Published online Apr 26, 2015. doi: 10.4330/wjc.v7.i4.178
Peer-review started: October 24, 2014
First decision: December 12, 2014
Revised: January 15, 2015
Accepted: January 30, 2015
Article in press: February 2, 2015
Published online: April 26, 2015
Processing time: 178 Days and 19.5 Hours
After very rapid advances in the development of the technique and devices, transcatheter aortic valve implantation (named TAVI or TAVR), is today a reality that is here to stay. It has become the minimally-invasive treatment option for high-risk and non-surgical patients with severe symptomatic aortic stenosis. Requiring the participation of a multidisciplinary team for its implementation, cardiac imaging plays an important role. From pre-assessment to determine the suitability of the patient, the access site, the type of device, to the guidance during the procedure, and ultimately the long term monitoring of the patient. Correct selection of the patient and device, correct placement of the stent-valve and early detection of complications are of paramount importance for procedural success and for patient outcome. Each technique has advantages and disadvantages, being the cardiologist who will determine the best approach according to the type of patient and the expertise of the center in each one of them. This article summarizes the last contributions of the most common used imaging techniques, in each step of the procedure.
Core tip: Cardiac imaging is of crucial importance in the whole process of transcatheter aortic valve implantation, from initial evaluation, intraprocedural guidance and post implantation evaluation and early detection of complications. Multiple techniques are available for this, and as the rapid development of new devices and equipments, the greater the necessity of being aware of these advances. We provide current data and tips for this purpose. This is the reason of this work.
- Citation: Feltes G, Núñez-Gil IJ. Practical update on imaging and transcatheter aortic valve implantation. World J Cardiol 2015; 7(4): 178-186
- URL: https://www.wjgnet.com/1949-8462/full/v7/i4/178.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i4.178
Since its beginnings 20 years ago, with first implantations in animals, transcatheter aortic valve implantation (TAVI or TAVR) has evolved substantially[1]. With the advancement of cardiovascular imaging, the application of the most innovative techniques acts jointly to obtain the best clinical results. At present, TAVI is a serious alternative treatment for inoperable or high risk patients with aortic stenosis (AS). In addition, is expected to expand quickly to other subgroups (intermediate risk and aortic regurgitation), since trial results are encouraging compared with medical treatment and cardiac surgery.
Several bioprosthesis types are available, being by far the most commonly used the self-expandable porcine Medtronic CoreValve (Medtronic Inc, Minneapolis, MN, United States), available in the sizes 23 mm, 26 mm, 29 mm, and 31 mm, and the balloon-expandable Edwards Sapien XT bovine valve (Edwards Lifesciences Inc, Irvine, CA, United States), available in multiple sizes: 20 mm, 23 mm, 26 mm, and 29 mm. Both models recently introduced their latest valve generations: Corevalve Evolut R and the Sapien 3, with several advantages and thinner sheaths-introducers (up to 14F). There are now being marketed other valves with different delivery systems, like for instance the Direct Flow valve, the Jena Valve or the Lotus valve, between others.
The preferred implantation route is usually transfemoral. If this is not possible because of patient characteristics, both valve types can be implanted via the subclavian artery, and the Edwards Sapien valve can be implanted via a transapical access.
Here we review the contribution of imaging techniques to the whole process of selection of patients and prosthesis, intraprocedural guidance and evaluation of deployment and complications.
Invasive cardiac catheterization used to be the standard for quantification of AS, but nowadays echocardiography is used for diagnostic purposes and in its replacement. Transthoracic echocardiography (TTE) allows evaluation of a calcified valve with restricted leaflet opening and quantification of peak and mean aortic valve (AV) gradient by applying the simplified Bernoulli equation (Δp = 4v2) to the maximal velocity recorded through the AV by continuous wave Doppler. Severe aortic stenosis is defined as a peak velocity > 4.0 m/s (peak gradient of 64 mmHg), a mean gradient > 40 mmHg, or valve area (AVA) < 1.0 cm2 (0.6 cm2/m2) whith normal left ventricular (LV) systolic function[2]. In cases of low gradient with small area, a dobutamine stress study (maximum dose 20 mcg/kg per minute), may be helpful to determine if the valve is truly severely stenotic, when the maximum jet velocity rises over 4 m/s with the dobutamine-induced increase in stroke volume, whereas the AVA remains less than 1.0 cm[2]. The AS is only mild to moderate in severity if stroke volume increases but there is a small rise in gradient (and therefore the valve area increases greatly), and thus other causes are the origin of LV dysfunction[3].
Particularly in the candidates selected for TAVI, other parameters than the severity of the stenosis and the ejection fraction must be evaluated previously to the intervention.
