Published online Apr 26, 2012. doi: 10.4330/wjc.v4.i4.121
Revised: March 26, 2012
Accepted: April 2, 2012
Published online: April 26, 2012
AIM: To validate the sleep-disordered breathing components of a portable electrocardiography and hemodynamic monitor to be used for sleep apnea screening.
METHODS: Sleep-disordered breathing (SDB) is associated with cardiovascular disease. Patients with existing cardiovascular disease may have unrecognized SDB or may develop SDB while under the care of a cardiologist. A screening device for SDB, easy to use and appealing to cardiologists, would assist in referral of appropriate patients for full polysomnography (PSG). A cardiac and respiratory monitor (CPAM) was attached to patients undergoing PSG and an apnea/hypopnea index (AHI) generated. The CPAM device produced respiration rate, snoring rate, individual apnea/hypopnea events and an SDB severity score (SDBSS). In addition to AHI, an expert over-reader annotated individual breaths, snores and SDB breathing events to which the automated algorithms were compared.
RESULTS: The test set consisted of data from 85 patients (age: 50.5 ± 12.4 years). Of these, 57 had a positive PSG defined as AHI ≥ 5.0 (mean: 30.0 ± 29.8, negative group mean: 1.5 ± 1.2). The sensitivity and specificity of the SDBSS compared to AHI was 57.9% and 89.3%, respectively. The correlation of snoring rate by CPAM compared to the expert over-reader was r = 0.58 (mean error: 1.52 snores/min), while the automated respiration rate had a correlation of r = 0.90 (mean error: 0.70 breaths/min).
CONCLUSION: This performance assessment shows that CPAM can be a useful portable monitor for screening and follow-up of subjects for SDB.
- Citation: Dillier R, Baumann M, Young M, Erne S, Schwizer B, Zuber M, Erne P. Continuous respiratory monitoring for sleep apnea screening by ambulatory hemodynamic monitor. World J Cardiol 2012; 4(4): 121-127
- URL: https://www.wjgnet.com/1949-8462/full/v4/i4/121.htm
- DOI: https://dx.doi.org/10.4330/wjc.v4.i4.121
As recognized by the American College of Cardiology in 2008, sleep-disordered breathing (SDB) is associated with cardiovascular disease including heart failure, hypertension and increased arrhythmias[1]. Central sleep apnea is a common comorbidity of heart failure and may contribute to readmission, poor response to treatment and heart failure progression. Effective treatment exists for SDB that may improve or reverse the cardiovascular consequences, making accurate diagnosis important. The prevalence of SDB ranges from 24% of men in a random sample of middle-aged adults[2] to 73% in patients with stable heart failure[3]. In addition, it has been estimated that 80%-90% of patients with obstructive sleep apnea (OSA) are undiagnosed[4]. SDB is typically diagnosed during overnight polysomnography (PSG), with technicians attending to both the patient and the equipment. These extensive studies are the accepted gold standard for diagnosis of sleep disorders. In 2007, the American Academy of Sleep Medicine (AASM) provided guidelines on the use of portable monitors to assess moderate to severe sleep apnea[5] for screening. In certain populations, such as those with heart failure, repeat studies are beneficial to evaluate the effectiveness of treatment, and a portable monitor for at-home studies would facilitate that process, especially if information concerning cardiac function was also available.
Standard PSG techniques have been adapted to portable monitors including electro-encephalography (EEG), electro-oculography (EOG), thoracic and abdominal effort, and oximetry. Novel technologies have been developed including one device that measures peripheral artery tone from a finger sensor to estimate changes in vascular flow[6] during apnea, whereas another incorporates actigraphy as a marker of sleep and wakefulness[7]. The performance of portable monitors compared to PSG has been reported and varies greatly[8]. To date, there have been no monitors available to assess both hemodynamic function and SDB simultaneously.
