Peer-review started: July 29, 2016
First decision: September 6, 2016
Revised: October 30, 2016
Accepted: November 21, 2016
Article in press: November 22, 2016
Published online: January 26, 2017
Processing time: 178 Days and 14.8 Hours
To investigate one-year outcomes after percutaneous mitral valve repair with MitraClip® in patients with severe mitral regurgitation (MR).
METHODS
Our study investigated consecutive patients with symptomatic severe MR who underwent MitraClip® implantation at the University Hospital Bergmannsheil from 2012 to 2014. The primary study end-point was all-cause mortality. Secondary end-points were degree of MR and functional status after percutaneous mitral valve repair.
The study population consisted of 46 consecutive patients (mean logistic EuroSCORE 32% ± 21%). The degree of MR decreased significantly (severe MR before MitraClip® 100% vs after MitraClip® 13%; P < 0.001), and the NYHA functional classes improved (NYHA III/IV before MitraClip® 98% vs after MitraClip® 35%; P < 0.001). The mortality rates 30 d and one year after percutaneous mitral valve repair were 4.3% and 19.5%, respectively. During the follow-up of 473 ± 274 d, 11 patients died (90% due to cardiovascular death). A pre-procedural plasma B-type natriuretic peptide level > 817 pg/mL was associated with all-cause mortality (hazard ratio, 6.074; 95%CI: 1.257-29.239; P = 0.012).
Percutaneous mitral valve repair with MitraClip® has positive effects on hemodynamics and symptoms. Despite the study patients’ multiple comorbidities and extremely high operative risk, one-year outcomes after MitraClip® are favorable. Elevated B-type natriuretic peptide levels indicate poorer mid-term survival.
Core tip: Percutaneous mitral valve repair with the MitraClip® device has positive effects on hemodynamics and symptoms. Despite the multiple comorbidities and extremely high operative risk of the study patients, mid-term outcomes after MitraClip® implantation are favorable. Elevated B-type natriuretic peptide (> 817 pg/mL) levels are indicative of poorer long-term survival.
- Citation: Gotzmann M, Sprenger I, Ewers A, Mügge A, Bösche L. One-year outcome of percutaneous mitral valve repair in patients with severe symptomatic mitral valve regurgitation. World J Cardiol 2017; 9(1): 39-46
- URL: https://www.wjgnet.com/1949-8462/full/v9/i1/39.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i1.39
Severe mitral regurgitation (MR) is a common valvular heart disease that has an unfavorable prognosis[1]. When possible, mitral valve repair is considered the optimal surgical treatment[1].
In the last few years, percutaneous mitral valve repair has developed into an alternative technique for patients with severe MR, with more than 20000 procedures worldwide[2,3]. However, the Everest II study is the only randomized trial that has compared percutaneous repair with heart surgery, and it mainly included patients with degenerative MR who did not have an elevated operative risk[2]. By contrast, the current European Society of Cardiology guidelines recommend applying MitraClip® only in symptomatic high-risk patients with severe functional MR (level of evidence IIb)[1].
In recent years, some registry studies[4-6] and several smaller studies[7-11] concerning MitraClip® have been published. However, relatively little is known about the long-term results of percutaneous mitral valve repair. Therefore, the aim of this study was to assess the one-year outcome after percutaneous mitral valve repair with MitraClip®.
We performed a retrospective study to evaluate the effects of percutaneous mitral valve repair using MitraClip® (Abbott Vascular, Menlo Park, California, United States) on symptoms, hemodynamics and outcomes. The primary study endpoint was all-cause mortality. The secondary endpoints were functional status and degree of MR after percutaneous mitral valve repair.
This study included consecutive patients with symptomatic severe MR who underwent MitraClip® implantation from May 2012 to December 2014 at the University Hospital Bergmannsheil. The risk of mitral valve surgery was estimated using the logistic EuroSCORE I[12] and the logistic EuroSCORE II[13]. All patient cases were discussed by the cardiology team. The decision to perform percutaneous mitral valve repair was based on: (1) a high EuroSCORE I (> 20%); and (2) serious comorbidities with a considerable risk for heart surgery [for example, porcelain aorta, left ventricular ejection fraction (LVEF) < 30%, previous chest radiation and severe chronic obstructive pulmonary disease]. The contraindications were active endocarditis, intracardiac thrombus, limited life expectancy (< 1 year) and unsuitable mitral valve morphology, according to the EVEREST criteria[2].
