Published online Nov 26, 2014. doi: 10.4330/wjc.v6.i11.1140
Revised: September 9, 2014
Accepted: October 1, 2014
Published online: November 26, 2014
Processing time: 274 Days and 6.3 Hours
Major bleeding is currently one of the most common non-cardiac complications observed in the treatment of patients with acute coronary syndrome (ACS). Hemorrhagic complications occur with a frequency of 1% to 10% during treatment for ACS. In fact, bleeding events are the most common extrinsic complication associated with ACS therapy. The identification of clinical characteristics and particularities of the antithrombin therapy associated with an increased risk of hemorrhagic complications would make it possible to adopt prevention strategies, especially among those exposed to greater risk. The international societies of cardiology renewed emphasis on bleeding risk stratification in order to decide strategy and therapy for patients with ACS. With this review, we performed an update about the ACS bleeding risk scores most frequently used in daily clinical practice.
Core tip: Bleeding is the main non-thrombotic complication associated with acute coronary syndrome. Bleeding implies a worse prognosis due itself directly (fatal bleeding, for example, intracranial bleeding) and indirectly (discontinuation of antithrombotic therapy). For this it is important to do an adequate bleeding risk stratification in all patients with acute coronary syndrome. In this review we analyze the different risk factors for bleeding, along with the bleeding risk scores currently available.
- Citation: Abu-Assi E, Raposeiras-Roubín S, García-Acuña JM, González-Juanatey JR. Bleeding risk stratification in an era of aggressive management of acute coronary syndromes. World J Cardiol 2014; 6(11): 1140-1148
- URL: https://www.wjgnet.com/1949-8462/full/v6/i11/1140.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i11.1140
The classic aim of acute coronary syndrome (ACS) therapy was to reduce mortality and to prevent or minimize ischemic complications. This was possible with percutaneous coronary intervention and with antithrombotic drugs[1]; however, these therapies have led to an increased risk of bleeding complications[2]. Until the recent past, bleeding was thought to be inherent to the modern therapeutic approach in ACS and percutaneous coronary intervention (PCI)[3]. Nowadays this consideration has been changed. Clinical trials have demonstrated that major bleeding has a strong impact on the risk of death, myocardial infarction and stroke in patients with ACS[4]. Therefore, a reduction in bleeding events translates into improved survival[1]. Because today we have a large arsenal of antiplatelet and anticoagulant drugs with different profile of efficacy and safety, it is important to make a proper selection of medication in order to balance the ischemic and hemorrhagic risk[5-8]. European and American Societies of Cardiology recommend bleeding risk stratification to guide ACS treatment [9-12].
Hemorrhagic complications occur with a frequency of 1% to 10% during treatment for ACS and after PCI[13]. The National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network Registry Get with the Guidelines (NCDR ACTION Registry-GWTG)[14] evaluated 72699 patients with non ST-segment elevation myocardial infarction (NSTEMI) and 48943 with ST-segment elevation myocardial infarction (STEMI). The reported major bleeding rate was approximately 9% among patients with NSTEMI and 12% among those patients with STEMI. Of note, the bleeding rates were significantly influenced by the presence of comorbidities, as well as by the use of invasive strategies in both NSTEMI and STEMI.
Bleeding rates depend mainly on the clinical setting and on the definition of bleeding events[15,16]. Since their initial development, both TIMI and GUSTO criteria have been applied to identify very significant bleeding in a wide range of clinical trials[17,18], but a myriad of other criteria have also been created[19] [the CURE[20], Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events (REPLACE)[21], STEEPLE[22], OASIS[6] and acute catheterization and urgent intervention triage strategy (ACUITY)[8] bleeding definitions] (Table 1).
