Published online May 26, 2011. doi: 10.4330/wjc.v3.i5.165
Revised: March 22, 2011
Accepted: March 29, 2011
Published online: May 26, 2011
With recent advancement in percutaneous endovascular management, most atherosclerotic peripheral arterial diseases are amenable for intervention. However, there is limited published literature about atherosclerotic axillary artery involvement and its endovascular management. We report two cases of atherosclerotic axillary artery stenosis, which were successfully managed with stent angioplasty using self expanding nitinol stents. The associated coronary artery disease was treated by percutaneous angioplasty and stenting. The long term follow-up revealed patent axillary stents in both cases.
- Citation: Vijayvergiya R, Yadav M, Grover A. Percutaneous endovascular management of atherosclerotic axillary artery stenosis: Report of 2 cases and review of literature. World J Cardiol 2011; 3(5): 165-168
- URL: https://www.wjgnet.com/1949-8462/full/v3/i5/165.htm
- DOI: https://dx.doi.org/10.4330/wjc.v3.i5.165
The successful percutaneous endovascular intervention of atherosclerotic peripheral arterial disease (PAD) of various locations is well described in the literature. However, there is very little published literature about atherosclerotic axillary artery stenosis and its endovascular management. We hereby describe two cases of atherosclerotic axillary artery stenosis in association with coronary artery disease (CAD), which were successfully managed by percutaneous stent angioplasty.
In March 2007, a 48-year-old chronic smoker, hypertensive, manual worker presented at the emergency room (ER) with acute anterior wall myocardial infarction. He was given intravenous streptokinase injection within 5 h
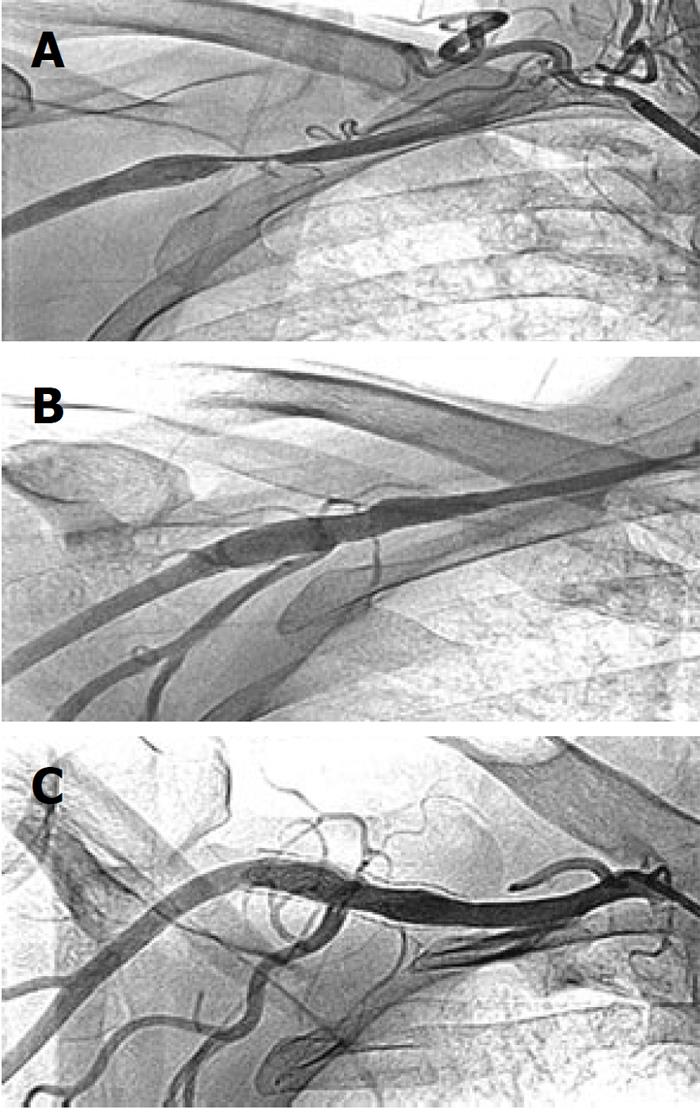
of the onset of chest pain. Post-thrombolysis he had mild, persistent chest pain and ST elevation in V1-V4 chest leads. The systolic blood pressure in the right upper limb was 80 mmHg, for which intravenous dopamine infusion was started in the ER. Later, it was realized that there was a disparity in systolic blood pressure in the two upper limbs - in the right arm blood pressure was 80 mmHg while in the left arm it was 130 mmHg. On further inquiry, he revealed a history of right upper limb claudication on doing manual work during the last 6 mo. There was no history of trauma of the right upper limb, or radiation therapy in the neck or chest region for any malignancy. The general physical examination was unremarkable other than feeble right upper limb arterial pulses. Cardiovascular examinations revealed a left ventricular third heart sound, but the rest of the systemic examination was normal. His 2-dimensional echocardiogram showed a left ventricular ejection fraction of 0.30, a hypokinetic