Published online Dec 26, 2015. doi: 10.4330/wjc.v7.i12.922
Peer-review started: July 12, 2015
First decision: August 15, 2015
Revised: October 2, 2015
Accepted: October 23, 2015
Article in press: October 27, 2015
Published online: December 26, 2015
Processing time: 180 Days and 21.2 Hours
AIM: To compare the utility of the partners-heart failure (HF) algorithm with the care alert strategy for remote monitoring, in guiding clinical actions oriented to treat impending HF.
METHODS: Consecutive cardiac resynchronization-defibrillator recipients were followed with biweekly automatic transmissions. After every transmission, patients received a phone contact in order to check their health status, eventually followed by clinical actions, classified as “no-action”, “non-active” and “active”. Active clinical actions were oriented to treat impending HF. The sensitivity, specificity, positive and negative predictive values and diagnostic accuracy of the partners-HF algorithm vs care alert in determining active clinical actions oriented to treat pre-HF status and to prevent an acute decompensation, were also calculated.
RESULTS: The study population included 70 patients with moderate to advanced systolic HF and QRS duration longer than 120 ms. During a mean follow-up of 8 ± 2 mo, 665 transmissions were collected. No deaths or HF hospitalizations occurred. The sensitivity and specificity of the partners-HF algorithm for active clinical actions oriented to treat impending HF were 96.9% (95%CI: 0.96-0.98) and 92.5% (95%CI: 0.90-0.94) respectively. The positive and negative predictive values were 84.6% (95%CI: 0.82-0.87) and 98.6% (95%CI: 0.98-0.99) respectively. The partners-HF algorithm had an accuracy of 93.8% (95%CI: 0.92-0.96) in determining active clinical actions. With regard to active clinical actions, care alert had a sensitivity and specificity of 11.05% (95%CI: 0.09-0.13) and 93.6% respectively (95%CI: 0.92-0.95). The positive predictive value was 42.3% (95%CI: 0.38-0.46); the negative predictive value was 71.1% (95%CI: 0.68-0.74). Care alert had an accuracy of 68.9% (95%CI: 0.65-0.72) in determining active clinical actions.
CONCLUSION: The partners-HF algorithm proved higher accuracy and sensitivity than care alert in determining active clinical actions oriented to treat impending HF. Future studies in larger populations should evaluate partners-HF ability to improve HF-related clinical outcomes.
Core tip: This is a multicenter observational registry that compared the utility of the partners-heart failure (HF) algorithm with the care alert strategy for remote monitoring, in guiding clinical actions oriented to treat impending HF in a population of 70 cardiac resynchronization therapy recipients followed over a mean follow-up period of 8 ± 2 mo. The partners-HF algorithm displayed high sensitivity (96.9%), specificity (92.5%), positive (84.6%) and negative (98.6%) predictive values for active clinical actions oriented to treat impending HF. The care alert exhibited lower sensitivity (11.1%), positive (42.3%) and negative (71.1%) predictive values.
- Citation: Calo’ L, Martino A, Tota C, Fagagnini A, Iulianella R, Rebecchi M, Sciarra L, Giunta G, Romano MG, Colaceci R, Ciccaglioni A, Ammirati F, Ruvo E. Comparison of partners-heart failure algorithm vs care alert in remote heart failure management. World J Cardiol 2015; 7(12): 922-930
- URL: https://www.wjgnet.com/1949-8462/full/v7/i12/922.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i12.922
Heart failure (HF) is a primary public health problem, with mortality and hospitalization rates of approximately 7.2% and 31.9% at one-year respectively[1]. Outpatient management, symptoms and daily weight often do not identify patients in time to prevent imminent HF. Modern implantable cardiac resynchronization therapy-defibrillators (CRT-D) with remote monitoring (RM) capabilities, continuously assess parameters, including heart rate, patient’s activity (PA), intra-thoracic impedance, atrial fibrillation (AF), ventricular arrhythmias (VA), shock therapy delivered and system integrity[2-5].
Previous studies have demonstrated the ability of individual device diagnostic data, to predict HF events, to reduce the time from clinical event to treatment, length of hospitalization and quantity of in-office visits[6-10].
Earlier studies have shown that implantable device-measured parameters, such as intra-thoracic impedance, AF burden, mean heart rate, heart rate variability (HRV), patient activity (PA), frequency of premature ventricular contractions (PVCs), VA episodes, implantable cardioverter defibrillators (ICD) shocks and percentage of pacing of cardiac resynchronization therapy (%CRT), individuate subjects at risk of HF and facilitate early interventions[6-12]. Variations of intrathoracic impedance[3] as well as HRV and PA[2] occurs nearly two weeks before HF exacerbation. Low HRV indicate a sympathetic dominance in cardiac autonomic control and may be associated with exacerbation of atrial and VAs[13]. A prolonged AF duration, a rapid ventricular rate (VR) during AF and an increase in the burden of PVCs reduce %CRT[14] and are warning signs of HF, together with ICD shocks[15].