| Trial | Definition |
| TIMI | Major bleeding: Intracranial hemorrhage or decrease of 5 g/dL in hemoglobin or 15% in hematocrit |
| Minor bleeding: Decrease of 3 g/dL in hemoglobin with known source of blood los sor decrease of 4 g/dL in hemoglobin withoun known source of blood loss | |
| GUSTO | Major bleeding: Fatal, intracranial, Retroperitoneal, intraocular leading to visión loss, or transfusion of 2 U |
| Minor bleeding: any clinically significant bleeding not meeting major criteria leading to study drug interruption, surgery, or transfusion of 1 U of blood | |
| ACUITY | Major bleeding: Intracranial or intraocular bleeding, hemorrhage at access site requiring intervention, hematoma ≥ 5 cm, decrease ≥ 4 g/dL of hemoglobin without overt bleeding source or ≥ 3 g/dL with source, reoperation for bleeding, or transfusion of blood product |
| Minor bleeding: any clinically significant bleeding not meeting major criteria | |
| CRUSADE | Major bleeding: intracranial hemorrhage, documented retroperitoneal bleed, hematocrit drop ≥ 12% (baseline to nadir), any red blood cell transfusion when baseline hematocrit was ≥ 28%, or any red blood cell transfusion when baseline hematocrit was < 28% with witnessed bleed |
| Minor bleeding: any clinically significant bleeding not meeting major criteria | |
| GRACE | Major bleeding: Life-threatening bleeding requiring transfusion of ≥ 2 U of packed red blood cells, bleeding resulting in absolute hematocrit decrease ≥ 10% or death hemorrhagic/subdural hematoma |
| Minor bleeding: any clinically significant bleeding not meeting major criteria | |
| BARC | Type 0: No bleeding |
| Type 1: Bleeding that is not actionable and does not cause the patient to seek unscheduled performance of studies, hospitalization, or treatment by a health care professional; may include episodes leading to self-discontinuation of medical therapy by the patient without consulting a health care professional | |
| Type 2: Any overt, actionable sign of bleeding (e.g., more bleeding than would be expected for a clinical circumstance, including bleeding found by imaging alone) that does not fit the criteria for type 3, 4, or 5 but does meet at least one of the following criteria: requiring nonsurgical, medical intervention by a health care professional; leading to hospitalization or increased level of care; or prompting evaluation | |
| Type 3a: Overt bleeding plus hemoglobin drop of 3-5 g/dL (provided hemoglobin drop is related to bleed), or any transfusion with overt bleeding | |
| Type 3b: Overt bleeding plus hemoglobin drop ≥ 5 g/dL (provided hemoglobin drop is related to bleed), or cardiac tamponade, or bleeding requiring surgical intervention for control (excluding dental/nasal/skin/hemorrhoid), or bleeding requiring intravenous vasoactive agents | |
| Type 3c: Intracranial bleeding (does not include microbleeds or hemorrhagic transformation, does include intraspinal), or subcategories confirmed by autopsy or imaging or lumbar puncture, or intraocular bleed compromising vision | |
| Type 4: Coronary artery bypass graft-related bleeding, or perioperative intracranial bleeding within 48 h, or reoperation after closure of sternotomy for the purpose of controlling bleeding, or transfusion of ≥ 5 U whole blood or packed red blood cells within a 48-h period, or chest tube output ≥ 2 L within a 24-h period | |
| Type 5 or fatal bleeding A: Probable fatal bleeding; no autopsy or imaging confirmation but clinically suspicious | |
| Type 5 or fatal bleeding B: Definite fatal bleeding; overt bleeding or autopsy or imaging confirmation |
The Bleeding Academic Research Consortium (BARC) convened in 2010 was idealized with the intention of reviewing the existing definitions and developing standards for the analysis of hemorrhagic complications[13]. Among the recommendations of the panel, the consensus around the challenge of creating a single definition of major bleeding to be adopted stands out since the analyzed population is extremely variable as to its characteristics, clinical profile, follow-up time length, and due to the constant temporary modifications in clinical therapy and treatment strategies considered appropriate at its time. Basing on this, BARC participants proposed 5 bleeding types (Table 1)[6].
Major bleeding is currently one of the most common non-cardiac complications observed in the treatment of ACS patients. The identification of clinical characteristics and particularities of the antithrombin therapy associated with an increased risk of hemorrhagic complications would make it possible to adopt prevention strategies, especially among those exposed to greater risk[15].
In this way, different studies exposed the main predictors of major bleeding in the treatment of ACS. The Global Registry of Acute Coronary Events (GRACE) investigators developed a risk score of major bleeding, basing on a registry with 24045 ACS patients, of which 933 (3.9%) developed an episode of major bleeding during hospitalization[23]. They identified 7 independent predictors of bleeding: age, female gender, prior bleeding, kidney dysfunction, fibrinolysis, glycoprotein IIb/IIIa inhibitors (GPI) use, and PCI. The most frequent bleeding sites were gastrointestinal (31.5%) and those related to the vascular access site (23.8%).
In the ACUITY study[22], authors identified 8 variables related to greater risk of bleeding were identified: female sex, anemia, advanced age, use of unfractionated heparin and IIb/IIIa inhibitors instead of isolated bivalirudin, elevated serum creatinine, increased leukocyte count, absence of previous PCI, prior stroke, ST-segment elevation ≥ 1 mm, and routine use of GPI.
In an analysis of the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines (CRUSADE) database[1], with 89134 high-risk NSTEMI patients and with a incidence of major bleeding of 9.4%, 8 variables were identified as independent predictors of major bleeding: female sex, peripheral vascular disease, diabetes, systolic blood pressure, heart rate, congestive heart failure, creatinine clearance, and hematocrit.
