Published online Jul 26, 2014. doi: 10.4330/wjc.v6.i7.638
Revised: January 26, 2014
Accepted: May 29, 2014
Published online: July 26, 2014
Processing time: 237 Days and 1.4 Hours
Calpains are ubiquitous non-lysosomal Ca2+-dependent cysteine proteases also present in myocardial cytosol and mitochondria. Numerous experimental studies reveal an essential role of the calpain system in myocardial injury during ischemia, reperfusion and postischemic structural remodelling. The increasing Ca2+-content and Ca2+-overload in myocardial cytosol and mitochondria during ischemia and reperfusion causes an activation of calpains. Upon activation they are able to injure the contractile apparatus and impair the energy production by cleaving structural and functional proteins of myocytes and mitochondria. Besides their causal involvement in acute myocardial dysfunction they are also involved in structural remodelling after myocardial infarction by the generation and release of proapoptotic factors from mitochondria. Calpain inhibition can prevent or attenuate myocardial injury during ischemia, reperfusion, and in later stages of myocardial infarction.
Core tip: Calpains, calcium-dependant cytosolic cysteine proteases, are essentially involved in the pathophysiology of myocardial infarction. Their inhibition has shown in animal experiments an enhanced tolerance towards ischemia, a reduction of myocardial infarction and reperfusion injury, and an improvement of the process of remodelling. The availability of specific calpain inhibitors offers new prophylactic and therapeutic possibilities for patients with myocardial infarction, revascularisation and coronary surgery.
- Citation: Neuhof C, Neuhof H. Calpain system and its involvement in myocardial ischemia and reperfusion injury. World J Cardiol 2014; 6(7): 638-652
- URL: https://www.wjgnet.com/1949-8462/full/v6/i7/638.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i7.638
Calpains are calcium-dependent, cytosolic cysteine proteases and are expressed as two “ubiquitous” isoenzymes (μ- and m-calpains) and several “tissue specific” isoforms (n-calpains). Their primary structure contains as well calmodulin-like calcium-binding proteins as well as papain protease-like components, reflected by the term calpain[1]. A non-lysosomal Ca2+-activated cysteine protease was isolated for the first time by Guroff[2] 1964 from rat brain. Calpains are meanwhile found in all cells of vertebrates that have been examined[2-5], in cells of invertebrates[6,7] and fungi[8], but not in bacteria and plants.
Besides their physiological functions they are also implicated in pathophysiological processes[4,9-12], especially with disturbed calcium homeostasis[4,13,14]. Thus, calpains were found to be involved in myocardial tissue damage resulting from ischemia and reperfusion[15,16]. Calpain inhibition on the other hand ameliorates, respectively, prevents these lesions in animal experiments with potential prophylactic and therapeutic implications even in clinical situations.
The following review will give an overview of the physiological and pathophysiological basis of the calpain system and finally focus on its role in myocardial ischemia, infarction and reperfusion and the effectiveness of calpain inhibition based on experimental studies.
The terms μ-calpain and m-calpain were first used by Cong et al[17] in 1989. They indicate the micromolar (μ-calpain) respectively millimolar (m-calpain) Ca2+-concentrations required for their activation. Thus, μ-calpain is activated in the presence of 3-50 μmol/L Ca2+ and m-calpain in the presence of 400-800 μmol/L Ca2+[17,18]. Meanwhile, more than 25 proteins with structural similarities were identified as calpains or calpain-like molecules. The genes assigned to 15 of these proteins are numerically named as CAPN1 up to CAPN15 and their coded molecules are named as calpain1 up to calpain15, correspondingly. Calpain1 as well as Calpain2 are biologically active as proteases not as monomers but only as dimers with an identical 30-kDa subunit each. Both biologically active calpains are usually called μ-calpain (calpain 1 + 30-kDa subunit) and m-calpain (calpain 2 + 30-kDa subunit), respectively[4,12].
According to Suzuki et al[19] calpains are subdivided into two main categories: (1) “typical” calpains with a calmodulin-like domain IV at their COOH-terminus; and (2) “atypical” calpains without this component. Typical calpains are μ-calpain, m-calpain and the calpains 5, 7, 10, 13 and 15 which are also named ”ubiquitous” calpains as they are present in almost all cells of vertebrates. In contrast to the ”ubiquitous” calpains the “tissue-specific” calpains are exclusively expressed in special cells and tissues, such as calpain 3 in skeletal muscle[20], calpain 6 in placenta and embryonic muscles[21], calpain 8 and 9 in the gastrointestinal tract[22], calpain 11 in the testis[23], and calpain 12 in hair follicles[24].
Domain structure ofμ- and m-calpain
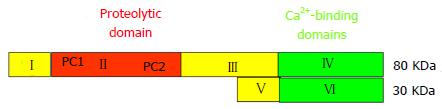
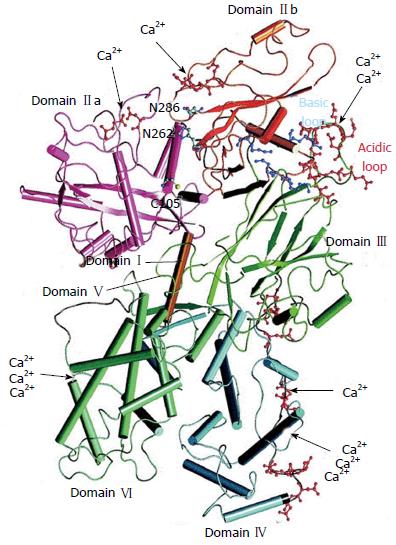
Both proteases μ- and m-calpain exist as dimers with two subunits of 80-kDa and 30-kDa each (Figure 1)[25,26]. The larger 80-kDa catalytic subunits of μ-calpain and m-calpain are coded in humans by different genes on chromosome 11 respectively chromosome 1[27]. On the base of their amino acid sequences they are composed of four regions/domains: (1) a N-terminal domain; (2) a catalytic CysPc protease domain consisting of two protease core regions PC1 and PC2; and (3) a C2-like Ca2+-regulated phospholipid-binding domain, and IV a Ca2+-binding penta-EF-hand domain[28-31] .
Domain I contains an amphipathic alpha-helix in the N-terminus of μ-calpain which was shown to be important in targeting and migrating of μ-calpain into the intermembrane space of mitochondria. Domain I of m-calpain, however, does not contain a similar N-terminal component[32].
Domain II represents the catalytic CysPc protease domain. It consists of two separate protease core domains PC1 with a cysteine (Cys) residue and PC2 with a histidine (His) residue and an asparagine (Asn) residue. These residues form a catalytic triade as known from cysteine proteases such as papain or cathepsin (Figure 2). Both core domains PC1 and PC2 have Ca2+-binding sites for a single Ca2+ by each[33,34].
Domain III is structurally related to C2 domains and can bind phospholipids in a Ca2+-dependent manner. It links the Ca2+-binding domains with the catalytic domain II and is supposed to be involved in the adjustment of the calpain activity via electrostatic interactions[35].
Domain IV shows a slight sequence homology to calmodulin (51%-54%) and has five Ca2+-binding COOH-terminal EF-hand motifs. The fifth motif binds to the corresponding EF-hand sequences of domain VI of the smaller 30 kDa subunit and, thus, contributes to the dimer formation of both calpain subunits[4,31,33,36].
The smaller regulatory 30 kDa subunit, responsible for the stability of the larger catalytic subunit, consists of the N-terminal Gly-rich domain V and the Ca2+-binding calmodulin-like penta-EF-hand domain VI. The long streches of Gly residues and an unordered structure of the amino acid sequence in domain V are supposed to bind to other molecules and structures.
The “calmodulin-like” domain VI is involved in Ca2+-binding and dimerization by their penta-EF-hand motifs, as also known from domain V of the 80-KDa subunit[4,31,37,38].
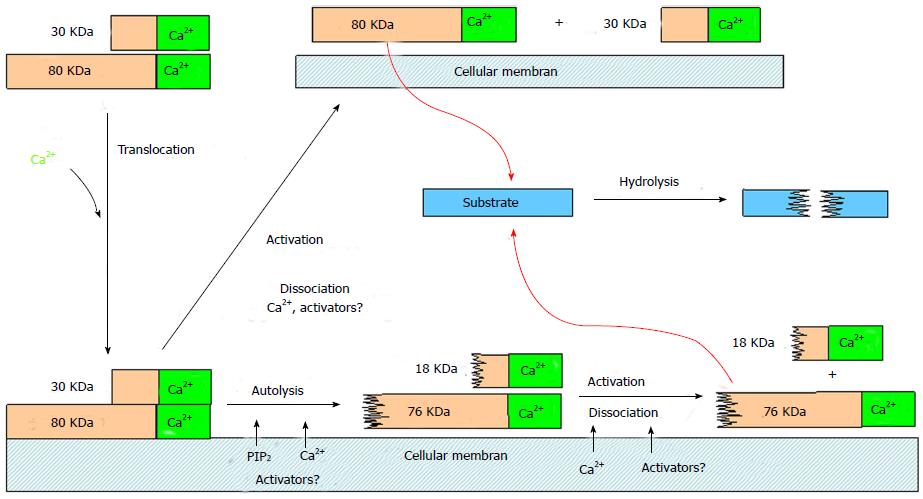
Increase of the intracellular Ca2+-concentration is the decisive trigger for calpain activation. The Ca2+-binding core domains PC1 and PC2 of domain II and the terminal EF-hand motifs of domain V and VI cause electrostatic conformational changes in these domains. By this electrostatic switch mechanism the PC1 and PC2 core domains approaches each other. Thus the distance of the Cys-residue from the αHis- and Asn-residues of the initially inactive catalytic triade shrinks from 10 to approximately 3.7 Å to form the proteolytic active centre[30,39]. Simultaneously, the change of conformation intensifies the affinity of calpain to membrane phospholipids and thus induces its translocation to the cell membranes (Figure 3)[40,41].
Immediately with the binding of Ca2+ the autolysis of both subunits of the calpain dimers happens by splitting off the NH2-terminal amino acids. The 80-kDa subunits of μ- and m-calpain are reduced by this process to active fragments of 76-kDa and 78-kDa, respectively, and both 30-kDa subunits are reduced to fragments of 18-kDa each[42-44]. The autolysis facilitates the dissociation and reassociation of the calpain dimers, but is not necessary for their activation, as the dissociated 80-kDa subunits are enzymatically full active[45].