Targeting the patient population with cardiovascular disease, we report the performance of the respiratory components of a new portable monitor capable of recording 48-h electrocardiography (ECG), heart sounds, snoring, body position and respiration [Cardiopulmonary Ambulatory Monitor (CPAM), Inovise Medical, Inc., Beaverton, OR, United States]. In addition to full Holter-type ECG analysis and heart rate variability, this device incorporates automated algorithms for quantification of diastolic heart sounds and systolic time intervals previously validated and shown to correlate with independent hemodynamic measurements of left ventricular (LV) function[9,10]. Parameters produced by CPAM include those to assess systolic and diastolic function[11]. For systolic function, the presence of a third heart sound has been shown to be associated with increased LV filling pressures and electromechanical activation time (Q-wave onset to S1 interval) correlating with reduced ejection fraction. For diastolic function the presence of a fourth heart sound (S4) has been shown to be associated with increased LV stiffness[12]. Moreover, we have previously shown that additional use of portable acoustic cardiography can improve cardiovascular diagnosis[13].
To test this device for SDB screening, we compared the results obtained with CPAM against standard PSG AHI and over-read of sound and respiration signals by trained personnel for snoring and respiration rate.
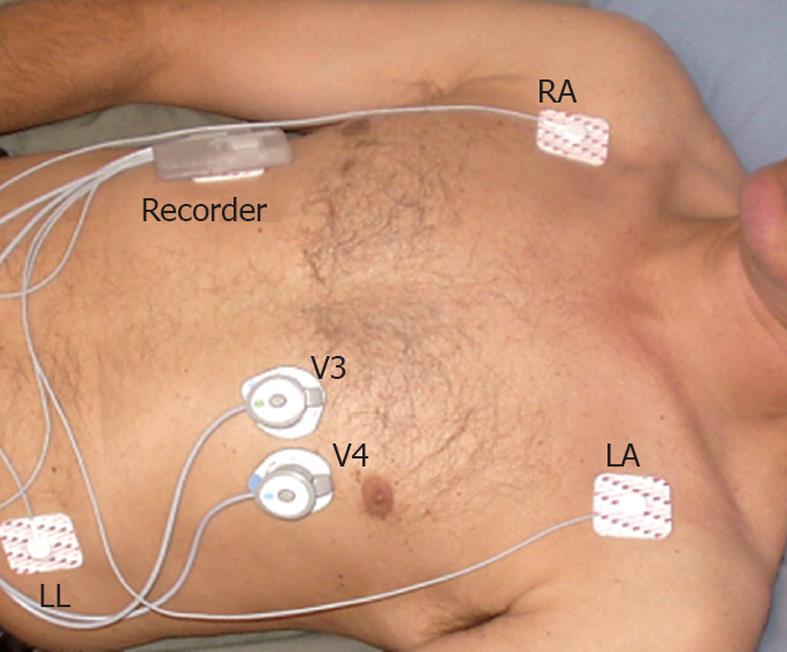
The cardiorespiratory monitor records three leads of ECG (leads I, II and a modified V4-RA) and two heart sound signals (V3 and V4 locations) (Figure 1); body position, snoring, and respiration. Body position and respiration are determined from a triaxial accelerometer within the main recorder unit. A wireless connection to a computer allows a preview of ECG and heart sound traces to confirm data quality. A removable micro SD card can store up to 48 h of data. The data are downloaded to a Windows PC application with automated algorithms for detection of respiration, including SDB events, snoring, body position, and activity level. The monitor uses previously validated automated algorithms for heart sound parameters and systolic time intervals[14] that were not part of this study.
The main recorder unit is secured in a holster that is placed on the upper chest wall and attached with two standard ECG snap-type electrodes. The triaxial accelerometer signals undergo several signal processing steps to create a pseudo-respiration signal. This signal is used to detect breaths for calculation of respiration rate and also to detect individual SDB events. The SDB, respiration and snoring algorithms were developed on learn portions of large datasets from apparently healthy subjects and from the learn set of data from this and other PSG studies. The algorithms were developed strictly on learning set data and tested infrequently on the separate testing datasets. The test data in this study were over-read and annotated by trained experts for individual breaths, snores and individual episodes of SDB. The data collected on patients during PSG studies had the additional independent results for apnea/hypopnea index (AHI).