In the REPLACE registry, female sex, anemia, and glomerular filtrate rate were also identified as independente predictor of bleeding[24]. Age > 55 years, low molecular weight heparin within 48 h pre-PCI, GPI, and intra-aortic balloon pump use were the other clinical variables associated with higher rate of major bleeding in the REPLACE trial.
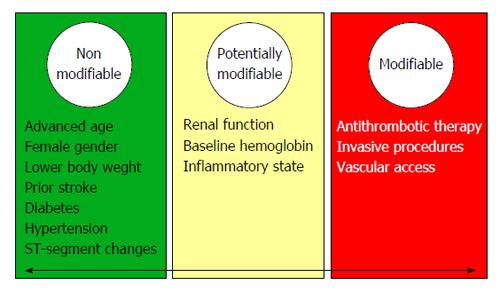
In a global way, bleeding risk factors can be categorized into nonmodifiable and modifiable groups[25]. Commonly reported bleeding risk factors in patients with ACS are summarized in Figure 1.
According to the non-modifiable risk factors it is important to remarked 2 clinical variables: advanced age and female sex. Advanced age would predispose to a greater risk of bleeding due to injuries located in the vessels and systemic diffuse vessel disease. In the GRACE registry encompassing the whole spectrum of ACS, the adjusted odds of having a major hemorrhage prior to discharge increased by about 30% per decade of age (OR = 1.28, 95%CI: 1.21-1.37)[6,23,26]. In relation to female sex, within the GRACE registry, women had a 43% higher likelihood of developing major bleeds in-hospital compared with men (adjusted OR = 1.43, 95%CI: 1.23-1.66)[6,23]. It is believed that the smaller body size as well as the lower vessel size, reduced creatinine clearance, higher prevalence of comorbidities, higher risk of drug overdosing, older age at the moment of admission, and a lower threshold for transfusion due to lower baseline levels of hemoglobin would justify the relationship between female sex and bleeding[15].
In relation with potentially modifiable factors, renal function is the most interesting. Santopinto et al[27] demonstrated that patients with moderate renal dysfunction were twice as likely to die (OR = 2.09, 95%CI: 1.55-2.81) and those patients with severe renal dysfunction are almost four times more likely to die (OR = 3.71, 95%CI: 2.57-5.37)[27]. Other potentially modifiable variable, with great interest in last years, is body mass index (BMI)[28]. Several epidemiologic studies have demonstrated that higher BMI was inversely associated with lower risk of mortality among patients with coronary artery disease (obesity-mortality paradox). As we know, the association between short-term death and BMI was affected not only by the ischemic risk but also by the major bleeding risk[25]. Recently, a meta-analysis have clarified the relationship between the risk of bleeding and BMI following PCI[29]. In this study, it was concluded that class I/II obese patients had the lowest risk of bleedings.
With regard to modifiable risk factors, two variables deserve a special mention: antithrombotic therapy and vascular access. Antithrombotic therapy would be influenced by pharmacodynamic and pharmacokinetic characteristics of the antithrombotic agents[15]. In this way, we can exemplify with the differences between fondaparinux, bivalirudin and enoxaparin, or the differences between clopidogrel, prasugrel, and ticagrelor. Fondaparinux and bivalirudin showed to reduce the rate of bleeding complications when compared with low molecular weight heparin and heparin sodium, with adequate antithrombotic ability (although fondaparinux have a slightly increased risk of catheter thrombosis in patients undergoing PCI). A critical aspect in the appropriate use of anticoagulant agents is dose adjustment according to the renal function. Current guidelines indicate dose reduction of enoxaparin to 1 mg/kg once daily in the case of severe renal failure (CrCl < 30 mL/min), and consider monitoring of anti-Xa activity[9,12]. Fondaparinux is contraindicated in severe renal failure (CrCl < 20 mL/min), ans is considered the drug of choice in patients with moderately reduced renal function (CrCl 30-60 mL/min). Regarding bivalirudin, patients with moderate renal impairment (30-59 mL/min) should receive an infusion of 1.75 mg/kg per hour, or 1 mg/kg per hour if the creatinine clearance is < 30 mL/min (0.25 mg/kg per hour if the patient is on haemodialysis). In presence of CrCl < 30 mL/min or eGFR is < 30 mL/min per 1.73 m2, unfractionated heparin infusion adjusted to activated partial thromboplastin time is the recommended anticoagulant, albeit fondaparinux could be maintained until CrCl < 20 mL/min.
For the vascular access, the use of radial access significantly reduces bleeding complications in PCI compared with femoral access[30]. Importantly, vascular closure devices should be used in patients without significant arterial calcification in order to obtain satisfactory results.
In addition to these clinical factors, current research is focused on meeting new bleeding risk indicators. In this sense, genetic factors have been associated to bleeding[26]. For example, in clopidogrel-treated patients, the gain-function variant CYP2C19*17 was associated with higher bleeding rate[6,31]. This is an area with great projection in the near future.