Each HF device diagnostic parameter, although validated in various studies, has several limitations. A previous study[16] showed that sensitivity values of individual parameters, ranged from 23.6% to 50.0%, whereas their combination displayed 65.4% sensitivity and 99.5% specificity for cardiovascular hospitalizations and deaths.
The partners-HF[11] is the largest cohort study to have evaluated the ability of combined HF device diagnostics, including Optivol™ Fluid index, AF duration, rapid VR during AF, low PA, high nocturnal heart rate (NHR), low HRV, low CRT pacing percentage, and ICD shocks, to identify patients at risk of acute HF in the subsequent 30 d. The retrospective analysis of the prospectively collected data of the partners-HF study, demonstrated that subjects with a positive partners-HF algorithm were at a greater risk (HR = 5.5; P < 0.001) of HF hospitalization during the next month.
The purpose of this multicenter, observational registry was to prospectively assess the utility of the partners-HF criteria, implemented within Discovery Link™, in guiding clinical actions oriented to treat pre-HF status and to prevent an acute decompensation in a population of HF individuals implanted with a Medtronic CRT-D device.
This study has been approved by our Institutional Review Board and is conform to the guiding principles of the Declaration of Helsinki.
Consecutive CRT-D candidates were enrolled by three Italian cardiology centers. The clinical status of the patients, including NYHA class, was initially assessed by the cardiologists involved in the project. All patients underwent implantation of a Medtronic CRT-D system (Model: Consulta™, Concerto™ II, VIVA XT™, PROTECTA XT™; Medtronic Inc., Minnesota) equipped with the CareLink Medtronic®-RM system for RM.
Inclusion criteria were: Left ventricular ejection fraction ≤ 35% + NYHA class II, III and ambulatory IV and broad QRS (> 120 ms if left bundle branch block was present, or otherwise > 150 ms + optimal pharmacological treatment for HF). Exclusion criteria were: acute coronary syndrome within 40 d, coronary artery revascularization within 3 mo, end-stage HF requiring inotropic support, ventricular assist devices or dialysis.
Each patient received a wireless CareLink Monitor which provided automatic transmission of clinical and technical parameters stored in the implanted device’s memory to a Service Center where information was decrypted, uploaded to a secure website and periodically accessed by the nurses. Patients were followed up for at least 6 mo. Data were prospectively collected between January 2012 and October 2012 and classified on the basis of both the care alert and the partners-HF algorithms at the same time. Automatic “scheduled” transmissions were programmed every 15 d. “Care alert”-triggered transmissions and transmissions activated manually by the patients were also collected. Patients were instructed to manually activate transmissions in case of occurrence or exacerbation of HF-related symptoms (including shortness of breath, dyspnea, orthopnea, asthenia, pre-syncope or syncope) or signs (including weight increase, peripheral edema enlargement).
The project, including data collection, was approved by the Hospital Ethics Committees of each cardiology center involved in the registry using the Medtronic Clinical Service Project®, and every individual enrolled gave written informed consent to enrolment in the registry.
The partners-HF application, based on the algorithm described by Whellan et al[11], was implemented within the Discovery Link™. The latter is a web environment enabling elaborated and aggregated information from the Medtronic CareLink Network® to be shown in interactive JavaScript charts. The partners-HF algorithm was adopted to process information in order to select the last transmission (including both manually and automatically triggered ones) from each device and to perform analysis. Statistics were calculated over the last 28 consecutive days[11]. Every two weeks, the first partners-HF profile of those patients who satisfied the partners-HF criteria was directly logged into the Discovery Link.
The partners-HF algorithm was considered positive in the following cases: Optivol™ Fluid index ≥ 100 or any 2 of the following criteria met during a one-month period of evaluation: Long AF duration, rapid VR during AF, Optivol™ fluid index ≥ 60, low PA, high nocturnal NHR, low HRV, low %CRT, and ICD shocks (Appendix).
The Carelink system automatically triggered alerts in case of shocks delivered or if the following clinical and technical parameters exceed a programmable threshold: OptiVol™ Fluid Monitoring Index (> 60), AF duration (> 24 h), VR rate during AF (> 100 bpm), lead impedance, integrity and battery voltage alert (out of predefined range).
