INTRODUCTION
Electrical storm (ES), also referred as arrhythmic storm, refers to a clinical condition characterized by 3 or more arrhythmia episodes of ventricular tachycardia (VT) or ventricular fibrillation (VF) leading to implantable cardioverter-defibrillator (ICD) therapies (Antitachycardia Pacing, ATP, or Direct Current shock, DC-shock), occurring over a single 24 h period[1]. ES represents an arrhythmic emergency that often affects patients at high risk of sudden cardiac death who previously underwent ICD implantation. In this setting ICD correctly interrupts VT/VF episodes; however ventricular arrhythmias, in terms of arrhythmogenic substrate, represent the gradual evolution of the underlying structural heart disease. In this review, we assess the most relevant epidemiological and clinical aspects of ES, with regard to the acute and long-term follow-up implications, focusing on novel therapeutic strategies of treatment.
CLINICAL CHARACTERIZATION
The term ES was introduced in the 1990s to describe an instability condition, highly malignant, characterized by repetitive episodes of ventricular arrhythmias[2]. Nowadays, ES implies several appropriate ICD interventions aimed at terminating the arrhythmic episodes.
ES has been reported in 10%-40% of patients in secondary prevention whereas the incidence of ES is lower (3.5%-4%) in primary prevention[3-16]. However, the correct incidence of ES is uncertain due to several confounding factors such as population considered, type of cardiomyopathy, pharmacological therapy undertaken and other variables. In addition, most of the studies concerning ES incidence were retrospective thus including only the patients who survived the arrhythmic event.
ES mainly affects patients with advanced dilated cardiomyopathy, both ischemic and non-ischemic, representing the gradual evolution of the underlying arrhythmic substrate; however, ES may affect patients with different types of structural heart disease, such as valvular or congenital heart disease, as well as patients without structural heart disease (i.e., Brugada syndrome)[17].
The most significant predictors of ES are severe reduction of left ventricle (LV) function, advanced age and previous VT/VF episodes[8,10,11,13,18,19]. Monomorphic ventricular tachycardia is the most common arrhythmia documented in ES patients. VT episodes, hemodynamically unstable and interrupted with ATP or DC-shock, are the rule, with evidence of multiple VT morphologies[20]. Anyway, clinical presentation of ES is variable[9].
Less commonly, ES has been recorded in patients in whom premature ventricular complexes are the trigger of VT/VF both in acute myocardial infarction and in absence of structural heart disease[16,21]. The latter are often patients with “primitive ventricular fibrillation”, in whom the trigger of the arrhythmia has not been documented, presenting with multiple VF episodes after ICD implantation, mostly refractory to pharmacological therapy. Therefore, in this setting, the identification of ES triggers may be of interest in preventing VT/VF episodes, particularly in case of electrolyte disorder[9,14]. The role of adrenergic system in maintaining ES is of special interest as well; in terms of acute event treatment[7,16]. Adrenergic activation seems to play a key role in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia due to the well known arrhythmic sensitivity to adrenergic stimulation; although in the majority of these patients the trigger is unkown[22].
ACUTE PRESENTATION, PROGNOSTIC RELEVANCE
ES is an arrhythmic emergency related to recurrent consecutive episodes of ventricular arrhythmia, with low likelihood of spontaneous termination.
The clinical scenario of ES is the result of a combination of various factors: in patients with structural heart disease affected by chronic heart failure, ES causes worsening of heart failure with high risk of pulmonary edema/cardiogenic shock. These events are much more frequent and severe, less stable the arrhythmic condition and the functional status are. ICD therapies even if allow arrhythmic episodes termination and prevent sudden cardiac death, do not play any role in stabilizing the clinical scenario. Moreover, the continuous intervention of ICD, implies unfavorable hemodynamic effects[3], also resulting in psychological distress, adrenergic hyperactivity, and patient discomfort[22,23].