The contemporary cardiology walks towards those predictive models that minimize as much as possible to morbidity and mortality resulting from cardiovascular disease[32]. This is to minimize the subjective component of clinical evaluation of a given patient. Therefore risk stratification is that characterizes modern clinical cardiologist[33]. Since patient’s admission, there are many factors that determine the patient’s prognosis in terms of mortality and morbidity. In this way, is necessary to go reassessing the patient risk at all times. Regarding acute coronary síndrome patient, particularly in relation to bleeding, there are a lot of variables that determine the hemorragic risk. The interaction between these variables is not easy to assess clinically. This is where lies the advantage of risk scores, which enable integration of all these variables providing a measure of risk that would not be possible otherwise. And this is the reason because of objective risk assessment provides superior risk discrimination when compared with physician-estimated risk[34]. Although there are several bleeding risk scores, there is no consensus about what is the best for bleeding risk assessment in daily clinical practice.
Contemporary bleeding risk scores (RS) (Table 2) in ACS comprise: REPLACE[24], CRUSADE[1], ACTION[35], and that derived by Mehran et al[36] from the combined dataset of ACUITY/HORIZONS-AMI trials. The CRUSADE risk score was developed to assess the in-hospital bleeding risk help during NSTEMI, whereas the ACTION and Mehran et al[36] models were derived from NSTEMI and STEMI patients. In addition to these risk models, the REPLACE proposes a stratification of the bleeding risk for patients submitted to PCI through femoral Access.
| Bleeding risk scores | Action | Mehran et al | CRUSADE | |||
| variables | Values | Points | Values | Points | Values | Points |
| Sex | Male | 0 | Male | 0 | Male | 0 |
| Female | 4 | Female | 8 | Female | 8 | |
| Age (yr) | ≤ 40 | 0 | < 50 | 0 | ||
| 41-50 | 1 | 50-59 | 3 | |||
| 51-60 | 2 | 60-69 | 6 | |||
| 61-70 | 3 | 70-79 | 9 | |||
| 71-80 | 4 | ≥ 80 | 12 | |||
| 81-90 | 5 | |||||
| ≥ 91 | 6 | |||||
| Weight (kg) | ≤ 50 | 5 | ||||
| 51-70 | 4 | |||||
| 71-100 | 3 | |||||
| 101-120 | 2 | |||||
| 121-140 | 1 | |||||
| ≥ 141 | 0 | |||||
| Systolic blood pressure (mmHg) | ≤ 90 | 4 | ≤ 90 | 10 | ||
| 91-100 | 3 | 91-100 | 8 | |||
| 101-120 | 2 | 101-120 | 5 | |||
| 121-140 | 1 | 121-180 | 1 | |||
| 141-170 | 0 | 181-200 | 3 | |||
| 171-200 | 1 | ≥ 201 | 5 | |||
| ≥ 201 | 2 | |||||
| Heart rate (BPM) | ≤ 40 | 0 | ≤ 70 | 0 | ||
| 41-60 | 2 | 71-80 | 1 | |||
| 61-70 | 3 | 81-90 | 3 | |||
| 71-80 | 5 | 91-100 | 6 | |||
| 81-100 | 6 | 101-110 | 8 | |||
| 101-110 | 8 | 111-120 | 10 | |||
| 111-120 | 9 | ≥ 121 | 11 | |||
| 121-130 | 11 | |||||
| 131-150 | 12 | |||||
| ≥ 151 | 14 | |||||
| Signs of heart failure | None | 0 | No | 0 | ||
| Killip 2-3 | 3 | Yes | 7 | |||
| Cardiogenic shock | 15 | |||||
| Diabetes mellitus | No | 0 | No | 0 | ||
| Yes | 3 | Yes | 6 | |||
| Prior vascular disease | No | 0 | No | 0 | ||
| Yes | 3 | Yes | 6 | |||
| Home warfarin use | No | 0 | ||||
| Yes | 2 | |||||
| Antithrombotic medications | Heparin plus GPI | 0 | ||||
| Bivalirudin | -5 | |||||
| ECG changes | No ST changes | 0 | No ST elevation | 0 | ||
| ST depresión | 3 | ST elevation | 6 | |||
| ST transient elevation | 7 | |||||
| ST elevation | ||||||
| Troponine I | Normal | 0 | ||||
| Raised | 6 | |||||
| Serum creatinine (mg/dL) | < 0.80 | 0 | < 1.00 | 0 | ||
| 0.80-1.59 | 1 | 1.00-1.19 | 2 | |||
| 1.60-1.99 | 2 | 1.20-1.39 | 3 | |||
| 2.00-2.99 | 4 | 1.40-1.59 | 5 | |||
| 3.00-3.99 | 6 | 1.60-1.79 | 6 | |||
| 4.00-4.99 | 8 | 1.80-1.99 | 8 | |||
| 5.00-5.99 | 10 | ≥ 2.00 | 10 | |||
| ≥ 6.00 | 11 | |||||
| On dialysis | 11 | |||||
| Creatinine clearance (mL/min) | ≤ 15.0 | 39 | ||||
| 15.1-30.0 | 35 | |||||