The patients participating in this study were prospectively enrolled at Luzerner Kantonsspital for PSG at the Center of Sleep Disorders. The main inclusion criterion was suspicion of sleep apnea. Exclusion criteria were: age < 18 years, decompensated heart failure, myocardial infarction within the last 6 mo, and significant chronic obstructive pulmonary disease. The study protocol was approved by the local medical ethics committee and all patients gave written informed consent. The anthropometric parameters of all patients were determined, and a brief medical history and all current medications were recorded. The ambulatory monitor was placed on the subject by research team members independent of the sleep study team not involved in the data analysis. Patients were fitted with the PSG equipment and the AUDICOR CPAM device between 20:00 and 22:00 h. The patients followed their personal routine and retired between 22:30 and midnight and were awakened between 05:00 and 07:00 h next morning. The PSG equipment was fully disconnected while the ambulatory monitor continued to record until the research team removed the device later in the morning. The ambulatory monitor data were downloaded and sent to one individual for automated analysis while PSG analysis was performed in the sleep laboratory according to usual standards by separate personnel.
PSG was performed in accordance with AASM 2007 standards[15] by trained and certified technicians. The following parameters were monitored: EEG (Fp1/Fp2, C3/C4, O1/O2 vs M1/M2, rate 500-2000 Hz), EOG, chin, submental and leg electromyograms, and ECG. Respiration was monitored with thoracic and abdominal effort (induction plethysmography) while airflow was monitored by nasal cannular (flow pressure transducer). A microphone was placed near the head of the bed and sound recorded for later snoring analysis. The body position was recorded using a position sensor and the patient monitored by an infrared camera. Oxygen saturation was monitored by a pulse oximeter applied to the finger. All these parameters were computerized using the Embla™ Recording System S7000 or N7000 (ResMed Embla™, ResMed Corp., San Diego, CA, United States). CO2 was measured using a transcutaneous partial pressure of CO2 (tcpCO2) sensor (TOSCA 500, Radiometer GmbH, Switzerland).
PSG: The PSG data were scored using AASM 2007 standards[15] by a certified Swiss sleep technician and physician certified by the Swiss Board of Sleep Medicine. Apnea was categorized into central, obstructive and mixed using the thermistor recordings or hypopnea (unclassified) using the cannula recordings. The criteria of hypopnea were 30% reduction of airflow and 4% oxygen desaturation or 50% airflow reduction and arousal. Sleep, arousal, periodic limb movements and respiration were analyzed using 30-s epochs with assistance from REMlogic software (Embla Systems, Thornton, CO, United States).
AUDICOR CPAM: Using the PSG report, the beginning and end of sleep were input to the software ignoring periods of intermittent wakefulness. The data were analyzed using the automated algorithms for snoring, respiration, individual timing and duration of SDB events and an SDB severity score. The timing and durations of the individual SDB events are used to create an SDB severity score (SDBSS). Apnea and hypopnea > 10 s in duration that met certain physiological timing constraints and occurred during quiescent periods were considered SDB events and were totaled and divided by the total sleep time. This score was developed by the manufacturer of the device on a learn set of data collected using AUDICOR CPAM from patients undergoing PSG, and was intended to correlate with the PSG AHI.
The test set of this study consisted of 85 patients (71 men) whose mean age was 50.5 ± 12.4 years (range: 22-81 years). There were 57 patients with a positive PSG (AHI ≥ 5.0), and the others, while negative for SDB, had other sleep disorders such as restless legs syndrome, periodic limb movements, narcolepsy or cataplexy syndrome. The positive SDB group had a mean AHI of 30.0 ± 29.8 events/h, whereas the negative SDB group had a mean AHI of 1.5 ± 1.2 events/h. Age was not significantly different, but body mass index was significantly higher in the positive SDB group (Table 1). Mean percent oxygen saturation, oxygen desaturation index, number of desaturations > 4%, apnea arousals, total arousals, and the SDBSS were all significantly different in the positive SDB group (AHI ≥ 5) compared to the negative SDB group (AHI < 5).
