. Active research is underway to test the feasibility of ischemia detection simultaneously using myocardial perfusion CT.
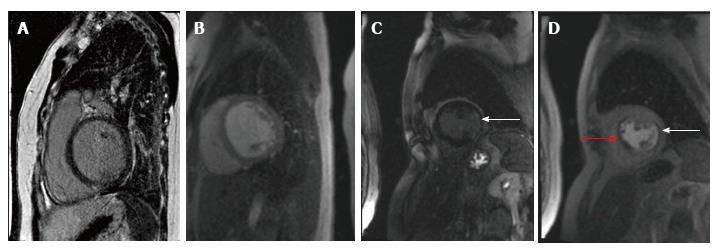
In evaluation of patients with LVD, CMR has distinct advantages over other modalities. Delayed enhancement CMR is the only technique that is able to directly visualize myocardial infarction in vivo. Subendocardial and transmural hyperenhancement corresponding to coronary perfusion territories is observed in CAD compared to mid-myocardial and epicardial hyperenhancement that may be found in non-ischemic cardiomyopathies[40]. Delayed enhancement CMR has 40 times higher spatial resolution compared to nuclear imaging[41]; it can detect small subendocardial infarcts that are likely to be missed by nuclear imaging[42]. CMR can also help in identifying the specific etiology of DCM[40].
CMR is now considered as an effective alternative to CA for CAD diagnosis in patients with heart failure (Figure 1, illustrative images). In one study, delayed enhancement CMR was shown to have sensitivity and specificity comparable to CA in differentiation between ischemic and non-ischemic cardiomyopathy[43]. In a recent study, it was named as a noninvasive gatekeeper to CA due to its accuracy and cost-effectiveness; delayed enhancement CMR had a sensitivity of 100%, specificity of 96%, and diagnostic accuracy of 97% for CAD detection, which were equivalent to CA[44,45]. One study clearly showed that the absence of CAD type hyperenhancement can reliably exclude myocardial infarction or severe CAD in patients with LVD and may obviate the need for CA[46]. With high sensitivity and specificity, CMR is now considered the gold standard for differentiation of ischemic and non-ischemic cardiomyopathy[47].
PET imaging can help determine if CAD is the etiology of LVD based on sequential Perfusion-Metabolic scan using flow tracer N-13 Ammonia (N-13 NH3) followed by metabolic tracer F-18 2 fluorodeoxyglucose (FDG). Two techniques have traditionally been used for reading PET scans: Visual analysis and Circumferential Profile Analysis[48]. On Visual analysis it was observed that patients with DCM had a homogenous distribution of blood flow on the N-13 NH3 and glucose metabolism on FDG in contrast to patients with CAD who exhibited LV segments with discrete blood flow reduction and enhanced or concordantly reduced glucose utilization. On Circumferential Profile Analysis ICMP patients had a regional reduction in N-13 NH3 Myocardial uptake[49]. DCM patients with left bundle branch block demonstrate selective uptake of FDG in the septum resulting in false positive results. PET imaging has limitations, including assumption of uniformity of myocardial thickness and decreased spatial resolution. Patchy fibrosis in DCM can falsely resemble a CAD pattern. The most important limiting factor is cost and availability. In most centers, PET is thus used for viability assessment in LVD patients to determine revascularization suitability after CA quantifies CAD burden. Combined PET-CT imaging has shown promise in low risk patients[50] and in the future may provide the combined functional and anatomic information to obviate the need for invasive CA.
