Published online Jan 26, 2016. doi: 10.4330/wjc.v8.i1.98
Peer-review started: June 24, 2015
First decision: August 25, 2015
Revised: September 19, 2015
Accepted: November 10, 2015
Article in press: November 11, 2015
Published online: January 26, 2016
Processing time: 212 Days and 8.9 Hours
AIM: To assess the impact of percutaneous cardiac support in cardiogenic shock (CS) complicating acute myocardial infarction (AMI), treated with percutaneous coronary intervention.
METHODS: We selected all of the studies published from January 1st, 1997 to May 15st, 2015 that compared the following percutaneous mechanical support in patients with CS due to AMI undergoing myocardial revascularization: (1) intra-aortic balloon pump (IABP) vs Medical therapy; (2) percutaneous left ventricular assist devices (PLVADs) vs IABP; (3) complete extracorporeal life support with extracorporeal membrane oxygenation (ECMO) plus IABP vs IABP alone; and (4) ECMO plus IABP vs ECMO alone, in patients with AMI and CS undergoing myocardial revascularization. We evaluated the impact of the support devices on primary and secondary endpoints. Primary endpoint was the inhospital mortality due to any cause during the same hospital stay and secondary endpoint late mortality at 6-12 mo of follow-up.
RESULTS: One thousand two hundred and seventy-two studies met the initial screening criteria. After detailed review, only 30 were selected. There were 6 eligible randomized controlled trials and 24 eligible observational studies totaling 15799 patients. We found that the inhospital mortality was: (1) significantly higher with IABP support vs medical therapy (RR = +15%, P = 0.0002); (2) was higher, although not significantly, with PLVADs compared to IABP (RR = +14%, P = 0.21); and (3) significantly lower in patients treated with ECMO plus IABP vs IABP (RR = -44%, P = 0.0008) or ECMO (RR = -20%, P = 0.006) alone. In addition, Trial Sequential Analysis showed that in the comparison of IABP vs medical therapy, the sample size was adequate to demonstrate a significant increase in risk due to IABP.
CONCLUSION: Inhospital mortality was significantly higher with IABP vs medical therapy. PLVADs did not reduce early mortality. ECMO plus IABP significantly reduced inhospital mortality compared to IABP.
Core tip: Meta-analyses from observational studies represent an area of innovation in statistical science. In the present review, we identified only a small number of randomized trials, which by themselves were underpowered to assess the efficacy of the support devices on inhospital mortality. To increase the power of the analysis we included observational data, which enabled us to add 14909 additional patients to the 890 from the randomized controlled trials selected. The results of the analysis showed that: (1) intra-aortic balloon pump (IABP) used alone was associated with significant increase in inhospital mortality compared to Medical therapy; (2) percutaneous left ventricular assist devices increased, although non significantly, the mortality as compared with IABP; and (3) extracorporeal membrane oxygenation (ECMO) plus IABP had significant protective effect compared to IABP or ECMO alone.
- Citation: Romeo F, Acconcia MC, Sergi D, Romeo A, Francioni S, Chiarotti F, Caretta Q. Percutaneous assist devices in acute myocardial infarction with cardiogenic shock: Review, meta-analysis. World J Cardiol 2016; 8(1): 98-111
- URL: https://www.wjgnet.com/1949-8462/full/v8/i1/98.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i1.98
Cardiogenic shock (CS) occurs in 5% to 15% of patients with acute myocardial infarction (AMI). Despite major technical advances the inhospital mortality of these patients continues to remain unacceptably high at over 40%[1-4]. To date immediate myocardial revascularization represents the only intervention of proven benefit. Emergency percutaneous coronary intervention (PCI) is recommended if coronary anatomy is amenable and emergency surgical revascularization is recommended in case coronary anatomy is not amenable for PCI (AHA/ACC and ESC/EACTS indication: Class I, Level B)[5-7]. In order to maintain hemodynamic stabilization before and/or after early revascularization, mechanical support with devices such as intra-aortic balloon pump (IABP), percutaneous left ventricular assist devices (PLVADs) and complete extracorporeal life support with extracorporeal membrane oxygenation (ECMO) are often considered[8]. It is known that IABP support provides significant benefit when used in association with thrombolysis; however, it is of no benefit when used in association with PCI[4,9,10].
It is of note that current guidelines do not recommend routine use of IABP in AMI patients with CS complicating AMI (AHA/ACC and ESC/EACTS indication: Class III, Level A), but IABP use may be considered in these patients when CS is secondary to mechanical complications (AHA/ACC indication: Class IIa, Level C). Further, it is recommended that the use of PLVADs should be restricted for short-term circulatory support (AHA/ACC and ESC/EACTS indication: Class IIb, Level C)[5-7].
Because the sickest patients are often excluded from randomized controlled trials (RCTs), only few RCTs of circulatory assist devices have been conducted thus far. On the other hand, there are some data from clinical observational studies[11-15].
We present here a meta-analysis of available data, based on RCTs and observational studies, on the use of support devices in AMI patients with CS undergoing PCI with regard to inhospital and late mortality.
We performed a systematic PubMed and the Cochrane Library literature search using the terms relating to the intervention of interest “IABP” or “IABC”, “Impella”, “Tandemheart”, “PLVADs”“ECMO” or “extracorporeal life support” or “ECLS” or “CPS” in the setting of CS in patients with AMI undergoing percutaneous coronary revascularization. We performed additional manual literature search through: (1) the reference lists of retrieved articles and published reviews; and (2) the abstracts presented at recent (last five years) International Conferences.
Two investigators independently examined the designs, patient populations and interventions used, aiming to include only studies designed to test the effect of the percutaneous support in patients with CS due to AMI and undergoing myocardial revascularization. The search was restricted to English-language journals and excluded studies on non-human subjects as well as articles unrelated to the topic.