Confusion still exists with regard to the Ca2+-concentration required for calpain activation. The in vitro concentrations for μ-calpain (3-50 μmol/L) and m-calpain (200-1000 μmol/L) to cause a half-maximal calpain activity are far above the physiological concentrations of 100-300 nmol/L necessary in living cells[46-48]. Additional mechanisms and factors are therefore supposed to contribute to the activation and activity in a physiological environment. Autolysis is known to increase the Ca2+-sensitivity of μ- and m-calpain for activation[19,49], however, the problem remains, that far higher Ca2+-concentrations are required to initiate autolysis as they occur in a physiological environment[50]. Autolysis normally happens in contact with biological membranes in presence of phospholipids such as PIP2 which considerably reduces the Ca2+-concentration necessary for autolysis[10,51]. Thus, in presence of PIP2 autolysis of μ-calpain already happens with 10-5-10-7 mol Ca2+.
In addition, activator proteins from rat brain lower the Ca2+-concentrations necessary for autolysis of μ-calpain to a tenth[52] and from rat skeletal muscle for autolysis of m-calpain from 400 μmol/L to 15 μmol/L[53]. Both activators are Ca2+-binding proteins combining with calpains and becoming effective upon contact with cell membranes. Further activator proteins are known which increase the catalytic activity of calpains against particular substrates twice[54], ten times[55] or twenty-five times[56] without influencing the required Ca2+-concentration.
Calpastatin is the only known specific endogenous inhibitor and regulator of μ- and m-calpain. In addition also H-kininogen and α2-macroglobulin are inhibiting calpain besides other proteases[57]. Human calpastatin is encoded by a single gene on chromosome 5[58] and expressed in several isoforms from 17.5 to 107 kDa[59-61]. It consists of four inhibitory domains I, II, III and IV, and one N-terminal domain L without inhibitory capability[62,63]. Each inhibitory unit inhibits one calpain molecule competitively by blocking the substrate access to the catalytic centre[64,65]. Calpastatin inhibits exclusively calpain and not other proteases[57]. Binding of calpastatin to calpain and its inhibition is Ca2+-dependent. The Ca2+-concentrations for this are lower as needed for the half-maximal proteolytic activity of μ- and m-calpain[66]. Calpains and calpastatin are found in physical proximity within the cells[67,68]. Therefore, mechanisms are necessary to enable calpain to perform its biological purpose, since calpastatin already binds to calpain with increasing Ca2+-concentrations. Thus, the translocation of calpain to the membranes could cause a spatial distance to calpastatin. Furthermore, special mechanisms/factors could lower the threshold for Ca2+ to activate calpain without influencing the binding of calpastatin[3]. With regard to activation and deactivation of calpain many questions are still open concerning a regulating, respectively, modifying role of substrate phosphorylation.
Localization ofμ- and m-calpain in cell and tissue
In all examined cells of vertebrates μ-calpain, m-calpain and calpastatin are found at least as the only constituents of the calpain system or they exist in various combinations with great varying patterns of distribution. Thus, human erythrocytes and platelets only contain μ-calpain, and smooth muscles of vessels and stomach predominantly contain m-calpain, whereas, in skeletal muscles and kidneys of the most representatives of vertebrates nearly equal amounts of μ- and m-calpain are found[67,69,70]. Both calpains as well as calpastatin are exclusively localized intracellular and apparently associated with subcellular structures. Thus, 93% of the μ-calpain are found in human red blood cells within the cytosol and 7% membrane associated[71]. Most of the μ-calpain, m-calpain and calpastatin is localized close to the Z-disc in the myofibrils of skeletal and cardiac muscle, smaller amounts are found in the I- and A-bands. In mitochondria and nuclei only a tenth, respectively, a fifth of calpains and calpastatin was identified compared to their concentration in the Z-disc region[67,72,73]. Calpain and calpastatin are normally localized with a close spatial proximity.
Normally, calpains have only access to intracellular substrates, whereby their cleavage decisively depends on the local activity of calpain and its inhibitor calpastatin. Many proteins are cleaved by calpains in vitro, but there is no conclusive evidence that they cannot also be splitted by calpain in vivo.
Calpain cleaves the cytoskeleton and membrane-associated proteins: adducin[74], ankyrin[75], caldesmon[9], cadherin[76,77], C-protein[78], desmin[79], dystrophin[80], the filamin/actin-binding proteins MAP1 and MAP2[81], myosin[82], the neurofilament-proteins NFH, NFM and NFL[83], NR2-subunit[84], the anchoring protein PSD-95 of NMDA-receptors[85], αII-spectrin[16], ß-spectrin[86], talin[87,88], titin[89], tropomyosin and troponin I[78], troponin T[90], vimentin[79,91], and vinculin[92].
Furthermore, kininases, phosphatases and transcription factors are cleaved, such as: EGF-rezeptor-kinase[93], myosin light-chain kinase[94], protein-kinase C[95], calcineurin[96], inositol-polyphosphat-4-phosphatase[97], protein-tyrosin-phosphatase-1B[98], the transcription factors c-Jun, c-Fos[99,100], and p53[101,102].
Physiological function ofμ- and m-calpain
Calpains are not seen to play an essential role in the intracellular protein digestion. In contrast to lysosomal proteases and the proteasome calpains split proteins by a limited proteolysis into large fragments with potential regulatory and signalling functions[4]. Many studies including experiments with transgenic mice indicate, that calpains are involved in the embryonic development and cell function[103-105], cytoskeletal/membrane attachments/cell motility[79,81,86-88,106], intracellular signal transduction[95,107-109], cell cycle[110,111], regulation of gene expression[99,101], apoptosis[112-115], and in the long-term potentiation of synaptic transmission[84,85,116].
A lacking synthesis of calpains or the dysregulation of the calpain activity disturbing the proteolysis of structural and regulatory proteins is found in a series of genetic and acquired diseases, such as: limb girdle muscular dystrophy (LGMD2A)[117,118], muscular dystrophy (type Duchenne and Becker)[119], diabetes mellitus (type 2)[120], gastric cancer[121], Alzheimer’s disease[122-125], multiple sclerosis[126,127], and cataract formation[127].
Many vital cell functions are regulated by the concentration of intracellular available Ca2+, such as muscle contraction, neurotransmitter release, glandular secretion, and intercellular communication[128,129]. And last but not least, calpains are Ca2+-activated proteases. Because of its key role, normally the Ca2+ concentration is controlled at different cellular levels via mitochondria, plasmalemma/sarcolemma and endoplasmatic reticulum. The transmembrane transport of ions is regulated actively, selectively and directionally-oriented by voltage gated ion channels, by ATP-consuming ion pumps (Na+-K+-ATPases, Ca2+-ATPases, proton-ATPases) and by the concentration gradient due to carrier proteins (Na+/H+-exchanger, Na+/HCO3- -symporter, Na+/Ca2+-exchanger)[130-133]. Failing of this control mechanisms may result in an excessive intracellular accumulation of Ca2+ (Ca2+-overload) with severe cellular dysfunction up to cell death[14,134,135].
Studies with isolated perfused mammalian hearts have shown an increasing cytosolic Ca2+ concentration during hypoxia in hearts of rabbits[136] and ferrets[137], during ischemia in hearts of rabbits[138] and rats[139], and during post-ischemic reperfusion in hearts of rats[140] and ferrets[141]. Severe burn trauma also augments the Ca2+ content in myocytes[142,143] and mitochondria[144] of rat hearts. The same effect can be observed upon exposure of isolated perfused rabbit hearts[145] and isolated rat cardiomyocytes[146,147] to hydroxyl free radicals. In analogy to the heart, a Ca2+-overload was also observed in rat brains[148,149] during hypoxia/ischemia and in the spinal cord[150] after traumatisation.
The underlying mechanisms and consequences of an imbalance in Ca2+ homeostasis are documented the most extensively in heart during hypoxia, ischemia and postischemic reperfusion. They are initiated by the decreasing ATP generation and developing acidosis resulting from oxygen deficiency. The activation of the Na+/H+-exchanger (NHE-1)[132,151,152], which causes the influx of Na+ into the cell for exchange with H+ in order to regulate pH, and the simultaneous inhibition of the Na+-K+-ATPase[153], due to lack of ATP, plays a key role in the intracellular Ca2+-overload. Thus, Na+ accumulates intracellular and lowers the transmembranous Na+ gradient, which is the driving force behind the Na+/Ca2+-exchanger by transporting Ca2+ out off the cell, resulting in Ca2+-accumulation. The Na+/Ca2+-exchanger which represents a bidirectional transport system is also able to transport Ca2+ in exchange with Na+ in a reverse mode into the cell[152,154,155]. Driving forces for this are the increasing intracellular Na+ concentration and depolarisation of the sarcolemma.
Today, disturbance of Ca2+-homeostasis is seen as the main triggering factor of cardial dysfunction and myocardial injury during ischemia and reperfusion, such as the myocardial stunning, a long-lasting reversible reduction of heart contraction after ischemia[156-158], or like the Ca2+-overload induced hypercontracture during reperfusion/reoxygenation[14,159-161], or the incidence of arrhythmias during reperfusion[162]. Other factors, such as reactive oxygen species or inflammation seem to play a minor role in these situations[163].
Many studies demonstrate as a consequence of an increasing intracellular Ca2+-concentration the activation of calpains, which cleave numerous functional and structural proteins, and thereby decisively contribute to ischemic and postischemic injury. Thus, the activation of the calpain system during hypoxia or ischemia is well documented in the myocardium of rats[164-167] and humans[168], as well as in the brain of rats[169-171]. In rat renal proximal tubules hypoxia induces the increase of μ-calpain activity[172], whereas calpain inhibition reduces the renal functional and structural damage following ischemia and reperfusion[173]. Hypoxia was also found to up-regulate the activity and gene expression of calpains in endothelial cells of the pulmonary artery[174].
Most studies on the implication of calpains for myocardial dysfunction and failure are based on experiments in isolated perfused mammalian hearts, in which the duration of perfusion stop (global ischemia) is restricted to enable at least a recovery with reperfusion.
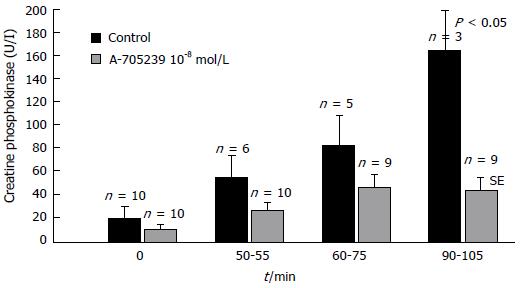
Global ischemia in isolated perfused rat hearts was found to induce a time-dependent translocation of m-calpain to the membrane initially not associated with calpain activation which occurred only during reperfusion and intracellular pH normalization[175]. Under comparable conditions, a loss of myofibrillar desmin, α-actinin, and spectrin was observed in guinea pig hearts, which was reduced by calpain inhibitor I[176]. Immunohistochemical studies revealed the proteolysis of calspectin and α-fodrin at the intercalated discs and the sarcolemma after postischemic reperfusion in rat hearts. Degradation of both proteins could be suppressed and myocardial function improved by calpain inhibitor I[16,177]. The inhibition of α-fodrin degradation associated with the attenuation of myocardial dysfunction could also be observed after cardioplegic cardiac arrest in rat hearts in the presence of calpain inhibitor SNJ-1945[178]. As a result of calpain activation, the essential Ca2+-handling proteins Ca2+-ATPase (SERCA2a) and the SERCA regulatory protein PLB were degraded upon global ischemia and reperfusion in a working rat heart preparation. Their degradation, the depression of cardiac performance and the release of lactate dehydrogenase, indicating the myocardial damage, could be significantly attenuated by calpain inhibition with calpain inhibitor III (MDL28170)[179]. As an indicator of myocardial tissue damage creatine phosphokinase and lactate dehydrogenase are released from myocytes into the perfusion fluid during reperfusion in concentrations dependent on the duration of ischemia (Figure 4). Calpains seem to be responsible or to contribute to these effects, as calpain inhibition with A-705239 significantly reduces the enzyme release[180].