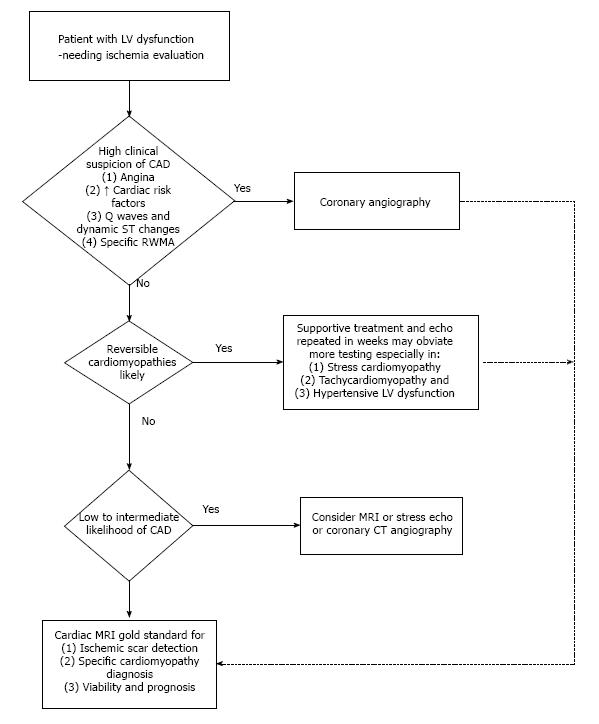
Identifying the etiology in patients with LVD is critical. The imaging modalities differ in their accuracy for CAD detection (Table 1). In patients with ischemic cardiomyopathy, adequate revascularization, especially if done early, significantly improves outcome. To achieve favorable risk-benefit ratio as well as cost effectiveness, we suggest a stepwise algorithm that incorporates patient demographics, clinical presentation and probability of CAD to determine the imaging approach for CAD detection. In patients with LVD and high index of suspicion for CAD, proceeding directly to CA would be prudent (Figure 2). When a reversible etiology such as stress cardiomyopathy or tachycardiomyopathy is likely, supportive treatment and repeat imaging in few weeks may obviate the need for invasive CA[51,52]. A sizeable proportion of patients with cardiomyopathy of undetermined etiology have a low to intermediate probability of CAD; here various imaging modalities may serve as the gatekeeper for CA. In our opinion, the wide availability of DSE or SPECT makes these modalities reasonable in those with low likelihood of CAD. CCT is also appropriate in low to intermediate risk groups[53]. Our algorithm for evaluation of LVD patients is outlined in Figure 2. CMR, if available, would arguably be the ideal test in the setting of LVD to identify CAD scar pattern; at the same time, CMR may establish the specific etiology in several non-ischemic cardiomyopathies (Table 2). Finally, even in patients with CA proven CAD, the CMR scar pattern will help differentiate true ischemic cardiomyopathy (embolic or recanalized coronary lesions) from coincidental CAD.
| Modality | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Diagnostic accuracy |
| SPECT | 80%-100% | 40%-50% | 90%-95% | 90%-95% | 75%-80% |
| DSE | 80%-85% | 60%-80% | 80%-90% | 45%-60% | 75%-80% |
| PET | 85%-90% | 80%-85% | 85%-90% | 80%-95% | 80%-85% |
| CCT | 70%-90% | 85%-90% | 90%-95% | 90%-95% | 90%-95% |
| CMR | 95%-100% | 90%-95% | 90%-95% | 90%-95% | 95%-100% |
| Modality | Advantages | Limitations |
| SPECT | Wide availability | Radiation |
| May miss left main and triple vessel disease | ||
| DSE | Wide availability | Inter-observer variability |
| Evaluates valves and pericardium | Nonspecific response to inotrope in LVD | |
| PET | Viability evaluation | Radiation |
| Quantifies myocardial blood flow | ||
| CCT | Anatomic information like invasive angiogram | Radiation |
| Iodinated contrast in renal dysfunction | ||
| CMR | Evaluates valves and pericardium Viability evaluation | Gadolinium in renal dysfunction |
| Determine etiology of DCM |
Incidental LVD is not uncommon in clinical practice. Numerous imaging modalities are available to help establish the etiology and guide management in this population. When the suspicion of CAD is high, proceeding directly to CA would be of highest clinical value eliminating the need for noninvasive testing. In other settings where noninvasive testing would be appropriate, an algorithmic imaging approach would optimize patient care.