After every transmission, all patients received a phone contact and their health status was checked by nurses experienced in HF. At time of enrollment, patients were instructed to measure frequently their body weight and to check their pulse in order to identify HF-related signs (increase of heart rate, weight and/or peripheral edema) and symptoms (increase in shortness of breath, cough and/or asthenia, reduction of exercise tolerance, needing use of extra pillows during the night). Data on vital status, symptoms, quality of life, adherence to pharmacological treatment, hospitalizations and mortality were collected by nurses at every phone contact. Pre-specified boundaries for weight, blood pressure, pulse and symptoms were previously established for every patient. Adjudication of impending HF was based on the development of early HF-related signs and symptoms (see above) and on the exceeding from the prespecified boundaries, but still not requiring hospitalization[12]. RM transmissions suggestive of worsening HF or device malfunctioning were submitted to physicians.
Clinical actions performed as a result of transmissions, according to each center’s clinical practice, were registered on a Medtronic Clinical Service®-form and were classified as follows: “no-action”, “non-active” and “active”. No action: (1) consisted on telephonic contact; (2) non-active clinical action; (3) consisted on clinical examination without pharmacological treatment modification (PTM). Active clinical actions included PTM during telephonic contact; or (4) during clinical examination. In the event of manual or care alert transmissions, physicians could decide either to undertake clinical action immediately or to wait until the first partners-HF data from those specific transmissions became available in the Discovery Link environment.
The aim of this study was to determine the sensitivity, specificity, positive and negative predictive values and diagnostic accuracy of the partners-HF algorithm and of care alert in determining active clinical actions oriented to treat pre-HF status and to prevent an acute decompensation. Analyses of sensitivity, specificity, predictivity and accuracy were performed with respect to overall active clinical actions (3 + 2) vs the sum of clinical actions and no actions (1 + 0).
Continuous variables are summarized as mean ± SD and categorical variables as counts and percentages. Positive transmissions by the partners-HF algorithm and/or care alert were considered true positive when they were associated with acute HF and/or with pharmacological treatment modification due to impending HF. Positive transmissions by the partners-HF algorithm and/or care alert were considered as false positive in the remaining cases. Negative transmissions by the partners-HF algorithm and/or care alert were considered true negative when they were not associated to acute HF or PTM due to pre-HF (see above), and as false negative when they were not. The sensitivity of the partners-HF algorithm and of care alert was calculated as the ratio between the number of true positive transmissions and the sum of true positive and false negative transmissions. Specificity was calculated as the ratio between true negatives and the sum of true negatives and false positives. Positive predictive value was calculated as the ratio between true positives and the sum of true positives and false positives. Negative predictive value was calculated as the ratio between true negatives and the sum of true negatives and false negatives. Accuracy was calculated as the ratio between the sum of true positive and true negative transmissions and total transmissions. All the tests were performed by means of R 2.11.1 for Windows.
The characteristics of the study population are presented in Table 1. Patients were predominantly males and had mostly a moderate to advanced HF. All patients had QRS duration longer than 120 ms. The relatively low (63.2) percentage of optimized pharmacological treatment is due to reduced aldosterone antagonists administration in patients affected by chronic kidney disease.
| Clinical characteristics of the study population | |
| Age (yr) | 70.3 ± 8.3 |
| Male (%) | 78.3 |
| EF (%) | 27.5 ± 6.5 |
| Etiology post-ischemic DC (%) | 63.2 |
| Idiopathic DC (%) | 33.7 |
| Valvular DC (%) | 2 |
| Congenital DC (%) | 1.1 |
| NYHA II (%) | 17.3 |
| III (%) | 78.6 |
| IV (%) | 4.1 |
| Optimized pharmacological treatment (%) | 63.21 |
| Prevention: Primary (%) | 73.7 |
| Secondary (%) | 18.3 |
| SVT (%) | 3.7 |
| Syncope (%) | 3.2 |
| Cardiac arrest (%) | 5.1 |
| AF permanent (%) | 16.3 |
| Persistent (%) | 7.4 |
| Paroxysmal (%) | 4.1 |
| Devices: Consulta™ CRT-D | 37.4 |
| Concerto™ II CRT-D (%) | 26.3 |
| Viva XT™ CRT-D (%) | 22.1 |
| Protecta XT™ CRT-D (%) | 14.2 |
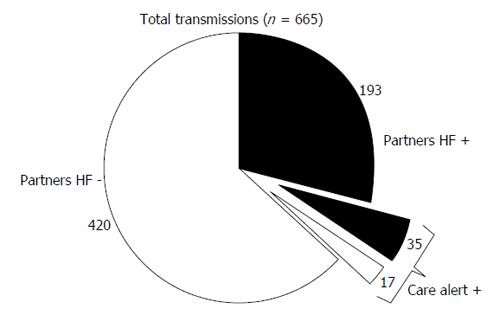
During a mean follow-up of 8 ± 2 mo, 665 transmissions were received from 70 patients. Transmissions were classified as follows: 52 (7.8%) care alert, 149 (22.4%) manual and 464 (69.8%) scheduled.