The most important aspect of anatomical screening includes assessment of the arterial vasculature and aortic valvar complex [left ventricular outflow tract (LVOT), aortic annulus, sinus of Valsalva, sinutubular junction and ascending aorta]. All this data will guide physicians to choose the most appropriate access route (subclavian, transfemoral, transaortic or apical) and transcatheter valve size, and it will help to be alert in the detection of potential complications during the procedure[4].
An annular size accurate evaluation is of utmost importance. Underestimation of its dimension could lead to selection and deployment of a smaller valve, with possible complications like paravalvular regurgitation, poor hemodynamics, valve migration and embolism. Overestimation of annular size and deployment of a larger valve can lead to incomplete unfolding (with the consequence of valvular and paravalvular regurgitation) or annular rupture. TAVIs are designed to be utilized in slightly smaller annuli than the prosthesis size[5]. The annular size and the correspondent prosthesis are listed in Table 1.
| Prosthesis type | Prosthesis size (mm) | Annular size (mm) | Introducer profile (F) | Minimun vessel diameter (mm) |
| Edwards Sapien | 23 | 18-22 | 22 | ≥ 7 |
| 26 | 21-25 | 24 | ≥ 8 | |
| Edwards Sapien XT | 20 | 16-19 | ||
| 23 | 18-22 | 16 | ≥ 6 | |
| 26 | 21-25 | 18 | ≥ 6.5 | |
| 29 | 24-27 | 20 | ≥ 7 | |
| Edwards Sapien 3 | 23 | 18-22 | 14 | ≥ 5.5 |
| 26 | 21-25 | 14 | ≥ 5.5 | |
| 29 | 24-28 | 16 | ≥ 6 | |
| CoreValve | 23 | 18-20 | 18 | ≥ 6 |
| 26 | 20-23 | 18 | ≥ 6 | |
| 29 | 23-26 | 18 | ≥ 6 | |
| 31 | 26-29 | 18 | ≥ 6 | |
| CoreValve evolut R | 23 | 18-20 | 12 | ≥ 6 |
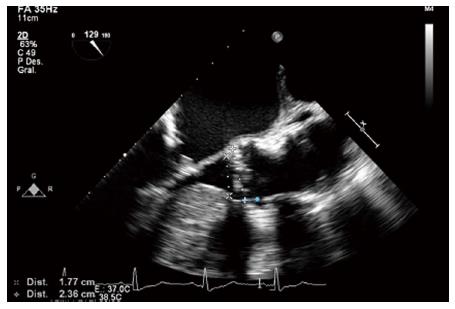
Aortic annulus can be evaluated using various techniques. Echocardiography is extensively available, repeatable, and easy to perform even taking into account that transesophageal echocardiography (TEE) is semi-invasive and usually requires sedation[6] (Figure 1). We use the sagital plane obtained from a 2-dimensional (2D) parasternal long axis image (TTE) or a mid-esophageal long axis (TEE) image among 120° and 140°, during early systole, measuring from the right coronary cusp to the left noncoronary commissure. To obtain measurements in the coronal and sagittal planes three-dimensional (3D) reconstructions and biplane imaging can be performed.
Multislice computed tomography (MSCT) can give appropriate measurements and may provide important additional information like the anatomy of the coronary arteries, the aortic valve anatomy and area, the plane of the valve and the amount and distribution of calcifications, but iodine injection and radiation are relative limitations. It is important to remember that the aortic annulus is not only a complex 3-dimensional structure, but also that its shape is oval and not circular in the vast majority of patients, as it was demonstrated in previous MSCT studies[7]. The aortic annulus plane is acquired by a reconstruction using two orthogonal planes, the short and long axis of the virtual basal ring, and measurements are taken from systolic phase reconstructions from 20% to 45% of the R-R interval. MSCT multiplanar reconstructions (MPRs) can provide coronal, sagittal and axial images of the aortic root, with accurate measuraments, almost always underestimated by 2D echocardiography.
On the other hand, cardiac magnetic resonance (CMR) permits an anatomic and functional evaluation of the aortic valve and aortic root, with most sequences in 2D and the selection of the imaging plane during the examination. However, whole heart, echo-gated 3D CMR with contrast allows obtaining images for multiplanar reconstruction and demonstrates the oval shape of the annulus with minimal and maximal diameters.
Every technique has its advantages and disadvantages. The annulus size is in general 1 mm smaller by TTE than by TEE, and the TEE measurement is 1 to 1.5 mm smaller than MSCT measurements[8,9]. Echocardiography (TTE or TEE) still is the most used technique to assess the aortic annulus, nevertheless, with the acquisition of more data in the near future, MSCT will probably become the first imaging modality to do this.