| 30.1-60.0 | 28 | |||||
| 60.1-90.0 | 17 | |||||
| 90.1-120.0 | 7 | |||||
| > 120 | 0 | |||||
| Baseline hemoglobin (g/dL) | < 5.0 | 17 | ||||
| 5.0-7.9 | 15 | |||||
| 8.0-9.9 | 13 | |||||
| 10.0-10.9 | 12 | |||||
| 11.0-13.9 | 9 | |||||
| 14.0-15.9 | 6 | |||||
| ≥ 16.0 | 2 | |||||
| Baseline hematocrit (%) | < 31.0 | 9 | ||||
| 31.0-33.9 | 7 | |||||
| 34.0-36.9 | 3 | |||||
| 37.0-39.9 | 2 | |||||
| ≥ 40.0 | 0 | |||||
| Anemia | No | 0 | ||||
| Yes | 6 | |||||
| White blood cell count (giga/L) | < 10.0 | 0 | ||||
| 10.0-11.9 | 2 | |||||
| 12.0-13.9 | 3 | |||||
| 14.0-15.9 | 5 | |||||
| 16.0-17.9 | 6 | |||||
| 18.0-19.9 | 8 | |||||
| ≥ 20.0 | 10 | |||||
Using data from the multicenter studies REPLACE-1 e 2[37,38], Nikolsky et al[24] proposed a bleeding RS for patients submitted to PCI through femoral access (http://www.bleedingriskscore.org). In multivariate analysis performed in 5395 patients, seven variables were identified as predictors of major bleeding: age, female sex, chronic kidney dysfunction, anemia, use of low-molecular-weight heparin, administration of GPI, and the use of intra-aortic balloon pump. Based on them, a risk score was constructed, with an adequate discrimination (C-statistic = 0.62). The main limitation is that this risk score was derived from a highly selective population undergoing PCI using the femoral approach.
More recently, investigators of the CRUSADE registry developed and validated a risk stratification tool for in-hospital major bleeding among NSTEMI patients[1]. Having a database constituted by 89134 patients, within 485 North American hospitals, the authors developed a bleeding risk score with those variables that resulted independent predictors of major bleeding: female sex, diabetes mellitus, peripheral artery disease, heart rate, systolic blood pressure, congestive heart failure, hematocrit, and creatinine clearance (http://www.crusadebleedingscore.org). Considering only the variables present at admission, the CRUSADE bleeding score is presented as an easily applicable and useful tool in predicting patient risk, in addition to the analysis of the risk of ischemic events, allowing a tailored therapeutic strategy, adapted to the individualized risk profile. Moreover, CRUSADE bleeding risk score was externally validated by Abu-Assi et al[39]. The CRUSADE score showed adequate calibration and excellent discriminatory powerful in the whole population and in the different treatment subgroups, except in patients treated with ≥ 2 antithrombotics who did not undergo cardiac catheterization (C-index = 0.56).
Mehran et al[36] using data from the ACUITY and the HORIZONS-AMI trials (17421 patients) developed a bleeding risk score. Six independent baseline predictors for major bleeding were identified: female sex, age, creatinine, white blood cell count, anemia, ST-segment-elevation. The risk score differentiated patients with a 30-d rate of non-CABG-related major bleeding ranging from 1% to over 40%. As a difference with the other bleeding risk scores, this one includes white blood cell count as a risk factor for major bleeding.
Using data from the ACTION trial in patients with STEMI and NSTEMI, an inhospital bleeding risk score was developed[35]. Twenty-two clinically variables were incorporated into the final regression model: heart rate, baseline hemoglobin, female gender, serum creatinine, age, electrocardiographic changes, heart failure or shock, diabetes, peripheral artery disease, body weight, systolic blood pressure, and home warfarin use. The rate of major bleeding in the overall population was 10.8%. The risk model discriminated well in the derivation (C-statistic = 0.73) and validation (C-statistic = 0.71) cohorts, with an optimal risk gradient: very low risk (3.9% of bleeding), low risk (7.3%), moderate risk (16.1%), high risk (29.0%), and very high risk (39.8%).