Of all transmissions, 228 (34.3%) fulfilled the partners-HF criteria. Positive partners-HF transmissions were classified as: scheduled (136; 59.6%), manual (57; 25%), and care alert (35; 15.4%). Of the 437 negative partners-HF transmissions, 328 (75.1%) were scheduled, 92 (21%) were manual and 17 (3.9%) were triggered by a care alert. Figure 1 shows the distribution of partners-HF positive and negative transmissions, contemporarily triggered or not by care alert. Overall, the “care alert” transmissions met the partners-HF criteria in 67.3% of cases (Figure 1).
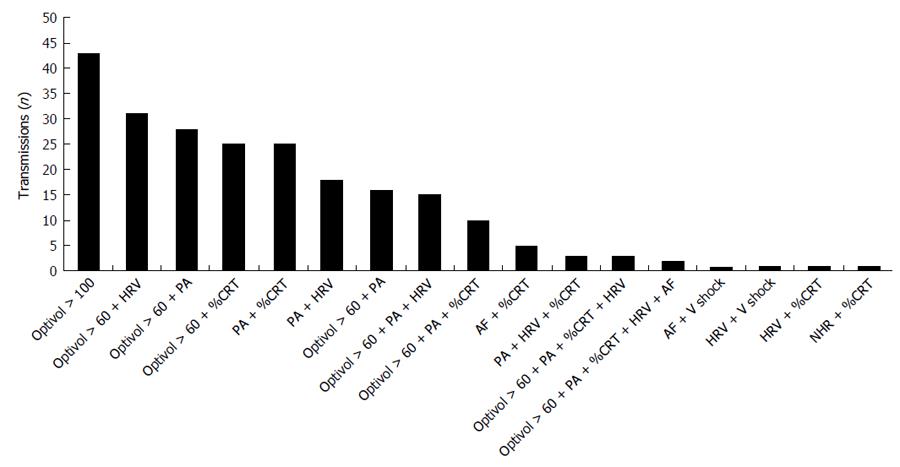
The most common reasons triggering a positive partners-HF transmission: Optivol fluid index ≥ 100 (18.8%) or optivol fluid index > 60 plus one of the following parameters: reduced HRV (13.6%), low PA (12.3%), or reduced %CRT (11%) (Figure 2). The 52 “care alert”-triggered transmissions (35 partners-HF positive and 17 partners-HF negative) were generated by OptiVol fluid index in 43 (82.7%) cases, AF duration and/or AF VR in 7 (13.5%) cases and shock for VAs in 2 (3.8%) cases.
During follow-up, no deaths or HF hospitalizations occurred. Of overall transmissions, 16 (2.4%) were followed by clinical examination and PTM, 183 (27.5%) by PTM during telephonic contact and 7 (1%) by clinical examination without PTM.
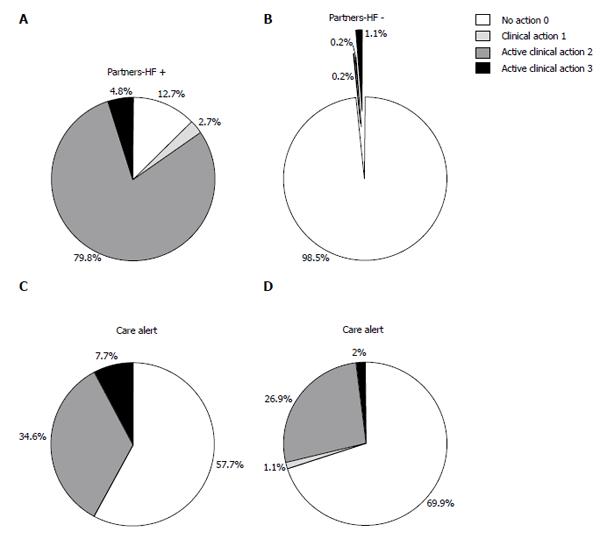
Of the 228 positive partners-HF transmissions, 11 (4.8%) were followed by clinical examination and PTM, 182 (79.8%) by PTM during telephonic contact and 6 (2.7%) by clinical examination without PTM (Figure 3A). PTM consisted of 19 drug dosage up-titrations and/or new treatment administrations during clinical examination and 188 during telephonic contact (Table 2). No pharmacological down titration was done.