The characteristics of the aortic valve, like the number of cusps, grade of calcification, thickness and mobility are important to predict the procedure success. Congenital or acquired bicuspid aortic valve stenosis were initially considered a contraindication for TAVI, nevertheless, several successful case reports have been documented and today is considered a relative issue[10-12]. It is frequently not easy to examine cusp anatomy in the severely calcified valves, but in these cases, MSCT or review of old echocardiograms may permit better evaluation of the underlying anatomy. With echocardiography important calcification might cause acoustic shadowing, so MSCT is nowadays the technique of choice in evaluating severity and showing the location of aortic cusp calcification. CMR is not a good choice because of the signal void caused by calcium. Large aortic valve calcifications raise the risk of gaps between the external face of the prosthesis and the patient’s native valve, allowing paravalvular regurgitation leaks. Also, the asymmetry, severity and the prosthesis “landing zone” calcification, may produce differences in the tension-force across the valve, with the consequent asymmetric deployment of the device and increased risk of obstruction of the coronary arteries ostium; and important sinotubular junction calcifications may cause limitation during balloon dilatation at the aortic end and may produce ventricular displacement of the prosthesis at the moment of unfolding[13].
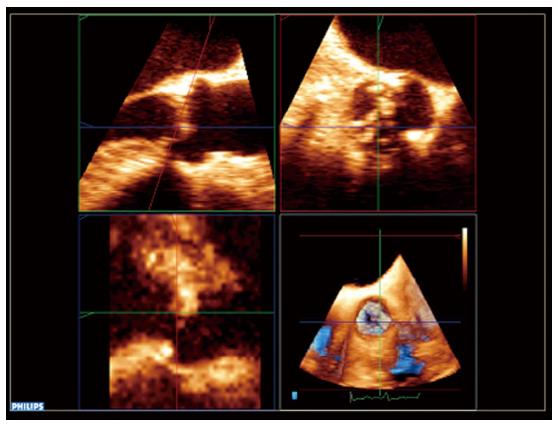
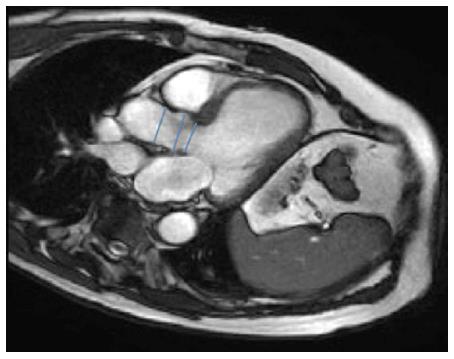
Another important issue to consider is the distance from the annulus to the coronary ostia, in order to avoid its compromise during the valve deployment. The distance to the right coronary ostia is easily determined with TEE (Figure 1), but not to the left coronary ostia, which requires 3D TEE (Figure 2). MSCT provides a more comprehensive assessment, showing an average annular-right coronary artery distance of 13.6 ± 2.8 mm and annular-left coronary artery distance of 13.4 ± 3.2 mm[5]. The distance between the aortic valve annular plane and the coronary ostia should be at least of ≥ 10-11 mm for both type of prosthesis.
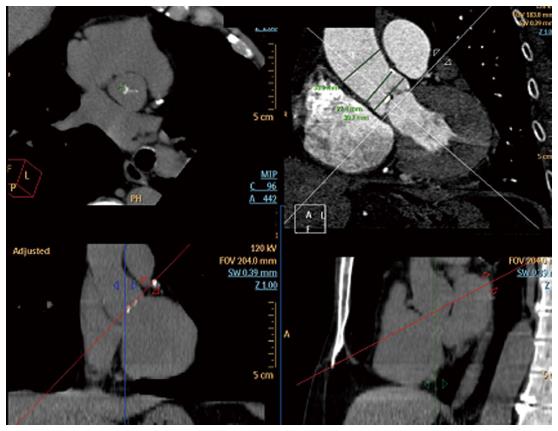
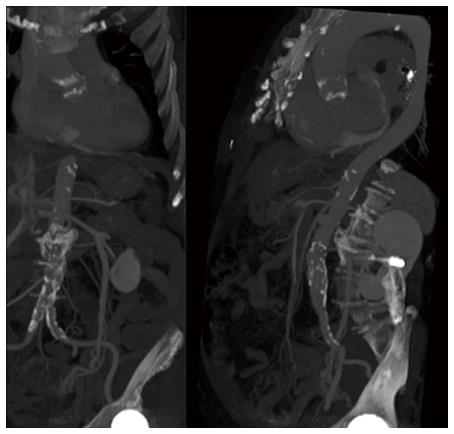
Evaluation of the aortic root and tubular portion of the aorta is as well essential, specially when using Core Valve, because its length is greater compared with regular valves, ranging from 52 mm (31-mm valve) to 55 mm (26-mm valve, i.e.,). It is advised that the dimensions of the tubular aorta measured at 45 mm above the annulus be 40 mm for the 26-mm valve and 43 mm for the 29-mm and 31-mm CoreValve prosthesis. MSCT can provide an excellent reconstruction and evaluation of the aortic sinus diameter, sinotubular junction, ascending and descending aorta and its iliofemoral branches (Figure 3). This is very important for the selection of the vascular access, because its complications