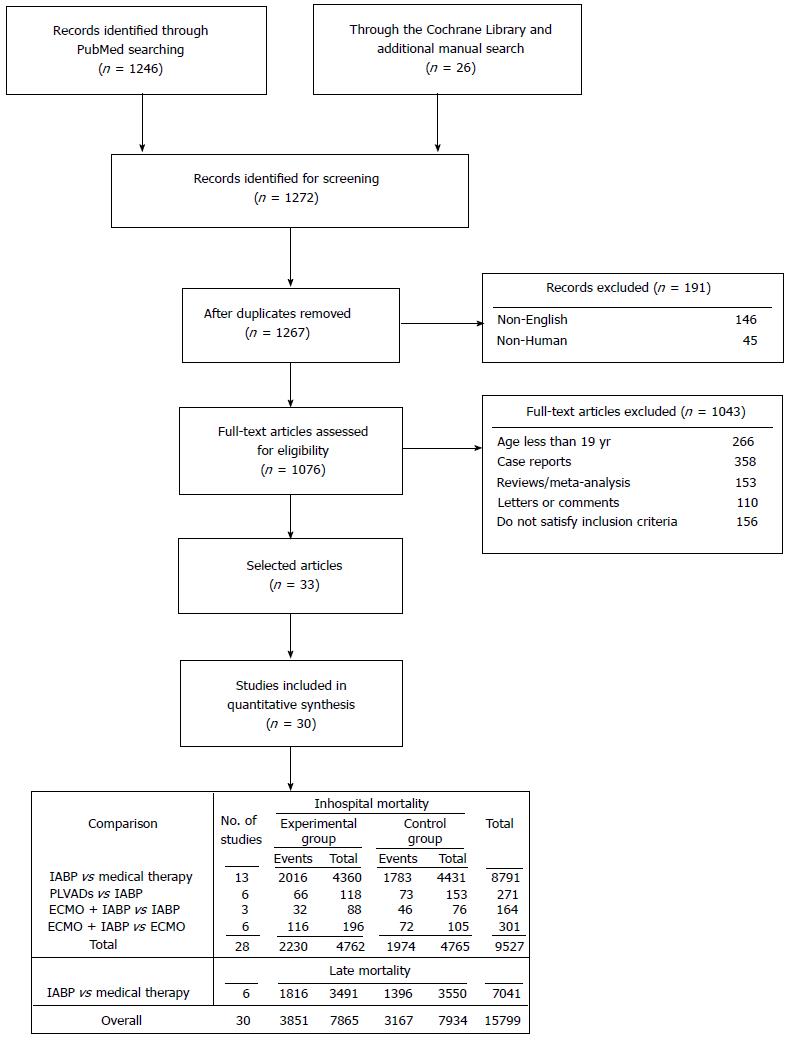
The study selection process is outlined in Figure 1. The exclusion criteria were data from registries or studies with lack of a control group, the absence of mortality data, the presence of different timing for the outcome or, more generally, insufficient data for risk estimation. Disagreements were resolved by asking the opinion of a third reviewer to reach consensus at each stage of the screening process. We selected all of the studies published from January 1st, 1997 to May 15st, 2015 that compared the following percutaneous mechanical support in patients with CS due to AMI undergoing myocardial revascularization: (1) IABP vs Medical therapy; (2) PLVADs vs IABP; and (3) ECMO plus IABP vs IABP or ECMO. CS was defined by: (1) a decrease in systolic blood pressure to ≤ 90 mmHg for more than 30 min, in the absence of hypovolemia, or requiring vasopressor support; (2) a reduction of cardiac index to 1.8 L/min per square meter without support or to 2.0–2.2 L/min per square meter with support; and (3) elevated left ventricular filling pressures[16,17]. Moreover, profound shock was defined as systolic blood pressure less than 75 mmHg-despite receiving an intravenous inotropic agent that was associated with altered mental status and respiratory failure[18]. The acronym PLVADs included the Impella®2.5 (Abiomed, Danvers, MA, United States) and the TandemHeart (Cardiac Assist Inc., Pittsburgh, PA, United States)[14,15]. The acronym of ECMO included a modified heart-lung machine, generally consisted of a centrifugal pump, a heat exchanger and a membrane oxygenator[15,18-22].
Primary and secondary endpoints: We evaluated the impact of the support devices on primary and secondary endpoints. Primary endpoint was the inhospital mortality due to any cause during the same hospital stay and secondary endpoint late mortality at 6-12 mo of follow-up.
Meta-analysis was performed separately for observational studies and RCTs comparing the following groups of patients: (1) IABP (experimental) vs Medical therapy (control); (2) PLVADs (experimental) vs IABP (control); (3) ECMO plus IABP (experimental) vs IABP (control); and (4) ECMO plus IABP (experimental) vs ECMO (control). We computed the risk ratio (RR) with 95%CI, using the Mantel-Haenszel random-effect model to take into account possible heterogeneity among the individual studies beyond that expected from chance, to point out the relative effect of the mechanical assist devices under study. We used the Forest plot to present the results graphically, to report the effect estimates for the individual studies together with the overall measure of effect. We computed the Cochran’s Q test and I2 statistics to quantify the homogeneity/heterogeneity among the selected studies within and between subgroups[23]. A Funnel Plot was designed as visual aid for detecting bias or systematic heterogeneity among the studies included in the meta-analysis (publication bias). A sensitivity analysis was then performed by repeating the meta-analysis after exclusion of the study(ies) falling out the 95%CI.
The meta-analysis was performed using Review Manager (RevMan) (Computer program) Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaborations, 2014[24].
We performed Trial Sequential Analysis using the program provide by “The Copenhagen Trial Unit, Center for Clinical Intervention Research CTU, Denmark; version 0.9 beta; available at http://www.ctu.dk/tsa” in order to assess if the studies enclosed in the meta-analysis reached the required number of participants (information size), and to construct the monitoring boundaries to detect significance and futility of the primary and secondary endpoints[25,26]. Trial Sequential Analysis was done using the effective difference in risks between the experimental (intervention risk) and control groups (basal risk) with a risk of a type I error of 5% and a power of 80%. The relative risk reductions (RRR) observed were linked to the number of patients to be treated (NNT) or to be harmed (NNH), to assess the clinical benefit or the detrimental effect corresponding to each level of RRR. All statistical tests were two-sided and α error of ≤ 0.05 was defined as statistically significant.
The statistical methods of this study were reviewed by Flavia Chiarotti, Biostatistician, Research Director from the Italian National Institute of Health.
One thousand two hundred and seventy-two records met the initial screening criteria. After detailed review, only 30 were selected[4,18,21,27-54]. There were 6 eligible RCTs[4,27-31] and 24 eligible observational studies[18,21,32-54] totaling 15799 patients. The main characteristics of the selected studies are reported in Table 1.