Cardiac muscle contraction is initiated by Ca2+via troponin/tropomyosin which are known as substrates of calpain. Therefore, their cleavage is supposed to be jointly responsible for myocardial dysfunction in ischemia/reperfusion injury. With regard to this, degradation of troponin T (TnT) was observed during ischemia/reperfusion of isolated perfused rat hearts and was reduced by calpain inhibition with PD150606 and PD151746[181]. In addition, “overexpression of calpastatin by gene transfer prevents troponin I (TnI) degradation and ameliorates contractile dysfunction in rat hearts subjected to global ischemia followed by reperfusion”[182].
Damage of mitochondria plays a central role in the pathophysiology of reperfusion injury via the impairment of oxidative metabolism, respectively, energy production and the generation and accumulation of metabolic products toxic to the myocytes. Cardiac mitochondria are located subsarcolemmal beneath the plasma membrane and interfibrillar between the myofibrils[183-185]. In animal and human hearts μ-calpain, m-calpain and calpain 10 are present in cytosol and in the intermembrane space of mitochondria[67,186-189]. Cytosolic calcium content is found to increase in hearts of rats and rabbits during myocardial ischemia and reperfusion and is made responsible for the subsequent activation of calpains[190,191]. The damage of Ca2+-handling proteins by direct cleaving or detaching the Na+/K+-ATPase and the Na+/Ca2+-exchanger from their binding ankyrin[174,192], and by proteolysis of sarcoplasmic reticulum Ca2+-ATPase (SERCA)[179,193] and Ryanodine receptor RyR)[194], sustains Ca2+-influx and calpain activation and aggravates myocardial injury. Thus, SERCA2a and the SERCA regulatory protein PLB were found to be degraded upon global ischemia and reperfusion in a working rat heart preparation. Their degradation, the depression of cardiac performance and the release of lactate dehydrogenase, indicating the myocardial damage, could be significantly attenuated by calpain inhibition with calpain inhibitor III (MDL28170)[179].
One of the most serious consequences of mitochondrial damage by calpains is the impairment of oxidative phosphorylation with loss of ATP generation. Damage to mitochondrial oxidative metabolism can be caused on various levels of the electron transport chain (ETC). In isolated renal cortical mitochondria from rats and rabbits calpain 10 was shown to cleave complex I subunits of the ETC, which could be prevented by pretreatment with calpeptin[195]. The impairment of mitochondrial respiration is documented in isolated perfused rabbit hearts[180,196]. State 3 respiration decreased significantly during 45 min of global ischemia and further decreased during 60 min of reperfusion, and this reaction could be significantly attenuated by addition of calpain inhibitor A-705239 to the perfusion fluid (Table 1).
| n | State 3 respiration (nmol O2/min per milligram) | State 4 respiration (nmol O2/min per milligram) | RCI (state3 rate): (state 4 rate) | Leak respiration(nmol O2/min per milligram) | Stimulation by cytochrome c % | |
| Control | ||||||
| Before ischemia | 4 | 6.4 ± 1.1 | 0.5 ± 0.1 | 12.5 ± 2.7 | 0.15 ± 0.07 | 6.0 ± 10.0 |
| Ischemia 45 min | 8 | 3.5 ± 1.4ac | 0.9 ± 0.3a | 4.4 ± 2.5a | 0.32 ± 0.14a | 10.0 ± 6.0 |
| Reperfusion 60 min | 4 | 2.6 ± 1.3ac | 0.9 ± 0.3a | 3.2 ± 2.1a | 0.43 ± 0.29 | 28.0 ± 16.0 |
| A-705239 treated hearts | ||||||
| Before ischemia | 4 | 6.8 ± 1.3 | 0.6 ± 0.1 | 12.4 ± 1.1 | 0.12 ± 0.06 | 16.0 ± 9.0 |
| Ischemia 45 min | 9 | 5.0 ± 0.8ac | 0.6 ± 0.2 | 8.2 ± 2.3ac | 0.20 ± 0.14a | 15.0 ± 13.0 |
| Reperfusion 60 min | 5 | 4.2 ± 1.2ac | 0.7 ± 0.2 | 6.4 ± 2.7a | 0.26 ± 0.24 | |
Reduced state 3 respiration reflects the impairment of the electron transport chain (ETC), above all complex I, which is an early target of myocardial ischemia[197].
Calpain inhibitor A-705239 administered before ischemia and reperfusion also attenuated the increase in permeability of the inner mitochondrial membrane (mitochondrial permeability transition), as reflected by the reduced state 4 respiration and leak-respiration[180].
Besides their deleterious effect on mitochondrial oxidative metabolism, calpains are also recognized to cause the generation and release of substances toxic to myocytes.
During reperfusion, mitochondria generate reactive oxygen species that lead to additional mitochondrial and myocyte injury[197-200].
Dependent on the degree of oxidative damage in concert with mitochondrial calcium overload and calpain activation, mitochondrial permeability transition can occur by formation of inner membrane pores[201,202]. Mitochondrial permeability transition can result in disruption of the outer mitochondrial membrane and the release of cytochrome c, a key step inducing apoptosis[203]. Cytochrome c is detectable in the cytosol of rabbit myocardium at 30 min of ischemia[204], whereas cytochrome c content decreases in subsarcolemmal mitochondria[205]. Mitochondrial calpain plays an important role in programmed cell death by generation or release of apoptotic factors in mitochondria during ischemia and reperfusion. Thus, the cleavage of Bid, a pro-apoptotic BH3-only Bcl-2 family member, is documented in isolated perfused adult rabbit hearts during ischemia/reperfusion, and in secondary in vitro studies recombinant Bid was cleaved by calpain to an active fragment that was able to mediate cytochrome c release[206]. It was also shown, that activated mitochondrial μ-calpain, mostly located in the intermembrane space, cleaves and releases apoptosis inducing factor (AIF) from isolated mouse heart mitochondria. Besides, mitochondrial μ-calpain activity increased in buffer perfused mouse hearts during ischemia/reperfusion whereas the mitochondrial AIF content decreased. Inhibition of mitochondrial μ-calpain using MDL-28170 preserved the AIF content within the mitochondria and reduced cardiac injury[186].
In contrast to models of global ischemia, in the experimental setting of partial ischemia by temporary occlusion of coronary arteries the duration of ischemia can be extended in time to enable irreversible myocardial damage to a restricted area with myocardial infarction without the risk of early global heart failure with reperfusion. In isolated perfused rat hearts it was shown, that during a 30 min occlusion of the left anterior descendent coronary artery calpain translocates to the cell membranes without being activated initially. Calpain activation, as indicated by the hydrolysis of α-fodrin, only started with the onset of reperfusion and could be prevented by calpain inhibition with MDL-28170, just as the infarct size could be reduced by 32%[175].
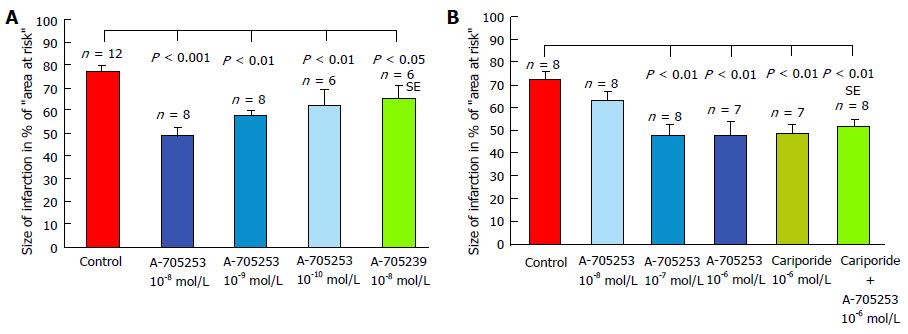
Inhibition of α-fodrin degradation and improvement of left ventricular function by calpain inhibitor SNJ-1945, administered 30 min before a gradual and partial coronary occlusion, was also found after mild ischemic-reperfusion in another study in rat hearts[207]. Protecting effects of calpain inhibition on myocardial injury could also be demonstrated by own experiments with inhibitor administration both before and during reperfusion. “Two novel calpain inhibitors (A-705239 and A-705253) were studied in isolated perfused rabbit hearts subjected to a 60 min occlusion of the ramus interventricularis of the left coronary artery (below the origin of the first diagonal branch), followed by 120 min of reperfusion[208,209]. The inhibitors were added to the perfusion fluid in various final concentrations from the beginning of the experiments before the coronary artery was blocked. The infarct size was significantly reduced in presence of both calpain inhibitors. The best effect was achieved with 10-8 mol/L A-705253 which reduced the infarcted area by 61.8 % (Figure 5A). In a second study in isolated perfused rabbit hearts subjected to a 60 min occlusion of the ramus interventricularis of the left coronary artery followed by 120 min of reperfusion calpain inhibitor A-705253 and/or the Na+/H+-exchange inhibitor cariporide® were added to the perfusion fluid at the beginning of reperfusion solely or in combination[210]. The infarct size was significantly reduced dose-dependently in presence of both inhibitors (Figure 5B). The best effect was achieved with 10-6 mol/L A-705253, which reduced the infarcted area by 33.6%. Cariporide® (10-6 mol/L) reduced the infarct size in the same extent. The combination of both inhibitors, however, didn’t further improve cardioprotection. Thus, the protective effect can be attributed exclusively to its influence on the calpain system, since the combination of both inhibitors didn’t augment the protective effect of sole calpain inhibition. The calpain inhibitor A-705253 is known to directly block the catalytic centre of activated calpains, whereas the Na+/H+-exchange inhibitor cariporide® prevents or reduces the ischemic intracellular Ca2+-overload and thus prevents or reduces the following calpain activation”. This is shown in postischemic perfused rat and rabbit hearts where reduced calpain activation[211] and calcium overload[212] were observed upon inhibition of Na+/H+-exchange. Even in patients undergoing coronary bypass surgery pretreatment with cariporide® reduced mortality and the risk of myocardial infarction[213], however, cerebrovascular events increased[214]. In accordance with the findings in rabbit hearts, also in pigs undergoing occlusion of the left anterior descending coronary artery for 45 min followed by 6 h of reperfusion infarct size was reduced by 35% and hemodynamic alterations attenuated using calpain inhibitor A-705253[215]. In experiments with isolated mouse hearts undergoing ischemia and reperfusion infarct size was decreased and ventricular function improved in calpain-1 kockout mice, whereas myocardial injury was greatly increased in transgenic mice hearts with calpain-1 overexpression[216].