| Parameter | All (n = 85) | Negative SDB | Positive SDB |
| (AHI < 5, n = 28) | (AHI≥5, n = 57) | ||
| Age (yr) | 50.5 ± 12.4 | 47.2 ± 12.3 | 52.1 ± 12.2 |
| Sex (% male) | 83.50% | 78.60% | 91.60% |
| Weight (kg) | 88.1 ± 18.9 | 82.1 ± 17.1 | 91.1 ± 19.2a |
| BMI (kg/m2) | 28.9 ± 5.5 | 26.9 ± 4.8 | 29.9 ± 5.6a |
| History of hypertension (%) | 34% | 39% | 21% |
| History of MI (%) | 6% | 11% | 2% |
| History of CAD (%) | 9% | 18% | 3% |
| AHI (events/h) | 20.6 ± 27.8 | 1.5 ± 1.2 | 30.0 ± 29.8a |
| Obstructive apnea (n) | 34.8 ± 89.3 | 0.9 ± 1.8 | 51.5 ± 105.3a |
| Central apnea (n) | 5.7 ± 22.3 | 1.6 ± 4.8 | 7.7 ± 26.9a |
| Mean % SaO2 (%) | 93.1 ± 2.7 | 93.9 ± 2.5 | 92.7 ± 2.7a |
| Oxygen desaturation index | 12.5 ± 19.6 | 1.1 ± 1.2 | 17.5 ± 21.8a |
| Desaturation > 4% (n) | 70.1 ± 120.3 | 6.2 ± 7.0 | 98.4 ± 135.7a |
| Apnea arousal (n) | 31.6 ± 79.3 | 1.8 ± 4.9 | 46.2 ± 93.6a |
| Total arousals (n) | 200.0 ± 137.8 | 166.1 ± 74.3 | 216.6 ± 158.0a |
| Awakening > 1 min (n) | 17.4 ± 32.7 | 11.0 ± 17.2 | 20.6 ± 37.9 |
| SDBSS | 9.3 ± 15.3 | 1.4 ± 3.7 | 13.2 ± 17.2a |
Expert over-readers annotated individual episodes of SDB for a total of 16 071 min in the test set. The product provided the clinician with a time-based graph of SDB events as well as duration of each event. The per-minute performance metrics represented the ability of the product to represent correctly these individual events. Performance metrics are presented in Tables 2 and 3 on a per-minute basis comparing the automated SDB detection algorithm to the expert over-reader. For the expert over-reader, a positive minute had at least one annotation of SDB > 10 s in length.
| Expert | |||
| Negative | Positive | ||
| Algorithm | Negative | 9782 | 413 |
| Positive | 398 | 1381 | |
| Mean | 95% CI | |
| Accuracy | 93.2 | 92.8-93.7 |
| Sensitivity | 77.0 | 75.0-78.9 |
| Specificity | 96.1 | 95.7-96.4 |
| Positive predictive value | 77.6 | 75.7-79.6 |
| Negative predictive value | 95.9 | 95.6-96.3 |
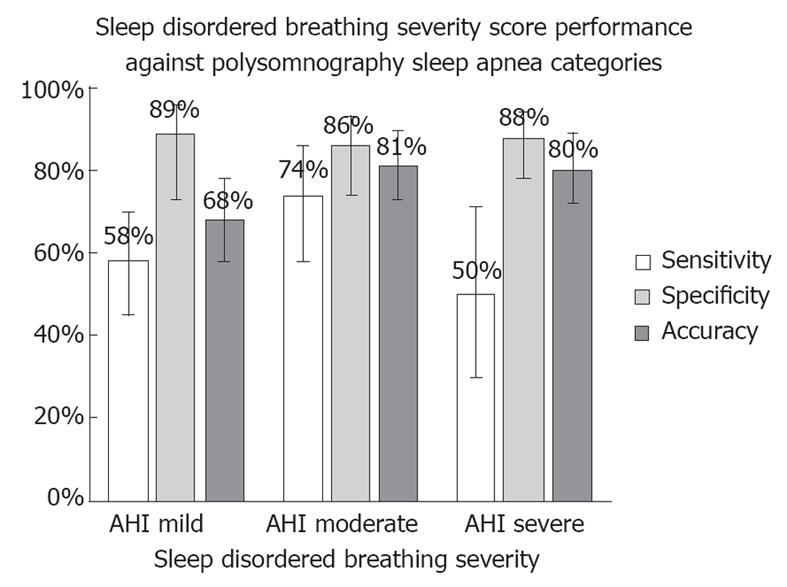
The AHI threshold recommended by AASM is 5 events/h (AHI ≥ 5). On this test set, the sensitivity and specificity of the SDBSS compared to positive PSG (AHI ≥ 5) was 57.9% and 89.3%, respectively (Figure 2 and Table 4). The performance was not significantly different between those patients with a final diagnosis of OSA (sensitivity 50.0%, specificity 89.3%) or central/mixed sleep apnea (sensitivity 69.6%, specificity 89.3%).