| Ref. | Setting | Study design | Etiology of CS | Cardiac arrest | Treatment | Period | No. of pts |
| IABP vs medical therapy | |||||||
| Anderson et al[32], 1997 (GUSTO-I) | United States, Europe | Obs.; multicenter | STEMI | No | PCI | 1990-1993 | 37 |
| Sanborn et al[33], 2000 (SHOCK Registry) | United States, Canada, Europe, New Zealand | Obs.; multicenter registry | AMI | No | PCI or CABG | 1993-1997 | 383 |
| Barron et al[34], 2001 (NRMI-2) | United States | Obs.; multicenter registry | AMI | No | PCI | 1994- < 2000 | 2990 |
| French et al[31], 2003 (SHOCK Trial 12-mo survival) | United States, Canada, Europe, New Zealand | RCT; multicenter | AMI | No | PCI or CABG | 1993-1998 | 152 |
| Vis et al[35,36], 2007 (AMC CS) | Europe | Obs.; single-center | STEMI | No | PCI | 1997-2005 | 292 |
| Gu et al[37], 2010 | Asia | Obs.; single-center | STEMI | No | PCI | 2003-2008 | 91 |
| Prondzinsky et al[27], 2010 (IABP-SHOCK) | Europe | RCT; single-center | AMI | No | PCI | 2003-2004 | 40 |
| Stub et al[38], 2011 | Europe | Obs.; multicenter registry | ACS | No | PCI | 2004-2010 | 410 |
| Zeymer et al[39], 2011 (Euro Heart Survey PCI) | Europe | Obs.; multicenter registry | STEMI or NSTEMI | No | PCI | 2005-2008 | 653 |
| Thiele et al[4], 2012 (IABP-SCHOCK II) | Europe | RCT; multicenter | AMI | No | PCI (95.8%), CABG (3.5%), PCI and CABG (0.7%) | 2009-2012 | 598 |
| Zeymer et al[40], 2013 (ALKK-PCI) | Europe | Obs.; multicenter registry | STEMI or NSTEMI | No | PCI | 2006-2011 | 1913 |
| Dziewierz et al[41], 2014 (EUROTRANSFER registry) | Europe | Obs.; multicenter registry | STEMI | No | PCI (49 pts), CABG (2 pts) | 2005-2007 | 51 |
| Kunadian et al[44], 2015 (BCIS registry) | Europe | Obs.; multicenter registry | ACS | No | PCI | 2005-2011 | 6120 |
| Kim et al[42], 2015 (KAMIR) | Asia | Obs.; multicenter registry | AMI | Yes | PCI | 2005-2014 | 1214 |
| Suzuki et al[43], 2015 (Tokyo CCU Network Scientific Council) | Asia | Obs.; multicenter registry | STEMI | No | PCI | 2009-2011 | 119 |
| PLVADs (TandemHeart, Impella® 2.5) vs IABP | |||||||
| Thiele et al[29], 20051 | Europe | RCT; single center | AMI | No | PCI (49 pts), CABG (2 pts) | 2000-2003 | 41 |
| Burkoff et al[28], 20061 | United States, Europe | RCT; multicenter | AMI (70%) | No | PCI (22 pts), CABG (3 pts) | 2002-2004 | 33 |
| Seyfarth et al[30], 20082 (ISAR-SHOCK) | Europe | RCT; two-center | AMI | No | PCI (22 pts) | 2004-2007 | 26 |
| Schwartz et al[46], 20121,2 | United States | Obs.; single center | 68% STEMI, 11% OHCA | Yes | PCI (63 pts), CABG (5 pts) | 2008-2010 | 76 |
| Shah et al[47], 20121,2 | United States | Obs.; single center | STEMI or UA/NSTEMI | No | PCI | 2007-2009 | 17 |
| Manzo-Silberman et al[45], 20132 | Europe | Obs.; single center registry | ACS (mainly), OHCA | Yes | PCI (54 pts) | 2007-2010 | 78 |
| ECMO plus IABP vs IABP | |||||||
| Sheu et al[18], 2010 | Asia | Obs.; single center | STEMI | No | PCI | 1993-2009 | 71 |
| Tsao et al[21], 2012 | Asia | Obs.; single center | AMI | No | PCI | 2004-2009 | 58 |
| Perazzolo Marra et al[48], 2013 | Europe | Obs.; single center | AMI | No | PCI | 2010-2012 | 35 |
| ECMO plus IABP vs ECMO | |||||||
| Yamauchi et al[49], 2009 | Asia | Obs.; single center | AMI | No | PCI | 2000-2007 | 16 |
| Chung et al[50], 2011 | Asia | Obs.; multicenter | AMI, INCA (14 pts) | Yes | PCI (7 pts), CABG (13 pts) | 2206-2009 | 20 |
| Kagawa et al[51], 2012 | Asia | Obs.; multicenter | ACS, INCA, OHCA | Yes | PCI | 2004-2011 | 73 |
| Aoyama et al[52], 2014 | Asia | Obs.; single center | AMI, INCA (2 pts, OHCA 7 pts) | Yes | PCI (34 pts), CABG (4 pts) | 1993-2000 | 38 |
| Park et al[53], 2014 | Asia | Obs.; single center | AMI | No | PCI (78 pts), PCI e/o CABG (10 pts), medical treatment (8 pts) | 2004-2011 | 96 |
| Kim et al[54], 2014 | Asia | Obs.; multicenter | ACS | No | PCI (53 pts), CABG (5 pts) | 2010-2013 | 58 |
In the comparison between IABP and Medical therapy, we analysed a total of 15063 patients (14273 from 12 observational studies[32-44] and 790 form 3 RCTs[4,27,31]). The data provided us by French et al[31] and Kunadian et al[44] contributed only for the analysis of the secondary outcome.
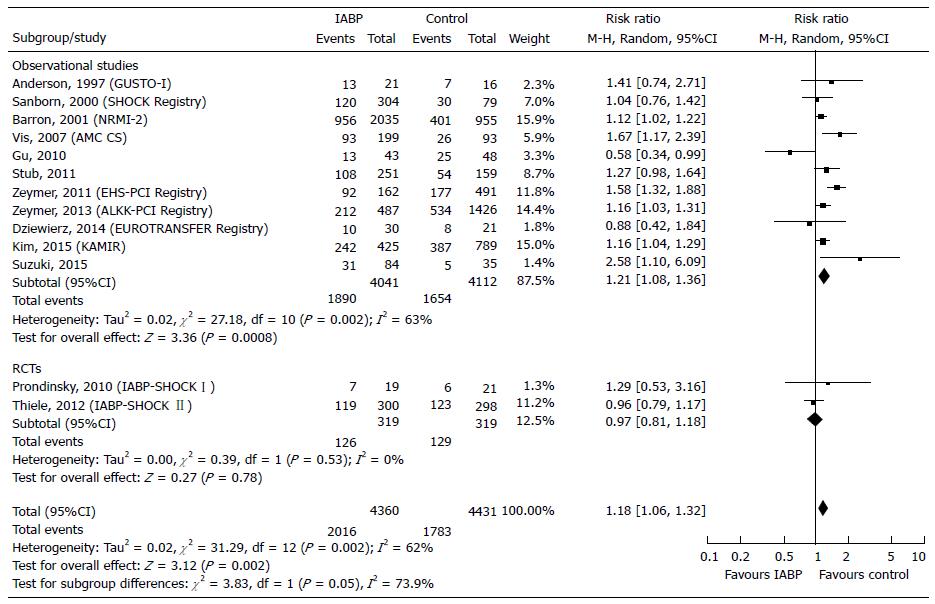
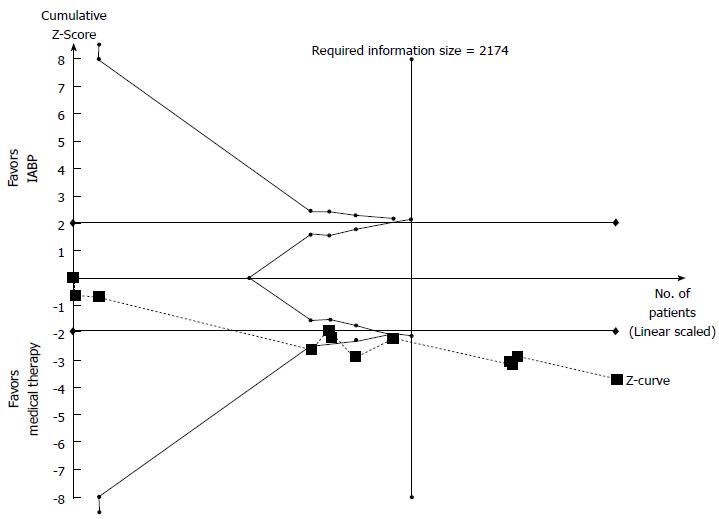
Primary endpoint: Primary endpoint was assessed in 8791 patients (8153 from 11 observational studies[32-43] and 638 from 2 RCTs[4,27]). The inhospital deaths occurred in 46.24% of patients in the experimental group and 40.24% of patients in the control. The NNH was 16 (6 more deaths every 100 patients treated with IABP). The overall analysis showed a significant risk increase (+18%, P = 0.002) in the IABP group (Figure 2). More specifically, we observed a significant risk increase in observational studies (RR = +21%, P = 0.0008) and a nonsignificant risk reduction in RCTs (RR = -3%%, P = 0.78) (Figure 2). The test for subgroup differences showed high heterogeneity among observational studies (I2 = 63%) and between observational and RCTs (I2 = 73.9%), providing a significantly different estimate of the IABP effect (Figure 2). In the Funnel plot, the studies by Gu et al[37] and by Zeymer et al[39] fell out of the 95%CI, thus appearing to be the potential source of bias. After the sensitivity analysis, heterogeneity decreased to a lower level among the observational (I2 = 19%), but persisted at high levels between observational studies and RCTs (I2 = 68.2%) (Table 2). Furthermore the overall risk in the experimental group slight decreased (RR = +15%) (Table 2). The NNH was equal to 18 (5 more deaths every 100 patients treated with IABP) (Table 3). Trial Sequential Analysis showed that the required number of participant was reached and the monitoring boundaries, constructed to detect significance, were crossed by the z-curves, demonstrating a detrimental effect of IABP (Table 3, Figure 3).