rates range from 5% to 25%[14], and are associated with a striking increase in early mortality risk. Its evaluation often begins with conventional angiography, but it proportionates a very limited data. MSCT provides many reconstructions, as 3D volume rendered imaging, curved multiplanar reformats, and maximum intensity projection images allowing evaluation of vessel size, minimal luminal diameter, calcification severity, plaque burden, vessel tortuosity and identification of high risk characteristics like dissections and complex atheroma[15] (Figure 4). Calcification of less than 180º or eccentric calcification usually do not cause procedural trouble like would nearly circumferential and luminal calcification do. Vascular complications and 30 d mortality can be predicted with the use of a sheath/femoral artery ratio of 1.05 or higher[16]. It is remarkable that the existence of significant aneurysmal dilatation in ascending aorta is a contraindication for the use of CoreValve.
Like MSCT, CMR can give exhaustive evaluation of the aortic valve, annulus, aortic root, coronary ostia, course of the thoracoabdominal aorta and luminal caliber of the iliofemoral branches, and LV function, with the advantage of not using ionizing radiation (Figure 5). Also, free-breathing noncontrast navigator-gated 3D whole-heart acquisition can be acquired, similar to the volumetric acquisition of CT[17].
Calcification in other areas, as dense calcification in the intertrigonal area rises the risk of paravalvular aortic regurgitation (AR) due to asymmetric unfolding of the valve[18]. The angle between the aorta and the LV and proximal septal hypertrophy are essential issues to consider when planning the procedure. A very prominent proximal septum is an important consideration to have in mind during the placement of the valve because can cause valve repositioning when stopping the pacing run[15]. LV function must be evaluated in order to minimize the number of pacing runs to prevent hemodynamic compromise in those with severe dysfunction. The degree of baseline aortic regurgitation is also very important, since balloon inflation might aggravate regurgitation and cause hemodynamic deterioration.
In conclusion, echocardiography (in all modalities, TTE, TEE, 2D and 3D), MSCT and CMR can be used to make a pre-implantation evaluation, but it is very important to remind that the imaging technique used might influence TAVI size selection and strategy.
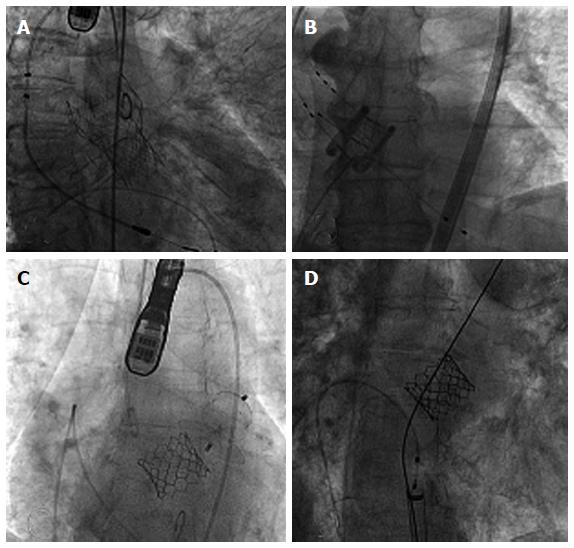
During the procedure, fluoroscopy and angiography are the principal techniques used to guide device placement (Figure 6). However, they involve several shortcomings, for example: (1) radiation use; (2) restricted 2D visualizations with scarce soft-tissue contrast, sometimes preventing early identification of complications (cardiac tamponade, etc.); and (3) the reiterated use of nephrotoxic contrast media to observe the aortic annulus and coronary ostia during the procedure.