Written informed consent was given by all patients to receive percutaneous mitral valve repair with the MitraClip®. The study was reviewed and approved by the Ruhr-University Bochum Institutional Review Board.
The pre-procedural examinations of study patients comprised an anamnesis, assessment of functional capacity (NYHA class), laboratory measurements, transthoracic and transesophageal echocardiography and coronary angiography. The diagnosis of coronary artery disease was made in patients with a coronary artery stenosis > 50% in the pre-procedural coronary angiography, previous myocardial infarction, coronary artery bypass grafting, or percutaneous coronary intervention.
We measured plasma B-type natriuretic peptide levels on the same day as clinical and echocardiographical examinations. By using a chemiluminescent immunoassay kit (Biosite Triage, San Diego, CA, United States) we analyzed the levels of plasma B-type natriuretic peptide.
All patients underwent transthoracic and transesophageal echocardiography (Vivid 7 or Vivid 9, General Electrics, Horton, Norway) according to the guidelines of the American Society of Echocardiography[14]. Using the modified Devereux formula left ventricular myocardial mass was calculated. The Simpson method (using 4- and 2-chamber views) was applied to measure LVEF[14]. According to the American Society of Echocardiography, MR and tricuspid regurgitation was graded as mild, moderate or severe[15]. In addition, MR was classified as degenerative, functional or mixed[15]. According to the current recommendations, systolic pulmonary pressure and tricuspid annular plane systolic excursion were measured[16].
Percutaneous mitral valve repair with the MitraClip® device was executed under general anesthesia without hemodynamic support using a femoral vein. The transseptal puncture and positioning of the device were performed under transesophageal echocardiography and fluoroscopic guidance. After positioning a clip, the remaining MR and mean transmitral gradient were assessed. If the reduction in MR was unsatisfactory, a second clip was positioned. A maximum mean transmitral gradient of 5 mmHg was accepted. Before and after implantation, left atrial pressure was measured invasively. The procedure and MitraClip® system have been previously described in detail[2,3].
Patients were treated with aspirin for 6 mo and with clopidogrel for 30 d[2]. When clinically indicated (mainly due to atrial fibrillation), patients also received oral anticoagulation.
The procedural and in-hospital difficulties were reported according to current recommendations[17]. Within 48 h after the MitraClip® procedure, a transthoracic echocardiography was performed.
Follow-up examinations of all surviving study patients were conducted 3 ± 2 mo after the MitraClip® implantation and comprised an assessment of functional capacity (NYHA class) and a transthoracic echocardiography. Any changes in MR, left atrial volume and diameter and left ventricular geometry and function were documented. The follow-up of patients was at least 12 mo and death, myocardial infarction, stroke and hospitalization due to heart failure were documented. During routine ambulatory visits follow-up information was obtained. In case of patients’ death, we contacted their physicians to get information about reasons of death.
Numerical values were expressed as mean ± SD. For the comparison of continuous variables we used unpaired t tests or Mann-Whitney U test, where appropriate. In order to compare categorical variables we performed χ2 analysis. To examine continuous variables before and after MitraClip® implantation, we applied paired Student’s t test (for normally distributed variables) or Wilcoxon test (for non-normally distributed variables). For categorical variables, we used McNemar’s test to measure changes before and after MitraClip® implantation. Univariate Cox proportional hazards model was performed to assess predictors of all-cause death. For this, all the parameters in Tables 1 and 2 were included in the analysis.
