Peer-review started: September 10, 2014
First decision: October 14, 2014
Revised: October 26, 2014
Accepted: November 7, 2014
Article in press: November 10, 2014
Published online: February 26, 2015
Processing time: 155 Days and 5.3 Hours
Transcatheter closure of the left atrial appendage has been developed as an alternative to chronic oral anticoagulation for stroke prevention in patients with atrial fibrillation, and as a primary therapy for patients with contraindications to chronic oral anticoagulation. The promise of this new intervention compared with warfarin has been supported by several, small studies and two pivotal randomized trial with the Watchman Device. The results regarding risk reduction for stroke have been favourable although acute complications were not infrequent. Procedural complications, which are mainly related to transseptal puncture and device implantation, include air embolism, pericardial effusions/tamponade and device embolization. Knowledge of nature, management and prevention of complications should minimize the risk of complications and allow transcatheter left atrial appendage closure to emerge as a therapeutic option for patients with atrial fibrillation at risk for cardioembolic stroke.
Core tip: Left atrial appendage (LAA)-Occlusion was developed as an alternative to chronic anticoagulation therapy in patients with nonvalvular atrial fibrillation. In two large randomized trials the principal concept of LAA-occlusion has been demonstrated to be noninferior to coumadine therapy, in longterm follow up being even superior to oral anticoagulation in terms of efficacy and some safety issues like bleeding complications. However the procedure is complex and knowledge of nature, management and prevention of complications should minimize the risk of the procedure and allow transcatheter left atrial appendage closure to emerge as a therapeutic option for patients with atrial fibrillation at risk for cardioembolic stroke.
- Citation: Möbius-Winkler S, Majunke N, Sandri M, Mangner N, Linke A, Stone GW, Dähnert I, Schuler G, Sick PB. Percutaneous left atrial appendage closure: Technical aspects and prevention of periprocedural complications with the watchman device. World J Cardiol 2015; 7(2): 65-75
- URL: https://www.wjgnet.com/1949-8462/full/v7/i2/65.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i2.65
Stroke is one of the leading causes of death and disability worldwide. Approximately 25% of all strokes have a cardioembolic origin. More than 90% of all left atrial thrombi in patients with non-rheumatic atrial fibrillation (AF) originate in the left atrial appendage (LAA)[1,2]. Oral anticoagulation therapy with the vitamin K antagonist warfarin including all its well-known limitations is standard for the prevention[3]. Only about 55% of patients with AF, who are indicated for warfarin therapy, are really treated, however these patients are only about 67% of the time in the therapeutic range[4,5]. Several major recent trials have demonstrated the superiority of the new oral anticoagulants Dabigatran, Rivaroxaban and Apixaban as compared to standard therapy with warfarin[6-8]. Nevertheless, even with these new agents bleeding complications were substantial and in part comparable to that observed with warfarin except for intracranial bleedings being less. A certain amount of patients discontinued drug therapy prematurely for adverse events, mainly gastrointestinal reasons for example in dabigatran patients.
Several studies have been conducted focusing on the development of novel therapeutic tools to prevent AF-related strokes as an alternative to medical treatment. The technique which has arguably shown the most promise is the percutaneous (transcatheter) exclusion of the LAA from systemic circulation.
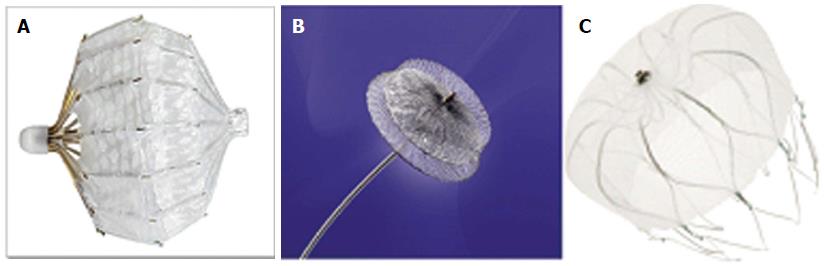
Three different devices specifically designed for occlusion of the LAA have been clinically evaluated: the Percutaneous LAA Transcatheter Occlusion system (PLAATO®), (ev3, Plymouth, MN), the WATCHMAN® system (Boston Scientific Corp., Natick, MA, United States), and the AMPLATZER® Cardiac Plug (St. Jude Medical, Inc., St. Paul, MN, United States) (Figure 1). Although safety and feasibility of the PLAATO device was demonstrated in several small non-randomized studies, the device was withdrawn from the market for commercial reasons. But even with the PLAATO device we have learned that there are specific complications associated with the implantation procedure such as device embolizations and cardiac tamponade[9-11]. Initial experience with the AMPLATZER® cardiac plug (ACP) has been published recently from a registry containing 132 successfully implanted devices[12]. To date, only the WATCHMAN® device has demonstrated superiority in long term follow up compared with chronic warfarin therapy in randomized, controlled trials[12-14]. In Europe, the latter two systems are approved for implantation, whereas in the United States both systems are under FDA investigation for potential approval. Despite being a lesser invasive procedure (than surgical ligation of the LAA), transcatheter LAA closure has been associated with potentially serious complications due to the necessity of transseptal puncture, manipulation of stiff wires and guide catheters in the left atrium and the release of the device in the LAA.