Nevertheless, other imaging modalities, like TEE, may overcome the lower soft tissue contrast resolution of fluoroscopy and do not use radiation or contrast. Specially 3D-TEE permits a good visualization of the guide wire path and allows a good assessment of the prosthesis position on the balloon, with respect to the native valve annulus and other structures. Using mid-esophagus long axis view is possible to observe the guide wire across the aortic valve in its delivery, retrograde (transaortic, transubclavian, transfemoral) or anterograde (transapical)[13]. For the adequate position of the prosthesis, TEE can be very useful. In the case of Sapien valve, about half of the prosthesis should be below and above the aortic annulus, and when using CoreValve, the nitinol stent must be well within the borders of the calcified native annulus. With 3D TEE it is feasible to observe ortogonal planes simultaneously, aortic valve’s views in long and short-axis in realtime, very helpfull for every step of the procedure (aortic valve pass, balloon inflation and prosthesis unfolding).
Intracardiac echocardiography has also been used for TAVI guidance, but imaging abilities are worse than with TEE[19].
In patients with preserved LV systolic function, it was described an improvement in diastolic function, evaluated minutes after deployment with TEE, evaluated through E wave deceleration time, E wave velocity and isovolumetric relaxation time[20].
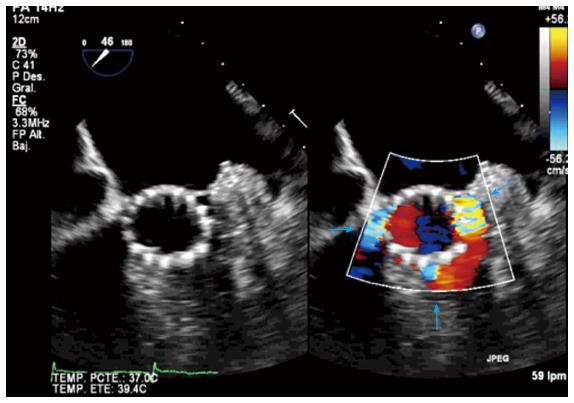
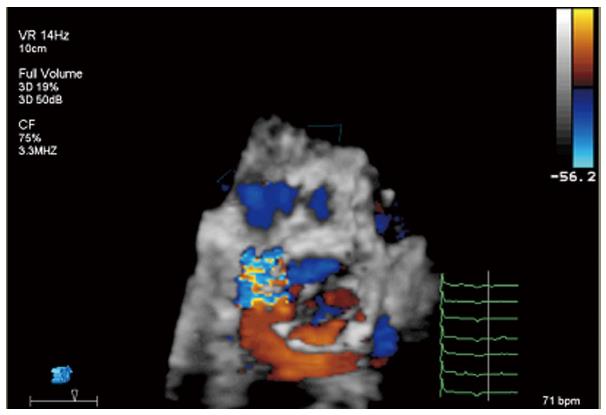
Immediately after deployment, it is very important to discard complications, for example AR, the most common one. This must be performed rapidly and in multiple echocardiographic views to permit possible reballooning or up delivery of a second prosthesis if the AR is severe and cannot be controlled in another way. It is of utmost importance to differentiate between paravalvular and valvular regurgitation (Figures 7 and 8). Because of irregular calcification in the native valve, small paravalvular leaks are usually observed due to gaps between the annulus and the device, particularly at the commissural areas.
In the case of paravalvular jets, the recommendations indicate that the rate of the circumference of the annulus occupied by the jet offers a guide to severity: < 10% mild, 10%-29% moderate, and ≥ 30% severe[21]. In a work of our group, we found that the vena contracta planimetry on 3D TTE was better correlated with AR volume than vena contracta width on 2D TTE [Kendall’s τ = 0.82 (P < 0.001) vs 0.66 (P < 0.001)]. The areas under the receiver operating characteristic curves were 0.96 for vena contracta planimetry and 0.35 for vena contracta width[22]. To avoid paravalvular leak, is required that the covered portion of the prosthesis must be well-apposed to the host valve and interleaflet triangles and the ventricular border of the device just under the hinge points of the AV. It is also common the presence of mild central valvular regurgitation that frequently resolves with removal of the guidewire; otherwise, it must be assessed looking for underexpansion of the prosthesis (Figure 9). Significant valvular AR is generally due to AV harm in the course of the procedure, too large a device for a little annulus with consequent valve distortion, or severe calcification of the patient’s AV producing deformation of the frame of the valve[23].
Obstruction of a coronary artery by the prosthesis or displaced calcium is another possible complication. This situation can be seen as regional hypokinesis, best evaluated from the transgastric view and, if it is possible, assessing the coronary arteries flow. If there is LVOT obstruction, this may cause hypotension because of rapid drop in afterload. Other causes must also be considered, like severe mitral regurgitation, pericardial tamponade, displacement of the device, air embolism, right ventricule perforation by a pacemaker lead, aortic dissection and vascular access bleeding.