| Comparison/subgroup | RR | |||||||
| Before | After | |||||||
| n | I2(%) | Estimate (95%CI) | P | n | I2(%) | Estimate (95%CI) | P | |
| Inhospital mortality | ||||||||
| IABP vs medical therapy | ||||||||
| Observational studies | 11 | 63 | 1.21 (1.08, 1.36) | 0.0008 | 9 | 19 | 1.17 (1.09, 1.26) | < 0.0001 |
| RCTs | 2 | 0 | 0.97 (0.81, 1.18) | 0.78 | 2 | 0 | 0.97 (0.81, 1.18) | 0.78 |
| Overall effect | 13 | 62 | 1.18 (1.06, 1.32) | 0.002 | 11 | 24 | 1.15 (1.07, 1.24) | 0.0002 |
| Test for subgroup differences1 | χ2 = 3.83, df = 1 (P = 0.05), I2 = 73.9% | χ2 = 3.14, df = 1 (P = 0.08), I2 = 68.2% | ||||||
| ECMO plus IABP vs ECMO | ||||||||
| Observational studies | 6 | 12 | 0.78 (0.65, 0.94) | 0.008 | 5 | 0 | 0.80 (0.68, 0.94) | 0.006 |
| Late mortality | ||||||||
| IABP vs medical therapy | ||||||||
| Observational studies | 3 | 90 | 0.92 (0.51, 1.67) | 0.78 | 2 | 60 | 1.16 (0.69, 1.95) | 0.57 |
| RCTs | 3 | 32 | 1.16 (0.86, 1.58) | 0.34 | 2 | 0 | 1.56 (0.97, 2.52) | 0.07 |
| Overall effect | 6 | 85 | 1.08 (0.82, 1.41) | 0.60 | 4 | 0 | 1.38 (1.30, 1.46) | < 0.00001 |
| Test for subgroup differences1 | χ2 = 0.48, df = 1 (P = 0.49), I2 = 0% | χ2 = 0.68, df = 1 (P = 0.41), I2 = 0% | ||||||
| Groups | Mortality rate (%) | RRR | Effect of experimental support | Trial Sequential Analysis | |||||||
| Experimental | Control | Experimental | Control | NNT | NNH | Harm1 | Benefit1 | Required information size | Results | ||
| Inhospital mortality | |||||||||||
| IABP2 | vs | Medical therapy2 | 45.99 | 40.62 | -13.22 | 18 | 5.37 | 2174 | Conclusive | ||
| PLVADs | vs | IABP | 55.93 | 47.71 | -17.23 | 12 | 8.22 | 1161 | Inconclusive | ||
| ECMO + IABP | vs | IABP | 36.36 | 60.53 | 39.92 | 5 | 24.16 | 150 | Conclusive | ||
| ECMO + IABP2 | vs | ECMO2 | 61.29 | 66.67 | 8.06 | 19 | 5.38 | Not calculable | Inconclusive | ||
| Late mortality | |||||||||||
| IABP | vs | Medical therapy | 52.02 | 39.32 | -32.28 | 7 | 12.70 | 5984 | Futility | ||
| IABP2 | vs | Medical therapy2 | 52.08 | 37.68 | -38.22 | 6 | 14.40 | 168 | Conclusive | ||
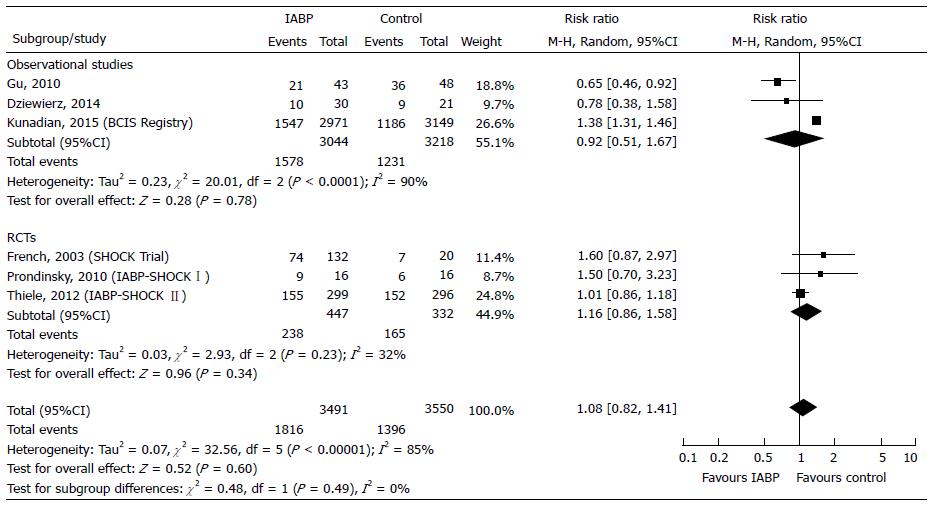
Secondary endpoint: The late mortality was assessed in 7041 patients (6262 from 3 observational studies[37,41,44] and 779 from 3 RCTs[4,27,31,55,56]). Mortality rate was higher, but not significantly, in the IABP group respect to control (52.02% vs 39.32%). IABP reduced mortality (-8%, P = 0.78) in observational studies and increased mortality (+16%, P = 0.34) in RCTs (Figure 4). In the Funnel plot the studies by Gu et al[37] and by Thiele et al[56] fell out of the 95%CI, appearing to be the potential source of bias. When we applied the sensitivity analysis by excluding the study by Gu et al[37] from observational studies and the study by Thiele et al[56] from RCTs, the overall I2 decreased from 85% to 0% (Table 2). Moreover, the test for subgroup differences showed that the heterogeneity between observational and RCTs was lower (I2 = 0%) and an overall significant detrimental effect of IABP was found (Table 2). Trial Sequential Analysis was performed: (1) by including all studies; and (2) by excluding the study by Gu et al[37] and that by Thiele et al[56] according to the sensitivity analysis (Table 3). With inclusion of all studies, there was a 32.28% mortality increase in the IABP group with about 13 more deaths every 100 treated patients. When studies by Gu et al[37] and Thiele et al[56] were excluded, IABP support resulted in a 38.22% risk increase, and Trial Sequential Analysis showed that data were sufficient to highlight the harmful effect of IABP support on the late mortality (Table 3).