| 1. | Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 470] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 2. | McDonagh TA, Morrison CE, Lawrence A, Ford I, Tunstall-Pedoe H, McMurray JJ, Dargie HJ. Symptomatic and asymptomatic left-ventricular systolic dysfunction in an urban population. Lancet. 1997;350:829-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 436] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 3. | Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1166] [Cited by in RCA: 1198] [Article Influence: 70.5] [Reference Citation Analysis (1)] |
| 4. | Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147-e239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4116] [Cited by in RCA: 4713] [Article Influence: 362.5] [Reference Citation Analysis (1)] |
| 5. | Bortman G, Sellanes M, Odell DS, Ring WS, Olivari MT. Discrepancy between pre- and post-transplant diagnosis of end-stage dilated cardiomyopathy. Am J Cardiol. 1994;74:921-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Ananthasubramaniam K, Dhar R, Cavalcante JL. Role of multimodality imaging in ischemic and non-ischemic cardiomyopathy. Heart Fail Rev. 2011;16:351-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Silva Marques J, Pinto FJ. Clinical use of multimodality imaging in the assessment of dilated cardiomyopathy. Heart. 2015;101:565-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Bulkley BH, Hutchins GM, Bailey I, Strauss HW, Pitt B. Thallium 201 imaging and gated cardiac blood pool scans in patients with ischemic and idiopathic congestive cardiomyopathy. A clinical and pathologic study. Circulation. 1977;55:753-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 134] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Tauberg SG, Orie JE, Bartlett BE, Cottington EM, Flores AR. Usefulness of thallium-201 for distinction of ischemic from idiopathic dilated cardiomyopathy. Am J Cardiol. 1993;71:674-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Dunn RF, Uren RF, Sadick N, Bautovich G, McLaughlin A, Hiroe M, Kelly DT. Comparison of thallium-201 scanning in idiopathic dilated cardiomyopathy and severe coronary artery disease. Circulation. 1982;66:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Wu YW, Yen RF, Chieng PU, Huang PJ. Tl-201 myocardial SPECT in differentiation of ischemic from nonischemic dilated cardiomyopathy in patients with left ventricular dysfunction. J Nucl Cardiol. 2003;10:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Bureau JF, Gaillard JF, Granier R, Ollivier JP. Diagnostic and prognostic criteria of chronic left ventricular failure obtained during exercise-201Tl imaging. Eur J Nucl Med. 1987;12:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Saltissi S, Hockings B, Croft DN, Webb-Peploe MM. Thallium-201 myocardial imaging in patients with dilated and ischaemic cardiomyopathy. Br Heart J. 1981;46:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Danias PG, Ahlberg AW, Clark BA, Messineo F, Levine MG, McGill CC, Mann A, Clive J, Dougherty JE, Waters DD. Combined assessment of myocardial perfusion and left ventricular function with exercise technetium-99m sestamibi gated single-photon emission computed tomography can differentiate between ischemic and nonischemic dilated cardiomyopathy. Am J Cardiol. 1998;82:1253-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Glamann DB, Lange RA, Corbett JR, Hillis LD. Utility of various radionuclide techniques for distinguishing ischemic from nonischemic dilated cardiomyopathy. Arch Intern Med. 1992;152:769-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Wallis DE, O’Connell JB, Henkin RE, Costanzo-Nordin MR, Scanlon PJ. Segmental wall motion abnormalities in dilated cardiomyopathy: a common finding and good prognostic sign. J Am Coll Cardiol. 1984;4:674-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 83] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Juillière Y, Marie PY, Danchin N, Gillet C, Paille F, Karcher G, Bertrand A, Cherrier F. Radionuclide assessment of regional differences in left ventricular wall motion and myocardial perfusion in idiopathic dilated cardiomyopathy. Eur Heart J. 1993;14:1163-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Greenberg JM, Murphy JH, Okada RD, Pohost GM, Strauss HW, Boucher CA. Value and limitations of radionuclide angiography in determining the cause of reduced left ventricular ejection fraction: comparison of idiopathic dilated cardiomyopathy and coronary artery disease. Am J Cardiol. 