| Total (n = 46) | Survivors (n = 35) | Non-survivors (n = 11) | P value | |
| Age (yr) | 76.5 ± 9.4 | 76.4 ± 9.5 | 76.9 ± 9.6 | 0.871 |
| Women, n (%) | 21 (46) | 16 (46) | 5 (45) | 0.988 |
| Body mass index (kg/m²) | 26.8 ± 4.3 | 26.8 ± 4.5 | 26.6 ± 3.7 | 0.928 |
| NYHA class | 0.818 | |||
| NYHA class II, n (%) | 1 (2) | 1 (3) | 0 (0) | |
| NYHA class III, n (%) | 27 (59) | 20 (57) | 7 (64) | |
| NYHA class IV, n (%) | 18 (39) | 14 (40) | 4 (36) | |
| Hypertension, n (%) | 31 (69) | 23 (66) | 8 (80) | 0.389 |
| Diabetes mellitus, n (%) | 21 (47) | 17 (49) | 4 (40) | 0.632 |
| Coronary artery disease, n (%) | 27 (60) | 22 (63) | 5 (50) | 0.464 |
| Previous coronary artery bypass grafting, n (%) | 15 (34) | 12 (35) | 3 (30) | 0.756 |
| Previous heart valve surgery, n (%) | 6 (13) | 5 (14) | 1 (10) | 0.725 |
| Previous stroke, n (%) | 5 (11) | 5 (14) | 0 (0) | 0.205 |
| Atrial fibrillation/flutter, n (%) | 33 (73) | 25 (71) | 8 (80) | 0.589 |
| Implanted PM/ICD/CRT-device, n (%) | 15 (34) | 9 (26) | 6 (60) | 0.067 |
| Chronic obstructive pulmonary disease, n (%) | 14 (31) | 12 (34) | 2 (20) | 0.389 |
| Peripheral artery disease, n (%) | 6 (13) | 4 (11) | 2 (18) | 0.482 |
| Logistic EuroSCORE I (%) | 31.8 ± 21.1 | 32.1 ± 21.4 | 30.9 ± 21.2 | 0.870 |
| Logistic EuroSCORE II (%) | 11.9 ± 9.5 | 12.0 ± 9.5 | 11.6 ± 9.98 | 0.885 |
| Laboratory parameters | ||||
| Creatinine (mg/dL) | 1.4 ± 0.5 | 1.4 ± 0.5 | 1.28 ± 0.39 | 0.476 |
| Hemoglobin (g/dL) | 12.1 ± 1.7 | 12.3 ± 1.6 | 11.4 ± 2.0 | 0.256 |
| Plasma BNP (pg/mL) (n = 36) | 1022 ± 897 | 793 ± 611 | 1710 ± 1264 | 0.006 |
| Echocardiography | ||||
| Mitral regurgitation etiology | ||||
| Organic, n (%) | 17 (37) | 14 (40) | 3 (27) | 0.446 |
| Functional, n (%) | 21 (46) | 16 (46) | 5 (45) | 0.988 |
| Mixed, n (%) | 8 (17) | 5 (14) | 3 (27) | 0.322 |
| Left atrial diameter (mm) | 51 ± 6 | 51 ± 6 | 50 ± 5 | 0.793 |
| Left atrial volume (mL) | 115 ± 43 | 110 ± 42 | 134 ± 45 | 0.118 |
| LV end-diastolic diameter (mm) | 55 ± 10 | 55 ± 10 | 57 ± 10 | 0.498 |
| LV end-systolic diameter (mm) | 42 ± 12 | 41 ± 11 | 45 ± 12 | 0.313 |
| LV end-diastolic volume (mL) | 159 ± 70 | 152 ± 62 | 181 ± 93 | 0.258 |
| LV end-systolic volume (mL) | 96 ± 63 | 88 ± 55 | 121 ± 82 | 0.151 |
| LV ejection fraction (%) | 42 ± 14 | 44 ± 14 | 37 ± 14 | 0.203 |
| MAPSE (mm) | 12 ± 3 | 13 ± 3 | 11 ± 3 | 0.163 |
| TAPSE (mm) | 17 ± 5 | 17 ± 4 | 18 ± 6 | 0.527 |
| Tricuspid regurgitation (moderate/severe), n (%) | 31 (67) | 21 (60) | 10 (91) | 0.070 |
| sPAP (mmHg) | 44 ± 12 | 44 ± 12 | 43 ± 14 | 0.844 |
| Before MitraClip | After MitraClip | P value | |
| NYHA class III and IV, n (%) | 43 (98) | 16 (35) | < 0.001 |
| Mitral regurgitation (severe) | 44 (100) | 6 (14) | < 0.001 |
| Left atrial diameter (mm) | 50 ± 6 | 49 ± 7 | 0.039 |
| Left atrial volume (mL) | 114 ± 43 | 102 ± 42 | 0.008 |
| LV end-diastolic diameter (mm) | 55 ± 10 | 54 ± 11 | 0.308 |
| LV end-systolic diameter (mm) | 42 ± 12 | 41 ± 12 | 0.367 |