No sufficient information is available to what extent polymorphonuclear leukocytes (PMN) contribute to ischemic/reperfusion injury. In one study in isolated rat hearts perfused with PMNs, exposed to 20 min of ischemia and followed by 45 min of reperfusion, calpain inhibition with Z-Leu-Leu-CHO reduced the adherence of PMNs to the vascular endothelium and improved ventricular function, however, controls without PMNs are missing[217]. Thus, with regard to the numerous experiments discussed in this review, which were all performed without PMNs in the perfusion fluid, polymorphonuclear leukocytes appear not to be essential for reperfusion injury.
Myocardial infarction is followed by a progressive structural remodelling of the heart, replacing and reconstructing the irreversibly damaged myocardium[218,219]. After the early phase of ischemia-induced myocyte necrosis a longer lasting myocyte death by apoptosis can be observed. Proapoptotic factors are generated and released from myocardial mitochondria already during ischemia and reperfusion which are considered to be essentially involved in remodelling after myocardial infarction[186,203,206]. Characteristics of apoptosis, DNA fragmentation and chromatin condensation, could be detected in isolated perfused rabbit hearts subjected to 30 min ischemia and 4 h reperfusion[220]. In ischemic/reperfused rat hearts undergoing 30 min coronary occlusion followed by 6 h reperfusion the administration of calpain inhibitor I (CAI) 10 min before reperfusion significantly reduced DNA fragmentation and infarct size[221]. Comparable results were achieved in mouse hearts with persistent coronary artery ligation for 4 d. Calpain inhibition with calpeptin was started 15 min before artery occlusion and continued during the observation time. Calpeptin administration reduced apoptotic cell death, as detected by TUNEL staining, and reduced infarct size and myocardial dysfunction[222]. The important contribution of calpains to the process of myocardial remodelling is also documented by a transgenic mouse model with cardiomyocyte-specific deletion of gene Capn4 (Capn4-ko) which is indispensable for μ- and m-calpain stability and activity. Mice were subjected to persistent left coronary artery ligation and followed up for 30 d. Deletion of Capn4 reduced infarct expansion, apoptosis, myocardial remodelling and dysfunction[223].
Numerous studies have shown an essential contribution of calpains in myocardial injury following ischemia and reperfusion. Proven prevention or attenuation of postischemic heart damage by calpain inhibition with various tested inhibitors could offer a novel prophylactic or therapeutic approach for patients with myocardial infarction, revascularisation and coronary surgery.
| 1. | Ohno S, Emori Y, Imajoh S, Kawasaki H, Kisaragi M, Suzuki K. Evolutionary origin of a calcium-dependent protease by fusion of genes for a thiol protease and a calcium-binding protein? Nature. 1984;312:566-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 268] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Guroff G. A neutral, calcium-activated proteinase from the soluble fraction of rat brain. J Biol Chem. 1964;239:149-155. [PubMed] |
| 3. | Mellgren RL. Canine cardiac calcium-dependent proteases: Resolution of two forms with different requirements for calcium. FEBS Lett. 1980;109:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 211] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731-801. [PubMed] |
| 5. | Dayton WR, Goll DE, Zeece MG, Robson RM, Reville WJ. A Ca2+-activated protease possibly involved in myofibrillar protein turnover. Purification from porcine muscle. Biochemistry. 1976;15:2150-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 298] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Beyette JR, Ma JS, Mykles DL. Purification and autolytic degradation of a calpain-like calcium-dependent proteinase from lobster (Homarus americanus) striated muscles. Comp Biochem Physiol B Biochem. 1993;104:95-99. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Pintér M, Friedrich P. The calcium-dependent proteolytic system calpain-calpastatin in Drosophila melanogaster. Biochem J. 1988;253:467-473. [PubMed] |
| 8. | Ojha M, Wallace CJ. Novel Ca2+-activated neutral protease from an aquatic fungus, Allomyces arbuscula. J Bacteriol. 1988;170:1254-1260. [PubMed] |
| 9. | Croall DE, DeMartino GN. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev. 1991;71:813-847. [PubMed] |
| 10. | Saido TC, Sorimachi H, Suzuki K. Calpain: new perspectives in molecular diversity and physiological-pathological involvement. FASEB J. 1994;8:814-822. [PubMed] |
| 11. | Carafoli E, Molinari M. Calpain: a protease in search of a function? Biochem Biophys Res Commun. 1998;247:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 278] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 12. | Sorimachi H, Ono Y. Regulation and physiological roles of the calpain system in muscular disorders. Cardiovasc Res. 2012;96:11-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Inserte J, Hernando V, Garcia-Dorado D. Contribution of calpains to myocardial ischaemia/reperfusion injury. Cardiovasc Res. 2012;96:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Garcia-Dorado D, Ruiz-Meana M, Inserte J, Rodriguez-Sinovas A, Piper HM. Calcium-mediated cell death during myocardial reperfusion. Cardiovasc Res. 2012;94:168-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 243] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 15. | Papp Z, van der Velden J, Stienen GJ. Calpain-I induced alterations in the cytoskeletal structure and impaired mechanical properties of single myocytes of rat heart. Cardiovasc Res. 2000;45:981-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Yoshida K, Inui M, Harada K, Saido TC, Sorimachi Y, Ishihara T, Kawashima S, Sobue K. Reperfusion of rat heart after brief ischemia induces proteolysis of calspectin (nonerythroid spectrin or fodrin) by calpain. Circ Res. 1995;77:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 134] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Cong J, Goll DE, Peterson AM, Kapprell HP. The role of autolysis in activity of the Ca2+-dependent proteinases (mu-calpain and m-calpain). J Biol Chem. 1989;264:10096-10103. [PubMed] |
| 18. | Suzuki K. Nomenclature of calcium dependent proteinase. Biomed Biochim Acta. 1991;50:483-484. [PubMed] |
| 19. | Suzuki K, Sorimachi H, Yoshizawa T, Kinbara K, Ishiura S. Calpain: novel family members, activation, and physiologic function. Biol Chem Hoppe Seyler. 1995;376:523-529. [PubMed] |
| 20. | Sorimachi H, Imajoh-Ohmi S, Emori Y, Kawasaki H, Ohno S, Minami Y, Suzuki K. Molecular cloning of a novel mammalian calcium-dependent protease distinct from both m- and mu-types. Specific expression of the mRNA in skeletal muscle. J Biol Chem. 1989;264:20106-20111. [PubMed] |
| 21. | Dear N, Matena K, Vingron M, Boehm T. A new subfamily of vertebrate calpains lacking a calmodulin-like domain: implications for calpain regulation and evolution. Genomics. 1997;45:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Sorimachi H, Ishiura S, Suzuki K. A novel tissue-specific calpain species expressed predominantly in the stomach comprises two alternative splicing products with and without Ca(2+)-binding domain. J Biol Chem. 1993;268:19476-19482. [PubMed] |
| 23. | Dear TN, Möller A, Boehm T. CAPN11: A calpain with high mRNA levels in testis and located on chromosome 6. Genomics. 1999;59:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Dear TN, Meier NT, Hunn M, Boehm T. Gene structure, chromosomal localization, and expression pattern of Capn12, a new member of the calpain large subunit gene family. Genomics. 2000;68:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Emori Y, Kawasaki H, Imajoh S, Kawashima S, Suzuki K. Isolation and sequence analysis of cDNA clones for the small subunit of rabbit calcium-dependent protease. J Biol Chem. 1986;261:9472-9476. [PubMed] |
| 26. | Imajoh S, Aoki K, Ohno S, Emori Y, Kawasaki H, Sugihara H, Suzuki K. Molecular cloning of the cDNA for the large subunit of the high-Ca2+-requiring form of human Ca2+-activated neutral protease. Biochemistry. 1988;27:8122-8128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 106] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Ohno S, Minoshima S, Kudoh J, Fukuyama R, Shimizu Y, Ohmi-Imajoh S, Shimizu N, Suzuki K. Four genes for the calpain family locate on four distinct human chromosomes. Cytogenet Cell Genet. 1990;53:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Suzuki K. The structure of the calpains and the calpain gene. In: Intracellular Calcium-dependent Proteolysis (edited by Mellgren RL, and Murachi T) CRC Press, Boca Raton, FL 1990; 25-35. |
| 29. | Hosfield CM, Elce JS, Davies PL, Jia Z. Crystal structure of calpain reveals the structural basis for Ca(2+)-dependent protease activity and a novel mode of enzyme activation. EMBO J. 1999;18:6880-6889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 262] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 30. | Strobl S, Fernandez-Catalan C, Braun M, Huber R, Masumoto H, Nakagawa K, Irie A, Sorimachi H, Bourenkow G, Bartunik H. The crystal structure of calcium-free human m-calpain suggests an electrostatic switch mechanism for activation by calcium. Proc Natl Acad Sci USA. 2000;97:588-592. [PubMed] |
| 31. | Maki M, Narayana SV, Hitomi K. A growing family of the Ca2+-binding proteins with five EF-hand motifs. Biochem J. 1997;328:718-720. [PubMed] |
| 32. | Badugu R, Garcia M, Bondada V, Joshi A, Geddes JW. N terminus of calpain 1 is a mitochondrial targeting sequence. J Biol Chem. 2008;283:3409-3417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Suzuki K, Hata S, Kawabata Y, Sorimachi H. Structure, activation, and biology of calpain. Diabetes. 2004;53 Suppl 1:S12-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 303] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 34. | Sorimachi H, Hata S, Ono Y. Impact of genetic insights into calpain biology. J Biochem. 2011;150:23-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Tompa P, Emori Y, Sorimachi H, Suzuki K, Friedrich P. Domain III of calpain is a ca2+-regulated phospholipid-binding domain. Biochem Biophys Res Commun. 2001;280:1333-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 115] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Ohno S, Emori Y, Suzuki K. Nucleotide sequence of a cDNA coding for the small subunit of human calcium-dependent protease. Nucleic Acids Res. 1986;14:5559. [PubMed] |
