Published online Jun 26, 2014. doi: 10.4330/wjc.v6.i6.502
Revised: April 11, 2014
Accepted: May 16, 2014
Published online: June 26, 2014
Processing time: 183 Days and 17.6 Hours
AIM: To conduct the first systematic test of the hypothesis that modulation of cardiac vagal tone is impaired in Lyme disease.
METHODS: The response of cardiac vagal tone to respiratory modulation was measured in 18 serologically positive Lyme disease patients and in 18 controls.
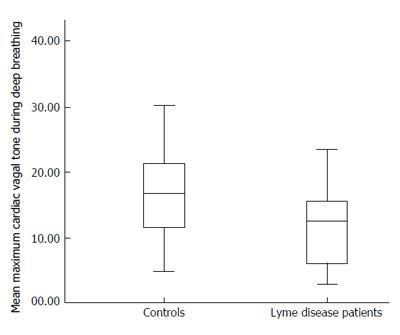
RESULTS: The two groups were matched in respect of age, sex, body mass, mean arterial blood pressure, mean resting heart rate and mean resting cardiac vagal tone. The mean maximum cardiac vagal tone during deep breathing in the Lyme disease patients [11.2 (standard error 1.3)] was lower than in the matched controls [16.5 (standard error 1.7); P = 0.02].
CONCLUSION: Respiratory modulation of cardiac vagal tone is impaired in Lyme disease, which suggests that Lyme disease may directly affect the vagus nerve or the brainstem.
Core tip: Given that immune dysfunction, postural orthostatic tachycardia syndrome, fatigue, cognitive dysfunction, orthostatic palpitations, syncope, and stress, which may occur in Lyme disease, are associated with parasympathetic activity and reduced modulation of cardiac vagal tone, we hypothesized that modulation of cardiac vagal tone is impaired in this disease. This was confirmed in our study of 18 patients and 18 matched controls. Cardiac vagal tone is reflexly generated through arterial baroreceptor stimulation, by the afferents of the latter facilitating cardiac vagal motoneuron discharge relaying through interneurons in the nucleus tractus solitarius, implying that Lyme disease may directly affect the vagus or brainstem.
- Citation: Puri BK, Shah M, Monro JA, Kingston MC, Julu PO. Respiratory modulation of cardiac vagal tone in Lyme disease. World J Cardiol 2014; 6(6): 502-506
- URL: https://www.wjgnet.com/1949-8462/full/v6/i6/502.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i6.502
Lyme disease or Lyme borreliosis is an arthropod-borne zoonosis caused by Borrelia spirochetes, the incidence of which has recently been increasing with the geographical spread of infected ticks and which was previously identified clinically in Europe as Garin-Bujadoux-Bannwarth syndrome and in the United States as Lyme arthritis[1-3]. There is growing evidence for the role of the autonomic nervous system in a wide range of diseases[4] and autonomic instability has been reported in Lyme disease[5] but has not, thus far, been systematically studied in this illness. It has recently been reported that a series of five female Lyme disease patients developed postural orthostatic tachycardia syndrome; they suffered from symptoms of fatigue, cognitive dysfunction, orthostatic palpitations and either near syncope or frank syncope[6]. Again, a case report has been published of a 16-year-old female patient with clinical, radiological and scintigraphic features consistent with reflex sympathetic dystrophy associated with Lyme disease[7].
Given that immune dysfunction, postural orthostatic tachycardia syndrome, fatigue, cognitive dysfunction, orthostatic palpitations, syncope, and stress, which may occur in Lyme disease[6-11], are associated with parasympathetic activity and reduced modulation of cardiac vagal tone (or the related measure of heart rate variability)[4,12-14], we hypothesized that modulation of cardiac vagal tone might be impaired in this illness. The aim of our study was to test this hypothesis by comparing the response of cardiac vagal tone to respiratory modulation in a sample of Lyme disease patients and matched controls. To the best of our knowledge, this was the first such study.