| Mean | 95% CI | |
| Accuracy | 68.2 | 58.3-78.1 |
| Sensitivity | 57.9 | 45.0-69.8 |
| Specificity | 89.3 | 72.8-96.3 |
| Positive predictive value | 91.7 | 82.6-100 |
| Negative predictive value | 51.0 | 37.0-65.0 |
The standard thresholds defined by the AASM on AHI for classification into mild, moderate and severe sleep apnea were: (1) mild, 5 ≤ AHI < 15; (2) moderate, 15 ≤ AHI < 30; and (3) severe, AHI ≥ 30. In Table 5, these three thresholds for SDBSS were implemented, yielding the performance statistics shown.
| Sensitivity | Specificity | Accuracy | |
| (95% CI) | (95% CI) | (95% CI) | |
| Mild | 57.9 | 89.3 | 68.2 |
| 5 ≤ AHI < 15 | (45.0-69.8) | (72.8-96.3) | (58.3-78.1) |
| Moderate | 74.3 | 86.0 | 81.2 |
| 15 ≤ AHI < 30 | (57.9-85.8) | (73.8-93.0) | (72.9-89.5) |
| Severe | 50.0 | 88.1 | 80.0 |
| AHI ≥ 30 | (29.0-71.0) | (78.2-93.8) | (71.5-88.5) |
We also sought to determine the impact of periodic limb movements on the performance of the CPAM device SDB detections. The SDB detection algorithm was not trained specifically on patients with and without periodic limb movement syndrome (PLMS). Therefore, the full dataset from this study (n = 111, 32 patients with final diagnosis of PLMS) was used to determine the impact of PLMS on performance. The sensitivity and specificity of SDB detection on patients with PLMS was 57.9% and 92.3% and those without PLMS was 60.4% and 90.0%, respectively, indicating no substantial performance degradation with PLMS.
The automated algorithmic snore detections generated event markers on the main AUDICOR CPAM summary screen and metrics in the physician’s report to assist categorization of SDB into obstructive or central by the clinician.
To test the ability of the device to detect snoring, the expert over-readers annotated individual snores visually from the collected sound signal. The automated algorithm of the ambulatory monitor to detect snoring was developed by the manufacturer strictly on learn datasets. On this test set, the correlation of snoring rate by the automated algorithm compared to the expert over-reader snoring rate was r = 0.58, mean error of 1.52 snores/min with 74% of the algorithm snoring rates within 10% of the expert over-reader snoring rate.
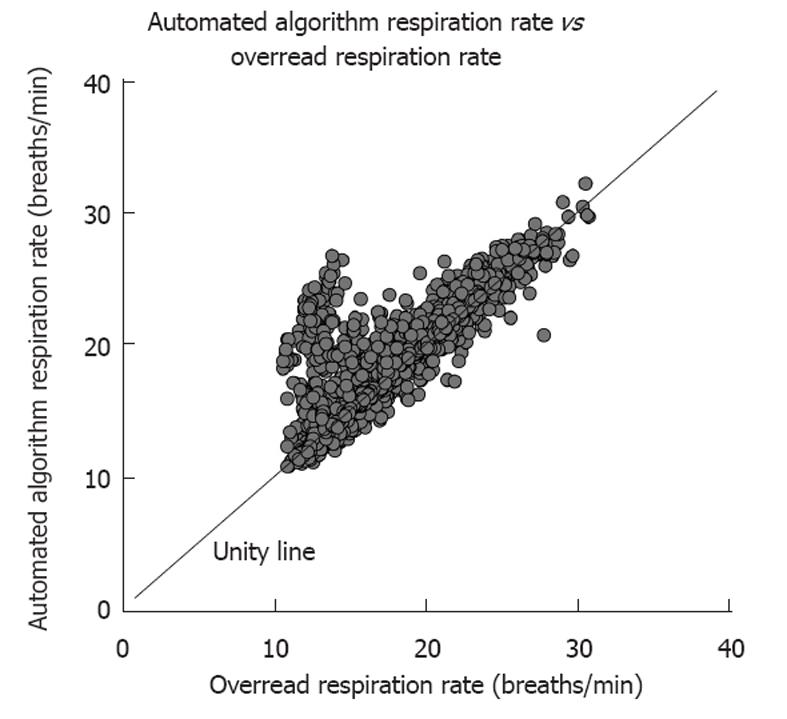
The CPAM product generated a trend of respiration rate, and to test this feature, the expert over-readers annotated individual breaths in the test dataset. The CPAM automated algorithm to detect individual breaths and respiration rate was developed strictly on learn datasets. The performance of the respiration rate by the automated algorithm compared to the expert over-reader respiration rate calculated on 4830 5-min intervals in this test dataset is shown in Figure 3 (r = 0.90, average error = 0.70 breaths/min). The respiration rate of the ambulatory monitor was within 2 breaths/min of the over-reader respiration rate 93% of the time.