| LV end-diastolic volume (mL) | 158 ± 69 | 159 ± 75 | 0.866 |
| LV end-systolic volume (mL) | 95 ± 63 | 95 ± 61 | 0.993 |
| LV ejection fraction (%) | 42 ± 15 | 44 ± 13 | 0.216 |
| Severe tricuspid regurgitation, n (%) | 12 (27) | 9 (20) | 0.763 |
| sPAP (mmHg) | 44 ± 13 | 27 ± 10 | 0.027 |
In order to determine cut-off values for plasma B-type natriuretic peptide (BNP), receiver operating characteristic (ROC) curves were performed. The Kaplan-Meier method was used for analysis of all-cause mortality. A comparison of survival between different groups of patients was assumed with log-rank tests. A P value < 0.05 was considered to be statistically significant. All probability values reported were two-sided. A statistical review of the study was performed by a biomedical statistician. The analyses were done using SPSS software (version 20.0; SPSS Inc., Chicago, IL, United States).
A total of 48 consecutive patients with native MR who underwent percutaneous mitral valve repair with the MitraClip® device were enrolled between May 2012 and December 2014. During the procedures, no patients died and no cases of stroke or myocardial infarction occurred. In one patient, positioning of the MitraClip® was not possible, and the procedure was thus abandoned. In another patient, leaflet detachment occurred after implantation of the clip. One day after the unsuccessful procedure, this patient received surgical mitral valve repair. Therefore, these two patients were not included in the analysis. The final study cohort consisted of 46 patients. Clip embolization, acute conversion to open surgery, pericardial tamponade, need for resuscitation, need for dialysis, respiratory failure and major bleeding did not occur after MitraClip® implantation.
The mean age of the study patients (21 women, 25 men) was 76.5 ± 9.4 years, and the mean logistic EuroSCORE I was 32% ± 21%. Plasma BNP was measured in 36 of the 46 patients. The baseline characteristics of the patients are provided in Table 1.
The mean procedure time was 143 ± 34 min (door-to-door time, including general anesthesia). One clip was implanted in 31 patients (67%), and two clips were implanted in 15 patients (33%). Immediately after the percutaneous mitral valve repair, transesophageal echocardiography revealed remaining severe MR in 2 patients (4%). There were no cases of post-procedural mitral stenosis (the transmitral gradient was ≤ 5 mmHg in all patients). The invasive hemodynamic measurements demonstrated a significant decrease in mean left atrial pressure (left atrial pressure before procedure 27 ± 10 mmHg vs left atrial pressure after procedure 23 ± 10 mmHg, P = 0.018).
The mean duration of hospitalization after MitraClip® procedure was 11 ± 8 d. Within 30 d after the MitraClip® implantation, 2 patients suffered from death due to heart failure. Follow-up examinations were done in the remaining 44 patients (the mean follow-up was 95 d after the percutaneous mitral valve repair).