1985;55:541-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Oakley C. Diagnosis and natural history of congested (dilated) cardiomyopathies. Postgrad Med J. 1978;54:440-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Gewirtz H, Grötte GJ, Strauss HW, O’Keefe DD, Akins CW, Daggett WM, Pohost GM. The influence of left ventricular volume and wall motion in myocardial images. Circulation. 1979;59:1172-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Freedman B, Dunn RF, Richmond DR, Kelly DT. Coronary artery spasm during exercise: treatment with verapamil. Circulation. 1981;64:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Klein GJ, Kostuk WJ, Boughner DR, Chamberlain MJ. Stress myocardial imaging in mitral leaflet prolapse syndrome. Am J Cardiol. 1978;42:746-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Bailey IK, Come PC, Kelly DT, Burow RD, Griffith LS, Strauss HW, Pitt B. Thallium-201 myocardial perfusion imaging in aortic valve stenosis. Am J Cardiol. 1977;40:889-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Loong CY, Anagnostopoulos C. Diagnosis of coronary artery disease by radionuclide myocardial perfusion imaging. Heart. 2004;90 Suppl 5:v2-v9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Lesser JR, Bae R, Flygenring B, Sharkey SS, Lindberg J, Schwartz RS. Balanced myocardial ischaemia: a case of “normal” stress Tc99 sestamibi scan and diagnosis. Heart. 2005;91:e53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20:1021-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 531] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 27. | Geleijnse ML, Fioretti PM, Roelandt JR. Methodology, feasibility, safety and diagnostic accuracy of dobutamine stress echocardiography. J Am Coll Cardiol. 1997;30:595-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 314] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 28. | Marcovitz PA, Armstrong WF. Accuracy of dobutamine stress echocardiography in detecting coronary artery disease. Am J Cardiol. 1992;69:1269-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 29. | Hennessy TG, Codd MB, Kane G, McCarthy C, McCann HA, Sugrue DD. Dobutamine stress echocardiography in the detection of coronary artery disease: importance of the pretest likelihood of disease. Am Heart J. 1997;134:685-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Mahajan N, Polavaram L, Vankayala H, Ference B, Wang Y, Ager J, Kovach J, Afonso L. Diagnostic accuracy of myocardial perfusion imaging and stress echocardiography for the diagnosis of left main and triple vessel coronary artery disease: a comparative meta-analysis. Heart. 2010;96:956-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | de Jong RM, Cornel JH, Crijns HJ, van Veldhuisen DJ. Abnormal contractile responses during dobutamine stress echocardiography in patients with idiopathic dilated cardiomyopathy. Eur J Heart Fail. 2001;3:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Cohen A, Chauvel C, Benhalima B, Guyon P, Desert I, Valty J. Is dobutamine stress echocardiography useful for noninvasive differentiation of ischemic from idiopathic dilated cardiomyopathy? Angiology. 1997;48:783-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Sharp SM, Sawada SG, Segar DS, Ryan T, Kovacs R, Fineberg NS, Feigenbaum H. Dobutamine stress echocardiography: detection of coronary artery disease in patients with dilated cardiomyopathy. J Am Coll Cardiol. 1994;24:934-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Yuda S, Khoury V, Marwick TH. Influence of wall stress and left ventricular geometry on the accuracy of dobutamine stress echocardiography. J Am Coll Cardiol. 2002;40:1311-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Vigna C, Russo A, De Rito V, Perna GP, Testa M, Lombardo A, Lanna P, Langialonga T, Salvatori MP, Fanelli R. Regional wall motion analysis by dobutamine stess echocardiography to distinguish between ischemic and nonischemic dilated cardiomyopathy. Am Heart J. 1996;131:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Senior R, Lahiri A. Enhanced detection of myocardial ischemia by stress dobutamine echocardiography utilizing the “biphasic” response of wall thickening during low and high dose dobutamine infusion. J Am Coll Cardiol. 