The following review highlights the potential procedural complications of transcatheter LAA closure with the Watchman-Device and discusses their nature, management and prevention.
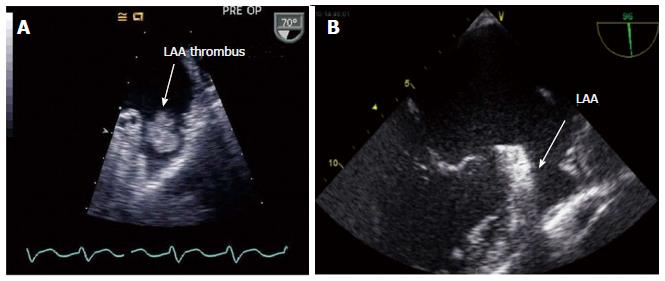
Before starting the procedure transesophageal echocardiography (TEE) has to be performed as the gold standard for thrombus detection within the LAA (Figure 2). Thrombi have been reported to be present in the LAA in 8%-15% of patients with AF lasting greater than 48 h[15]. Understanding the anatomy of the LAA is also critical for procedural safety. To facilitate successful implantation, width and depth of the LAA as well as number and position of different lobes are important to know. Studies have shown that the width of the LAA orifice can vary from 15-35 mm and length from 20-45 mm[16] with various anatomical configurations.
Implantation of the LAA closure devices should be usually performed under conscious sedation or even general anaesthesia. This is necessary to reduce unintended movement of the patient to avoid perforation of the LA/LAA and for tolerance of TEE, which is crucial for guidance of the procedure with the Watchman-Device. Usually a combination of midazolam and propofol is used, although alternative regimens may be possible. Implantation of the device is performed via the right femoral vein through a transseptal puncture with a small sheath in the femoral artery for pressure control or even management of air embolism as outlined below, which might not be mandatory.
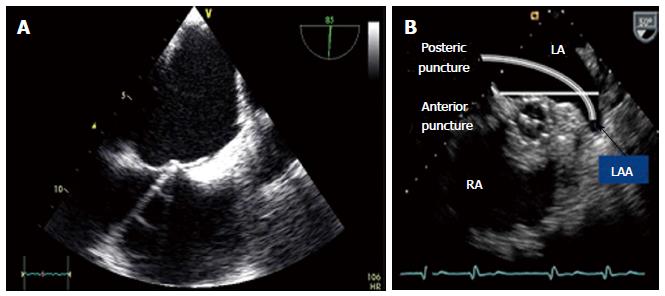
Transseptal puncture is described elsewhere[17]. Transseptal puncture should be ideally performed under pressure control and with TEE and fluoroscopic guidance. To enhance successful implantation of the LAA closure device the site of transseptal puncture should be at the posterior atrial septum. The more anterior or inferior the LAA is located, the higher transseptal puncture should be performed. The more cranial the axis of the LAA, the more inferior puncture site should be. This can be easily controlled by TEE in the bicaval view (90°) for cranio-caudal orientation and in the 45° view for anterior/posterior direction (Figure 3). Anterior puncture of the septum likewise going through an open PFO should be avoided due to the impossibility to turn the guiding catheter adequately to an anterior located axis of the LAA. An atrial septal defect may be used having enough space for turning the guide. After successful transseptal puncture heparin is given using a dosage of 100 U/kg body weight to achieve an ACT between 200 and 300 s. Heparin could be applied already before transseptal puncture, which, however might lead to an increased risk of bleeding during the transseptal procedure.