No reproducible data on acute mortality in ES are available. It should be reminded that acute mortality represents a common cause of death in patients with severe structural heart disease, in most of cases as cardiogenic shock or electromechanical dissociation in the setting of unmanageable arrhythmias[3]. Literature data show that patients with ES present an increased risk of sudden arrhythmic or cardiac death in the mid-term follow up[8-10,16,24]; furthermore, these findings were not confirmed just in few papers[7,13]. The MADIT II trial showed that patients with VT episodes interrupted by ICD have a significantly higher risk of sudden and non-sudden cardiac death. Moreover, patients who have survived ventricular arrhythmias have an increased risk of worsening heart failure and of mortality related to it[25]. Specifically, in the MADIT II trial, Sesselberg et al[11] have shown that ES is the most important independent predictor of mid-term cardiac death (increased risk of 7-fold), resulting particularly significant in the first 3 mo after ES (increasing risk of 18-fold). The results of SCD-HeFT trial are comparable, in addition, Poole et al[26] observed that not only appropriate shocks - directly related with arrhythmic events - but also inappropriate shocks impact on an increased mortality. More specifically the authors reported a significant increase of death in patients with appropriate (HR = 5.68; P < 0.001) and inappropriate shocks (HR = 1.98; P = 0.002). In particular, multiple shocks were associated with a 8-fold risk of death (HR = 8.23; P < 0.001).
These findings support the hypothesis that the recurrence of frequent arrhythmic events (and even more ES) strongly impacts on the evolution of patients’ clinical history, particularly by worsening the cardiac function. In this setting multiple shocks could have their own etiopathogenetic role related to repeated myocardial injury.
DIAGNOSIS AND CLINICAL MANAGEMENT
Patients with ES require a diagnostic evaluation of their structural heart disease, the type of arrhythmia and the presence of clinical triggers. The most common triggering factors are acute myocardial ischemia, electrolytic disorders and adverse drug effects. Identification of triggers is a key point: sometimes it allows the suppression of arrhythmias through simple therapeutic interventions, such as in case of hypokalaemia. Acute myocardial ischemia must be accurately identified and excluded through clinical and non-invasive diagnostic parameters. However, in most patients with coronary artery disease and previous history of myocardial infarction presenting with ES, myocardial ischemia is just a secondary effect of the arrhythmias. Myocardial ischemia should therefore be interpreted and consequently treated with the aid of pharmacological and/or interventional therapies in the presence of acute coronary syndrome. In the majority of cases, however, ES represents the evolution of an arrhythmogenic substrate in patients with previous VT/VF episodes.
Therapeutic interventions first depend on the arrhythmic pattern and on the hemodynamic stability of patients. ICD interrogation is the preliminary diagnostic step to evaluate the appropriateness of shock delivery and arrhythmic parameters (heart rate, electrogram analysis, trigger). ICD reprogramming is mandatory in order to both limit ICD shock delivery and attempt VT/VF interruption with antitachycardia pacing[27,28]. The accuracy of the diagnosis of ventricular arrhythmia may only occasionally show interpretative troubles in single-chamber ICD recipients in whom the comparison between basal and arrhythmic electrograms should be carried out carefully privileging reading from multiple recording channels.
ES patients require hospitalization. A continuous ECG and vital signs monitoring must be performed in the Coronary Intensive Care Unit or in a dedicated Emergency Arrhythmia Unit. During the evaluation phase, the possibility to document and characterize different morphologies of VT responsible for the clinical scenario is relevant, also with regard to a possible ablative treatment[20].
Hemodynamic and metabolic evaluations are needed in order to perform urgent interventions through intravenous therapies, such as inotropic agents or hydro-electrolytic infusion.
In the acute setting, prevention of arrhythmic recurrence should be as efficient as possible, by means of: (1) amiodarone is the first choice drug, unless contraindicated (presence of hyperthyroidism, long QT interval)[29]; (2) beta-blocker administration plays an important role because of its antiarrhythmic and antiadrenergic effect. Beta-blockers administration should be limited in patients with labile hemodynamic compensation or severe reduction of LV function; (3) lidocaina and azimilide are second choice drugs, useful in case of contraindications to previous medications[14,30]; (4) verapamile should be used as drug of choice in case of premature ventricular beats originating from His-Purkinje system; and (5) finally, atrial or sequential atrio-(bi)ventricular pacing are useful to avoid bradycardia[31,32].