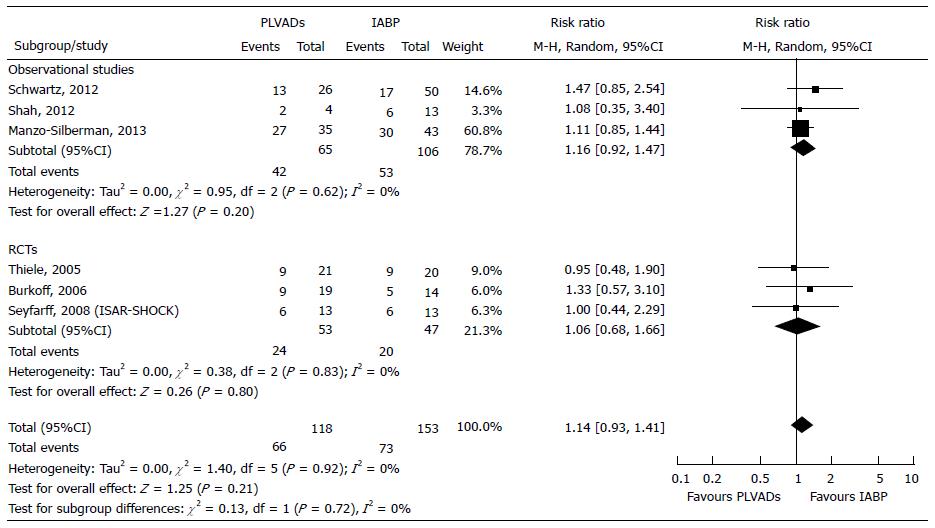
We compared the effect of PLVADs vs IABP in 271 patients; 171 from 3 observational studies[45-47] and 100 from 3 RCTs[28-30].
Primary endpoint: The overall inhospital mortality increased although not significantly, in PLVADs group compared to IABP group, both in the observational studies (+16%, P = 0.20) and the RCTs (+6%, P = 0.80) (Figure 5). The test for subgroup differences did not show significant differences between observational studies and RCTs (χ2 = 0.13, P = 0.72, I2 = 0%). Indeed, in the Forest plot the confidence intervals overlapped, P values of the χ2 tests were all greater than 0.10 and the I2 statistics were all equal to zero, showing the homogeneity among the studies within both observational and RCTs (Figure 5). In the Funnel plot, all studies were enclosed into 95%CI and the larger studies were plotted at the central top of the graph, demonstrating a convergence in risk estimation while increasing the sample size. RRR equaled -17.23%; when translated into clinical terms, use of PLVADs resulted 8 more deaths every 100 patients treated. For appropriate Trial Sequential Analysis, more patients would have to be included (Table 3).
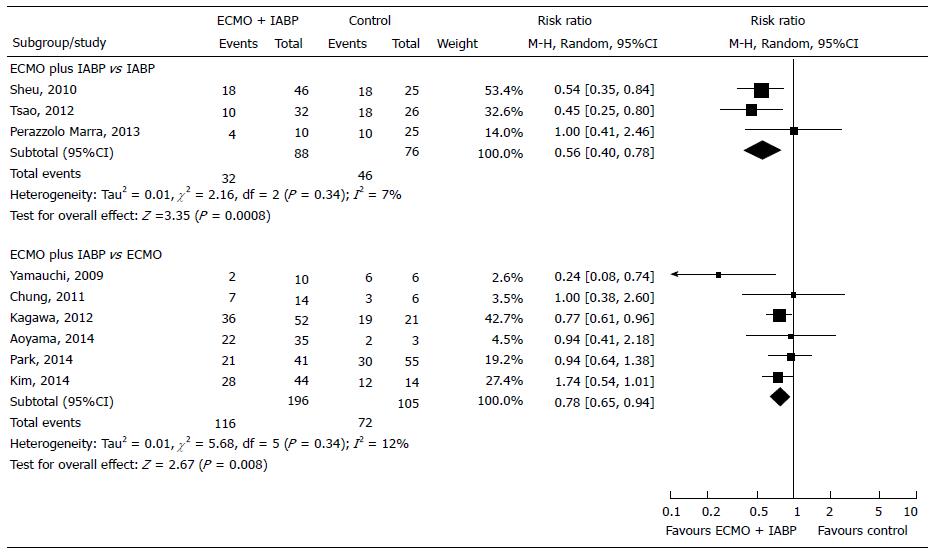
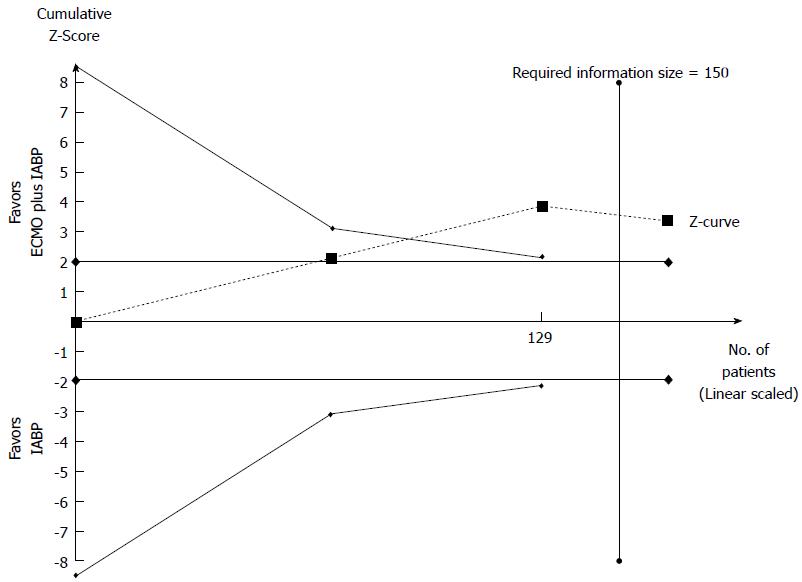
Primary endpoint: We compared the effect of ECMO plus IABP vs IABP in 164 patients from 3 observational studies[18,21,48]. We did not find any RCTs on the topic. In the Forest plot the χ2 test and the I2 statistics detected the absence of significant heterogeneity (I2 = 7%). In the Funnel plot analysis, all studies within 95%CI were included. The inhospital mortality was higher when IABP was used alone rather than in combination with ECMO (60.53% vs 36.36%, respectively). ECMO plus IABP group showed a 44% reduction in mortality (Figure 6). The observed RRR was 39.92%, which means that there were 24 fewer deaths for every 100 treated patients. Trial Sequential Analysis showed that the cumulative Z-curve crossed the alpha-spending boundaries, demonstrating that a significant RRR was obtained when ECMO support was used in association with IABP (Figure 7). The required numbers of patients was reached and the meta-analysis could be considered conclusive (Table 3, Figure 7).
Primary endpoint: We compared the effect of ECMO plus IABP vs IABP in 301 patients from 6 observational studies[49-54]. We did not find any RCTs that analyzed this topic. We found a significantly lower inhospital mortality (RR = -22%, P = 0.008) in the group of patients treated with ECMO plus IABP compared to ECMO alone (Figure 6). In the Funnels plot analysis, only the study by Yamauchi et al[49] could be a potential source of bias. After the sensitivity analysis I2 decreased to 0% while the significant effect of ECMO plus IABP vs ECMO remained substantially unchanged (RR = -20%, P = 0.006) (Table 2). Despite these results, Trial Sequential Analysis could not be performed because of the small number of patients included (Table 3).
All recent reviews on the use of support devices in AMI patient with CS undergoing PCI thus far show lack of a meta-analytic estimates[11-15], probably because the results were based mainly on registry data.