| Active clinical actions | Partners-HF + | Care alert + |
| Pharmacological treatment modification during telephonic contact | ||
| Diuretic dosage increase | 120 | 25 |
| BB dosage increase | 57 | 22 |
| AAD administration | 2 | 2 |
| ACE-I and ARA dosage increase | 4 | 0 |
| OAC administration | 5 | 5 |
| Clinical examination and pharmacological treatment modification | ||
| Diuretic dosage increase | 9 | 4 |
| BB dosage increase | 8 | 3 |
| AAD administration | 1 | 1 |
| ACE-I and ARA dosage increase | 1 | 1 |
| Anti-platelet administration | 0 | 0 |
Of the 437 negative partners-HF transmissions, 5 (1.1%) were followed by clinical examination and PTM, 1 (0.2%) by PTM during telephonic contact and 1 (0.2%) by clinical examination without PTM (Figure 3B). PTM consisted of 7 drug dosage up-titrations and/or new treatment administrations during clinical examination and 2 telephonic PTM, made as a consequence of a single care alert transmission. In these cases, diuretic and beta-blocker dosages were increased; no pharmacological down titration was reported.
Of the 52 care alert transmissions, 4 (7.7%) were followed by clinical examination and PTM and 18 (34.6%) by PTM during telephonic contact (Figure 3C). PTM consisted of 9 drug dosage up-titrations and/or new treatment administrations during clinical examination and 54 without in-office clinical examinations (Table 2).
Clinical actions following negative care alert transmissions consisted of 12 (1.9%) clinical examinations and PTM, 165 (27%) PTM during telephonic contact, 7 (1.1%) clinical examinations without PTM and 429 (70%) telephone contacts alone. PTM consisted of 17 drug dosage up-titrations and/or new treatment administrations during clinical examination and 136 without in-office clinical examinations.
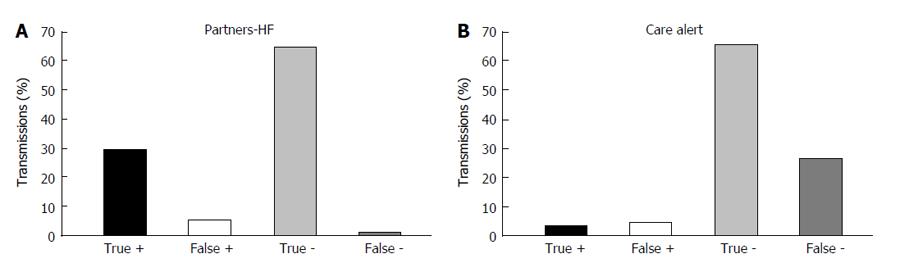
True positive, true negative, false positive and false negative partners-HF transmissions with respect to active clinical actions are depicted in Figure 4A. The sensitivity and specificity of the partners-HF algorithm for active clinical actions (classes 2-3) were 96.9% (95%CI: 0.96-0.98) and 92.5% (95%CI: 0.90-0.94) respectively (Table 3). The positive and negative predictive values were 84.6% (95%CI: 0.82-0.87) and 98.6% (95%CI: 0.98-0.99) respectively. The partners-HF algorithm had an accuracy of 93.8% (95%CI: 0.92-0.96) in determining active clinical actions (Table 3).
| Active clinical actions (2-3) | Non-active/no clinical actions (0-1) | Total transmissions | |
| Positive partners-HF transmissions | 193 | 35 | 228 |
| Negative partners-HF transmissions | 6 | 431 | 437 |
| Positive care alert transmissions | 22 | 30 | 52 |
| Negative care alert transmissions | 177 | 436 | 613 |
| Overall transmissions followed by an action | 199 | 466 | 665 |
| Appendix partners-HF algorithm | |||
| Parameters | Criterion | ||
| Fluid index | ≥ 60 d | ||
| AT/AF duration | ≥ 6 h and not persistent AT/AF | ||
| VR during AT/AF | AT/AF ≥ 24 h and VR ≥ 90 bpm | ||
| Patient activity | < 1 h over 1 wk | ||
| NHR | ≥ 85 bpm for 7 consecutive days | ||
| HRV | < 60 ms for 7 consecutive days | ||
| %CRT pacing | < 90% for 5 of 7 d | ||
| Shock (s) | ≥ 1 shock |
Care alert true positive, true negative, false positive and false negative transmissions with respect to active clinical actions are depicted in Figure 4B. With regard to active clinical actions (classes 2-3), care alert had a sensitivity and specificity of 11.05% (95%CI: 0.09-0.13) and 93.6% respectively (95%CI: 0.92-0.95). The positive predictive value was 42.3% (95%CI: 0.38-0.46); the negative predictive value was 71.1% (95%CI: 0.68-0.74). Care alert had an accuracy of 68.9% (95%CI: 0.65-0.72) in determining active clinical actions (Table 3).