As we have shown above, all bleeding RS have shown good discrimination and calibration. The question is: Which RS should be recommended for the management of patients with ACS? Perhaps for that question we first must be sure about the reliability of a given predictive model in our population. The REPLACE bleeding RS was designed for a femoral approach, so now, in times of radial access, its usefulness is less. The other 3 scores (CRUSADE, ACTION and Mehran et al[36]) were compared recently by the group of Abu Assi on a patient population with ACS (STEMI and NSTEMI)[40], being the greatest accuracy obtained with the CRUSADE method, even in patients with STEMI.
Although any score cannot replace the clinical evaluation, data from our study suggests that CRUSADE score represents an useful objective clinical tool which could lead to improvements in ACS care[40].
The risk of developing bleeding complications continues after discharge. About 5% of patients develop bleeding complications throught the first yeart after hospital discharge being on dual antiplatelet therapy (DAPT)[41]. There is no risk model to estimate risk of bleeding after discharge in ACS patients. Using data from the REACH registry[42], a risk score was built although in a stable scenario (not in the ACS setting). Because the CRUSADE risk score performed well among patients taking DAPT, this risk model may be used for bleeding risk stratification in ACS on DAPT after hospital discharge.
The main clinical implication of RS is to pave the way for a decision concerning the best antithrombotic strategy to be used aiding individual evaluation for risk of ischemic or hemorrhagic events.
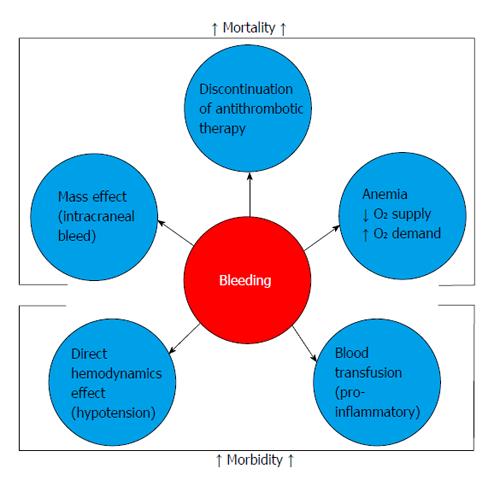
Collectively, innumerous studies have shown a robust association between the occurrence of major bleeding and the necessity of blood cell transfusion with greater mortality in patients admitted with ACS or submitted to PCI (Figure 2). Subherwal et al[1] demonstrated an association between bleeding and inhospital mortality. Mehran et al[36] showed that major bleeding was an independent predictor of a 3.2-fold increase in mortality.
Although it is coherent to justify the association between major bleeding and mortality by the coexistence of comorbidities and risk factors in the population common to the occurrence of these outcomes, today an accumulation of evidence is observed that points to direct or indirect influence of bleeding as a greater determinant of subsequent adverse ischemic events. The localization (intracranial) or the intensity (gastrointestinal, retroperitoneal) of the bleeding may itself result in death. However, other consequences may exhibit harmful effects to the ACS patients or those submitted to invasive coronary procedures[43].
The reduction of major bleeding, a relatively common complication in the current ACS scenario and possibly underestimated in randomized clinical trials, may be translated in better short- and long-term outcomes. Nowadays, its prevention represents a goal to be reached in the treatment of patients with ACS, through the balance between the risks and benefits of the pharmacological and invasive strategies offered. Appropriate risk stratification allows properly select those patients at increased risk of bleeding, focusing on them the efforts to reduce bleeding complications.
| 1. | Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK, Wang TY, Gibler WB, Ohman EM, Roe MT. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation. 2009;119:1873-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 750] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 2. | Anderson JL. Stopping the hemorrhage: a new baseline bleeding score brings us a step closer for patients with non-ST-elevation myocardial infarction. Circulation. 2009;119:1846-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Bassand JP. Acute Coronary Syndromes and Percutaneous Coronary Interventions: impact of bleeding and blood transfusion. Hamostaseologie. 2009;29:381-387. [PubMed] |
| 4. | Mehran R, Pocock S, Nikolsky E, Dangas GD, Clayton T, Claessen BE, Caixeta A, Feit F, Manoukian SV, White H. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient-level pooled analysis of the REPLACE-2 (randomized evaluation of PCI linking angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC Cardiovasc Interv. 2011;4:654-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 320] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 5. | Cannon CP, Harrington RA, James S, Ardissino D, Becker RC, Emanuelsson H, Husted S, Katus H, Keltai M, Khurmi NS. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet. 2010;375:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 466] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 6. | Steg PG, Jolly SS, Mehta SR, Afzal R, Xavier D, Rupprecht HJ, López-Sendón JL, Budaj A, Diaz R, Avezum A. Low-dose vs standard-dose unfractionated heparin for percutaneous coronary intervention in acute coronary syndromes treated with fondaparinux: the FUTURA/OASIS-8 randomized trial. JAMA. 2010;304:1339-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Montalescot G, Wiviott SD, Braunwald E, Murphy SA, Gibson CM, McCabe CH, Antman EM. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet. 2009;373:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 811] [Cited by in RCA: 748] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 8. | Stone GW, White HD, Ohman EM, Bertrand ME, Lincoff AM, McLaurin BT, Cox DA, Pocock SJ, Ware JH, Feit F. Bivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial. Lancet. 2007;369:907-919. [PubMed] |