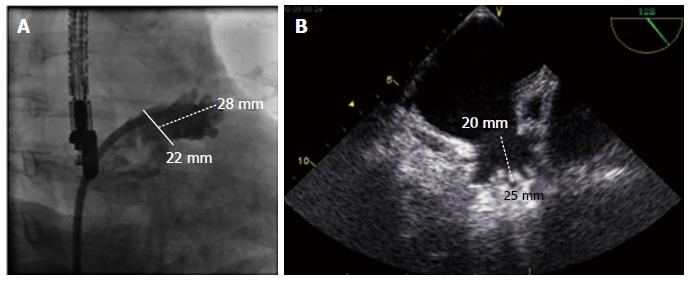
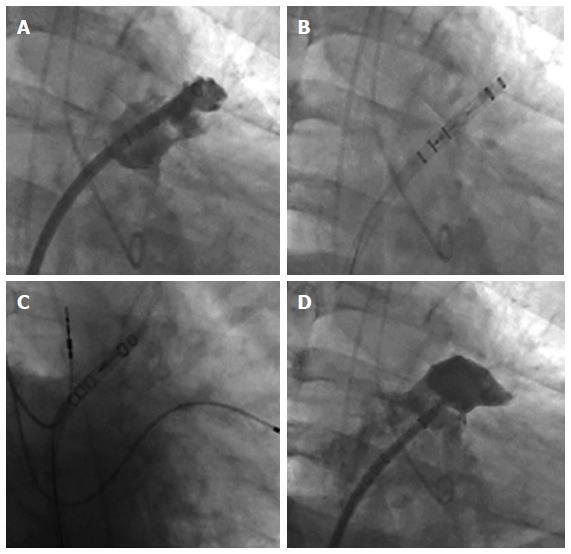
Device implantation has been described previously[18] After exchange of the transseptal sheath over a stiff wire located best in the left upper pulmonary vein, the guiding catheter can be introduced. Wire positioning in the LAA with a loop may be also possible, however there is some risk of perforation. Two different curved sheaths (single and double curve) are available for implantation of the Watchman® device. For the Watchman device in more than 90% of patients the double curved sheath is used. To avoid undersizing of the LAA, the mean filling pressure of the left atrium should be in the high normal range (> 10 mmHg). For Watchman implantation a 4 or 5 French standard Pigtail catheter should be placed through the sheath. By turning the guiding catheter counter clockwise the pigtail can be advanced into the LAA under TEE and fluoroscopic guidance. The access sheath is then advanced slowly and carefully over the pigtail catheter into the LAA, thus at least reducing the risk of perforation. The marker bands on the access sheath of the Watchman® Device will help to determine the landing zone of the cover of the device at the site of the left atrium. The most suitable projections for angiographic visualization of the LAA seem to be RAO 30° with caudal 25° and cranial 20° for measurement of the orifice width and length of the LAA (Figure 4). Ideally the angiographic measurements will match the echocardiographic measurements in different angles from 0° to 135°, although they may vary by several mm depending on calibration methods.
Devices should be prepared according to the instructions for use. Careful flushing of the device and retrograde bleeding out of the access sheath is essential to avoid air embolism. Slight pressure on the patient’s abdomen will increase venous pressure thus increasing backflow of blood through the sheath. A saline pressure infusion over the side branch of the guiding cath may be helpful.
The Watchman device should be advanced in the sheath until the marker of the device catheter matches the most distal marker on the access sheath. The next step is to pull back the access sheath over the device until device catheter and access sheath are connected. At this point the device should remain in position and forward pushing of the device must be strictly avoided in terms of the risk of LAA injury or perforation with subsequent cardiac tamponade. The device is deployed by retracting the sheath and device catheter simultaneously while the device is held in place. Small amounts of contrast injections may help to visualize the relation between the tip of the device and the LAA wall during deployment (Figure 5).
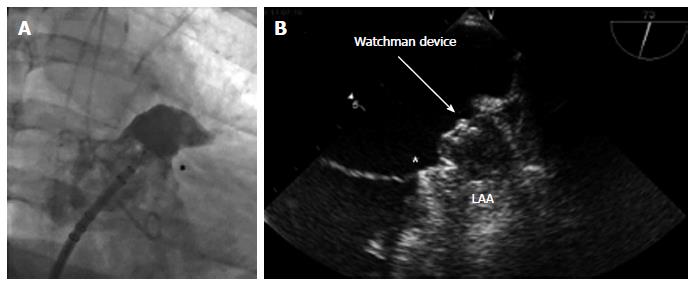
Once the device is deployed within the LAA, correct positioning of the Watchman device at the LAA ostium must be demonstrated by echocardiography and angiography (Figure 6). To avoid embolism of the Watchman Device, there are four release criteria that should be evaluated before release: Position, Anchor, Size and Seal (PASS).