Cardiovascular disease and SDB are common comorbidities. An overnight technician-attended sleep study with multi-parameter PSG provides the standard measurement of AHI used to diagnose SDB. However, the desire for a less expensive, more timely and convenient alternative for screening patients and to use for repeat studies has grown and various devices have been developed. There are different classes of portable sleep monitors based upon the number of channels of data collected and the type of sensors/technology used in the assessment[16]. A single device capable of assessing SDB and cardiac function such as CPAM is desirable, particularly for cardiologists following the progression of diseases such as heart failure with central sleep apnea, and to understand the efficacy of treatment over time.
Other portable monitors have reported performance of SDB detection with moderate to quite good accuracy[17], using a variety of thresholds on the PSG AHI, ranging from 5 to 40 events/h. In a study of 75 stable heart failure patients comparing PSG to a home study with a portable monitor, Quintana-Gallego found diagnostic accuracies of 78.6%, 84% and 80% using AHI thresholds of 5, 10 and 15, respectively[18]. In another study of 37 patients comparing contemporaneous PSG and a multichannel portable monitor to detect correctly the presence of SDB (defined as AHI ≥ 15), the reported sensitivity was 96% and the specificity was 83%[19]. The AUDICOR CPAM monitor has similar performance to these devices.
Growing evidence shows that over time SDB contributes to LV dysfunction and the American College of Cardiology has provided guidelines for diagnosis and treatment of SDB in the context of cardiovascular disease[1]. In heart failure, there is a high prevalence of central sleep apnea, whose effect may lead to further decline of LV function, but since symptoms overlap, underdiagnosis of CSA is common. The German Sleep Society has recommended that a sleep study be performed on every patient with congestive heart failure and LV ejection fraction < 40%, irrespective of the presence of sleep-related symptoms[20]. Treatment for SDB within the context of heart failure could lead to improved quality of life and better prognosis[21], especially if repeat sleep study confirms effectiveness of treatment. OSA has been shown to be associated with hypertension, LV dysfunction, arrhythmias and increased sympathetic tone. In one study, LV diastolic function was assessed by echocardiography in 68 OSA patients. Abnormal diastolic relaxation patterns were common in OSA patients and more severe sleep apnea was associated with longer isovolumic relaxation times[22]. Alchanatis et al[23] found that OSA patients had lower LV ejection fraction, reduced LV peak emptying rate, lower LV peak filling rate, and delayed time to peak filling rate as compared to controls. The authors conclude that OSA patients without any cardiovascular disease tend to develop both LV systolic and diastolic dysfunction that can be reversed after 6 mo of CPAP.
AUDICOR CPAM has the ability to detect abnormal diastolic heart sounds and assess systolic function through systolic time intervals. Until recently, the ability to do long-duration simultaneous ECG and heart sound recording has not been available. We performed CPAM ambulatory monitoring in 128 asymptomatic subjects[24] and found the third heart sound was significantly more prevalent in patients aged < 40 years and more pronounced during sleep in this age group. The fourth heart sound was meanwhile significantly more prevalent in patients aged > 40 years and more pronounced during sleep in this age group, reflecting the change in diastolic filling patterns with increasing age. In addition, time intervals reflecting systolic function showed less circadian variation and less worsening with age in this asymptomatic population. We also performed CPAM ambulatory monitoring on 67 heart failure patients and 63 asymptomatic controls with no history of heart failure[25]. The heart failure group had significantly greater prevalence of the third heart sound and prolongation of the electromechanical activation time, indicating poorer systolic function and worse prognosis[26]. Now that the ability of CPAM to detect SDB has been determined, studies can begin to use it to evaluate LV function and assess changes in hemodynamic function in the presence of SDB and over the course of its treatment.