| 9. | O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362-e425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 1159] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 10. | Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE, Ettinger SM, Fesmire FM, Ganiats TG, Lincoff AM. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/Non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2012;126:875-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 370] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 11. | Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999-3054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2431] [Cited by in RCA: 2516] [Article Influence: 167.7] [Reference Citation Analysis (0)] |
| 12. | Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3540] [Cited by in RCA: 3732] [Article Influence: 266.6] [Reference Citation Analysis (0)] |
| 13. | Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736-2747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2595] [Cited by in RCA: 3666] [Article Influence: 244.4] [Reference Citation Analysis (0)] |
| 14. | Kadakia MB, Desai NR, Alexander KP, Chen AY, Foody JM, Cannon CP, Wiviott SD, Scirica BM. Use of anticoagulant agents and risk of bleeding among patients admitted with myocardial infarction: a report from the NCDR ACTION Registry--GWTG (National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network Registry--Get With the Guidelines). JACC Cardiovasc Interv. 2010;3:1166-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | de Andrade PB, Tebet MA, Maia da Silva FS, Athanazio de Andrade MV, Labrunie A, Piva E Mattos LA. Major bleeding in acute coronary syndromes. J Invasive Cardiol. 2011;23:485-490. [PubMed] |
| 16. | Bassand JP. Bleeding and transfusion in acute coronary syndromes: a shift in the paradigm. Heart. 2008;94:661-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Chesebro JH, Knatterud G, Roberts R, Borer J, Cohen LS, Dalen J, Dodge HT, Francis CK, Hillis D, Ludbrook P. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: A comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation. 1987;76:142-154. [PubMed] |
| 18. | Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921-1929. [PubMed] |
| 19. | Steinhubl SR, Kastrati A, Berger PB. Variation in the definitions of bleeding in clinical trials of patients with acute coronary syndromes and undergoing percutaneous coronary interventions and its impact on the apparent safety of antithrombotic drugs. Am Heart J. 2007;154:3-11. [PubMed] |
| 20. | Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774-782. [PubMed] |
| 21. | Feit F, Voeltz MD, Attubato MJ, Lincoff AM, Chew DP, Bittl JA, Topol EJ, Manoukian SV. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 Trial. Am J Cardiol. 2007;100:1364-1369. [PubMed] |
| 22. | Koestenberger M, Nagel B, Ravekes W, Avian A, Heinzl B, Fritsch P, Fandl A, Rehak T, Gamillscheg A. Left ventricular long-axis function: reference values of the mitral annular plane systolic excursion in 558 healthy children and calculation of z-score values. Am Heart J. 2012;164:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Moscucci M, Fox KA, Cannon CP, Klein W, López-Sendón J, Montalescot G, White K, Goldberg RJ. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J. 2003;24:1815-1823. [PubMed] |
| 24. | Nikolsky E, Mehran R, Dangas G, Fahy M, Na Y, Pocock SJ, Lincoff AM, Stone GW. Development and validation of a prognostic risk score for major bleeding in patients undergoing percutaneous coronary intervention via the femoral approach. Eur Heart J. 2007;28:1936-1945. [PubMed] |
| 25. | Pham PA, Pham PT, Pham PC, Miller JM, Pham PM, Pham SV. Implications of bleeding in acute coronary syndrome and percutaneous coronary intervention. Vasc Health Risk Manag. 2011;7:551-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Steg PG, Huber K, Andreotti F, Arnesen H, Atar D, Badimon L, Bassand JP, De Caterina R, Eikelboom JA, Gulba D. Bleeding in acute coronary syndromes and percutaneous coronary interventions: position paper by the Working Group on Thrombosis of the European Society of Cardiology. Eur Heart J. 2011;32:1854-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 276] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 27. | Santopinto JJ, Fox KA, Goldberg RJ, Budaj A, Piñero G, Avezum A, Gulba D, Esteban J, Gore JM, Johnson J. Creatinine clearance and adverse hospital outcomes in patients with acute coronary syndromes: findings from the global registry of acute coronary events (GRACE). Heart. 2003;89:1003-1008. [PubMed] |