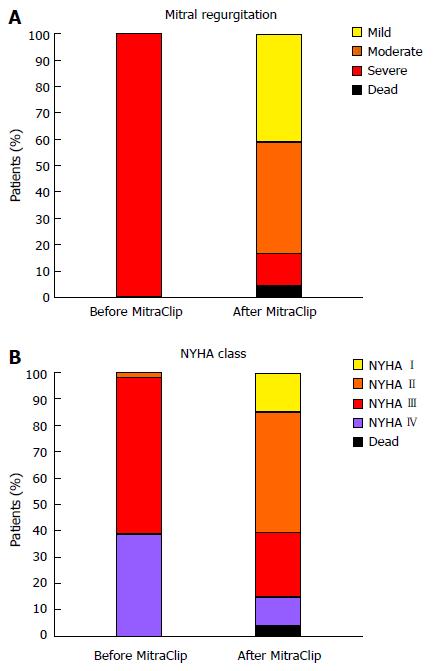
Prior to the procedure, 98% of our study population was considered NYHA functional class III or IV. After the percutaneous mitral valve repairs, the NYHA functional classes and degree of MR improved significantly (both P < 0.001) (Figure 1). Additionally, the left atrial diameter, left atrial volume and systolic pulmonary artery pressure decreased. By contrast, the left ventricular dimensions and LVEF remained unchanged (Table 2). In our study, there were no differences in clinical course after mitral valve repair between patients with organic and with functional MR.
The all-cause mortality rate 30 d after percutaneous mitral valve repair was 4.3% (n = 2), and this rate increased to 19.5% (n = 9) at one year. In the first year after the MitraClip® implantation, no cases of myocardial infarction occurred, but 2 patients suffered from ischemic stroke. One of these patients had atrial fibrillation and received aspirin and coumarin at the time of stroke. This patient died within the first year. The other patient was in sinus rhythm and received monotherapy with aspirin. Hospitalization due to heart failure occurred in 16 patients (35% of study patients), and of these patients, 7 died within the first year after MitraClip® implantation.
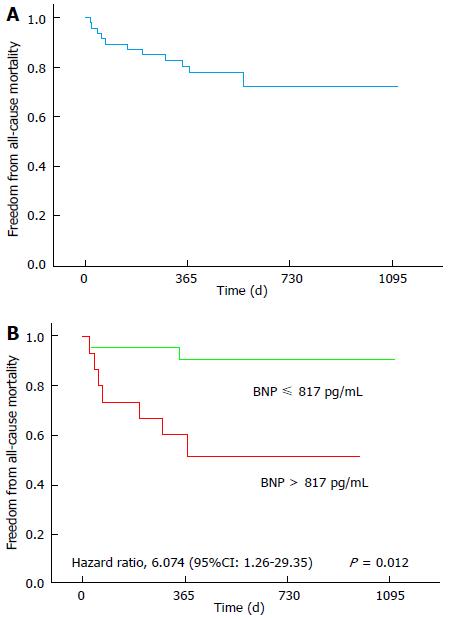
During the follow-up of 473 ± 274 d, the primary endpoint (all-cause mortality) was attained in 11 patients (24%). Reasons for death included heart failure (n = 7), myocardial infarction (n = 1), unknown causes (n = 2) and malignancy (n = 1). Kaplan-Meier curves were created for the analysis of all-cause mortality (Figure 2A).
In the univariate Cox analysis, only plasma BNP was significantly associated to the primary study endpoint. Patients who died after percutaneous mitral valve repair had significantly higher pre-procedural BNP levels compared to patients who survived (Table 1). The ability of BNP to predict all cause-mortality was assessed using ROC curve analysis. Using a cut-off point of BNP > 817 pg/mL, the sensitivity was 78% and the specificity was 70% [area under the ROC curve (AUC) = 0.778, (95%CI: 0.621-0.936; P < 0.001)]. A pre-procedural plasma BNP level > 817 pg/mL was associated with all-cause mortality (HR = 6.074; 95%CI: 1.257-29.239; P = 0.012) (Figure 2B).
The main result of this study was an improvement in hemodynamics (Figure 1A and Table 2) and symptoms (Figure 1B) in patients with severe symptomatic MR after receiving percutaneous mitral valve repair with the MitraClip® system. However, the relatively high one-year mortality rate (19.5%) observed in the present study must be mentioned (Figure 2A). Preexisting co-morbidities of these selected patients who had a very high mean logistic EuroSCORE I of 31.8% (Table 1) is the most likely explanation. Several previous studies reported comparable mortality rates after MitraClip® implantation[3-11].