In this registry we observed that: (1) The partners-HF algorithm has high sensitivity (96.9%), specificity (92.5%) and diagnostic accuracy (93.8%) in identifying patients with early HF-related symptoms and signs (pre-HF), at risk of acute HF, who benefit from active clinical actions; (2) The care alert displays good specificity (93.5%) but very low sensitivity (11.1%) in identifying patients with pre-HF who benefit from active clinical actions; (3) Of all the CRT-D remote transmissions, 34.3% fulfilled the partners-HF criteria and 7.8% were triggered by a care alert. Positive partners-HF transmissions also determined a care alert in 15.4% of cases, and care alert transmissions met partners criteria in 67.3% of cases; (4) The most common reasons triggering a Positive partners-HF transmission were: Optivol fluid index ≥ 100 (18.8%) or optivol fluid index > 60 plus one of the following parameters: reduced HRV (13.6%), low PA (12.3%), or reduced %CRT (11%); and (5) The most common active clinical action was HF-therapy titration, particularly of diuretics and beta-blockers, and the introduction of oral anticoagulation in patients with asymptomatic AF.
Despite advances in treatment of HF, it is still a major cause of cardiovascular mortality and hospitalization, especially in the early period after hospital discharge[1]. Prevention of HF relapses is important not only to reduce HF mortality and morbidity, but also health care costs[1]. Cardiac implantable electronic devices have nowadays remote monitoring capabilities that allow clinicians to have remote access to the complete device diagnostic information.
Earlier studies have shown that implantable device-measured variables, including intra-thoracic impedance, AF burden, mean heart rate, HRV, PA, frequency of PVCs, VA episodes, ICD shocks and %CRT, individuate subjects at risk of HF and facilitate early interventions[6-10]. Intrathoracic impedance[3], HRV and PA[2] reduction, occurs nearly two weeks before HF exacerbation. Low HRV indicate a sympathetic dominance in cardiac autonomic control and may be associated with exacerbation of atrial and VAs[13]. A prolonged AF duration, a rapid VR during AF and an increase in the burden of PVCs reduce %CRT[14] and are warning signs of HF, together with ICD shocks[15].
Although validated in various studies, the use of each device parameter in HF patients, is restricted by some limitations. In particular, variations of intrathoracic impedance may be related to lung inflammation; increased AF burden and prolonged AF duration are not useful in subjects with permanent AF; reduced mean heart rate, HRV or patient activity may reflect difficulty walking secondary to orthopedic diseases. Consequently, there is great interest in combining HF device diagnostic parameters for the management of CRT-D recipients.
The partners-HF[11] was a large cohort study exploring the ability of the partners-HF criteria algorithm to dynamically stratify patients’ risks of HF. A cohort of 694 CRT-D recipients with advanced HF (NYHA III-IV) was prospectively evaluated in 100 centers. The retrospective evaluation of diagnostic CRT-D data demonstrated that subjects with a positive partners-HF algorithm had greater risk of hospitalization due to HF in the next month (adjusted HR = 5.5; P < 0.001). Moreover, the study demonstrated that increasing the frequency of reviewing the HF device diagnostics from quarterly (90 d) to monthly (30 d) but not to semimonthly (15 d), improved the ability to identify individuals at higher HF risk.
The prospective, multicenter observational Home Monitoring in CRT (Home-CARE) study[16] followed up for 1 year 377 CRT-D recipients who had been hospitalized for HF at least once within the 12 mo before enrollment. The following data were automatically retrieved every 24 h by the Home Monitoring (Biotronik, Berlin, Germany) algorithm: Mean heart rate, heart rate at rest, PA, frequency of PVCs, HRV, right ventricular pacing impedance, and painless shock impedance. The retrospective sensitivity values of individual parameters ranged from 23.6% to 50.0%, whereas their combination displayed 65.4% sensitivity and 99.5% specificity for cardiovascular hospitalizations and deaths.