| 28. | Lin GM, Li YH, Lin CL, Wang JH, Han CL. Relation of body mass index to bleeding events among patients with percutaneous coronary intervention: a meta-analysis. Int J Cardiol. 2013;168:4831-4835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Das SR, Alexander KP, Chen AY, Powell-Wiley TM, Diercks DB, Peterson ED, Roe MT, de Lemos JA. Impact of body weight and extreme obesity on the presentation, treatment, and in-hospital outcomes of 50,149 patients with ST-Segment elevation myocardial infarction results from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol. 2011;58:2642-2650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 30. | Jolly SS, Cairns J, Yusuf S, Niemela K, Steg PG, Worthley M, Ferrari E, Cantor WJ, Fung A, Valettas N. Procedural volume and outcomes with radial or femoral access for coronary angiography and intervention. J Am Coll Cardiol. 2014;63:954-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Sibbing D, Koch W, Gebhard D, Schuster T, Braun S, Stegherr J, Morath T, Schömig A, von Beckerath N, Kastrati A. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation. 2010;121:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 421] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 32. | Raposeiras-Roubín S, Abu-Assi E, Cabanas-Grandío P, Agra-Bermejo RM, Gestal-Romarí S, Pereira-López E, Fandiño-Vaquero R, Álvarez-Álvarez B, Cambeiro C, Rodríguez-Cordero M. Walking beyond the GRACE (Global Registry of Acute Coronary Events) model in the death risk stratification during hospitalization in patients with acute coronary syndrome: what do the AR-G (ACTION [Acute Coronary Treatment and Intervention Outcomes Network] Registry and GWTG [Get With the Guidelines] Database), NCDR (National Cardiovascular Data Registry), and EuroHeart Risk Scores Provide? JACC Cardiovasc Interv. 2012;5:1117-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Halim SA, Rao SV. Bleeding and acute coronary syndromes: defining, predicting, and managing risk and outcomes. Curr Drug Targets. 2011;12:1831-1835. [PubMed] |
| 34. | Chew DP, Junbo G, Parsonage W, Kerkar P, Sulimov VA, Horsfall M, Mattchoss S. Perceived risk of ischemic and bleeding events in acute coronary syndromes. Circ Cardiovasc Qual Outcomes. 2013;6:299-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Mathews R, Peterson ED, Chen AY, Wang TY, Chin CT, Fonarow GC, Cannon CP, Rumsfeld JS, Roe MT, Alexander KP. In-hospital major bleeding during ST-elevation and non-ST-elevation myocardial infarction care: derivation and validation of a model from the ACTION Registry®-GWTG™. Am J Cardiol. 2011;107:1136-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 36. | Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ, Parise H, Fahy M, Manoukian SV, Feit F. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol. 2010;55:2556-2566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 510] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 37. | Lincoff AM, Bittl JA, Kleiman NS, Sarembock IJ, Jackman JD, Mehta S, Tannenbaum MA, Niederman AL, Bachinsky WB, Tift-Mann J. Comparison of bivalirudin versus heparin during percutaneous coronary intervention (the Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events [REPLACE]-1 trial). Am J Cardiol. 2004;93:1092-1096. [PubMed] |
| 38. | Lincoff AM, Kleiman NS, Kereiakes DJ, Feit F, Bittl JA, Jackman JD, Sarembock IJ, Cohen DJ, Spriggs D, Ebrahimi R. Long-term efficacy of bivalirudin and provisional glycoprotein IIb/IIIa blockade vs heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary revascularization: REPLACE-2 randomized trial. JAMA. 2004;292:696-703. [PubMed] |
| 39. | Abu-Assi E, Gracía-Acuña JM, Ferreira-González I, Peña-Gil C, Gayoso-Diz P, González-Juanatey JR. Evaluating the Performance of the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines (CRUSADE) bleeding score in a contemporary Spanish cohort of patients with non-ST-segment elevation acute myocardial infarction. Circulation. 2010;121:2419-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Abu-Assi E, Raposeiras-Roubin S, Lear P, Cabanas-Grandío P, Girondo M, Rodríguez-Cordero M, Pereira-López E, Romaní SG, González-Cambeiro C, Alvarez-Alvarez B. Comparing the predictive validity of three contemporary bleeding risk scores in acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2012;1:222-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 41. | Mehran R, Baber U, Steg PG, Ariti C, Weisz G, Witzenbichler B, Henry TD, Kini AS, Stuckey T, Cohen DJ. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet. 2013;382:1714-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 489] [Article Influence: 37.6] [Reference Citation Analysis (1)] |
| 42. | Ducrocq G, Wallace JS, Baron G, Ravaud P, Alberts MJ, Wilson PW, Ohman EM, Brennan DM, D’Agostino RB, Bhatt DL. Risk score to predict serious bleeding in stable outpatients with or at risk of atherothrombosis. Eur Heart J. 2010;31:1257-1265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 43. | Doyle BJ, Rihal CS, Gastineau DA, Holmes DR. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol. 2009;53:2019-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 318] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
P- Reviewer: Berenguer AB, Lin GM, Sethi A S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