Sedation is pivotal to stabilize patients with ES, but hemodynamic and/or respiratory instability can limit the use of sedation drugs, such as benzodiazepine. In these cases mechanical ventilation with oro-tracheal intubation are absolutely required in refractory forms of ES. In some cases mechanical ventilation allows safer drugs administration otherwise not tolerated.
ROLE OF CATHETER ABLATION
The role of catheter ablation (CA) in patients with VT is becoming more and more relevant, as a definite treatment of multiple forms of arrhythmias and a complementary intervention in cases of high electrical instability, thus improving prognosis and quality of life in patients with advanced forms of heart disease. This observation creates the rationale to investigate the possibility to apply CA in patients with frequently recurring ventricular arrhythmias and ES[33].
Preliminary reports regarding the role of CA in the treatment of ES are limited to patients with specific clinical characteristics and/or small case series. Silva et al[34] reported a success rate of 80% in an ES population with recurrent hemodynamic stable VT; Schreieck et al[35] reported acute success in most of cases of a selected population undergoing CA of hemodynamic unstable arrhythmias guided by substrate mapping. Also Bänsch et al[16] described CA in patients with acute myocardial infarction and ES in whom VF was triggered by premature ventricular contractions, targeted by CA.
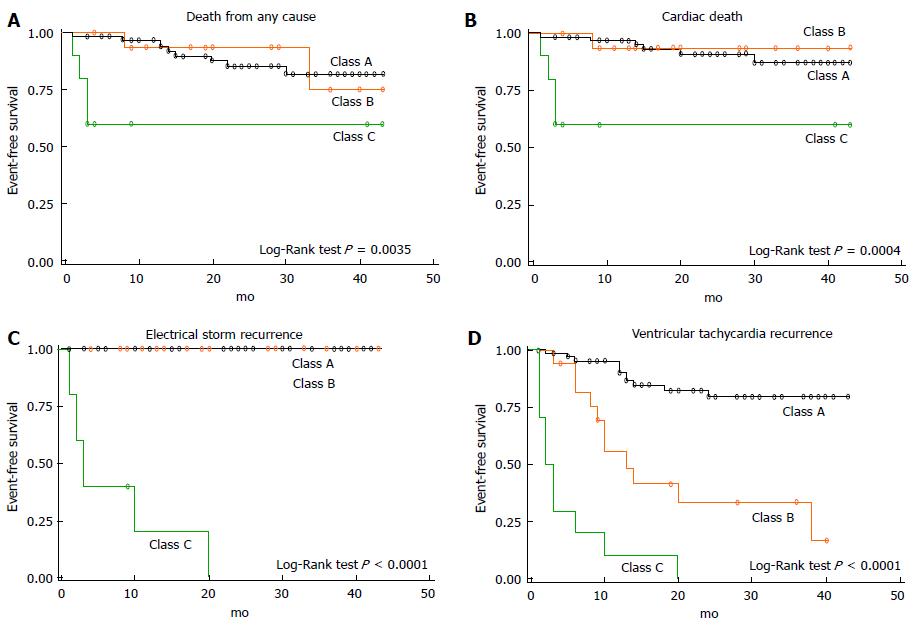
The ability to assess the feasibility and effectiveness of CA in a wider ES population arises from more recent experiences, which better represent the profile of patients with complex, hemodynamically non-tolerated, drug-refractory ventricular arrhythmias, mostly in the setting of structural heart disease with severe impairment of left ventricular function. In this population, Carbucicchio et al[20] have described for the first time VT suppression in 90% of patient undergoing one or more CA procedures with or without the use of haemodynamic mechanical support. Moreover, the authors have shown that non-inducibility of VT at the end of the procedure was predictive of no recurrence of ES or VT at 2 years follow-up; accordingly, CA survival was improved in arrhythmia-free patients (Figure 1). This experience once again shows that ES represents a turning point in the natural history of patients with dilated cardiomyopathy and ventricular arrhythmias and that the treatment of arrhythmic burden plays a favourable effect on the clinical history of these patients both in terms of arrhythmic death and acute heart failure. More recent studies have confirmed that CA of ES is effective in reducing mortality in the middle-term follow up[18,36].
Figure 1 Kaplan-Meier survival analysis after catheter ablation of electrical storm.