Some studies have demonstrated favorable effects of RM in improving HF treatment, with potential benefits on clinical outcomes[6-10]. However, few and inconclusive data are available on the RM use in routine clinical practice and its impact on HF clinical outcomes. The Home Guide[17] registry proved RM highly effective in detecting clinical events, excluding deaths, with a sensitivity and a positive predictive values of 89% and 97%, respectively. RM sensitivity for atrial and VAs and device-related issues was > 90%, while it was < 35% for stroke, syncope and acute coronary syndromes and displayed an intermediate sensitivity (59%) for HF detection. Interestingly, 3 out of 4 events needing clinical intervention were asymptomatic and were effectively detected by RM, allowing a prompt reaction.
In our study the most common clinical reaction to partners-HF transmissions was drug therapy adjustment, while HF therapy titration and oral anticoagulation introduction in patients with asymptomatic AF were the most prevalent therapy interventions.
This is the first multicenter observational registry prospectively assessing the clinical utility of partners-HF algorithm for risk stratification of HF patients in clinical practice. Remote monitoring of CRT-D recipients trough partners-HF algorithm, was not compared with usual care and this registry was not powered to explore the impact of the partners-HF algorithm on HF-hospitalizations and mortality. Our results prove that the partners-HF has significant diagnostic accuracy in determining active clinical actions oriented to treat pre-HF status and to prevent an acute decompensation. Given the high positive and very high negative predictive values, clinicians could contact only patients with positive partners-HF transmissions, thus avoiding a significant number of unnecessary telephone contacts.
Care alert displays very low sensitivity and a poor ability to identify patients needing active clinical action oriented to treat pre-HF status. Moreover, given its low positive predictive value, clinicians should be aware that an active clinical action oriented to preventing acute HF may be not necessary in case of care alert triggered transmissions.
Another important aspect is that this prospective analysis was conducted in patients with advanced HF (82.7%: NYHA classes III/IV; mean EF: 27.5% ± 6.5%). This may explain the high percentage of manual and care alert transmissions collected and the high prevalence of positive partners-HF transmissions (35%). Considering that no HF hospitalization occurred in a population of advanced HF during a 6 mo follow-up, the partners-HF algorithm appears to be a powerful tool to identify and consequently treat pre-HF status in order to prevent acute decompensation.
According to the partners-HF study[11], positive partners transmissions were mostly triggered by the optivol fluid index, alone or in combination with low HRV, low PA or a low %CRT. The weight of each partners-HF criterion in the risk stratification of HF patients was not considered in this registry. A combined algorithm of HF diagnostic parameters could be utilized to stratify patients into high, medium and low risk of HF by using a specific risk stratification score, calculated by attributing a specific weight to each partners-HF criterion on the basis of its ability to detect pre-HF status. Finally, whether therapeutic interventions based on the partners-HF algorithm are effective in improving outcomes in HF, was not investigated.
The partners-HF algorithm proved to be a powerful predictor of a pre-HF status and was able to guide clinical actions oriented to avoiding acute HF. Future larger randomized prospective trials should be performed to confirm our results, to develop and validate a dynamic HF risk score based on the partners-HF algorithm and to ascertain whether the use of this algorithm for RM can improve the main clinical outcomes of HF patients.
Heart failure (HF) is a principal cause of death hospitalization and health care costs. The partners-HF algorithm retrospectively identified cardiac resynchronization-defibrillator (CRT-D) recipients at risk of HF relapses in the subsequent 30 d. However no studies have validated this algorithm prospectively and have compared it with the care alert strategy, that is commonly adopted for CRT-remote monitoring.
Remote monitoring has emerged as a useful tool to prevent HF relapses, and to reduce cardiac hospitalization and mortality.
This is the first multicenter observational registry prospectively assessing the clinical utility of partners-HF algorithm for risk stratification of HF patients in clinical practice.
The authors’ prospective study showed that the partners-HF algorithm has significant diagnostic accuracy in determining active clinical actions oriented to prevent HF relapses. Moreover, it has a high positive and a high negative predictive value, allowing clinicians to contact only patients with positive partners-HF transmissions, thus avoiding a significant number of unnecessary telephone contacts.
Remote monitoring: Wireless remote monitoring of cardiac electronic devices, including cardiac defibrillators and CRT.