Other imaging techniques in vogue at the moment are fusion imaging modalities, like C-arm computed tomography with valve landmark detection and automatic aorta segmentation, that tries to make simpler the procedure using 3D over fluoroscopic imaging[24]. CMR capacities are real-time image without restricted scan plane orientation and incomparable soft-tissue contrast, with concomitant display of the prosthesis; and it seems convenient over X-ray angiography and fluoroscopy during all of complete procedure. It allows on-line monitoring cardiac performance, instant recognition of vascular and cardiac harm, and real-time orientation for axial placement and unfolding of the device[25].
After TAVI implantation, the imaging evaluation will contribute to determine the valve hemodynamic condition, like effective valve area and gradients; the presence and quantity of valvular and paravalvular regurgitation; the effect of the procedure in ventricular function, hypertrophy, etc. and the recognition of long term problems like device migration, endocarditis, ventricular perforation, mitral valve impingement and thrombus formation.
Echocardiography remains the technique of choice for this, because of its wide availability, avoidance of ionizing radiation, together with real time hemodynamic and structural assessment. MSCT and CMR can also provide and excellent anatomic detail and detection of complications like pseudoaneurysm of the root or the apex. Postprocedural evaluation of remaining AR by CMR might have a possible part in TAVI patients[26]. Nevertheless, CMR is a time-intensive method, and this could be an important factor, especially in older patients. In cases of renal disfunction the advantages for the use of gadolinium must be greater than the risks of nephrogenic systemic fibrosis[27]. Also CMR is not warranted in patients with defibrillators, pacemakers or intracranial aneurysm clips, even though the prosthesis used now are CMR compatible.
Echocardiography is most common used tool to evaluate the changes described after TAVI, like reductions in LV mass[28], recovery in EF[29], amelioration in diastolic function, and reduction of mitral regurgitation[30]. Although, CMR evaluation of LV mass provides a greatly precise calculation[31].
Determination of mean and peak transvalvular pressure gradients and the calculation of effective orifice area are easily obtained with continuous wave Doppler with TTE, not forgetting suprasternal notch and right parasternal windows to confirm that maximum gradients are catched. CoreValve and Edward Sapien valves have very good flow features with mean gradients of 10-15 mmHg, with relatively stable gradients[32] or little rise in mean transvalvular gradient (3.8%/year) and a low decrease in valve area (0.06 cm2/year)[33].
Follow-up echocardiograms should recognize the existence, position, and severity of valvular and paravalvular AR, using all the possible views in case of eccentrical jets. Paravalvular regurgitation is generally produced by imperfect device apposition to the host annulus because of to remaining calcium, undersized prosthesis, or too low position of the valve[34], so imaging in multiple planes is necessary. Valvular and paravalvular AR affect LV hemodynamic condition, determined by raised volume burden, consequently affecting chamber dilatation, LV performance, and progress to pulmonary hypertension, so total AR should routinely be calculated combining information from color and spectral Doppler. Three-dimensional TTE allows quantification of AR with greater accuracy than 2D TTE. CMR might be the technique of election in cases of severe AR, or disagreement in gradients with echocardiography[35].
Although it is always difficult to predict the future, TAVI seems a truly promising therapeutic alternative with settled indications nowadays and expanding indications (intermediate risk, aortic regurgitation, valve in valve[36], etc.). What seems clear is that cardiovascular imaging will be needed in this field in order to achieve all its potential objetives.
To sum up, although at the begining multiple test and measurements were required, as experience grows, patients and devices selection are improving, with a more rational imaging algorithm, based in local expertise and availability.