Position: To confirm that the device is properly positioned, ensure that the plane of maximum diameter of the device is at or just distal to the orifice of the LAA.
Anchor: To confirm the device is anchored in place, withdraw the access sheath/delivery catheter assembly 1-2 cm from the face of the device. After injecting a small puff of contrast gently retract and push the deployment knob to see the combined movement of the device and the LAA tissue.
Size: To confirm the correct device size, measure the plane of the maximum diameter of the device using TEE in the 4 standard views 0, 45, 90, and 135 degrees, ensuring the threaded insert is visible. The device size should be 80%-92% of the nominal diameter.
Seal: Using colour Doppler, ensure that all of the lobes are distal of the device and are sealed. If there is a gap visible between the wall of LAA and the device with more than 3 mm, the device should be repositioned.
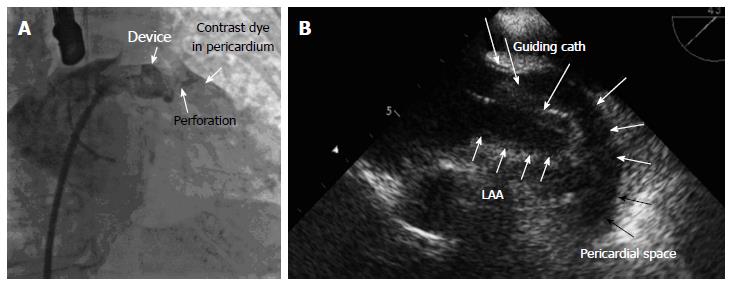
Pericardial effusion as acute/subacute cardiac tamponade or as an asymptomatic effusion is one of the most serious complications in LAA-occlusion procedures[12,19]. The transseptal puncture itself[19], manipulation of stiff wires, guiding catheters and the device itself within the left atrium and the thin-walled LAA, as well as too aggressive movement of the device during stability testing may result in LAA wall injury leading to pericardial effusions.
In the Protect AF trial[12], the rate of pericardial effusion occurring within 7 d of Watchman implantation was 4.5%, 3.3% of patients required pericardiocentesis. These complications are mainly observed at the beginning of the learning curve and became less frequent with more experience[12]. In the CAP registry, where experienced operators implanted Watchman® devices after the randomized trial was completed, the rate of pericardial effusions decreased to 2.2%[20] and could be held in the same range in the PREVAIL-trial with adequate training of new operators[21-23].
Several techniques to avoid pericardial effusions are: (1) TEE guidance and pressure monitoring to ensure a safe transseptal puncture at the correct site before advancing the sheath; (2) use of a 4 or 5 F. pigtail catheter inside the access sheath or a looped wire in the LAA to facilitate a safe movement of the guiding into the LAA; (3) slow and careful movements of the catheter and device manipulation within the left atrium; and (4) stability (tug-) testing after device implantation should be performed under TEE-control and fluoroscopy with injection of small amounts of contrast dye.
Cardiac tamponade with hypotension requires an aggressive approach with pericardiocentesis and reversal of anticoagulation (Figure 7). In case of recurrent tamponade, surgery is needed. Therefore early detection of an effusion by TEE is very important before hemodynamic deterioration arises from cardiac tamponade. Though most pericardial effusions occur early, subacute and late effusions are also possible. Therefore monitoring over a period of 48 h including 6 h close heart rate and pressure control and performance of trans-thoracic echocardiography at least before discharge to rule out pericardial effusion and to proof stable position of the device is recommended. Subacute effusions may arise from the anchors of the devices due to the thickness of only 0.5-0.8 mm of myocardial tissue of the LAA. If the effusion results in tamponade, pericardiocentesis and sometimes surgery is required. In case of late pericardial effusions also inflammatory processes may play a role, probably due to chronic injury of the hooks to the pericardium. In these cases anti-inflammatory therapy with non-steroidal antirheumatics like ASA, ibuprofen, diclofenac or even steroid therapy may be required at least for a certain period of time.