| 37. | Xie X, Dwyer MD, Swenson L, Parker MH, Botfield MC. Crystal structure of calcium-free human sorcin: a member of the penta-EF-hand protein family. Protein Sci. 2001;10:2419-2425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Lin GD, Chattopadhyay D, Maki M, Wang KK, Carson M, Jin L, Yuen PW, Takano E, Hatanaka M, DeLucas LJ. Crystal structure of calcium bound domain VI of calpain at 1.9 A resolution and its role in enzyme assembly, regulation, and inhibitor binding. Nat Struct Biol. 1997;4:539-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 138] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Moldoveanu T, Hosfield CM, Lim D, Elce JS, Jia Z, Davies PL. A Ca(2+) switch aligns the active site of calpain. Cell. 2002;108:649-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 252] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 41. | Hayashi M, Suzuki H, Kawashima S, Saido TC, Inomata M. The behavior of calpain-generated N- and C-terminal fragments of talin in integrin-mediated signaling pathways. Arch Biochem Biophys. 1999;371:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | McCelland P, Lash JA, Hathaway DR. Identification of major autolytic cleavage sites in the regulatory subunit of vascular calpain II. A comparison of partial amino-terminal sequences to deduced sequence from complementary DNA. J Biol Chem. 1989;264:17428-17431. [PubMed] |
| 43. | Zimmerman UJ, Schlaepfer WW. Two-stage autolysis of the catalytic subunit initiates activation of calpain I. Biochim Biophys Acta. 1991;1078:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Brown N, Crawford C. Structural modifications associated with the change in Ca2+ sensitivity on activation of m-calpain. FEBS Lett. 1993;322:65-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Yoshizawa T, Sorimachi H, Tomioka S, Ishiura S, Suzuki K. A catalytic subunit of calpain possesses full proteolytic activity. FEBS Lett. 1995;358:101-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Harkins AB, Kurebayashi N, Baylor SM. Resting myoplasmic free calcium in frog skeletal muscle fibers estimated with fluo-3. Biophys J. 1993;65:865-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 193] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Konishi M, Berlin JR. Ca transients in cardiac myocytes measured with a low affinity fluorescent indicator, furaptra. Biophys J. 1993;64:1331-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Maravall M, Mainen ZF, Sabatini BL, Svoboda K. Estimating intracellular calcium concentrations and buffering without wavelength ratioing. Biophys J. 2000;78:2655-2667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 324] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 49. | Baki A, Tompa P, Alexa A, Molnár O, Friedrich P. Autolysis parallels activation of mu-calpain. Biochem J. 1996;318:897-901. [PubMed] |
| 50. | Tompa P, Baki A, Schád E, Friedrich P. The calpain cascade. Mu-calpain activates m-calpain. J Biol Chem. 1996;271:33161-33164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Melloni E, Michetti M, Salamino F, Minafra R, Pontremoli S. Modulation of the calpain autoproteolysis by calpastatin and phospholipids. Biochem Biophys Res Commun. 1996;229:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Melloni E, Michetti M, Salamino F, Pontremoli S. Molecular and functional properties of a calpain activator protein specific for mu-isoforms. J Biol Chem. 1998;273:12827-12831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 53. | Michetti M, Viotti PL, Melloni E, Pontremoli S. Mechanism of action of the calpain activator protein in rat skeletal muscle. Eur J Biochem. 1991;202:1177-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Shiba E, Ariyoshi H, Yano Y, Kawasaki T, Sakon M, Kambayashi J, Mori T. Purification and characterization of a calpain activator from human platelets. Biochem Biophys Res Commun. 1992;182:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Takeyama Y, Nakanishi H, Uratsuji Y, Kishimoto A, Nishizuka Y. A calcium-protease activator associated with brain microsomal-insoluble elements. FEBS Lett. 1986;194:110-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 56. | DeMartino GN, Blumenthal DK. Identification and partial purification of a factor that stimulates calcium-dependent proteases. Biochemistry. 1982;21:4297-4303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Crawford C. Protein and peptide inhibitors of calpain. Boca Raton, FL: CRC 1990; 75-89. |
| 58. | Inazawa J, Nakagawa H, Misawa S, Abe T, Minoshima S, Fukuyama R, Maki M, Murachi T, Hatanaka M, Shimizu N. Assignment of the human calpastatin gene (CAST) to chromosome 5 at region q14----q22. Cytogenet Cell Genet. 1990;54:156-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | Takano E, Kitahara A, Sasaki T, Kannagi R, Murachi T. Two different molecular species of pig calpastatin. Structural and functional relationship between 107 kDa and 68 kDa molecules. Biochem J. 1986;235:97-102. [PubMed] |
| 60. | Wang LF, Wei SG, Miao SY, Liu QY, Koide SS. Calpastatin gene in human testis. Biochem Mol Biol Int. 1994;33:245-251. [PubMed] |
| 61. | Cong M, Thompson VF, Goll DE, Antin PB. The bovine calpastatin gene promoter and a new N-terminal region of the protein are targets for cAMP-dependent protein kinase activity. J Biol Chem. 1998;273:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 62. | Takano E, Maki M, Mori H, Hatanaka M, Marti T, Titani K, Kannagi R, Ooi T, Murachi T. Pig heart calpastatin: identification of repetitive domain structures and anomalous behavior in polyacrylamide gel electrophoresis. Biochemistry. 1988;27:1964-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 146] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Maki M, Takano E, Mori H, Sato A, Murachi T, Hatanaka M. All four internally repetitive domains of pig calpastatin possess inhibitory activities against calpains I and II. FEBS Lett. 1987;223:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | Emori Y, Kawasaki H, Imajoh S, Minami Y, Suzuki K. All four repeating domains of the endogenous inhibitor for calcium-dependent protease independently retain inhibitory activity. Expression of the cDNA fragments in Escherichia coli. J Biol Chem. 1988;263:2364-2370. [PubMed] |
| 65. | Betts R, Weinsheimer S, Blouse GE, Anagli J. Structural determinants of the calpain inhibitory activity of calpastatin peptide B27-WT. J Biol Chem. 2003;278:7800-7809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Otsuka Y, Goll DE. Purification of the Ca2+-dependent proteinase inhibitor from bovine cardiac muscle and its interaction with the millimolar Ca2+-dependent proteinase. J Biol Chem. 1987;262:5839-5851. [PubMed] |
| 67. | Kumamoto T, Kleese WC, Cong JY, Goll DE, Pierce PR, Allen RE. Localization of the Ca(2+)-dependent proteinases and their inhibitor in normal, fasted, and denervated rat skeletal muscle. Anat Rec. 1992;232:60-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 68. | Barnoy S, Zipser Y, Glaser T, Grimberg Y, Kosower NS. Association of calpain (Ca(2+)-dependent thiol protease) with its endogenous inhibitor calpastatin in myoblasts. J Cell Biochem. 1999;74:522-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 69. | Taylor RG, Christiansen JA, Goll DE. Immunolocalization of the calpains and calpastatin in human and bovine platelets. Biomed Biochim Acta. 1991;50:491-498. [PubMed] |
| 70. | Thompson VF, Goll DE. Purification ofµ-calpain, m-calpain, and calpastatin 68, from animal tissues. Calpain Methods and Protocols, edited by Elce, JS. Totowa, N. Humana Press. Meth Mol Biol. 2000;144:3-16. |
| 71. | Samis JA, Elce JS. Immunogold electron-microscopic localization of calpain I in human erythrocytes. Thromb Haemost. 1989;61:250-253. [PubMed] |
| 72. | Lane RD, Mellgren RL, Mericle MT. Subcellular localization of bovine heart calcium-dependent protease inhibitor. J Mol Cell Cardiol. 1985;17:863-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Yoshimura N, Murachi T, Heath R, Kay J, Jasani B, Newman GR. Immunogold electron-microscopic localisation of calpain I in skeletal muscle of rats. Cell Tissue Res. 1986;244:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Scaramuzzino DA, Morrow JS. Calmodulin-binding domain of recombinant erythrocyte beta-adducin. Proc Natl Acad Sci USA. 1993;90:3398-3402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 75. | Harada K, Fukuda S, Kunimoto M, Yoshida K. Distribution of ankyrin isoforms and their proteolysis after ischemia and reperfusion in rat brain. J Neurochem. 1997;69:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Covault J, Liu QY, el-Deeb S. Calcium-activated proteolysis of intracellular domains in the cell adhesion molecules NCAM and N-cadherin. Brain Res Mol Brain Res. 1991;11:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 77. | Sato N, Fujio Y, Yamada-Honda F, Funai H, Wada A, Kawashima S, Awata N, Shibata N. Elevated calcium level induces calcium-dependent proteolysis of A-CAM (N-cadherin) in heart--analysis by detergent-treated model. Biochem Biophys Res Commun. 1995;217:649-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 78. | Dayton WR, Goll DE, Stromer MH, Reville WJ, Zeece MG, Robson . M. Some properties of a Ca2 -activated protease that may be involved in myofibrillar protein turnover. Rifkin, D.B., Shaw, E). NY: Cold Spring Harbor Laboratory 1975; 551-577. |
| 79. | Nelson WJ, Traub P. Proteolysis of vimentin and desmin by the Ca2 -activated proteinase specific for these intermediate filaments. Mol Cell Biol. 1983;3:1146-1156. [RCA] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 80. | Yoshida M, Suzuki A, Shimizu T, Ozawa E. Proteinase-sensitive sites on isolated rabbit dystrophin. J Biochem. 1992;112:433-439. [PubMed] |
| 81. | Davies PJ, Wallach D, Willingham MC, Pastan I, Yamaguchi M, Robson RM. Filamin-actin interaction. Dissociation of binding from gelation by Ca2+-activated proteolysis. J Biol Chem. 1978;253:4036-4042. [PubMed] |
| 82. | Pemrick SM, Grebenau RC. Qualitative analysis of skeletal myosin as substrate of Ca2+-activated neutral protease: comparison of filamentous and soluble, native, and L2-deficient myosin. J Cell Biol. 1984;99:2297-2308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 83. | Kamakura K, Ishiura S, Suzuki K, Sugita H, Toyokura Y. Calcium-activated neutral protease in the peripheral nerve, which requires microM order Ca2+, and its effect on the neurofilament triplet. J Neurosci Res. 1985;13:391-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 84. | Guttmann RP, Baker DL, Seifert KM, Cohen AS, Coulter DA, Lynch DR. Specific proteolysis of the NR2 subunit at multiple sites by calpain. J Neurochem. 2001;78:1083-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 85. | Vinade L, Petersen JD, Do K, Dosemeci A, Reese TS. Activation of calpain may alter the postsynaptic density structure and modulate anchoring of NMDA receptors. Synapse. 2001;40:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 86. | Löfvenberg L, Backman L. Calpain-induced proteolysis of beta-spectrins. FEBS Lett. 1999;443:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 87. | Hemmings L, Rees DJ, Ohanian V, Bolton SJ, Gilmore AP, Patel B, Priddle H, Trevithick JE, Hynes RO, Critchley DR. Talin contains three actin-binding sites each of which is adjacent to a vinculin-binding site. J Cell Sci. 1996;109:2715-2726. [PubMed] |