| 1. | Cribier A. Development of transcatheter aortic valve implantation (TAVI): a 20-year odyssey. Arch Cardiovasc Dis. 2012;105:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2682] [Cited by in RCA: 2659] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 3. | Blais C, Burwash IG, Mundigler G, Dumesnil JG, Loho N, Rader F, Baumgartner H, Beanlands RS, Chayer B, Kadem L. Projected valve area at normal flow rate improves the assessment of stenosis severity in patients with low-flow, low-gradient aortic stenosis: the multicenter TOPAS (Truly or Pseudo-Severe Aortic Stenosis) study. Circulation. 2006;113:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 197] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 4. | Piazza N, Lange R, Martucci G, Serruys PW. Patient selection for transcatheter aortic valve implantation: patient risk profile and anatomical selection criteria. Arch Cardiovasc Dis. 2012;105:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Holmes DR, Mack MJ, Kaul S, Agnihotri A, Alexander KP, Bailey SR, Calhoon JH, Carabello BA, Desai MY, Edwards FH. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59:1200-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 567] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 6. | Moss RR, Ivens E, Pasupati S, Humphries K, Thompson CR, Munt B, Sinhal A, Webb JG. Role of echocardiography in percutaneous aortic valve implantation. JACC Cardiovasc Imaging. 2008;1:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Hamdan A, Guetta V, Konen E, Goitein O, Segev A, Raanani E, Spiegelstein D, Hay I, Di Segni E, Eldar M. Deformation dynamics and mechanical properties of the aortic annulus by 4-dimensional computed tomography: insights into the functional anatomy of the aortic valve complex and implications for transcatheter aortic valve therapy. J Am Coll Cardiol. 2012;59:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Leipsic J, Gurvitch R, Labounty TM, Min JK, Wood D, Johnson M, Ajlan AM, Wijesinghe N, Webb JG. Multidetector computed tomography in transcatheter aortic valve implantation. JACC Cardiovasc Imaging. 2011;4:416-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 9. | Messika-Zeitoun D, Serfaty JM, Brochet E, Ducrocq G, Lepage L, Detaint D, Hyafil F, Himbert D, Pasi N, Laissy JP. Multimodal assessment of the aortic annulus diameter: implications for transcatheter aortic valve implantation. J Am Coll Cardiol. 2010;55:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 330] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 10. | Chiam PT, Chao VT, Tan SY, Koh TH, Lee CY, Tho VY, Sin YK, Chua YL. Percutaneous transcatheter heart valve implantation in a bicuspid aortic valve. JACC Cardiovasc Interv. 2010;3:559-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Delgado V, Tops LF, Schuijf JD, van der Kley F, van de Veire NR, Schalij MJ, Bax JJ. Successful deployment of a transcatheter aortic valve in bicuspid aortic stenosis: role of imaging with multislice computed tomography. Circ Cardiovasc Imaging. 2009;2:e12-e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Raja Y, Holloway B, Doshi SN. Symmetrical expansion of an Edwards Sapien valve in a congenitally bicuspid aortic valve. Heart. 2011;97:1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Zamorano JL, Gonçalves A, Lang R. Imaging to select and guide transcatheter aortic valve implantation. Eur Heart J. 2014;35:1578-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Généreux P, Head SJ, Van Mieghem NM, Kodali S, Kirtane AJ, Xu K, Smith C, Serruys PW, Kappetein AP, Leon MB. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. J Am Coll Cardiol. 2012;59:2317-2326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 471] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 15. | Bloomfield GS, Gillam LD, Hahn RT, Kapadia S, Leipsic J, Lerakis S, Tuzcu M, Douglas PS. A practical guide to multimodality imaging of transcatheter aortic valve replacement. JACC Cardiovasc Imaging. 2012;5:441-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 16. | Hayashida K, Lefèvre T, Chevalier B, Hovasse T, Romano M, Garot P, Mylotte D, Uribe J, Farge A, Donzeau-Gouge P. Transfemoral aortic valve implantation new criteria to predict vascular complications. JACC Cardiovasc Interv. 2011;4:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 443] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 17. | Koos R, Altiok E, Mahnken AH, Neizel M, Dohmen G, Marx N, Kühl H, Hoffmann R. Evaluation of aortic root for definition of prosthesis size by magnetic resonance imaging and cardiac computed tomography: implications for transcatheter aortic valve implantation. Int J Cardiol. 2012;158:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Delgado V, Ng AC, Shanks M, van der Kley F, Schuijf JD, van de Veire NR, Kroft L, de Roos A, Schalij MJ, Bax JJ. Transcatheter aortic valve implantation: role of multimodality cardiac imaging. Expert Rev Cardiovasc Ther. 2010;8:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Bartel T, Bonaros N, Müller L, Friedrich G, Grimm M, Velik-Salchner C, Feuchtner G, Pedross F, Müller S. Intracardiac echocardiography: a new guiding tool for transcatheter aortic valve replacement. J Am Soc Echocardiogr. 