This is a valuable research, because status of clinical actions is very important for patient’s therapy and outcomes. Herein the traits of the partners-HF algorithm vs care alert in determining active clinical actions were explored and observed the effect of different methods on treatment or prevent heart failure.
| 1. | Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Leiro MC, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Metra M. EURObservational Research Programme: the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail. 2010;12:1076-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 317] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 2. | Adamson PB, Smith AL, Abraham WT, Kleckner KJ, Stadler RW, Shih A, Rhodes MM. Continuous autonomic assessment in patients with symptomatic heart failure: prognostic value of heart rate variability measured by an implanted cardiac resynchronization device. Circulation. 2004;110:2389-2394. [PubMed] |
| 3. | Yu CM, Wang L, Chau E, Chan RH, Kong SL, Tang MO, Christensen J, Stadler RW, Lau CP. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841-848. [PubMed] |
| 4. | Sarkar S, Koehler J, Crossley GH, Tang WH, Abraham WT, Warman EN, Whellan DJ. Burden of atrial fibrillation and poor rate control detected by continuous monitoring and the risk for heart failure hospitalization. Am Heart J. 2012;164:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, Reddy RK, Marchlinski FE, Yee R, Guarnieri T. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009-1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1224] [Cited by in RCA: 1155] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 6. | Bourge RC, Abraham WT, Adamson PB, Aaron MF, Aranda JM, Magalski A, Zile MR, Smith AL, Smart FW, O’Shaughnessy MA. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol. 2008;51:1073-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 412] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 7. | Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. 2011;57:1181-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 383] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 8. | De Ruvo E, Gargaro A, Sciarra L, De Luca L, Zuccaro LM, Stirpe F, Rebecchi M, Sette A, Lioy E, Calò L. Early detection of adverse events with daily remote monitoring versus quarterly standard follow-up program in patients with CRT-D. Pacing Clin Electrophysiol. 2011;34:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Landolina M, Perego GB, Lunati M, Curnis A, Guenzati G, Vicentini A, Parati G, Borghi G, Zanaboni P, Valsecchi S. Remote monitoring reduces healthcare use and improves quality of care in heart failure patients with implantable defibrillators: the evolution of management strategies of heart failure patients with implantable defibrillators (EVOLVO) study. Circulation. 2012;125:2985-2992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 259] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 10. | Varma N, Epstein AE, Irimpen A, Schweikert R, Love C. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation. 2010;122:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 428] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 11. | Whellan DJ, Ousdigian KT, Al-Khatib SM, Pu W, Sarkar S, Porter CB, Pavri BB, O’Connor CM. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure) study. J Am Coll Cardiol. 2010;55:1803-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 288] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 12. | Weintraub A, Gregory D, Patel AR, Levine D, Venesy D, Perry K, Delano C, Konstam MA. A multicenter randomized controlled evaluation of automated home monitoring and telephonic disease management in patients recently hospitalized for congestive heart failure: the SPAN-CHF II trial. J Card Fail. 2010;16:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Jhanjee R, Templeton GA, Sattiraju S, Nguyen J, Sakaguchi S, Lu F, Ermis C, Milstein S, Van Heel L, Lurie KG. Relationship of paroxysmal atrial tachyarrhythmias to volume overload: assessment by implanted transpulmonary impedance monitoring. Circ Arrhythm Electrophysiol. 2009;2:488-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Boriani G, Gasparini M, Landolina M, Lunati M, Proclemer A, Lonardi G, Iacopino S, Rahue W, Biffi M, DiStefano P. Incidence and clinical relevance of uncontrolled ventricular rate during atrial fibrillation in heart failure patients treated with cardiac resynchronization therapy. Eur J Heart Fail. 2011;13:868-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Boveda S, Marijon E, Jacob S, Defaye P, Winter JB, Bulava A, Gras D, Albenque JP, Combes N, Pavin D. Incidence and prognostic significance of sustained ventricular tachycardias in heart failure patients implanted with biventricular pacemakers without a back-up defibrillator: results from the prospective, multicentre, Mona Lisa cohort study. Eur Heart J. 2009;30:1237-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Sack S, Wende CM, Nägele H, Katz A, Bauer WR, Barr CS, Malinowski K, Schwacke H, Leyva F, Proff J. Potential value of automated daily screening of cardiac resynchronization therapy defibrillator diagnostics for prediction of major cardiovascular events: results from Home-CARE (Home Monitoring in Cardiac Resynchronization Therapy) study. Eur J Heart Fail. 2011;13:1019-1027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Ricci RP, Morichelli L, D’Onofrio A, Calò L, Vaccari D, Zanotto G, Curnis A, Buja G, Rovai N, Gargaro A. Effectiveness of remote monitoring of CIEDs in detection and treatment of clinical and device-related cardiovascular events in daily practice: the HomeGuide Registry. Europace. 2013;15:970-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
P- Reviewer: Cornelis J, Kosmas P, Tang JM S- Editor: Qiu S L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/