anterior wall and septum, and no mitral regurgitation. His routine biochemistry, including fasting blood sugar, renal and liver function tests, were within normal limits. His fasting lipid profile revealed total cholesterol 117 mg%, HDL 26 mg%, LDL 67 mg%, and triglycerides 101 mg%. Forty-eight hours after admission, he underwent coronary and peripheral angiography via the trans-femoral route. Coronary angiography revealed 90% proximal left anterior descending (LAD) tubular bifurcation stenosis; left main, left circumflex and dominant right coronary arteries (RCA) were normal. A peripheral angiogram revealed 90% eccentric right axillary artery stenosis (Figure 1A). Bilateral subclavian, renal, common and internal carotid arteries were normal.
Following written informed consent, he was taken for coronary and peripheral intervention. The left coronary artery was cannulated with a Judkins Left 3, 6F guide catheter, the proximal LAD lesion was stented with a 3 mm × 18 mm Bx Sonic stent (Cordis Co., Miami, Florida). TIMI-3 flow was achieved in the LAD. Then, the right subclavian artery was cannulated with a 7F sheath (Cook, Bloomington, Indiana, USA) and the axillary artery lesion was crossed with a 0.014 inch All Track guide wire (ATW) (Cordis). The lesion was pre-dilated with a 4 mm × 20 mm balloon. An 8 mm × 40 mm PRECISE™ self expanding nitinol stent (Cordis) was deployed across the lesion. It was post dilated with a 7 mm × 20 mm OptaPro balloon (Cordis). Brisk flow was achieved in the axillary artery (Figure 1B). The systolic blood pressure in both upper limbs became equal following the intervention. At follow-up, his right upper limb claudication symptom was absent. However, he had class II dyspnea on exertion, which was attributed to his low left ventricular ejection fraction (0.25). He was managed with optimal doses of diuretics, angiotensin converting enzyme inhibitors and β-blockers.
In January 2008, at 10 mo of follow-up, he underwent a diagnostic angiogram. Coronary angiogram revealed 100% diffuse in-stent restenosis of the proximal LAD bare metal stent, with grade III retrograde filling of the distal LAD from intra-coronary collaterals; the rest of the coronary arteries were normal. The left ventricular angiogram in RAO 30° view showed an ejection fraction of 0.25, antero-lateral and apex region akinetic. The axillary stent was patent with brisk flow across it (Figure 1C). A stress Thallium test was performed following the angiogram, which revealed scarred non-viable anterior wall of the left ventricle myocardium, and a resting ejection fraction of 0.25. He was continued on optimal medical treatment. At 4 years follow-up in February 2011, he was doing well with no right upper limb symptoms and equal blood pressure in both upper limbs.