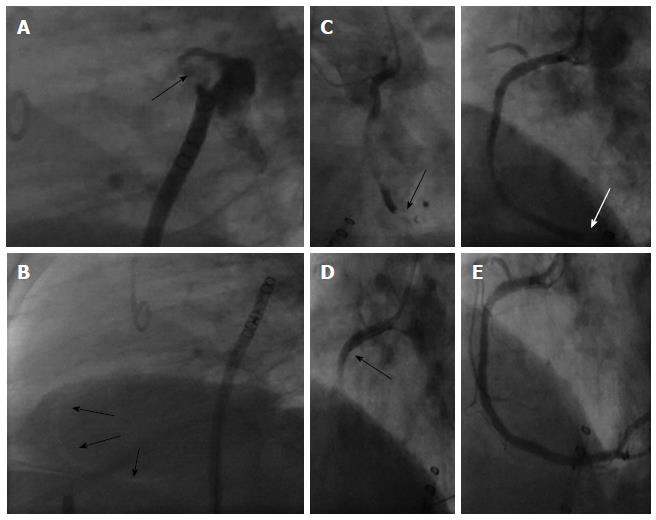
Air embolism is frequently a clinically silent event. However, acute coronary ischemia, stroke, hypotension, cardiac arrest and/or death are all possible outcomes. Holmes et al[12] report, that 5 of 449 patients with implantation of a Watchman® device suffered from a periprocedural stroke. The most common cause was air embolism, which is usually short-lived. Air emboli may enter the left atrium due to accidental injection of air, trapped air despite of flushing of the catheter or by air intrusion driven by a gradient between atmospheric and intracardiac pressure with a deep inspiration of the patient[24]. Therefore the left atrial pressure should be increased to normal or slightly high mean pressure using saline infusions. High pressure infusion through the side arm of the guiding catheter conversely can even lead to air embolism due to the Venturi-effect when opening the stop cock.
The management of air embolism is largely supportive. Hyperbaric oxygen has been shown to be of benefit in up to 80% of cases of cerebral air embolism, but controlled trials have not been performed yet[25]. However, as cerebral air embolism is mostly short-lived, there are usually no sequelae to be expected in longterm follow up.
Air embolism to the coronary circulation is most common in the right coronary artery because of the anterior position of the ostium (Figure 8). It often resolves within several minutes. However, marked ST-segment elevation, hypotension and ventricular arrhythmias may result, leading to cardiogenic shock. Aspiration of the air with an aspiration device (e.g., EXPORT Catheter, Medtronic Inc. Minneapolis, MN, United States) or rigorous contrast dye injection into the coronary artery may be helpful in selected cases (Figure 8). In this case the arterial access may be helpful to advance a right coronary catheter quickly to the aortic rout or even having a right coronary diagnostic catheter already in place during transseptal puncture to mark the aortic rout Also Trendelenburg position is recommended; as air trapped anywhere within the heart (e.g., the LAA) might be dislodged leading to another air embolism. If there is a large amount of air within the left or right atrium, the LAA or in the ascending aorta, this may also be aspirated through the sheath or via a coronary multipurpose or right coronary catheter. Aspiration of air should be performed before catecholamines are given for pressure management. This can protect from air movement into the brain circulation.
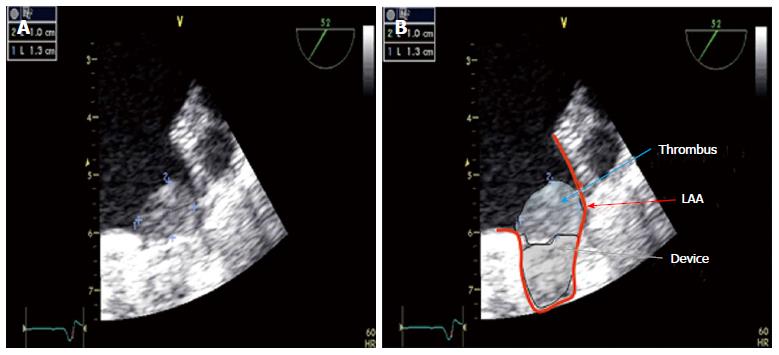
During the procedure an activated clotting time of about 250 s should be aimed at using heparin and continious flushing of the guiding catheter is recommended to avoid thrombus formation within the catheter. In case of new thrombus formation in the LAA, the operator must decide if it is prudent to continue the procedure. If a good position of the delivery sheath has been achieved, implantation might be possible, especially if it is deemed to be more dangerous to retract the device and sheath. The membrane of the device, once deployed, is able to catch a thrombus in the LAA.