AUDICOR CPAM is able to detect SDB with reasonable accuracy. The ambulatory monitor should assist in screening and follow-up of patients at home and in hospital, and may be particularly appealing to cardiologists who can additionally assess contemporaneous cardiac function. Although the automatically generated SDBSS alone is useful, the device is best used with physician over-read to assess the potential relationships of SDB to its hemodynamic consequences.
The present study was conducted solely in the sleep laboratory and further validation of the device performance is needed in at-home environments. The study population was Caucasian and further study on a more diverse group of patients should be completed. Additional testing of heart failure patients and those with associated respiratory or cardiac conditions is important.
Given the high prevalence of known SDB and an estimated 80%-90% being undiagnosed, there is a need for simplified diagnostic devices that do not require special facilities and highly trained personnel. We conclude that AUDICOR CPAM can adequately identify subjects with SDB and may be a useful screening device. In addition, the ambulatory monitor can quantify and detect LV dysfunction, which may be present with SDB, particularly in the setting of heart failure.
The authors would like to thank Peter Bauer and Patti Arand of Inovise Medical, Inc. for their assistance.
The relationship between sleep-disordered breathing (SDB) and detrimental altered cardiac dynamics has been demonstrated. There exists a need for a portable ambulatory monitor capable of simultaneously assessing both SDB and cardiac hemodynamics.
Due to the association between SDB and cardiac consequences, the ability to screen for and prioritize patients for full polysomnography (PSG) would be beneficial. A novel device [cardiac and respiratory monitor (CPAM)] is available that is capable of assessing both cardiac hemodynamics and SDB. In this study, the authors demonstrated that the CPAM device had adequate performance to be used for screening of SDB.
The CPAM device evaluated in this study is unique in its ability to assess both cardiac hemodynamics and SDB simultaneously. The performance of the cardiac assessment portion of CPAM has been investigated and published previously. This study reports the first evaluation of the SDB assessment components of the device.
The ability to screen and prioritize patients, particularly those with existing cardiac disease such as heart failure, for full PSG would be beneficial. In addition, follow-up assessments after modifications to either cardiac or SDB treatment could assist in optimizing treatment. The performance reported of the SDB assessment components of the device under study indicates its use for screening is feasible.
SDB is a term that describes a group of disorders characterized by abnormal respiratory patterns such as pauses or reduced quantity of ventilation, including obstructive and central sleep apnea.
The authors aim to validate a portable monitor incorporating a detection method for heart sounds, holter-ecg analysis and detection of sleep-disordered breathing. The study was designed as a prospective cross-sectional mono-centric trial with 85 persons undergoing polysomnography and ambulatory cardiorespiratory monitoring with the CPAM.
| 1. | Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ. Sleep apnea and cardiovascular disease: an American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008;118:1080-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 650] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 2. | Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230-1235. [PubMed] |
| 3. | Ferreira S, Marinho A, Patacho M, Santa-Clara E, Carrondo C, Winck J, Bettencourt P. Prevalence and characteristics of sleep apnoea in patients with stable heart failure: Results from a heart failure clinic. BMC Pulm Med. 2010;10:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705-706. [PubMed] |
| 5. | Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, Hudgel D, Sateia M, Schwab R. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737-747. [PubMed] |
| 6. | Zou D, Grote L, Peker Y, Lindblad U, Hedner J. Validation a portable monitoring device for sleep apnea diagnosis in a population based cohort using synchronized home polysomnography. Sleep. 2006;29:367-374. [PubMed] |