2011;24:966-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Gonçalves A, Marcos-Alberca P, Almeria C, Feltes G, Rodríguez E, Hernández-Antolín RA, Garcia E, Maroto L, Fernandez Perez C, Silva Cardoso JC. Acute left ventricle diastolic function improvement after transcatheter aortic valve implantation. Eur J Echocardiogr. 2011;12:790-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Zoghbi WA, Chambers JB, Dumesnil JG, Foster E, Gottdiener JS, Grayburn PA, Khandheria BK, Levine RA, Marx GR, Miller FA. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2009;22:975-1014; quiz 1082-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 909] [Cited by in RCA: 977] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 22. | Gonçalves A, Almeria C, Marcos-Alberca P, Feltes G, Hernández-Antolín R, Rodríguez E, Cardoso JC, Macaya C, Zamorano JL. Three-dimensional echocardiography in paravalvular aortic regurgitation assessment after transcatheter aortic valve implantation. J Am Soc Echocardiogr. 2012;25:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Zahn R, Schiele R, Kilkowski C, Zeymer U. Severe aortic regurgitation after percutaneous transcatheter aortic valve implantation: on the importance to clarify the underlying pathophysiology. Clin Res Cardiol. 2010;99:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | John M, Liao R, Zheng Y, Nöttling A, Boese J, Kirschstein U, Kempfert J, Walther T. System to guide transcatheter aortic valve implantations based on interventional C-arm CT imaging. Med Image Comput Comput Assist Interv. 2010;13:375-382. [PubMed] |
| 25. | Kahlert P, Parohl N, Albert J, Schäfer L, Reinhardt R, Kaiser GM, McDougall I, Decker B, Plicht B, Erbel R. Towards real-time cardiovascular magnetic resonance guided transarterial CoreValve implantation: in vivo evaluation in swine. J Cardiovasc Magn Reson. 2012;14:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Sherif MA, Abdel-Wahab M, Beurich HW, Stöcker B, Zachow D, Geist V, Tölg R, Richardt G. Haemodynamic evaluation of aortic regurgitation after transcatheter aortic valve implantation using cardiovascular magnetic resonance. EuroIntervention. 2011;7:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Juluru K, Vogel-Claussen J, Macura KJ, Kamel IR, Steever A, Bluemke DA. MR imaging in patients at risk for developing nephrogenic systemic fibrosis: protocols, practices, and imaging techniques to maximize patient safety. Radiographics. 2009;29:9-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Tzikas A, Geleijnse ML, Van Mieghem NM, Schultz CJ, Nuis RJ, van Dalen BM, Sarno G, van Domburg RT, Serruys PW, de Jaegere PP. Left ventricular mass regression one year after transcatheter aortic valve implantation. Ann Thorac Surg. 2011;91:685-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Di Bello V, Giannini C, De Carlo M, Delle Donne MG, Nardi C, Palagi C, Cucco C, Dini FL, Guarracino F, Marzilli M. Acute improvement in arterial-ventricular coupling after transcatheter aortic valve implantation (CoreValve) in patients with symptomatic aortic stenosis. Int J Cardiovasc Imaging. 2012;28:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Durst R, Avelar E, McCarty D, Poh KK, Friera LF, Llano MF, Chu J, Anumandla AK, Rodriguez LL, Mack MJ. Outcome and improvement predictors of mitral regurgitation after transcatheter aortic valve implantation. J Heart Valve Dis. 2011;20:272-281. [PubMed] |
| 31. | Myerson SG, Montgomery HE, World MJ, Pennell DJ. Left ventricular mass: reliability of M-mode and 2-dimensional echocardiographic formulas. Hypertension. 2002;40:673-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 121] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Ye J, Cheung A, Lichtenstein SV, Nietlispach F, Albugami S, Masson JB, Thompson CR, Munt B, Moss R, Carere RG. Transapical transcatheter aortic valve implantation: follow-up to 3 years. J Thorac Cardiovasc Surg. 2010;139:1107-1113, 1113.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Buellesfeld L, Gerckens U, Schuler G, Bonan R, Kovac J, Serruys PW, Labinaz M, den Heijer P, Mullen M, Tymchak W. 2-year follow-up of patients undergoing transcatheter aortic valve implantation using a self-expanding valve prosthesis. J Am Coll Cardiol. 2011;57:1650-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 34. | Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, Krucoff MW, Mack M, Mehran R, Miller C. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. Eur Heart J. 2011;32:205-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 486] [Cited by in RCA: 531] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 35. | Altiok E, Frick M, Meyer CG, Al Ateah G, Napp A, Kirschfink A, Almalla M, Lotfi S, Becker M, Herich L. Comparison of two- and three-dimensional transthoracic echocardiography to cardiac magnetic resonance imaging for assessment of paravalvular regurgitation after transcatheter aortic valve implantation. Am J Cardiol. 2014;113:1859-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Núñez-Gil IJ, Gonçalves A, Rodríguez E, Cobiella J, Marcos-Alberca P, Maroto L, Fernandez-Golfin C, Carnero M, Macaya C, Zamorano JL. Transapical mitral valve-in-valve implantation: a novel approach guided by three-dimensional transoesophageal echocardiography. Eur J Echocardiogr. 2011;12:335-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Gotzmann M, Lin SL S- Editor: Ji FF L- Editor: A E- Editor: Wu HL