The arterial blood pressure, resting cardiac rate, resting cardiac vagal tone, and the cardiac vagal tone following deep breathing were measured in 18 serologically positive Lyme disease patients who were undergoing routine clinical investigation, and in 18 normal controls; all subjects were asked to refrain from any caffeine-containing beverages from midnight before testing. For inclusion in this study, the Lyme disease patients, who were selected from outpatient attendees, were required to be IgM positive for Lyme disease and to be aged 18 years or over. The control subjects were identified as subjects who were not suffering from any autonomic nervous system dysfunction (dysautonomia) or from any condition that might directly or indirectly affect the autonomic nervous system, and were recruited from hospital staff, and from friends and family of staff members. The exclusion criteria for the normal controls included: under 18 years of age; subjects with dysautonomia; taking medications that affect the autonomic nervous system, such as stimulants, tricyclic antidepressants, anti-histaminergic medication, calcium-channel blockers, beta-adrenoceptor blocking drugs, beta agonists, monoamine oxidase inhibitors, levodopa, anti-psychotic drugs, clonidine and vasopressin; medical problems that affect the autonomic nervous system, such as neurodegenerative disorders, peripheral neuropathies, diabetes, connective tissue disease and infectious diseases. The clinical stage and presentation of the patients were early, presumed localized and without cardiac involvement (that is, without clinical evidence of Lyme carditis). As there has been no previous work in this area, it was not possible to calculate the value of beta, and therefore calculate the statistical power, for this study. Written informed consent was obtained and a local research ethics committee approved the study. The study was carried out in accordance with the Declaration of Helsinki.
Resting cardiac rate and cardiac vagal tone were measured in real time using the NeuroScope Model 300BA (Brainstem Autonomic Function Monitor) (Medifit Instruments Ltd, London, United Kingdom) as described by Little et al[15] and during a 10-s cycle of deep breathing as described by Julu et al[16]. In particular, the non-invasive cardiac vagal tone was measured on a continuous, beat-to-beat basis and was defined as pulse-synchronized phase shifts in consecutive cardiac cycles; it is essentially a form of pulse interval variability which is quantified continuously from the electrocardiogram. The index of cardiac vagal tone was measured and quantified in arbitrary units of a linear vagal scale; the minimum value of this scale is zero, which corresponds to full atropinisation of human subjects.
Arterial blood pressure was measured using the Ohmeda 2300 Finapres (Ohmeda, Engleswood, CO, United States).
Continuous variables for which data did not differ significantly from normality and for which the two groups did not have significantly different variances were compared between the Lyme disease and control groups using independent samples t-tests (equal variances), while the discrete nominal variable (sex) was compared between groups using Fisher’s exact probability test. The software package IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp, Armonk, NY, United States) was used for the statistical analyses.
The main findings are shown in Table 1. The two groups were matched in respect of age, sex, body mass, mean arterial blood pressure, mean resting heart rate, and mean resting cardiac vagal tone.
| Lyme disease patients n = 18 mean (standard error) | Controls n = 18 mean (standard error) | P-value | |
| Age, yr | 35.6 (3.7) | 44.7 (3.9) | 0.10 |
| Sex | 7 males, 11 females | 7 males, 11 females | 1.00 |
| Body mass | 25.0 (1.2) kg | 24.6 (0.80) kg | 0.44 |
| Arterial blood pressure | 74.1 (3.9) mmHg | 66.9 (4.4) mmHg | 0.23 |
| Resting cardiac rate | 72.0 (3.1) min-1 | 63.7 (4.5) min-1 | 0.14 |
| Resting cardiac vagal tone | 4.7 (0.8) | 5.7 (1.1) | 0.46 |
| Maximum cardiac vagal tone during deep breathing | 11.2 (1.3) | 16.5 (1.7) | 0.02 |
Details of the heart rate for each of the 18 patients before, during, and following the deep breathing procedure are provided in Table 2.
| Patient number | Heart rate at 20 beats before the deep breathing procedure | Heart rate 20 beats before the end of the deep breathing procedure | Heart rate two minutes after the deep breathing procedure |
| 1 | 71.8 | 80.5 | 76.0 |
| 2 | 90.8 | 90.7 | 83.1 |
| 3 | 89.8 | 120.7 | 83.5 |
| 4 | 68.0 | 73.6 | 66.0 |
| 5 | 70.3 | 86.4 | 66.3 |
| 6 | 90.5 | 83.6 | 90.6 |
| 7 | 69.0 | 68.6 | 70.2 |
| 8 | 64.4 | 65.5 | 61.7 |
| 9 | 141.8 | 125.9 | 137.8 |
| 10 | 69.5 | 76.1 | 70.3 |
| 11 | 71.9 | 71.6 | 74.5 |
| 12 | 69.4 | 71.6 | 71.4 |
| 13 | 74.6 | 78.1 | 79.7 |
| 14 | 68.4 | 69.9 | 66.7 |
| 15 | 68.7 | 77.2 | 68.3 |
| 16 | 62.0 | 72.9 | 65.3 |
| 17 | 69.2 | 72.1 | 67.5 |
| 18 | 63.0 | 69.1 | 68.3 |
Corresponding details of the heart rate for each of the 18 control subjects before, during, and following the deep breathing are provided in Table 3.