Meta-analyses of data from observational studies represent an area of innovation in statistical science. This analysis can be performed when the question of interest cannot be answered by a review of randomized controlled trials. Even though observational studies are prone to bias (including confounding variables), strategies to adjust for unmeasured confounding variables can be adopted[23]. In the present review, we identified only a small number of randomized trials, which by themselves were underpowered to assess the efficacy of the support devices on inhospital mortality. To increase the power of the analysis we included observational data, which enabled us to add 14909 additional patients to the 890 from the RCTs selected. Further, to avoid bias we used the Funnel plot analysis, the Cochran’s Q test and I2 statistics to test differences between groups and subgroups. The sensitivity analysis allowed us to make comparisons not affected by excessive heterogeneity.
First, in the comparison between IABP vs Medical therapy, the analysis confirmed that IABP support was associated with a significant increase inhospital mortality (Figure 2). The results of RCTs were marginal probably because of the small sample size and the results could be considered a chance occurrence (Figures 2 and 4). When we included the data from observational studies and applied the sensitivity analysis the results were affected only low heterogeneity (I2 = 19%). Trial Sequential Analysis showed that the Z-curves surpassed not only the conventional boundaries but also the alpha-spending boundaries, constructed to control for type 1 error as the source of bias. Thus, the meta-analysis can be considered conclusive in terms of showing a detrimental effect of IABP (Figure 3). With regard to late mortality, we did not identify any difference in both observational studies or in RCTs. However, after sensitivity analysis a significantly higher late mortality was observed in IABP-treated patients and was confirmed by Trial Sequential Analysis, that was conclusive (Table 3).
Second, relative the comparison between IABP vs PLVADs, recently reported studies have failed to show a hemodynamic or survival benefit of mechanical support in AMI patients with CS and undergoing PCI. The meta-analysis by Cheng et al[57] dates back to 2009, performed on 3 RCTs and included 100 patients, showed that although PLVADs provided superior haemodynamic support in patients with CS compared to IABP, the use of these more powerful devices did not significantly improve early survival. Afterwards only observational studied were performed on this topic. O’Neill et al[58] suggested that early initiation of hemodynamic support prior to PCI with Impella 2.5 was associated with more complete revascularization and improved survival in the setting of refractory CS complicating AMI.
In our analysis, the PLVADs increased, although non significantly, the mortality as compared with IABP. The Trial Sequential Analysis showed that 1161 patients will need be analyzed in order to demonstrate its detrimental effect. Our meta-analysis was as such inconclusive and additional perspective investigations would be needed to definitive conclusion.
Third, relative to comparisons of ECMO plus IABP vs IABP or ECMO plus IABP vs ECMO, the meta-analysis showed a significant protective effect of ECMO plus IABP on inhospital mortality compared to IABP or ECMO used alone (Figure 6). Moreover, Trial Sequential Analysis showed that in the comparison ECMO plus IABP vs IABP the required numbers of patients was reached and the meta-analysis could be considered conclusive (Figure 7).
The main limitation of this meta-analysis is the inclusion of the observational studies, since they are viewed as having less validity than RCTs, due to the absence of randomization. Indeed, we cannot exclude that CS was more severe in the IABP group compared to Medical therapy in some observational studies included in our meta-analysis. However, we repeated the analysis, including only the observational studies, between IABP vs control group, selected according to the same severity of shock. The results were substantially unchanged (RR = 1.11, 95%CI = 1.02 to 1.21), significantly in favour of Medical therapy. The heterogeneity was absent (I2 = 0%). If RCTs were added to the analysis, the heterogeneity appeared equally low (I2 = 38%). Moreover, RCTs conducted to assess the role of haemodynamic support in patients with CS complicating AMI reported in the scientific literature are few, perhaps due to ethical issues and feasibility, involving randomization of very severely sick patients. Thus, the inclusion of well-performed observational studies may be acceptable to allow for risk estimation in such situations. Concato et al[59] analyzed published meta-analyses based on randomized clinical trials and observational studies that examined identical clinical topics and found that the average results of well-designed observational studies (with either a cohort or a case-control design) were markedly similar to those of the RCTs. Therefore, an integrated approach should be adopted using both experimental and observational studies, as long as well-designed and conducted. Finally, “discarding observational evidence when randomised trials are available is missing an opportunity. Conversely, abandoning plans for randomised trials in favour of quick and dirty observational designs is poor science[60]”.
Another limitation was the lack of the analysis of the baseline characteristics (such as age, gender, race, etc.) that are recognized markers of risk. Unfortunately, these data available at baseline were not reported in the outcome.
The results of our meta-analysis showed that in AMI patients with CS and undergoing PCI: (1) the inhospital mortality was significantly higher with IABP support vs Medical therapy; (2) PLVADs increased, although non significantly, the mortality as compared with IABP; and (3) ECMO plus IABP had significant protective effect compared to IABP or ECMO alone. Trial Sequential Analysis of data on inhospital mortality in IABP vs control and ECMO plus IABP vs IABP showed that the analyses were sufficient to highlight the harmful effect of IABP and further studies would no longer be needed. Based on the results we can conclude that in CS complicating AMI: (1) routinely use of IABP and PLVADs is not recommended; and (2) the beneficial effect of the reduction inhospital mortality provided by ECMO plus IABP could be attributed to the synergistic action of the two devices in supporting the failing heart. IABP decreasing afterload and myocardial oxygen consumption, can avoid the negative effects on myocardial protection that can occur when using ECMO alone.
Despite major technical advances the inhospital mortality of patients with cardiogenic shock (CS) complicating AMI continues to remain high. To support the failing heart [intra-aortic balloon pump (IABP)], percutaneous left ventricular assist devices (PLVADs) and extracorporeal membrane oxygenation (ECMO) are used. Unfortunaletely randomized controlled trials (RCTs) on this issue are performed in small numbers, perhaps due to ethical issues and feasibility, involving randomization of patients with CS.
The question of impact of cardiac support percutaneous devices cannot be answered by a review of RCTs alone. Meta-analyses of observational studies increase the power of the analysis by adding more data to the RCTs to have more comprehensive results.
In the present study, the authors investigated the impact of IABP, PLVADs and ECMO on inhospital mortality and late survival in patients with CS complicating acute myocardial infarction (AMI) undergoing percutaneous coronary intervention (PCI). Meta-analysis of observational studies in addition to the RCTs enabled them to increase the power of the analysis.
The results of the meta-analysis allow us to understand the impact of percutaneous cardiac support with IABP, PLVAD and ECMO in patients with CS complicating AMI undergoing PCI.
This is a systematic review and meta-analysis of observational studies and RCTs.