Additionally, our study suggests that pre-procedural plasma BNP is an independent predictor of all-cause mortality after percutaneous mitral valve repair (Figure 2B).
Since the first description of percutaneous mitral valve repair with the MitraClip® device, this technique has now widely used. Today, it is a recognized treatment for high-risk patients with severe and symptomatic MR[1].
Several studies demonstrate symptomatic benefits of percutaneous mitral valve repair in terms of changes in NYHA functional class[3-11,18,19]. Vakil et al[18] found that three-quarters of patients were in NYHA class I or II after MitraClip® implantation. Our study confirmed a significant enhancement in NYHA class after MitraClip® implantation (Figure 1B and Table 2).
Other than the fact that percutaneous mitral valve repair is beneficial for treating the symptoms of valvular heart failure, some studies have additionally demonstrated favorable effects of repair on reverse cardiac remodeling[2,8,10,19].
Taramasso et al[19] revealed an enhancement in LVEF after MitraClip® implantation in patients with heart failure. By contrast, Feldman et al[2] reported a reduction in left ventricular volumes and LVEF after MitraClip®. These different findings may be explained by the fact that patients with functional and degenerative MR have different types of reverse remodeling. In patients with degenerative MR, end-systolic volume remains stable, whereas left ventricular end-diastolic volume decreases. In contrast, in patients with functional MR, a significant decrease in left ventricular end-diastolic and end-systolic volume is observed[20]. Therefore, LVEF decreases slightly in degenerative MR[2] and remains constant or increased in functional MR[19,20].
In our study, the proportion of patients with functional and degenerative MR was approximately equal (Table 1). There were no differences in clinical course after mitral valve repair between patients with organic and functional MR. Potentially due to the relatively small number of patients, we observed that the LVEF, the left ventricular end-systolic and end-diastolic volumes remained nearly constant (Table 2).
Our study demonstrated a clear reduction in the severity of MR (Figure 1A), a reduction in left atrial volume and size and a decline in systolic pulmonary artery pressure (Table 2).
The all-cause mortality rate 30 d after percutaneous mitral valve repair was 4.3%, and this rate was 19.5% at one year. During the follow-up of 473 ± 274 d, 11 patients (24%) died. Cardiovascular mortality accounted for the majority of the deaths at follow-up (90%). Patients’ various cardiac and non-cardiac diseases may explain the relatively high long-term mortality. Similar one-year mortality rates have been reported in several other studies[3-11]. However, it should be emphasized that the patients in our study had an extremely high operative risk (mean logistic EuroSCORE I > 31%). Thus, the risk profile of our patient cohort was less favorable than those of most other studies[3-11]. Our study suggests that even patients with an extremely high operative risk can be successfully treated with the MitraClip® system.
Plasma BNP levels have a prognostic impact in patients undergoing surgical mitral surgery[21]. In patients undergoing percutaneous mitral valve repair with the MitraClip® device, Taramasso et al[19] found that pre-procedural pro-BNP levels (pro-BNP level ≥ 1600 pg/mL) were an independent predictor of mortality. Furthermore, Neuss et al[22] demonstrated that among patients with end-stage heart failure and NT-proBNP values > 10000 pg/mL, the mortality rate was extremely high, despite a successful percutaneous mitral valve repair with MitraClip®. Our results are in line with these studies. Although pre-procedural measurements of plasma BNP were performed in only 36 of the 46 patients, we found that BNP levels > 817 pg/mL were related with a considerably higher long-term mortality rate (Figure 2B). Notably, plasma BNP was the only independent predictor of long-term mortality. On the other hand, our study suggests that patients with plasma BNP levels ≤ 817 pg/mL have favorable long-term results after MitraClip® implantation (Figure 2B).