If the sheath has not yet been maneuvered into the LAA, aspiration of blood and possible thrombus through the side port should be attempted followed by removal of the sheath for an outside flushing.
If thrombus formation is observed outside the access sheath in the left atrium, the only option is to withdraw the sheath back into the right atrium applying pressure on both proximal carotid arteries to minimize the risk of cerebral thromboembolism followed by close neurological monitoring.
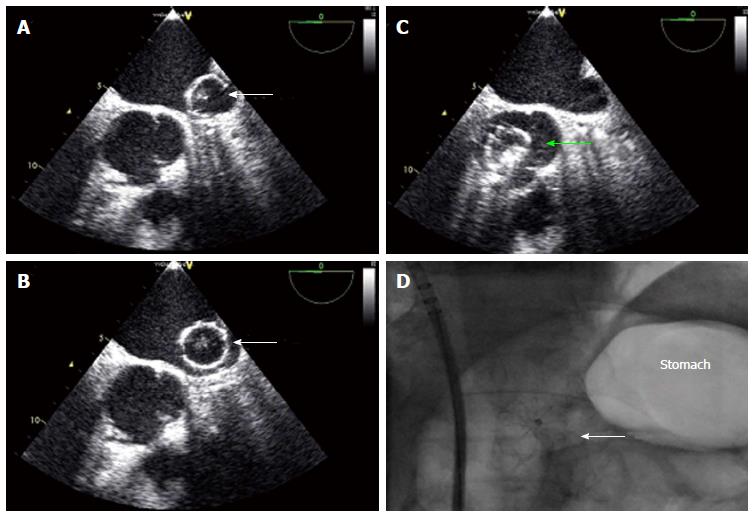
Percutaneous closure of the LAA may be complicated by immediate or late device embolization. Device embolization occurs in about 0.2% of cases with the Watchman® device (Figure 9)[12] Selection of patients with favorable LAA morphology and appropriate device sizing are crucial to prevent embolization. Negative predictors are: large LAA ostial size, use of undersized devices, short LAA length for the Watchman and unusual LAA morphologies.
The Watchman-Device must be fully expanded and compressed by at least 10%-30% of its original size. If the device is too deep in the LAA and therefore not fully expanded, the device must be partially recaptured and repositioned. If the device is too proximal, a complete recapture and exchange of the device is necessary.
The above mentioned Device release criteria should be fulfilled before release.
Routine post procedural TTE to exclude a new or expanding pericardial effusion and as a screen for device embolization is mandatory one to two days after implantation, even if the patient is asymptomatic[12,14]. The best way to visualize a LAA-occluder is from subxiphoidal. If the device cannot be seen, we recommend fluoroscopy to confirm correct position of the device.
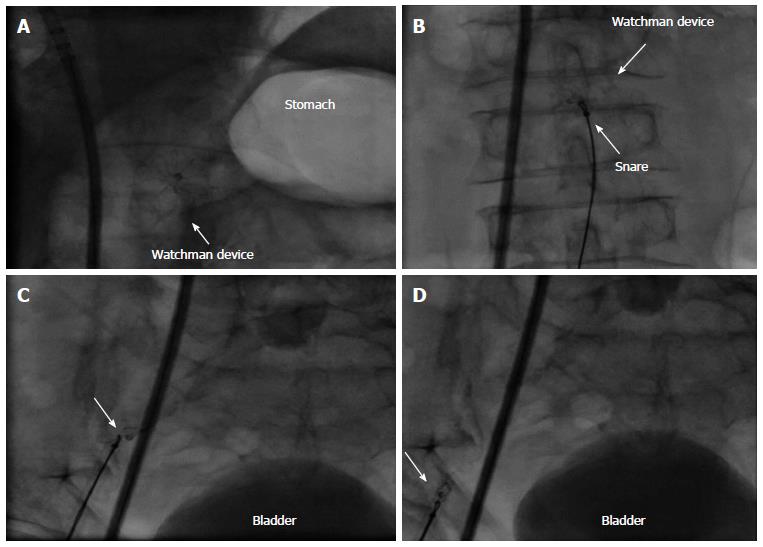
Embolized devices that are found in the left atrium or left ventricle may be safely moved antegradely across the mitral and aortic valve by using coronary catheters. Snaring of devices within the heart itself is challenging and dangerous. However, retrieval from the descending aorta using a snare or bioptome through an appropriately sized retrieval sheath (min. 14 Fr.) (Figure 10) is both safer and more feasible. In rare cases of embolisation of larger devices, surgical retrieval might be necessary even through the groin. Therefore, close collaboration with cardiac/vascular surgery ensures open access retrieval, if the percutaneous maneuver fails.