In this study, the authors collected the data from 30 published research papers (total 15799 patients) and used meta-analysis to analyze in hospital and late mortality of percutaneous mechanical support. This is an interesting study. The findings in this study have the potential to help the clinical doctor work out the guideline for reducing mortality in acute myocardial infarction complicated by cardiogenic shock.
| 1. | Aissaoui N, Puymirat E, Tabone X, Charbonnier B, Schiele F, Lefèvre T, Durand E, Blanchard D, Simon T, Cambou JP. Improved outcome of cardiogenic shock at the acute stage of myocardial infarction: a report from the USIK 1995, USIC 2000, and FAST-MI French nationwide registries. Eur Heart J. 2012;33:2535-2543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 2. | Goldberg RJ, Spencer FA, Gore JM, Lessard D, Yarzebski J. Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspective. Circulation. 2009;119:1211-1219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 544] [Cited by in RCA: 510] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 3. | Jeger RV, Radovanovic D, Hunziker PR, Pfisterer ME, Stauffer JC, Erne P, Urban P. Ten-year trends in the incidence and treatment of cardiogenic shock. Ann Intern Med. 2008;149:618-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 281] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 4. | Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1652] [Cited by in RCA: 2028] [Article Influence: 144.9] [Reference Citation Analysis (0)] |
| 5. | Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK, Kern M, Garratt KN, Goldstein JA, Dimas V. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care (Endorsed by the American Heart Association, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention). J Card Fail. 2015;21:499-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541-2619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3295] [Cited by in RCA: 3423] [Article Influence: 285.3] [Reference Citation Analysis (7)] |
| 7. | O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1953] [Cited by in RCA: 2321] [Article Influence: 165.8] [Reference Citation Analysis (0)] |
| 8. | Graf T, Thiele H. Mechanical support in cardiogenic shock. Herz. 2015;40:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Sjauw KD, Konorza T, Erbel R, Danna PL, Viecca M, Minden HH, Butter C, Engstrøm T, Hassager C, Machado FP. Supported high-risk percutaneous coronary intervention with the Impella 2.5 device the Europella registry. J Am Coll Cardiol. 2009;54:2430-2434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 182] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 10. | Romeo F, Acconcia MC, Sergi D, Romeo A, Muscoli S, Valente S, Gensini GF, Chiarotti F, Caretta Q. The outcome of intra-aortic balloon pump support in acute myocardial infarction complicated by cardiogenic shock according to the type of revascularization: a comprehensive meta-analysis. Am Heart J. 2013;165:679-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Thiele H, Ohman EM, Desch S, Eitel I, de Waha S. Management of cardiogenic shock. Eur Heart J. 2015;36:1223-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 340] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 12. | Gilotra NA, Stevens GR. Temporary mechanical circulatory support: a review of the options, indications, and outcomes. Clin Med Insights Cardiol. 2014;8:75-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Greenwood JC, Herr DL. Mechanical circulatory support. Emerg Med Clin North Am. 2014;32:851-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Khan MH, Corbett BJ, Hollenberg SM. Mechanical circulatory support in acute cardiogenic shock. F1000Prime Rep. 2014;6:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Werdan K, Gielen S, Ebelt H, Hochman JS. Mechanical circulatory support in cardiogenic shock. Eur Heart J. 2014;35:156-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 345] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 16. | Alexander JH, Reynolds HR, Stebbins AL, Dzavik V, Harrington RA, Van de Werf F, Hochman JS. Effect of tilarginine acetate in patients with acute myocardial infarction and cardiogenic shock: the TRIUMPH randomized controlled trial. JAMA. 2007;297:1657-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 284] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 17. | Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1988] [Cited by in RCA: 2120] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 18. | Sheu JJ, Tsai TH, Lee FY, Fang HY, Sun CK, Leu S, Yang CH, Chen SM, Hang CL, Hsieh YK. Early extracorporeal membrane oxygenator-assisted primary percutaneous coronary intervention improved 30-day clinical outcomes in patients with ST-segment elevation myocardial infarction complicated with profound cardiogenic shock. Crit Care Med. 2010;38:1810-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 266] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 19. | Tharmaratnam D, Nolan J, Jain A. Management of cardiogenic shock complicating acute coronary syndromes. Heart. 2013;99:1614-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Cove ME, MacLaren G. Clinical review: mechanical circulatory support for cardiogenic shock complicating acute myocardial infarction. Crit Care. 2010;14:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Tsao NW, Shih CM, Yeh JS, Kao YT, Hsieh MH, Ou KL, Chen JW, Shyu KG, Weng ZC, Chang NC. Extracorporeal membrane oxygenation-assisted primary percutaneous coronary intervention may improve survival of patients with acute myocardial infarction complicated by profound cardiogenic shock. J Crit Care. 2012;27:530.e1-530.11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Kim H, Lim SH, Hong J, Hong YS, Lee CJ, Jung JH, Yu S. Efficacy of veno-arterial extracorporeal membrane oxygenation in acute myocardial infarction with cardiogenic shock. Resuscitation. 2012;83:971-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Higgins JPT, Green S, editors . Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011]. The Cochrane Collaboratio. 2011; Available from: http://www.cochrane-handbook.org. |
| 24. | Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboratio 2014; . |
| 25. | Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for trial sequential analysis (TSA). Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen, Denmark. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboratio 2011; 1-115 Available from: http://www.ctu.dk/tsa. |
| 26. | Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med Res Methodol. 2009;9:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 653] [Cited by in RCA: 768] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 27. | Prondzinsky R, Lemm H, Swyter M, Wegener N, Unverzagt S, Carter JM, Russ M, Schlitt A, Buerke U, Christoph A. Intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP SHOCK Trial for attenuation of multiorgan dysfunction syndrome. Crit Care Med. 2010;38:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 245] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 28. | Burkhoff D, Cohen H, Brunckhorst C, O’Neill WW. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006;152:469.e1-469.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 438] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 29. | Thiele H, Sick P, Boudriot E, Diederich KW, Hambrecht R, Niebauer J, Schuler G. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26:1276-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 503] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 30. | Seyfarth M, Sibbing D, Bauer I, Fröhlich G, Bott-Flügel L, Byrne R, Dirschinger J, Kastrati A, Schömig A. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52:1584-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 853] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 31. | French JK, Feldman HA, Assmann SF, Sanborn T, Palmeri ST, Miller D, Boland J, Buller CE, Steingart R, Sleeper LA. Influence of thrombolytic therapy, with or without intra-aortic balloon counterpulsation, on 12-month survival in the SHOCK trial. Am Heart J. 2003;146:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Anderson RD, Ohman EM, Holmes DR, Col I, Stebbins AL, Bates ER, Stomel RJ, Granger CB, Topol EJ, Califf RM. Use of intraaortic balloon counterpulsation in patients presenting with cardiogenic shock: observations from the GUSTO-I Study. Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries. J Am Coll Cardiol. 1997;30:708-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 110] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Sanborn TA, Sleeper LA, Bates ER, Jacobs AK, Boland J, French JK, Dens J, Dzavik V, Palmeri ST, Webb JG. Impact of thrombolysis, intra-aortic balloon pump counterpulsation, and their combination in cardiogenic shock complicating acute myocardial infarction: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK? J Am Coll Cardiol. 