Class A indicates catheter ablation success, defined as suppression of each ventricular tachycardia (VT) morphology; Class B indicates partial success, defined as suppression of each clinical VT; Class C indicates failure, defined as persistence of one or more clinical VT (reprinted from Carbucicchio et al[20]).
Regarding management of patients with ES or with recurrent VT following points must be taken in account: (1) clinical management in this setting is highly demanding. It requires an experienced Intensive Care Unit staff and a multidisciplinary approach that includes anesthesiological and psychological support; (2) advanced CA strategies in these patients are particularly complex (Figure 2). Obviously, the use of electroanatomical mapping (EAM) to guide CA is mandatory, and a substrate-guided approach is commonly more efficient, limiting activation mapping manoeuvres[37]. An epicardial approach should be preferred in all patients with non-ischemic cardiomyopathy to minimize recurrences. In patients with unstable VT or very depressed cardiac function, or in those presenting with cardiogenic shock, hemodynamic mechanical support allows patients stabilization and enhances efficacy and safety of CA, and can be used both during intraprocedurally as in the post-procedural period[38]; and (3) in selected patients, requiring concomitant surgical indications or in whom a percutaneous approach is not feasible, surgical ablation guided by EAM (endo- and/or epicardial) may be taken into account, in an experienced and multidisciplinary setting.
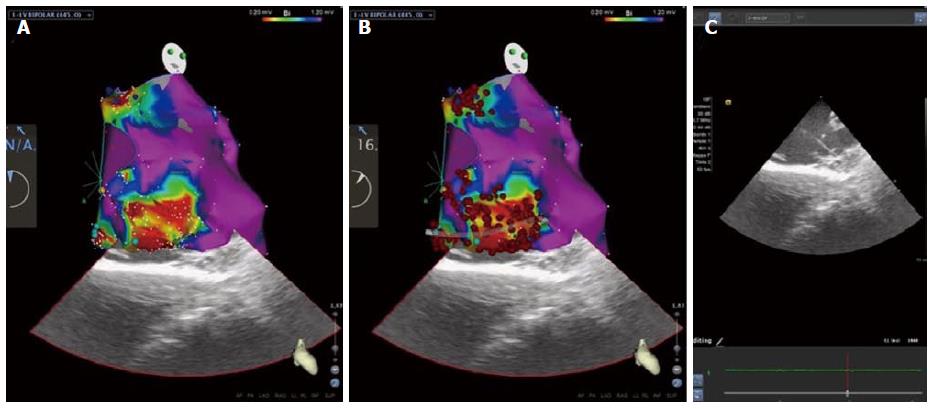
Figure 2 Modern approach of mapping and ablation for the treatment of electrical storm: High-density electroanatomical mapping of the left ventricle using CARTOSOUND contact-force technology (Biosense Webster, Diamond Bar, CA, United States).
A: Two distinct peri-valvular scars, in the mitro-aortic continuity and below the mitral annulus, in the setting of idiopathic cardiomyopathy are visualized. Scars are characterized by dense scar (abnormal bipolar electrograms < 0.20 mV), surrounded by a border zone (0.20-1.20 mV) in which late potentials, are tagged (dark and light blue dots); B: Radiofrequency ablation lines are dragged for dechanneling and isolation of all proarhythmic sites; C: Intracardiac echocardiography imaging is aiming at substrate characterization and correct positioning of the catheter during mapping and ablation.
CONCLUSION
ES is an “extreme” ventricular arrhythmia affecting ICD patients with structural heart disease and is a major predictor of cardiac death in the short-term follow-up. Problems related to the treatment of ES patients are complex, depending on the type of patient as well as on the treatment of cardiac emergency, and require high standard facilities and specialized skills.
CA for the treatment of ES is particularly promising and should be considered the elective form of treatment to achieve long-term rhythm stabilization and to prevent heart failure. The possibility to modify the arrhythmic substrate by CA in an early phase, thus preventing critical situations deriving from repetitive ICD interventions, looks promising, but necessitates further corroborations.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Falconi M, Said SAM, Sakabe K S- Editor: Ji FF L- Editor: A E- Editor: Wu HL