The main limitation of our study is the relatively small number of patients and the fact that this study was conducted only at one center. However, the percutaneous repair of the mitral valve is a relatively new technique. There are still few long-term results of MitraClip® implantation. Another important limitation is the absence of a control group in our study. A comparison with conservatively treated patients, or patients undergoing heart surgery would give a more rigorous investigation of the effects of percutaneous mitral valve repair. As our study examined relatively few patients, the associations found in this paper must still be considered preliminary.
Mitral regurgitation (MR) is the most common heart valve disease and the second most common reason for heart valve surgery. When feasible, mitral valve repair is considered the optimal surgical treatment. However, a considerable portion of patients with severe MR cannot receive surgical treatment because of their co-morbidities and high surgical risks. In the last few years, percutaneous mitral valve repair has become an alternative technique for patients with severe MR.
In the last few years, percutaneous mitral valve repair has become an alternative technique for patients with severe MR, with more than 20000 procedures worldwide. However, the Everest II study is the only randomized trial that has compared percutaneous repair with heart surgery, and it mainly included patients with degenerative MR and without an elevated operative risk. By contrast, the current European Society of Cardiology guidelines recommend applying the MitraClip® only in symptomatic high-risk patients with severe functional MR (level of evidence IIb). In recent years, some registry studies and several smaller studies concerning MitraClip® have been published. However, relatively little is known about the mid- and long-term results of percutaneous mitral valve repair.
The aim of the present study was to assess the mid-term outcomes after percutaneous mitral valve repair with MitraClip®. The study demonstrates that percutaneous mitral valve repair with MitraClip® had positive effects on hemodynamics and symptoms. Despite the multiple comorbidities and extremely high operative risk of the study patients, the mid-term outcome after MitraClip® was favorable. Elevated BNP levels were indicative of a poorer long-term survival.
The data in this study suggest that patients with an extremely high risk can also be successfully treated with the MitraClip® system. In addition, pre-procedural plasma BNP levels > 817 pg/mL were associated with significantly higher long-term mortality. On the other hand, the study suggests that patients with plasma BNP levels ≤ 817 pg/mL had favorable long-term results after MitraClip®.
MR is a valvular heart disease that is characterized by an abnormal systolic blood flow into the left atrium and a volume overload of the left ventricle. The reasons for this condition include defects of the valve (usually referred to as degenerative mitral valve regurgitation) or heart failure with dilatation of the ventricle (usually called functional mitral valve regurgitation). Mitral valve repair is the standard therapy. Percutaneous mitral valve repair has emerged as an alternative technique.
Good article, well written, interesting for the reader, with a useful “take home message”.
| 1. | Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2682] [Cited by in RCA: 2658] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 2. | Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1496] [Cited by in RCA: 1721] [Article Influence: 114.7] [Reference Citation Analysis (0)] |
| 3. | Glower DD, Kar S, Trento A, Lim DS, Bajwa T, Quesada R, Whitlow PL, Rinaldi MJ, Grayburn P, Mack MJ. Percutaneous mitral valve repair for mitral regurgitation in high-risk patients: results of the EVEREST II study. J Am Coll Cardiol. 2014;64:172-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 348] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 4. | Maisano F, Franzen O, Baldus S, Schäfer U, Hausleiter J, Butter C, Ussia GP, Sievert H, Richardt G, Widder JD. Percutaneous mitral valve interventions in the real world: early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J Am Coll Cardiol. 2013;62:1052-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 670] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 5. | Nickenig G, Estevez-Loureiro R, Franzen O, Tamburino C, Vanderheyden M, Lüscher TF, Moat N, Price S, Dall’Ara G, Winter R. Percutaneous mitral valve edge-to-edge repair: in-hospital results and 1-year follow-up of 628 patients of the 2011-2012 Pilot European Sentinel Registry. J Am Coll Cardiol. 