1995;26:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 75] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Schinkel AF, Bax JJ, Geleijnse ML, Boersma E, Elhendy A, Roelandt JR, Poldermans D. Noninvasive evaluation of ischaemic heart disease: myocardial perfusion imaging or stress echocardiography? Eur Heart J. 2003;24:789-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Ghostine S, Caussin C, Habis M, Habib Y, Clément C, Sigal-Cinqualbre A, Angel CY, Lancelin B, Capderou A, Paul JF. Non-invasive diagnosis of ischaemic heart failure using 64-slice computed tomography. Eur Heart J. 2008;29:2133-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Andreini D, Pontone G, Bartorelli AL, Agostoni P, Mushtaq S, Bertella E, Trabattoni D, Cattadori G, Cortinovis S, Annoni A. Sixty-four-slice multidetector computed tomography: an accurate imaging modality for the evaluation of coronary arteries in dilated cardiomyopathy of unknown etiology. Circ Cardiovasc Imaging. 2009;2:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Senthilkumar A, Majmudar MD, Shenoy C, Kim HW, Kim RJ. Identifying the etiology: a systematic approach using delayed-enhancement cardiovascular magnetic resonance. Heart Fail Clin. 2009;5:349-367, vi. [PubMed] [DOI] [Full Text] |
| 41. | Wu E, Judd RM, Vargas JD, Klocke FJ, Bonow RO, Kim RJ. Visualisation of presence, location, and transmural extent of healed Q-wave and non-Q-wave myocardial infarction. Lancet. 2001;357:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 518] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 42. | Wagner A, Mahrholdt H, Thomson L, Hager S, Meinhardt G, Rehwald W, Parker M, Shah D, Sechtem U, Kim RJ. Effects of time, dose, and inversion time for acute myocardial infarct size measurements based on magnetic resonance imaging-delayed contrast enhancement. J Am Coll Cardiol. 2006;47:2027-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 43. | McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54-59. [PubMed] [DOI] [Full Text] |
| 44. | Assomull RG, Shakespeare C, Kalra PR, Lloyd G, Gulati A, Strange J, Bradlow WM, Lyne J, Keegan J, Poole-Wilson P. Role of cardiovascular magnetic resonance as a gatekeeper to invasive coronary angiography in patients presenting with heart failure of unknown etiology. Circulation. 2011;124:1351-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 45. | Karamitsos TD, Francis JM, Myerson S, Selvanayagam JB, Neubauer S. The role of cardiovascular magnetic resonance imaging in heart failure. J Am Coll Cardiol. 2009;54:1407-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 308] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 46. | Soriano CJ, Ridocci F, Estornell J, Jimenez J, Martinez V, De Velasco JA. Noninvasive diagnosis of coronary artery disease in patients with heart failure and systolic dysfunction of uncertain etiology, using late gadolinium-enhanced cardiovascular magnetic resonance. J Am Coll Cardiol. 2005;45:743-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 47. | Valle-Muñoz A, Estornell-Erill J, Soriano-Navarro CJ, Nadal-Barange M, Martinez-Alzamora N, Pomar-Domingo F, Corbí-Pascual M, Payá-Serrano R, Ridocci-Soriano F. Late gadolinium enhancement-cardiovascular magnetic resonance identifies coronary artery disease as the aetiology of left ventricular dysfunction in acute new-onset congestive heart failure. Eur J Echocardiogr. 2009;10:968-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Mody FV, Brunken RC, Stevenson LW, Nienaber CA, Phelps ME, Schelbert HR. Differentiating cardiomyopathy of coronary artery disease from nonischemic dilated cardiomyopathy utilizing positron emission tomography. J Am Coll Cardiol. 1991;17:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Boff GM, Zanco P, Della Valentina P, Cardaioli P, Thiene G, Chioin R, Dalla Volta S. Positron emission tomography is a useful tool in differentiating idiopathic from ischemic dilated cardiomyopathy. Int J Cardiol. 2000;74:67-74; discussion 75-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 50. | Sampson UK, Dorbala S, Limaye A, Kwong R, Di Carli MF. Diagnostic accuracy of rubidium-82 myocardial perfusion imaging with hybrid positron emission tomography/computed tomography in the detection of coronary artery disease. J Am Coll Cardiol. 2007;49:1052-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 51. |
Lishmanov A, Chockalingam P, Senthilkumar A, Chockalingam A.
Tachycardia-induced cardiomyopathy: evaluation and therapeutic options. |
| 52. | Chockalingam A, Xie GY, Dellsperger KC. Echocardiography in stress cardiomyopathy and acute LVOT obstruction. Int J Cardiovasc Imaging. 2010;26:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O’Gara P, Rubin GD, Kramer CM, Berman D, Brown A. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56:1864-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 727] [Cited by in RCA: 706] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kettering K, Teragawa H, Said SAM, Soliman EZ S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