| 88. | Muguruma M, Nishimuta S, Tomisaka Y, Ito T, Matsumura S. Organization of the functional domains in membrane cytoskeletal protein talin. J Biochem. 1995;117:1036-1042. [PubMed] |
| 89. | Suzuki A, Kim K, Ikeuchi Y. Proteolytic cleavage of connectin/titin. Adv Biophys. 1996;33:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 90. | Ho CY, Stromer MH, Robson RM. Identification of the 30 kDa polypeptide in post mortem skeletal muscle as a degradation product of troponin-T. Biochimie. 1994;76:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 118] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 91. | Fischer S, Vandekerckhove J, Ampe C, Traub P, Weber K. Protein-chemical identification of the major cleavage sites of the Ca2+ proteinase on murine vimentin, the mesenchymal intermediate filament protein. Biol Chem Hoppe Seyler. 1986;367:1147-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 92. | Franco SJ, Rodgers MA, Perrin BJ, Han J, Bennin DA, Critchley DR, Huttenlocher A. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol. 2004;6:977-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 442] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 93. | Gregoriou M, Willis AC, Pearson MA, Crawford C. The calpain cleavage sites in the epidermal growth factor receptor kinase domain. Eur J Biochem. 1994;223:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 94. | Ito M, Tanaka T, Nunoki K, Hidaka H, Suzuki K. The Ca2+ -activated protease (calpain) modulates Ca2+/calmodulin dependent activity of smooth muscle myosin light chain kinase. Biochem Biophys Res Commun. 1987;145:1321-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 95. | Kishimoto A, Mikawa K, Hashimoto K, Yasuda I, Tanaka S, Tominaga M, Kuroda T, Nishizuka Y. Limited proteolysis of protein kinase C subspecies by calcium-dependent neutral protease (calpain). J Biol Chem. 1989;264:4088-4092. [PubMed] |
| 96. | Tallant EA, Brumley LM, Wallace RW. Activation of a calmodulin-dependent phosphatase by a Ca2+-dependent protease. Biochemistry. 1988;27:2205-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 97. | Norris FA, Atkins RC, Majerus PW. Inositol polyphosphate 4-phosphatase is inactivated by calpain-mediated proteolysis in stimulated human platelets. J Biol Chem. 1997;272:10987-10989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 98. | Frangioni JV, Oda A, Smith M, Salzman EW, Neel BG. Calpain-catalyzed cleavage and subcellular relocation of protein phosphotyrosine phosphatase 1B (PTP-1B) in human platelets. EMBO J. 1993;12:4843-4856. [PubMed] |
| 99. | Hirai S, Kawasaki H, Yaniv M, Suzuki K. Degradation of transcription factors, c-Jun and c-Fos, by calpain. FEBS Lett. 1991;287:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 100. | Pariat M, Salvat C, Bébien M, Brockly F, Altieri E, Carillo S, Jariel-Encontre I, Piechaczyk M. The sensitivity of c-Jun and c-Fos proteins to calpains depends on conformational determinants of the monomers and not on formation of dimers. Biochem J. 2000;345 Pt 1:129-138. [PubMed] |
| 101. | Gonen H, Shkedy D, Barnoy S, Kosower NS, Ciechanover A. On the involvement of calpains in the degradation of the tumor suppressor protein p53. FEBS Lett. 1997;406:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 102. | Kubbutat MH, Vousden KH. Proteolytic cleavage of human p53 by calpain: a potential regulator of protein stability. Mol Cell Biol. 1997;17:460-468. [PubMed] |
| 103. | Arthur JS, Elce JS, Hegadorn C, Williams K, Greer PA. Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol Cell Biol. 2000;20:4474-4481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 272] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 104. | Azam M, Andrabi SS, Sahr KE, Kamath L, Kuliopulos A, Chishti AH. Disruption of the mouse mu-calpain gene reveals an essential role in platelet function. Mol Cell Biol. 2001;21:2213-2220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 188] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 105. | Dutt P, Croall DE, Arthur JS, Veyra TD, Williams K, Elce JS, Greer PA. m-Calpain is required for preimplantation embryonic development in mice. BMC Dev Biol. 2006;6:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 106. | Taylor RG, Geesink GH, Thompson VF, Koohmaraie M, Goll DE. Is Z-disk degradation responsible for postmortem tenderization? J Anim Sci. 1995;73:1351-1367. [PubMed] |
| 107. | Pfaff M, Du X, Ginsberg MH. Calpain cleavage of integrin beta cytoplasmic domains. FEBS Lett. 1999;460:17-22. [PubMed] |
| 108. | Fox JEB, Saido TC. Calpain in signal transduction. In: Calpain: Pharmacology and Toxicology of Calcium-Dependent Protease, edited by Wang KKK and Yuen P-W, Philadelphia, PA: Taylor and Francis 1999; 103-126. |
| 109. | Glading A, Chang P, Lauffenburger DA, Wells A. Epidermal growth factor receptor activation of calpain is required for fibroblast motility and occurs via an ERK/MAP kinase signaling pathway. J Biol Chem. 2000;275:2390-2398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 211] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 110. | Watanabe N, Vande Woude GF, Ikawa Y, Sagata N. Specific proteolysis of the c-mos proto-oncogene product by calpain on fertilization of Xenopus eggs. Nature. 1989;342:505-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 200] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 111. | Santella L, Kyozuka K, Hoving S, Munchbach M, Quadroni M, Dainese P, Zamparelli C, James P, Carafoli E. Breakdown of cytoskeletal proteins during meiosis of starfish oocytes and proteolysis induced by calpain. Exp Cell Res. 2000;259:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 112. | Kidd VJ, Lahti JM, Teitz T. Proteolytic regulation of apoptosis. Semin Cell Dev Biol. 2000;11:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 113. | Polster BM, Basañez G, Etxebarria A, Hardwick JM, Nicholls DG. Calpain I induces cleavage and release of apoptosis-inducing factor from isolated mitochondria. J Biol Chem. 2005;280:6447-6454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 317] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 114. | Knepper-Nicolai B, Savill J, Brown SB. Constitutive apoptosis in human neutrophils requires synergy between calpains and the proteasome downstream of caspases. J Biol Chem. 1998;273:30530-30536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 115. | Wolf BB, Goldstein JC, Stennicke HR, Beere H, Amarante-Mendes GP, Salvesen GS, Green DR. Calpain functions in a caspase-independent manner to promote apoptosis-like events during platelet activation. Blood. 1999;94:1683-1692. [PubMed] |
| 116. | Lynch G. Memory and the brain: unexpected chemistries and a new pharmacology. Neurobiol Learn Mem. 1998;70:82-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 81] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 117. | Richard I, Broux O, Allamand V, Fougerousse F, Chiannilkulchai N, Bourg N, Brenguier L, Devaud C, Pasturaud P, Roudaut C. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell. 1995;81:27-40. [PubMed] |
| 118. | Ono Y, Shimada H, Sorimachi H, Richard I, Saido TC, Beckmann JS, Ishiura S, Suzuki K. Functional defects of a muscle-specific calpain, p94, caused by mutations associated with limb-girdle muscular dystrophy type 2A. J Biol Chem. 1998;273:17073-17078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 119. | Tidball JG, Spencer MJ. Calpains and muscular dystrophies. Int J Biochem Cell Biol. 2000;32:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 120. | Horikawa Y, Oda N, Cox NJ, Li X, Orho-Melander M, Hara M, Hinokio Y, Lindner TH, Mashima H, Schwarz PE. Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nat Genet. 2000;26:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1007] [Cited by in RCA: 936] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 121. | Yoshikawa Y, Mukai H, Hino F, Asada K, Kato I. Isolation of two novel genes, down-regulated in gastric cancer. Jpn J Cancer Res. 2000;91:459-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 122. | Grynspan F, Griffin WR, Cataldo A, Katayama S, Nixon RA. Active site-directed antibodies identify calpain II as an early-appearing and pervasive component of neurofibrillary pathology in Alzheimer’s disease. Brain Res. 1997;763:145-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 115] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 123. | Tsuji T, Shimohama S, Kimura J, Shimizu K. m-Calpain (calcium-activated neutral proteinase) in Alzheimer’s disease brains. Neurosci Lett. 1998;248:109-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 124. | Nixon RA, Mohan P. Calpains in the pathogenesis of Alzheimer’s disease. In: Calpain: Pharmacology and Toxicology of Calcium-dependent Protease, edited by Wang KKW and Yeun P-W, Philadelphia, PA; Taylor & Francis 1999; 267-291. |
| 125. | Shields DC, Schaecher KE, Saido TC, Banik NL. A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proc Natl Acad Sci USA. 1999;96:11486-11491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 130] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 126. | Shields DC, Banik NL. Pathophysiological role of calpain in experimental demyelination. J Neurosci Res. 1999;55:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 127. | Shearer TR, Ma H, Shih M, Fukiage C, Azuma M. Calpains in the lens and cataractogenesis. Methods Mol Biol. 2000;144:277-285. [PubMed] |
| 128. | Scarpa A, Malmstrom K, Chiesi M, Carafoli E. On the problem of the release of mitochondrial calcium by cyclic AMP. J Membr Biol. 1976;29:205-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 129. | Rasmussen H. The calcium messenger system (1). N Engl J Med. 1986;314:1094-1101. [PubMed] |
| 130. | Schultz SG. A century of (epithelial) transport physiology: from vitalism to molecular cloning. Am J Physiol. 1998;274:C13-C23. [PubMed] |
| 131. | Schultz SG. Basic principles of membrane transport. Cambride: University Press 1980; 1-144. |
| 132. | Leem CH, Lagadic-Gossmann D, Vaughan-Jones RD. Characterization of intracellular pH regulation in the guinea-pig ventricular myocyte. J Physiol. 1999;517:159-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 201] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 133. | Zucchi R, Ronca F, Ronca-Testoni S. Modulation of sarcoplasmic reticulum function: a new strategy in cardioprotection? Pharmacol Ther. 2001;89:47-65. [PubMed] |
| 134. | Schanne FA, Kane AB, Young EE, Farber JL. Calcium dependence of toxic cell death: a final common pathway. Science. 1979;206:700-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1075] [Article Influence: 22.9] [Reference Citation Analysis (1)] |
| 135. | Fleckenstein A, Frey M, Fleckenstein-Grün G. Consequences of uncontrolled calcium entry and its prevention with calcium antagonists. Eur Heart J. 1983;4 Suppl H:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 136. | Lakatta EG, Nayler WG, Poole-Wilson PA. Calcium overload and mechanical function in posthypoxic myocardium: biphasic effect of pH during hypoxia. Eur J Cardiol. 1979;10:77-87. [PubMed] |