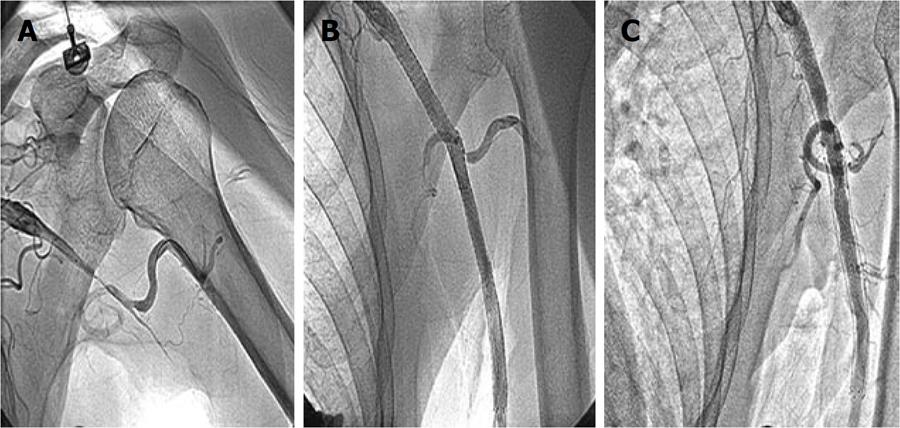
In July 2009, a 65-year-old non-smoker, normotensive, non-diabetic, right handed gentleman presented with left upper limb claudication on doing minimal manual work during the last 3 mo. He also complained of class II dyspnea on exertion of the same duration. There was no history of chest pain, syncope, left upper limb numbness or discoloration. There was no history of radiation therapy in the neck or chest region for any malignancy. On general physical examination, his left brachial and radial pulses were not palpable and blood pressure in left upper limb was not recordable. The left upper limb was warm and viable with no discoloration, epilation, brittle nails or gangrenous changes. Other arterial pulses were well palpable. Systemic examination was unremarkable. His ECG was within normal limits, 2-dimensional echocardiography revealed no regional wall motion abnormality, and his left ventricular ejection fraction was 0.60. His routine biochemistry, including blood sugar, liver and renal function tests, were normal. The fasting lipid profile was total cholesterol 121 mg%, HDL 32 mg%, LDL 65 mg% and triglycerides 102 mg%. He was subjected to cardiac catheterization and a peripheral angiogram. Coronary angiography performed via the right trans-femoral route, revealed 50% diffuse stenosis of major obtuse marginal 1, 70% diffuse stenosis of the proximal-distal LAD, dominant RCA having mid cutoff with grade III antegrade filling of the distal RCA. A peripheral angiogram revealed total cutoff of the left axillary artery at the level of the head of the humerus (Figure 2A). Bilateral carotid, subclavian and renal arteries were normal.
Following a written informed consent, he was taken-up for coronary and peripheral interventions. The left coronary artery was cannulated with an Extra Back-Up 3.5, 6F (Medtronic) guide catheter via right trans-femoral approach. The LAD lesion was crossed with a 0.014 inch ATW coronary guide wire (Cordis), pre- dilated with a 2 mm × 20 mm Sprinter (Medtronic) balloon, and stented with 3.5 mm × 28 mm and 3.5 mm × 18 mm Multi-Link Vision (Abbott Vascular, Santa Clara, CA, USA) stents at 14 atms. The whole stented LAD segment was post-dilated with a 3.5 mm × 15 mm non-compliant Sprinter (Medtronic) balloon at 18 atms. TIMI-3 flow was achieved in LAD. Thereafter, the left subclavian artery was cannulated with a Judkins Right 3.5, 7F coronary guide catheter, and the totally occluded axillary-brachial segment was crossed with a 0.014 inch All Track coronary guide wire (Cordis) with a 2.5 mm × 20 mm balloon support. After successful crossing of the lesion with the guide wire, it was dilated with a 2.5 mm × 20 mm followed by a 3.5 mm × 28 mm balloon. There was a long segment dissection across the occluded axillary-brachial artery, which was stented with two 8 mm × 80 mm and 8 mm × 60 mm SMART® CONTROL self expanding nitinol stents (Cordis). The whole stented segment was post-dilated with a 7 mm × 20 mm OptaPro balloon (Cordis). Brisk flow was achieved in the left upper limb (Figure 2B). The blood pressure in both the upper limbs became equal. On follow-up, his claudication symptom of the left upper limb had been relieved.
However, 5 mo later in November 2009, he presented with angina on rest and dynamic ST-T changes in anterior chest leads. A check angiogram revealed 90% in-stent restenosis of the LAD. The left axillary stent was patent (Figure 2C). He was advised to undergo coronary artery bypass surgery for underlying triple vessel disease. At 20 mo of follow-up in February 2011, his left brachial and radial arteries were well palpable, blood pressure in both upper limbs was equal and ultrasound Doppler showed a patent axillary stent.