| Patient number | Heart rate at 20 beats before the deep breathing procedure | Heart rate 20 beats before the end of the deep breathing procedure | Heart rate two minutes after the deep breathing procedure |
| 1 | 86.8 | 88.7 | 90.9 |
| 2 | 52.8 | 70.2 | 52.0 |
| 3 | 67.0 | 74.9 | 69.5 |
| 4 | 73.7 | 83.7 | 75.8 |
| 5 | 62.9 | 63.9 | 54.3 |
| 6 | 65.0 | 73.4 | 61.8 |
| 7 | 65.2 | 69.2 | 65.6 |
| 8 | 73.8 | 78.0 | 76.0 |
| 9 | 64.5 | 70.6 | 53.8 |
| 10 | 66.6 | 66.7 | 64.2 |
| 11 | 63.4 | 72.6 | 66.7 |
| 12 | 62.9 | 69.8 | 63.1 |
| 13 | 75.4 | 73.5 | 79.0 |
| 14 | 78.8 | 81.8 | 72.1 |
| 15 | 70.6 | 81.2 | 66.4 |
| 16 | 76.0 | 70.9 | 74.9 |
| 17 | 88.0 | 92.3 | 81.8 |
| 18 | 75.9 | 74.8 | 68.3 |
The mean (± standard error) maximum cardiac vagal tone during deep breathing in the Lyme disease patients (11.2 ± 1.3) was significantly lower than that in the matched controls (16.5 ± 1.7; P = 0.02); these data are illustrated in Figure 1.
This study has demonstrated impairment of respiratory modulation of cardiac vagal tone in Lyme disease. This is an original finding which has not previously been described.
At the outset, it should be noted that our results demonstrate impaired respiratory modulation of cardiac vagal tone in Lyme disease; this is not the same as showing changed cardiac vagal tone per se. Indeed, the resting cardiac vagal tone in the Lyme disease patients was found not to differ significantly from that in the matched control group. Therefore, in attempting to provide an explanation for our results, it will not suffice simply to look for causes of altered (e.g., reduced) cardiac vagal tone in the patient group.
The cause of the observed abnormalities might be vagal nerve changes resulting from Lyme disease. It is also worth bearing in mind that, since cardiac vagal tone is reflexly generated through arterial baroreceptor stimulation, by the afferents of the latter facilitating cardiac vagal motoneuron discharge relaying through interneurons in the nucleus tractus solitarius[15,17,18], the results are also compatible with the possibility that Lyme disease might affect the brainstem. Indeed, given that the dorsal nucleus of the vagus nerve, the nucleus ambiguus, the nucleus tractus solitarius, and the spinal trigeminal nucleus, which give rise to or receive axons of the vagus nerve, are located in the medulla oblongata, these two possibilities are not mutually exclusive.
The causative Borrelia bacteria are able to undergo pleomorphic changes, including into a cystic form; indeed, it has been suggested that this may at least in part account for some cases of antibiotic resistance and recurrence of Lyme disease[19]. It may be that this cystic form is often to be found in the brainstem in affected patients. However, this is unlikely to be an explanation here because this is believed to occur only in chronic Lyme neuroborreliosis.
It should be noted that there was no evidence that the patients were suffering from other disorders, such as Epstein-Barr viral infection, which might have caused false-positive serological results.
Finally, from Table 1 it can be seen that the mean resting cardiac rate in the controls (63.7 min-1) was slightly (but not statistically significantly) lower than that in the patients (72.0 min-1), while the mean resting cardiac vagal tone (5.7) was slightly (but not statistically significantly) higher than that in the patients (4.7). These findings might reflect the fact that the control group, in contrast to the Lyme patient group, were more physically fit; eight of the control subjects regularly engaged in exercise (sports, gymnasium attendance, or regular walking).
Lyme disease or Lyme borreliosis is an arthropod-borne zoonosis caused by Borrelia spirochetes, the incidence of which has recently been increasing with the geographical spread of infected ticks and which was previously identified clinically in Europe as Garin-Bujadoux-Bannwarth syndrome and in the United States as Lyme arthritis.
Respiratory modulation of cardiac vagal tone is impaired in Lyme disease, which suggests that Lyme disease may directly affect the vagus nerve or the brainstem.