| 7. | Elbaz M, Roue GM, Lofaso F, Quera Salva MA. Utility of actigraphy in the diagnosis of obstructive sleep apnea. Sleep. 2002;25:527-531. [PubMed] |
| 8. | Kuna ST. Portable-monitor testing: an alternative strategy for managing patients with obstructive sleep apnea. Respir Care. 2010;55:1196-1215. [PubMed] |
| 9. | Marcus GM, Gerber IL, McKeown BH, Vessey JC, Jordan MV, Huddleston M, McCulloch CE, Foster E, Chatterjee K, Michaels AD. Association between phonocardiographic third and fourth heart sounds and objective measures of left ventricular function. JAMA. 2005;293:2238-2244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Roos M, Toggweiler S, Jamshidi P, Zuber M, Kobza R, Meier R, Erne P. Noninvasive detection of left ventricular systolic dysfunction by acoustic cardiography in cardiac failure patients. J Card Fail. 2008;14:310-319. [PubMed] |
| 11. | Shah SJ, Michaels AD. Hemodynamic correlates of the third heart sound and systolic time intervals. Congest Heart Fail. 2006;12 Suppl 1:8-13. [PubMed] |
| 12. | Shah SJ, Nakamura K, Marcus GM, Gerber IL, McKeown BH, Jordan MV, Huddleston M, Foster E, Michaels AD. Association of the fourth heart sound with increased left ventricular end-diastolic stiffness. J Card Fail. 2008;14:431-436. [PubMed] |
| 13. | Zuber M, Erne P. Acoustic cardiography to improve detection of coronary artery disease with stress testing. World J Cardiol. 2010;2:118-124. [PubMed] |
| 14. | Erne P. Beyond auscultation--acoustic cardiography in the diagnosis and assessment of cardiac disease. Swiss Med Wkly. 2008;138:439-452. [PubMed] |
| 15. | Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine 2007; . |
| 16. | Ferber R, Millman R, Coppola M, Fleetham J, Murray CF, Iber C, McCall V, Nino-Murcia G, Pressman M, Sanders M. Portable recording in the assessment of obstructive sleep apnea. ASDA standards of practice. Sleep. 1994;17:378-392. [PubMed] |
| 17. | Ghegan MD, Angelos PC, Stonebraker AC, Gillespie MB. Laboratory versus portable sleep studies: a meta-analysis. Laryngoscope. 2006;116:859-864. [PubMed] |
| 18. | Quintana-Gallego E, Villa-Gil M, Carmona-Bernal C, Botebol-Benhamou G, Martínez-Martínez A, Sánchez-Armengol A, Polo-Padillo J, Capote F. Home respiratory polygraphy for diagnosis of sleep-disordered breathing in heart failure. Eur Respir J. 2004;24:443-448. [PubMed] |
| 19. | Cunnington D, Garg H, Teichtahl H. Accuracy of an ambulatory device for the diagnosis of sleep disordered breathing. Indian J Sleep Med. 2009;4:143-148. |
| 20. | Schulz R, Blau A, Börgel J, Duchna HW, Fietze I, Koper I, Prenzel R, Schädlich S, Schmitt J, Tasci S. Sleep apnoea in heart failure. Eur Respir J. 2007;29:1201-1205. [PubMed] |
| 21. | Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol. 2007;49:2028-2034. [PubMed] |
| 22. | Fung JW, Li TS, Choy DK, Yip GW, Ko FW, Sanderson JE, Hui DS. Severe obstructive sleep apnea is associated with left ventricular diastolic dysfunction. Chest. 2002;121:422-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 196] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 23. | Alchanatis M, Tourkohoriti G, Kosmas EN, Panoutsopoulos G, Kakouros S, Papadima K, Gaga M, Jordanoglou JB. Evidence for left ventricular dysfunction in patients with obstructive sleep apnoea syndrome. Eur Respir J. 2002;20:1239-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Dillier R, Zuber M, Arand P, Erne S, Erne P. Assessment of systolic and diastolic function in asymptomatic subjects using ambulatory monitoring with acoustic cardiography. Clin Cardiol. 2011;34:384-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Dillier R, Zuber M, Arand P, Erne S, Erne P. Assessment of systolic and diastolic function in heart failure using ambulatory monitoring with acoustic cardiography. Ann Med. 2011;43:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Chao TF, Sung SH, Cheng HM, Yu WC, Wang KL, Huang CM, Chen CH. Electromechanical activation time in the prediction of discharge outcomes in patients hospitalized with acute heart failure syndrome. Intern Med. 2010;49:2031-2037. [PubMed] |
Peer reviewer: Tienush Rassaf, MD, Professor, University Hospital Düsseldorf, Department of Cardiology, Pulmonology, Angiology, Moorenstr 5, 40225 Düsseldorf, Germany
S- Editor Cheng JX L- Editor Kerr C E- Editor Li JY