To avoid thrombus formation on the device for the Watchman® device, aspirin 100 mg/d and warfarin (INR goal 2-3x normal) should be administered until 45 d TEE control according to the PROTECT AF-trial[12]. If the device is still in position, the LAA occluded and no or minimal flow (residual jet < 5 mm) around the device, oral anticoagulation can be stopped and clopidogrel 75 mg/d should be added up to 6 mo after implantation. After another TEE-control aspirin alone should be administered lifelong at least according to the actual study results, though there are some centers that even stop aspirin therapy after three to six months. However, there are no data about long term results without any antiplatelet therapy. Within the Protect AF trial, patients having a residual gap < 5 mm were not likely to develop more stroke/TIA than patients without a gap, as a recent publication by Viles-Gonzalez was able to demonstrate[26]. If the 45 d TEE demonstrates a jet > 5 mm, warfarin must be continued with another TEE after 3 mo. If there is no change, the implant is deemed to have failed and the patient should remain on chronic oral anticoagulation therapy. If the gap has decreased to < 5 mm, therapy can be changed to ASA and clopidogrel.
In case of absolute or relative contraindications for warfarin therapy, another regimen recommends to give ASA and Clopidogrel immediately after implantation for 6 mo followed by ASA alone, which was similar effective in a small registry trial called ASAP[27]. If ASA could be even stopped at 6 mo or some time later is not clear yet. There are no data really supporting continuation or discontinuation of ASA lifelong except the experience of PROTECT AF.
In some cases, thrombus formation on the device may be detected by TEE (Figure 11). In these cases, low molecular weight heparin or oral anticoagulation should be restarted for another 4-8 wk. A repeat TEE will direct further treatment as described above.
Percutaneous closure of the left atrial appendage has been shown to be feasible with promising results in terms of reducing the rate of stroke and hemorrhagic complications, and has become an alternative therapy to standard anticoagulation therapy in patients with atrial fibrillation in Europe and other countries. The actual accepted indications for the use of LAA-occlusion therapy to be considered is published in an EHRA/EAPCI expert consensus statement[28]. Main indications are real contraindications for oral anticoagulation even for NOACs, patient refusal despite adequate information about different anticoagulation modalities, increased risk for bleeding due to a high HASBLED-score, need for prolonged triple therapy for example after coronary stenting, increased bleeding risk not reflected by the HASBLED-score and severe renal failure as a contraindication to NOACs. An individual risk benefit evaluation for each patient should be performed. FDA-approval may be expected in the beginning of the year 2015.
As a new invasive procedure, transcatheter LAA closure has several device- and procedure-specific complications, mainly pericardial effusions with or without tamponade, air embolism with subsequent stroke or device embolization. To minimize these complications, the procedure should be performed only by operators experienced in transseptal puncture and structural heart interventions. Additionally, TEE guidance by an experienced echocardiographer is important to ensure a complication-free and successful procedure. Profound knowledge of the nature, management and prevention of complications is essential to optimize the outcome of transcatheter LAA closure.