2014;64:875-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 362] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 6. | Puls M, Lubos E, Boekstegers P, von Bardeleben RS, Ouarrak T, Butter C, Zuern CS, Bekeredjian R, Sievert H, Nickenig G. One-year outcomes and predictors of mortality after MitraClip therapy in contemporary clinical practice: results from the German transcatheter mitral valve interventions registry. Eur Heart J. 2016;37:703-712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 377] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 7. | Capodanno D, Adamo M, Barbanti M, Giannini C, Laudisa ML, Cannata S, Curello S, Immè S, Maffeo D, Bedogni F. Predictors of clinical outcomes after edge-to-edge percutaneous mitral valve repair. Am Heart J. 2015;170:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Boerlage-vanDijk K, Wiegerinck EM, Araki M, Meregalli PG, Bindraban NR, Koch KT, Vis MM, Piek JJ, Tijssen JG, Bouma BJ. Predictors of outcome in patients undergoing MitraClip implantation: An aid to improve patient selection. Int J Cardiol. 2015;189:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Triantafyllis AS, Kortlandt F, Bakker AL, Swaans MJ, Eefting FD, van der Heyden JA, Post MC, Rensing BW. Long-term survival and preprocedural predictors of mortality in high surgical risk patients undergoing percutaneous mitral valve repair. Catheter Cardiovasc Interv. 2016;87:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Lesevic H, Sonne C, Braun D, Orban M, Pache J, Kastrati A, Schömig A, Mehilli J, Barthel P, Ott I. Acute and Midterm Outcome After MitraClip Therapy in Patients With Severe Mitral Regurgitation and Left Ventricular Dysfunction. Am J Cardiol. 2015;116:749-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Velazquez EJ, Samad Z, Al-Khalidi HR, Sangli C, Grayburn PA, Massaro JM, Stevens SR, Feldman TE, Krucoff MW. The MitraClip and survival in patients with mitral regurgitation at high risk for surgery: A propensity-matched comparison. Am Heart J. 2015;170:1050-1059.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Roques F, Nashef SA, Michel P, Gauducheau E, de Vincentiis C, Baudet E, Cortina J, David M, Faichney A, Gabrielle F. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg. 1999;15:816-822; discussion 822-823. [PubMed] |
| 13. | Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, Lockowandt U. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41:734-744; discussion 744-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1572] [Cited by in RCA: 2252] [Article Influence: 160.9] [Reference Citation Analysis (0)] |
| 14. | Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440-1463. [PubMed] |
| 15. | Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777-802. [PubMed] |
| 16. | Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685-713; quiz 786-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4670] [Cited by in RCA: 5486] [Article Influence: 342.9] [Reference Citation Analysis (0)] |
| 17. | Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33:2403-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 903] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 18. | Vakil K, Roukoz H, Sarraf M, Krishnan B, Reisman M, Levy WC, Adabag S. Safety and efficacy of the MitraClip® system for severe mitral regurgitation: a systematic review. Catheter Cardiovasc Interv. 2014;84:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Taramasso M, Maisano F, Latib A, Denti P, Buzzatti N, Cioni M, La Canna G, Colombo A, Alfieri O. Clinical outcomes of MitraClip for the treatment of functional mitral regurgitation. EuroIntervention. 2014;10:746-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Grayburn PA, Foster E, Sangli C, Weissman NJ, Massaro J, Glower DG, Feldman T, Mauri L. Relationship between the magnitude of reduction in mitral regurgitation severity and left ventricular and left atrial reverse remodeling after MitraClip therapy. Circulation. 2013;128:1667-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 21. | Hwang IC, Kim YJ, Kim KH, Lee SP, Kim HK, Sohn DW, Oh BH, Park YB. Prognostic value of B-type natriuretic peptide in patients with chronic mitral regurgitation undergoing surgery: mid-term follow-up results. Eur J Cardiothorac Surg. 2013;43:e1-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Neuss M, Schau T, Schoepp M, Seifert M, Hölschermann F, Meyhöfer J, Butter C. Patient selection criteria and midterm clinical outcome for MitraClip therapy in patients with severe mitral regurgitation and severe congestive heart failure. Eur J Heart Fail. 2013;15:786-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Amiya E, De Maria E, Tan XR S- Editor: Ji FF L- Editor: A E- Editor: Wu HL