| 137. | Kihara Y, Grossman W, Morgan JP. Direct measurement of changes in intracellular calcium transients during hypoxia, ischemia, and reperfusion of the intact mammalian heart. Circ Res. 1989;65:1029-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 180] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 138. | Mohabir R, Lee HC, Kurz RW, Clusin WT. Effects of ischemia and hypercarbic acidosis on myocyte calcium transients, contraction, and pHi in perfused rabbit hearts. Circ Res. 1991;69:1525-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 139. | Steenbergen C, Murphy E, Levy L, London RE. Elevation in cytosolic free calcium concentration early in myocardial ischemia in perfused rat heart. Circ Res. 1987;60:700-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 307] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 140. | Seki S, Horikoshi K, Takeda H, Izumi T, Nagata A, Okumura H, Taniguchi M, Mochizuki S. Effects of sustained low-flow ischemia and reperfusion on Ca2+ transients and contractility in perfused rat hearts. Mol Cell Biochem. 2001;216:111-119. [PubMed] |
| 141. | Marban E, Kitakaze M, Koretsune Y, Yue DT, Chacko VP, Pike MM. Quantification of [Ca2+]i in perfused hearts. Critical evaluation of the 5F-BAPTA and nuclear magnetic resonance method as applied to the study of ischemia and reperfusion. Circ Res. 1990;66:1255-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 127] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 142. | Xia Z, Horton JW, Tang H, Yang Y. Metabolic disorder in myocardiac intracellular free calcium after thermal injury. Burns. 2001;27:453-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 143. | White DJ, Maass DL, Sanders B, Horton JW. Cardiomyocyte intracellular calcium and cardiac dysfunction after burn trauma. Crit Care Med. 2002;30:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 144. | Liang WY, Tang LX, Yang ZC, Huang YS. Calcium induced the damage of myocardial mitochondrial respiratory function in the early stage after severe burns. Burns. 2002;28:143-146. [PubMed] |
| 145. | Josephson RA, Silverman HS, Lakatta EG, Stern MD, Zweier JL. Study of the mechanisms of hydrogen peroxide and hydroxyl free radical-induced cellular injury and calcium overload in cardiac myocytes. J Biol Chem. 1991;266:2354-2361. [PubMed] |
| 146. | Corretti MC, Koretsune Y, Kusuoka H, Chacko VP, Zweier JL, Marban E. Glycolytic inhibition and calcium overload as consequences of exogenously generated free radicals in rabbit hearts. J Clin Invest. 1991;88:1014-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 147. | Gen W, Tani M, Takeshita J, Ebihara Y, Tamaki K. Mechanisms of Ca2+ overload induced by extracellular H2O2 in quiescent isolated rat cardiomyocytes. Basic Res Cardiol. 2001;96:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 148. | Semenov DG, Samoilov MO, Zielonka P, Lazarewicz JW. Responses to reversible anoxia of intracellular free and bound Ca(2+) in rat cortical slices. Resuscitation. 2000;44:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 149. | Vannucci RC, Brucklacher RM, Vannucci SJ. Intracellular calcium accumulation during the evolution of hypoxic-ischemic brain damage in the immature rat. Brain Res Dev Brain Res. 2001;126:117-120. [PubMed] |
| 150. | Zhang Y, Hou S, Wu Y. Changes of intracellular calcium and the correlation with functional damage of the spinal cord after spinal cord injury. Chin J Traumatol. 2002;5:40-42. [PubMed] |
| 151. | Wu ML, Vaughan-Jones RD. Interaction between Na+ and H+ ions on Na-H exchange in sheep cardiac Purkinje fibers. J Mol Cell Cardiol. 1997;29:1131-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 152. | Mentzer RM, Lasley RD, Jessel A, Karmazyn M. Intracellular sodium hydrogen exchange inhibition and clinical myocardial protection. Ann Thorac Surg. 2003;75:S700-S708. [PubMed] |
| 153. | Griese M, Perlitz V, Jüngling E, Kammermeier H. Myocardial performance and free energy of ATP-hydrolysis in isolated rat hearts during graded hypoxia, reoxygenation and high Ke+-perfusion. J Mol Cell Cardiol. 1988;20:1189-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 154. | Tani M, Neely JR. Role of intracellular Na+ in Ca2+ overload and depressed recovery of ventricular function of reperfused ischemic rat hearts. Possible involvement of H+-Na+ and Na+-Ca2+ exchange. Circ Res. 1989;65:1045-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 513] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 155. | Pierce GN, Meng H. The role of sodium-proton exchange in ischemic/reperfusion injury in the heart. Na(+)-H+ exchange and ischemic heart disease. Am J Cardiovasc Pathol. 1992;4:91-102. [PubMed] |
| 156. | Heyndrickx GR, Millard RW, McRitchie RJ, Maroko PR, Vatner SF. Regional myocardial functional and electrophysiological alterations after brief coronary artery occlusion in conscious dogs. J Clin Invest. 1975;56:978-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 680] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 157. | Bolli R, Marbán E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79:609-634. [PubMed] |
| 158. | Kim SJ, Peppas A, Hong SK, Yang G, Huang Y, Diaz G, Sadoshima J, Vatner DE, Vatner SF. Persistent stunning induces myocardial hibernation and protection: flow/function and metabolic mechanisms. Circ Res. 2003;92:1233-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 159. | Siegmund B, Schlack W, Ladilov YV, Balser C, Piper HM. Halothane protects cardiomyocytes against reoxygenation-induced hypercontracture. Circulation. 1997;96:4372-4379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 160. | Inserte J, Garcia-Dorado D, Ruiz-Meana M, Padilla F, Barrabés JA, Pina P, Agulló L, Piper HM, Soler-Soler J. Effect of inhibition of Na(+)/Ca(2+) exchanger at the time of myocardial reperfusion on hypercontracture and cell death. Cardiovasc Res. 2002;55:739-748. [PubMed] |
| 161. | Piper HM, Meuter K, Schäfer C. Cellular mechanisms of ischemia-reperfusion injury. Ann Thorac Surg. 2003;75:S644-S648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 258] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 162. | Lakatta EG, Guarnieri T. Spontaneous myocardial calcium oscillations: are they linked to ventricular fibrillation? J Cardiovasc Electrophysiol. 1993;4:473-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 163. | Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 164. | Iizuka K, Kawaguchi H, Yasuda H. Calpain is activated during hypoxic myocardial cell injury. Biochem Med Metab Biol. 1991;46:427-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 165. | Iizuka K, Kawaguchi H, Yasuda H. Calpain is activated by beta-adrenergic receptor stimulation under hypoxic myocardial cell injury. Jpn Circ J. 1991;55:1086-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 166. | Yoshida K, Yamasaki Y, Kawashima S. Calpain activity alters in rat myocardial subfractions after ischemia or reperfusion. Biochim Biophys Acta. 1993;1182:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 167. | Sandmann S, Prenzel F, Shaw L, Schauer R, Unger T. Activity profile of calpains I and II in chronically infarcted rat myocardium--influence of the calpain inhibitor CAL 9961. Br J Pharmacol. 2002;135:1951-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 168. | Kunimatsu M, Tada T, Narita Y, Ozaki Y, Liu ZQ, Shearer TR, Sasaki M. Activation of calpain in myocardial infarction: an immunohistochemical study using a calpain antibody raised against active site histidine-containing peptide. Cardiovasc Pathol. 1999;8:7-15. [PubMed] |
| 169. | Ostwald K, Hagberg H, Andiné P, Karlsson JO. Upregulation of calpain activity in neonatal rat brain after hypoxic-ischemia. Brain Res. 1993;630:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 170. | Liebetrau M, Staufer B, Auerswald EA, Gabrijelcic-Geiger D, Fritz H, Zimmermann C, Pfefferkorn T, Hamann GF. Increased intracellular calpain detection in experimental focal cerebral ischemia. Neuroreport. 1999;10:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 171. | Neumar RW, Meng FH, Mills AM, Xu YA, Zhang C, Welsh FA, Siman R. Calpain activity in the rat brain after transient forebrain ischemia. Exp Neurol. 2001;170:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 172. | Edelstein CL, Yaqoob MM, Alkhunaizi AM, Gengaro PE, Nemenoff RA, Wang KK, Schrier RW. Modulation of hypoxia-induced calpain activity in rat renal proximal tubules. Kidney Int. 1996;50:1150-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 173. | Chatterjee PK, Todorovic Z, Sivarajah A, Mota-Filipe H, Brown PA, Stewart KN, Mazzon E, Cuzzocrea S, Thiemermann C. Inhibitors of calpain activation (PD150606 and E-64) and renal ischemia-reperfusion injury. Biochem Pharmacol. 2005;69:1121-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 174. | Zhang J, Patel JM, Block ER. Hypoxia-specific upregulation of calpain activity and gene expression in pulmonary artery endothelial cells. Am J Physiol. 1998;275:L461-L468. [PubMed] |
| 175. | Hernando V, Inserte J, Sartório CL, Parra VM, Poncelas-Nozal M, Garcia-Dorado D. Calpain translocation and activation as pharmacological targets during myocardial ischemia/reperfusion. J Mol Cell Cardiol. 2010;49:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 176. | Matsumura Y, Saeki E, Inoue M, Hori M, Kamada T, Kusuoka H. Inhomogeneous disappearance of myofilament-related cytoskeletal proteins in stunned myocardium of guinea pig. Circ Res. 1996;79:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 177. | Yoshikawa Y, Hagihara H, Ohga Y, Nakajima-Takenaka C, Murata KY, Taniguchi S, Takaki M. Calpain inhibitor-1 protects the rat heart from ischemia-reperfusion injury: analysis by mechanical work and energetics. Am J Physiol Heart Circ Physiol. 2005;288:H1690-H1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 178. | Yoshikawa Y, Zhang GX, Obata K, Ohga Y, Matsuyoshi H, Taniguchi S, Takaki M. Cardioprotective effects of a novel calpain inhibitor SNJ-1945 for reperfusion injury after cardioplegic cardiac arrest. Am J Physiol Heart Circ Physiol. 2010;298:H643-H651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 179. | French JP, Quindry JC, Falk DJ, Staib JL, Lee Y, Wang KK, Powers SK. Ischemia-reperfusion-induced calpain activation and SERCA2a degradation are attenuated by exercise training and calpain inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H128-H136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 180. | Neuhof C, Götte O, Trumbeckaite S, Attenberger M, Kuzkaya N, Gellerich F, Möller A, Lubisch W, Speth M, Tillmanns H. A novel water-soluble and cell-permeable calpain inhibitor protects myocardial and mitochondrial function in postischemic reperfusion. Biol Chem. 2003;384:1597-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 181. | Zhang Z, Biesiadecki BJ, Jin JP. Selective deletion of the NH2-terminal variable region of cardiac troponin T in ischemia reperfusion by myofibril-associated mu-calpain cleavage. Biochemistry. 