Cardiac vagal tone is reflexly generated through arterial baroreceptor stimulation, by the afferents of the latter facilitating cardiac vagal motoneuron discharge relaying through interneurons in the nucleus tractus solitarius, implying that Lyme disease may directly affect the vagus or brainstem.
The topic is relatively new and there are few data about the topic of the study about (respiratory modulation of cardiac vagal tone in Lyme disease). This finding seems very interesting.
P- Reviewers: Al-Biltagi M, Ong HT, Zielinski T S- Editor: Wen LL L- Editor: Wang TQ E- Editor: Liu SQ
| 1. | Halperin JJ, Baker P, Wormser GP. Common misconceptions about Lyme disease. Am J Med. 2013;126:264 e1-264 e7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Steere AC, Malawista SE, Hardin JA, Ruddy S, Askenase W, Andiman WA. Erythema chronicum migrans and Lyme arthritis. The enlarging clinical spectrum. Ann Intern Med. 1977;86:685-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 410] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 3. | Steere AC, Malawista SE, Snydman DR, Shope RE, Andiman WA, Ross MR, Steele FM. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three connecticut communities. Arthritis Rheum. 1977;20:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 722] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 4. | Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141:122-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1236] [Cited by in RCA: 1484] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 5. | Burman M, Nguyen HL, Murthy V, Sen Gupta P, Davies C, Wragg A, Peterson D, Chowdhury TA. Severe orthostatic hypotension in a diabetic patient may not be due to diabetic autonomic neuropathy. Clin Med. 2011;11:290-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Kanjwal K, Karabin B, Kanjwal Y, Grubb BP. Postural orthostatic tachycardia syndrome following Lyme disease. Cardiol J. 2011;18:63-66. [PubMed] |
| 7. | Gila L, Guerrero A, Astarloa R, Martí P, Gutiérrez JM. [Reflex sympathetic dystrophy. A new manifestation of Lyme disease?]. Enferm Infecc Microbiol Clin. 1990;8:32-35. [PubMed] |
| 8. | Kaplan RF, Jones-Woodward L, Workman K, Steere AC, Logigian EL, Meadows ME. Neuropsychological deficits in Lyme disease patients with and without other evidence of central nervous system pathology. Appl Neuropsychol. 1999;6:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Sigal LH. Lyme disease: a review of aspects of its immunology and immunopathogenesis. Annu Rev Immunol. 1997;15:63-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 89] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Singh SK, Girschick HJ. Lyme borreliosis: from infection to autoimmunity. Clin Microbiol Infect. 2004;10:598-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Vartiovaara I. Living with Lyme. Lancet. 1995;345:842-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. Int J Psychophysiol. 2003;48:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 473] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 13. | Hayano J, Sakakibara Y, Yamada A, Yamada M, Mukai S, Fujinami T, Yokoyama K, Watanabe Y, Takata K. Accuracy of assessment of cardiac vagal tone by heart rate variability in normal subjects. Am J Cardiol. 1991;67:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 482] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 14. | Kim DH, Lipsitz LA, Ferrucci L, Varadhan R, Guralnik JM, Carlson MC, Fleisher LA, Fried LP, Chaves PH. Association between reduced heart rate variability and cognitive impairment in older disabled women in the community: Women’s Health and Aging Study I. J Am Geriatr Soc. 2006;54:1751-1757. [PubMed] |
| 15. | Little CJ, Julu PO, Hansen S, Reid SW. Real-time measurement of cardiac vagal tone in conscious dogs. Am J Physiol. 1999;276:H758-H765. [PubMed] |
| 16. | Julu PO, McCarron MO, Hansen S, Barnes A, Jamal GA, Ballantyne JP. Selective defect of baroreflex blood pressure buffering with intact cardioinhibition in a woman with familial aniridia. Neurology. 1997;49:1705-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Kollai M, Jokkel G, Bonyhay I, Tomcsanyi J, Naszlady A. Relation between baroreflex sensitivity and cardiac vagal tone in humans. Am J Physiol. 1994;266:H21-H27. [PubMed] |
| 18. | Spyer KM. Central nervous integration of cardiovascular control. J Exp Biol. 1982;100:109-128. [PubMed] |
| 19. | Sapi E, Kaur N, Anyanwu S, Luecke DF, Datar A, Patel S, Rossi M, Stricker RB. Evaluation of in-vitro antibiotic susceptibility of different morphological forms of Borrelia burgdorferi. Infect Drug Resist. 2011;4:97-113. [PubMed] |