2000;36:1123-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 186] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 34. | Barron HV, Every NR, Parsons LS, Angeja B, Goldberg RJ, Gore JM, Chou TM. The use of intra-aortic balloon counterpulsation in patients with cardiogenic shock complicating acute myocardial infarction: data from the National Registry of Myocardial Infarction 2. Am Heart J. 2001;141:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 201] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 35. | Vis MM, Sjauw KD, van der Schaaf RJ, Baan J, Koch KT, DeVries JH, Tijssen JG, de Winter RJ, Piek JJ, Henriques JP. In patients with ST-segment elevation myocardial infarction with cardiogenic shock treated with percutaneous coronary intervention, admission glucose level is a strong independent predictor for 1-year mortality in patients without a prior diagnosis of diabetes. Am Heart J. 2007;154:1184-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Vis MM, Sjauw KD, van der Schaaf RJ, Koch KT, Baan J, Tijssen JG, Piek JJ, de Winter RJ, Henriques JP. Prognostic value of admission hemoglobin levels in ST-segment elevation myocardial infarction patients presenting with cardiogenic shock. Am J Cardiol. 2007;99:1201-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Gu J, Hu W, Xiao H, Feng X, Chen Y, Zhang D. Intra-aortic balloon pump improves clinical prognosis and attenuates C-reactive protein level in acute STEMI complicated by cardiogenic shock. Cardiology. 2010;117:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Stub D, Chan W, Clark DJ, Ajani AE, Andrianopoulos N, Brennan A, Loane P, Black A, New G, Shaw JA. Are intra-aortic balloon pumps harmful in cardiogenic shock? Eur Heart J. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboratio 2011; 723 Poster text [Last access on June 3rd 2015] Available from: http://spo.escardio.org/eslides/view.aspx?eevtid=48&fp=P4162. |
| 39. | Zeymer U, Bauer T, Hamm C, Zahn R, Weidinger F, Seabra-Gomes R, Hochadel M, Marco J, Gitt A. Use and impact of intra-aortic balloon pump on mortality in patients with acute myocardial infarction complicated by cardiogenic shock: results of the Euro Heart Survey on PCI. EuroIntervention. 2011;7:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Zeymer U, Hochadel M, Hauptmann KE, Wiegand K, Schuhmacher B, Brachmann J, Gitt A, Zahn R. Intra-aortic balloon pump in patients with acute myocardial infarction complicated by cardiogenic shock: results of the ALKK-PCI registry. Clin Res Cardiol. 2013;102:223-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Dziewierz A, Siudak Z, Rakowski T, Kleczyński P, Zasada W, Dudek D. Impact of intra-aortic balloon pump on long-term mortality of unselected patients with ST-segment elevation myocardial infarction complicated by cardiogenic shock. Postepy Kardiol Interwencyjnej. 2014;10:175-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Kim HK, Jeong MH, Ahn Y, Sim DS, Chae SC, Kim YJ, Hur SH, Seong IW, Hong TJ, Choi DH. Clinical outcomes of the intra-aortic balloon pump for resuscitated patients with acute myocardial infarction complicated by cardiac arrest. J Cardiol. 2016;67:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Suzuki M, Sumiyoshi T, Miyachi H, Yamashita J, Yamasaki M, Miyauchi K, Yamamoto T, Nagao K, Tomoike H, Takayama M. Effect of Coronary Thrombectomy in Cardiogenic Shock Complicating ST-Segment Elevation Myocardial Infarction. Am J Cardiol. 2015;115:1649-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Kunadian V, Qiu W, Ludman P, Redwood S, Curzen N, Stables R, Gunn J, Gershlick A. Outcomes in patients with cardiogenic shock following percutaneous coronary intervention in the contemporary era: an analysis from the BCIS database (British Cardiovascular Intervention Society). JACC Cardiovasc Interv. 2014;7:1374-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 45. | Manzo-Silberman S, Fichet J, Mathonnet A, Varenne O, Ricome S, Chaib A, Zuber B, Spaulding C, Cariou A. Percutaneous left ventricular assistance in post cardiac arrest shock: comparison of intra aortic blood pump and IMPELLA Recover LP2.5. Resuscitation. 2013;84:609-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 46. | Schwartz BG, Ludeman DJ, Mayeda GS, Klonera RA, Economides C, Burstein S. Treating Refractory Cardiogenic Shock With the TandemHeart and Impella Devices: A Single Center Experience. Cardiol Res. 2012;3:54-66. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Shah R, Thomson A, Atianzar K, Somma K, Mehra A, Clavijo L, Matthews RV, Shavelle DM. Percutaneous left ventricular support for high-risk PCI and cardiogenic shock: who gets what? Cardiovasc Revasc Med. 2012;13:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Perazzolo Marra M, Gasparetto N, Salotti C, Prevedello F, Marzari A, Bianco R, Tarantini G, Gerosa G, Iliceto S, Cacciavillani L. Clinical impact of mechanical supports for management of post-infarction cardiogenic shock: a balance between survival and hemorrhagic complications in a single tertiary centre. Eur Heart J. 2013;34 (suppl 1). [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 49. | Yamauchi T, Masai T, Takeda K, Kainuma S, Sawa Y. Percutaneous cardiopulmonary support after acute myocardial infarction at the left main trunk. Ann Thorac Cardiovasc Surg. 2009;15:93-97. [PubMed] |
| 50. | Chung ES, Lim C, Lee HY, Choi JH, Lee JS, Park KH. Results of Extracorporeal Membrane Oxygenation (ECMO) Support before Coronary Reperfusion in Cardiogenic Shock with Acute Myocardial Infarction. Korean J Thorac Cardiovasc Surg. 2011;44:273-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Kagawa E, Dote K, Kato M, Sasaki S, Nakano Y, Kajikawa M, Higashi A, Itakura K, Sera A, Inoue I. Should we emergently revascularize occluded coronaries for cardiac arrest?: rapid-response extracorporeal membrane oxygenation and intra-arrest percutaneous coronary intervention. Circulation. 2012;126:1605-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 52. | Aoyama N, Imai H, Kurosawa T, Fukuda N, Moriguchi M, Nishinari M, Nishii M, Kono K, Soma K, Izumi T. Therapeutic strategy using extracorporeal life support, including appropriate indication, management, limitation and timing of switch to ventricular assist device in patients with acute myocardial infarction. J Artif Organs. 2014;17:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Park TK, Yang JH, Choi SH, Song YB, Hahn JY, Choi JH, Sung K, Lee YT, Gwon HC. Clinical impact of intra-aortic balloon pump during extracorporeal life support in patients with acute myocardial infarction complicated by cardiogenic shock. BMC Anesthesiol. 2014;14:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 54. | Kim DK, Seo GW, Song PS, Kim KH, Kim DI, Jin HY, Jang JS, Yoon HJ, Nam CW. Impact of concomitant use of intra-aortic balloon pump during percutaneous cardiopulmonary support in patients with cardiogenic shock complicating acute myocardial infarction. Eurointervention (EuroPCR Abstracts and Poster 2014), Poster text. Available from: http://www.pcronline.com/eurointervention/AbstractsEuroPCR2014/132/#sthash.4rWHEr1q.dpuf. |
| 55. | Unverzagt S, Buerke M, de Waha A, Haerting J, Pietzner D, Seyfarth M, Thiele H, Werdan K, Zeymer U, Prondzinsky R. Intra-aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock. Cochrane Database Syst Rev. 2015;3:CD007398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 56. | Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, de Waha A, Richardt G, Hennersdorf M, Empen K. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013;382:1638-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 703] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 57. | Cheng JM, den Uil CA, Hoeks SE, van der Ent M, Jewbali LS, van Domburg RT, Serruys PW. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J. 2009;30:2102-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 355] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 58. | O’Neill WW, Schreiber T, Wohns DH, Rihal C, Naidu SS, Civitello AB, Dixon SR, Massaro JM, Maini B, Ohman EM. The current use of Impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: results from the USpella Registry. J Interv Cardiol. 2014;27:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 306] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 59. | Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2245] [Cited by in RCA: 2269] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 60. | Ioannidis JP, Haidich AB, Lau J. Any casualties in the clash of randomised and observational evidence? BMJ. 2001;322:879-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Lin GM, Li Y S- Editor: Ji FF L- Editor: A E- Editor: Wu HL