2006;45:11681-11694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 182. | Maekawa A, Lee JK, Nagaya T, Kamiya K, Yasui K, Horiba M, Miwa K, Uzzaman M, Maki M, Ueda Y. Overexpression of calpastatin by gene transfer prevents troponin I degradation and ameliorates contractile dysfunction in rat hearts subjected to ischemia/reperfusion. J Mol Cell Cardiol. 2003;35:1277-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 183. | Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia--reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001;33:1065-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 537] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 184. | Palmer JW, Tandler B, Hoppel CL. Biochemical differences between subsarcolemmal and interfibrillar mitochondria from rat cardiac muscle: effects of procedural manipulations. Arch Biochem Biophys. 1985;236:691-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 185. | Piper HM, Sezer O, Schleyer M, Schwartz P, Hütter JF, Spieckermann PG. Development of ischemia-induced damage in defined mitochondrial subpopulations. J Mol Cell Cardiol. 1985;17:885-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 186. | Chen Q, Paillard M, Gomez L, Ross T, Hu Y, Xu A, Lesnefsky EJ. Activation of mitochondrial μ-calpain increases AIF cleavage in cardiac mitochondria during ischemia-reperfusion. Biochem Biophys Res Commun. 2011;415:533-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 187. | Ma H, Fukiage C, Kim YH, Duncan MK, Reed NA, Shih M, Azuma M, Shearer TR. Characterization and expression of calpain 10. A novel ubiquitous calpain with nuclear localization. J Biol Chem. 2001;276:28525-28531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 188. | Kar P, Samanta K, Shaikh S, Chowdhury A, Chakraborti T, Chakraborti S. Mitochondrial calpain system: an overview. Arch Biochem Biophys. 2010;495:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 189. | Ozaki T, Yamashita T, Ishiguro S. Mitochondrial m-calpain plays a role in the release of truncated apoptosis-inducing factor from the mitochondria. Biochim Biophys Acta. 2009;1793:1848-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 190. | Ataka K, Chen D, Levitsky S, Jimenez E, Feinberg H. Effect of aging on intracellular Ca2+, pHi, and contractility during ischemia and reperfusion. Circulation. 1992;86:II371-II376. [PubMed] |
| 191. | Grover GJ, Dzwonczyk S, Sleph PG. Ruthenium red improves postischemic contractile function in isolated rat hearts. J Cardiovasc Pharmacol. 1990;16:783-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 192. | Li ZP, Burke EP, Frank JS, Bennett V, Philipson KD. The cardiac Na+-Ca2+ exchanger binds to the cytoskeletal protein ankyrin. J Biol Chem. 1993;268:11489-11491. [PubMed] |
| 193. | Singh RB, Chohan PK, Dhalla NS, Netticadan T. The sarcoplasmic reticulum proteins are targets for calpain action in the ischemic-reperfused heart. J Mol Cell Cardiol. 2004;37:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 194. | Pedrozo Z, Sánchez G, Torrealba N, Valenzuela R, Fernández C, Hidalgo C, Lavandero S, Donoso P. Calpains and proteasomes mediate degradation of ryanodine receptors in a model of cardiac ischemic reperfusion. Biochim Biophys Acta. 2010;1802:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 195. | Arrington DD, Van Vleet TR, Schnellmann RG. Calpain 10: a mitochondrial calpain and its role in calcium-induced mitochondrial dysfunction. Am J Physiol Cell Physiol. 2006;291:C1159-C1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 196. | Trumbeckaite S, Neuhof C, Zierz S, Gellerich FN. Calpain inhibitor (BSF 409425) diminishes ischemia/reperfusion-induced damage of rabbit heart mitochondria. Biochem Pharmacol. 2003;65:911-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 197. | Otani H, Tanaka H, Inoue T, Umemoto M, Omoto K, Tanaka K, Sato T, Osako T, Masuda A, Nonoyama A. In vitro study on contribution of oxidative metabolism of isolated rabbit heart mitochondria to myocardial reperfusion injury. Circ Res. 1984;55:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 198. | Shlafer M, Gallagher KP, Adkins S. Hydrogen peroxide generation by mitochondria isolated from regionally ischemic and nonischemic dog myocardium. Basic Res Cardiol. 1990;85:318-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 199. | Veitch K, Hombroeckx A, Caucheteux D, Pouleur H, Hue L. Global ischaemia induces a biphasic response of the mitochondrial respiratory chain. Anoxic pre-perfusion protects against ischaemic damage. Biochem J. 1992;281:709-715. [PubMed] |
| 200. | Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiariello M. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem. 1993;268:18532-18541. [PubMed] |
| 201. | Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233-249. [PubMed] |
| 202. | Kushnareva YE, Sokolove PM. Prooxidants open both the mitochondrial permeability transition pore and a low-conductance channel in the inner mitochondrial membrane. Arch Biochem Biophys. 2000;376:377-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 203. | Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6842] [Cited by in RCA: 6847] [Article Influence: 244.5] [Reference Citation Analysis (0)] |
| 204. | 72nd Scientific Sessions of the American Heart Association. Atlanta, Georgia, USA. November 7-10, 1999. Abstracts. Circulation. 1999;100:IA-V, I1-928. [PubMed] |
| 205. | Lesnefsky EJ, Chen Q, Slabe TJ, Stoll MS, Minkler PE, Hassan MO, Tandler B, Hoppel CL. Ischemia, rather than reperfusion, inhibits respiration through cytochrome oxidase in the isolated, perfused rabbit heart: role of cardiolipin. Am J Physiol Heart Circ Physiol. 2004;287:H258-H267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 206. | Chen M, He H, Zhan S, Krajewski S, Reed JC, Gottlieb RA. Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J Biol Chem. 2001;276:30724-30728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 277] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 207. | Takeshita D, Tanaka M, Mitsuyama S, Yoshikawa Y, Zhang GX, Obata K, Ito H, Taniguchi S, Takaki M. A new calpain inhibitor protects left ventricular dysfunction induced by mild ischemia-reperfusion in in situ rat hearts. J Physiol Sci. 2013;63:113-123. [PubMed] |
| 208. | Lubisch W, Beckenbach E, Bopp S, Hofmann HP, Kartal A, Kästel C, Lindner T, Metz-Garrecht M, Reeb J, Regner F. Benzoylalanine-derived ketoamides carrying vinylbenzyl amino residues: discovery of potent water-soluble calpain inhibitors with oral bioavailability. J Med Chem. 2003;46:2404-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 209. | Neuhof C, Fabiunke V, Deibele K, Speth M, Möller A, Lubisch W, Fritz H, Tillmanns H, Neuhof H. Reduction of myocardial infarction by calpain inhibitors A-705239 and A-705253 in isolated perfused rabbit hearts. Biol Chem. 2004;385:1077-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 210. | Neuhof C, Fabiunk V, Speth M, Möller A, Fritz F, Tillmanns H, Neuhof H, Erdogan A. Reduction of myocardial infarction by postischemic administration of the calpain inhibitor A-705253 in comparison to the Na(+)/H(+) exchange inhibitor Cariporide in isolated perfused rabbit hearts. Biol Chem. 2008;389:1505-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 211. | Chen M, Won DJ, Krajewski S, Gottlieb RA. Calpain and mitochondria in ischemia/reperfusion injury. J Biol Chem. 2002;277:29181-29186. [PubMed] |
| 212. | Cun L, Ronghua Z, Bin L, Jin L, Shuyi L. Preconditioning with Na+/H+ exchange inhibitor HOE642 reduces calcium overload and exhibits marked protection on immature rabbit hearts. ASAIO J. 2007;53:762-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 213. | Boyce SW, Bartels C, Bolli R, Chaitman B, Chen JC, Chi E, Jessel A, Kereiakes D, Knight J, Thulin L. Impact of sodium-hydrogen exchange inhibition by cariporide on death or myocardial infarction in high-risk CABG surgery patients: results of the CABG surgery cohort of the GUARDIAN study. J Thorac Cardiovasc Surg. 2003;126:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 214. | Mentzer RM, Bartels C, Bolli R, Boyce S, Buckberg GD, Chaitman B, Haverich A, Knight J, Menasché P, Myers ML. Sodium-hydrogen exchange inhibition by cariporide to reduce the risk of ischemic cardiac events in patients undergoing coronary artery bypass grafting: results of the EXPEDITION study. Ann Thorac Surg. 2008;85:1261-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 215. | Khalil PN, Neuhof C, Huss R, Pollhammer M, Khalil MN, Neuhof H, Fritz H, Siebeck M. Calpain inhibition reduces infarct size and improves global hemodynamics and left ventricular contractility in a porcine myocardial ischemia/reperfusion model. Eur J Pharmacol. 2005;528:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 216. | Kang MY, Zhang Y, Matkovich SJ, Diwan A, Chishti AH, Dorn GW. Receptor-independent cardiac protein kinase Calpha activation by calpain-mediated truncation of regulatory domains. Circ Res. 2010;107:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 217. | Ikeda Y, Young LH, Lefer AM. Attenuation of neutrophil-mediated myocardial ischemia-reperfusion injury by a calpain inhibitor. Am J Physiol Heart Circ Physiol. 2002;282:H1421-H1426. [PubMed] |
| 218. | Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981-2988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1182] [Cited by in RCA: 1305] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 219. | Letavernier E, Zafrani L, Perez J, Letavernier B, Haymann JP, Baud L. The role of calpains in myocardial remodelling and heart failure. Cardiovasc Res. 2012;96:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 220. | Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994;94:1621-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1022] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 221. | Iwamoto H, Miura T, Okamura T, Shirakawa K, Iwatate M, Kawamura S, Tatsuno H, Ikeda Y, Matsuzaki M. Calpain inhibitor-1 reduces infarct size and DNA fragmentation of myocardium in ischemic/reperfused rat heart. J Cardiovasc Pharmacol. 1999;33:580-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 222. | Mani SK, Balasubramanian S, Zavadzkas JA, Jeffords LB, Rivers WT, Zile MR, Mukherjee R, Spinale FG, Kuppuswamy D. Calpain inhibition preserves myocardial structure and function following myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;297:H1744-H1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 223. | Ma J, Wei M, Wang Q, Li J, Wang H, Liu W, Lacefield JC, Greer PA, Karmazyn M, Fan GC. Deficiency of Capn4 gene inhibits nuclear factor-κB (NF-κB) protein signaling/inflammation and reduces remodeling after myocardial infarction. J Biol Chem. 2012;287:27480-27489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
P- Reviewer: Coelho AMM, Kusmic C, Tagarakis G S- Editor: Ji FF L- Editor: A E- Editor: Wu HL