| 1. | Kirshner HS. Differentiating ischemic stroke subtypes: risk factors and secondary prevention. J Neurol Sci. 2009;279:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1232] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 3. | Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace. 2010;12:1360-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 953] [Cited by in RCA: 1031] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 4. | Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the United States. J Manag Care Pharm. 2009;15:244-252. [PubMed] |
| 5. | Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999;131:927-934. [PubMed] |
| 6. | Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7917] [Cited by in RCA: 8202] [Article Influence: 482.5] [Reference Citation Analysis (0)] |
| 7. | Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6075] [Cited by in RCA: 6695] [Article Influence: 446.3] [Reference Citation Analysis (9)] |
| 8. | Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6519] [Cited by in RCA: 7058] [Article Influence: 470.5] [Reference Citation Analysis (2)] |
| 9. | Ostermayer SH, Reisman M, Kramer PH, Matthews RV, Gray WA, Block PC, Omran H, Bartorelli AL, Della Bella P, Di Mario C. Percutaneous left atrial appendage transcatheter occlusion (PLAATO system) to prevent stroke in high-risk patients with non-rheumatic atrial fibrillation: results from the international multi-center feasibility trials. J Am Coll Cardiol. 2005;46:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 335] [Article Influence: 16.0] [Reference Citation Analysis (2)] |
| 10. | Ussia GP, Mulè M, Cammalleri V, Scarabelli M, Barbanti M, Immè S, Mangiafico S, Marchese A, Galassi AR, Tamburino C. Percutaneous closure of left atrial appendage to prevent embolic events in high-risk patients with chronic atrial fibrillation. Catheter Cardiovasc Interv. 2009;74:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Block PC, Burstein S, Casale PN, Kramer PH, Teirstein P, Williams DO, Reisman M. Percutaneous left atrial appendage occlusion for patients in atrial fibrillation suboptimal for warfarin therapy: 5-year results of the PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) Study. JACC Cardiovasc Interv. 2009;2:594-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 181] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 12. | Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1498] [Cited by in RCA: 1668] [Article Influence: 98.1] [Reference Citation Analysis (0)] |
| 13. | Reddy V. PROTECT AF: The Mortality Effects of LAA Closure vs. Warfarin for Stroke Prophylaxis in A-fib. Denver, Colorado: Heart Rhythm 2013; . |
| 14. | Holmes DR. Left Atrial Appendage Occlusion: Results & Future Predictions. San Francisco, CA: TCT 2013; . |
| 15. | Al-Saady NM, Obel OA, Camm AJ. Left atrial appendage: structure, function, and role in thromboembolism. Heart. 1999;82:547-554. [PubMed] |
| 16. | Veinot JP, Harrity PJ, Gentile F, Khandheria BK, Bailey KR, Eickholt JT, Seward JB, Tajik AJ, Edwards WD. Anatomy of the normal left atrial appendage: a quantitative study of age-related changes in 500 autopsy hearts: implications for echocardiographic examination. Circulation. 1997;96:3112-3115. [PubMed] |
| 17. | Earley MJ. How to perform a transseptal puncture. Heart. 2009;95:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Sick P. Left Atrial Appendage Closure with the Watchman® Device. Percutaneous Interventions for Congenital Heart Disease. 1st ed. Florida, US: CRC Press 2007; . |
| 19. | Bayard YL, Omran H, Neuzil P, Thuesen L, Pichler M, Rowland E, Ramondo A, Ruzyllo W, Budts W, Montalescot G. PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) for prevention of cardioembolic stroke in non-anticoagulation eligible atrial fibrillation patients: results from the European PLAATO study. EuroIntervention. 2010;6:220-226. [PubMed] |
| 20. | Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation. 2011;123:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 643] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 21. | Landmesser U, Holmes DR. Left atrial appendage closure: a percutaneous transcatheter approach for stroke prevention in atrial fibrillation. Eur Heart J. 2012;33:698-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Holmes D. Randomized Trial of LAA Closure vs Warfarin for Stroke/ Thromboembolic Prevention in Patients with Non-valvular Atrial Fibrillation (PREVAIL). Beijing, China: CIT, CNCC 2013; . |
| 23. | Holmes D. Randomized Trial of LAA Closure vs Warfarin for Stroke/ Thromboembolic Prevention in Patients with Non-valvular Atrial Fibrillation (PREVAIL) [Abstract]. San Francisco, US: ACC 2013; . |
| 24. | Franzen OW, Klemm H, Hamann F, Koschyk D, von Kodolitsch Y, Weil J, Meinertz T, Baldus S. Mechanisms underlying air aspiration in patients undergoing left atrial catheterization. Catheter Cardiovasc Interv. 2008;71:553-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Murphy BP, Harford FJ, Cramer FS. Cerebral air embolism resulting from invasive medical procedures. Treatment with hyperbaric oxygen. Ann Surg. 1985;201:242-245. [PubMed] |
| 26. | Viles-Gonzalez JF, Kar S, Douglas P, Dukkipati S, Feldman T, Horton R, Holmes D, Reddy VY. The clinical impact of incomplete left atrial appendage closure with the Watchman Device in patients with atrial fibrillation: a PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) substudy. J Am Coll Cardiol. 2012;59:923-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 450] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 27. | Reddy VY, Möbius-Winkler S, Miller MA, Neuzil P, Schuler G, Wiebe J, Sick P, Sievert H. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol. 2013;61:2551-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 584] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 28. | Meier B, Blaauw Y, Khattab AA, Lewalter T, Sievert H, Tondo C, Glikson M. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion. EuroIntervention. 2015;10:1109-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Gi-Byoung N, Ozaydin M S- Editor: Qi Y L- Editor: A E- Editor: Wu HL