The axillary artery is the continuation of the subclavian artery, commences at the outer border of the first rib, and ends at the lower border of the tendon of the Teres major muscle, where it continues as the brachial artery[1]. The commonly reported etiologies of axillary artery stenosis are Takayasu’s aorto-arteritis[2], giant cell arteritis[3], radiation induced arteritis[4] and crutch related injuries[5]. Though atherosclerosis is known to involve the arterial bed at various sites, it is uncommon to encounter atherosclerotic axillary artery stenosis in clinical practice[6-8]. We have reported two cases of atherosclerotic axillary artery stenosis - the first case had short segment isolated axillary artery stenosis, while the second case had diffuse, long segment axillary-brachial occlusion. The associated CAD in both cases suggests atherosclerosis as a common etiology. In the absence of systemic symptoms, non-ostial involvement of coronaries, and absence of other major artery involvement, which are classic of Takayasu’s and giant cell arteritis, the inflammatory etiology in these two cases is very unlikely. Both cases required intervention to relieve the symptoms of claudication in the affected upper limb. There are reports of percutaneous transluminal angioplasty of the axillary artery stenosis from 1990 onwards[8-11]; however the limited success rate, early and late re-occlusion are a few of the limitations of plain angioplasty, which has improved over the decade with stent angioplasty. In 1994, McBride et al[12] reported the first case of stent angioplasty in radiation induced axillary artery stenosis and in 2000, Oran et al[5] reported another case of stent angioplasty in crutch related axillary artery stenosis. There is only one original article about atherosclerotic stent angioplasty of the axillary artery stenosis published by Müller-Hülsbeck et al[6] in 2007. As the axillary artery is located at the mobile shoulder joint, the self expanding stent instead of balloon expandable stent is preferable as the earlier one is more flexible, compressible, and non-deformable in comparison to the balloon expandable stent[13].
The limited published literature about axillary artery disease and its endovascular management is possibly because more attention is given by the endovascular specialist to critical limb ischemia of lower limbs and proximal subclavian artery disease in comparison to the distal arterial bed of the upper limb. Another reason may be that ther is a good collateral circulation across the shoulder joint and adequate distal flow in the limb, which results into fewer symptoms. Both our cases had long term patency of axillary stents. Though a 1 year patency rate of > 90% has been reported for self expanding stents of the iliac artery[14], similar studies are required for short and long term outcomes of stent angioplasty of the axillary artery.
One interesting observation in our cases was that there was significant in-stent restenosis of coronary bare metal stents. Though the risk factors for in-stent restenosis like bare metal stents, long lesions (in case 2), smaller diameter of stents (3 mm stent in case 1) were there, an associated PAD is also being considered as one of the risk factors for repeat target vessel revascularization in CAD patients[15].
In conclusion, this is a report about two uncommon cases of atherosclerotic axillary artery stenosis, which were successfully treated with endovascular stents and had a favorable long term outcome.
| 1. | Gray H. Anatomy of the Human Body. Philadelphia: Lea & Febiger 1918; 1-58734-102-6 Available from: http://education.yahoo.com/reference/gray/subjects/subject/149. |
| 2. | Ikuta K, Torimoto Y, Shindo M, Sato K, Kohgo Y. Atypical Takayasu arteritis with solitary stenosis in the short segment of right axillary artery. Rheumatol Int. 2010;30:1635-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Amann-Vesti BR, Koppensteiner R, Rainoni L, Pfamatter T, Schneider E. Immediate and long-term outcome of upper extremity balloon angioplasty in giant cell arteritis. J Endovasc Ther. 2003;10:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Cavendish JJ, Berman BJ, Schnyder G, Kerber C, Mahmud E, Turi ZG, Blanchard D, Tsimikas S. Concomitant coronary and multiple arch vessel stenoses in patients treated with external beam radiation: pathophysiological basis and endovascular treatment. Catheter Cardiovasc Interv. 2004;62:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Oran I, Parildar M, Memis A. Crutch-Induced Axillobrachial Artery Stenosis: Management With Vascular Stent. Int J Angiol. 2000;9:31-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Müller-Hülsbeck S, Both M, Charalambous N, Schäfer PJ, Heller M, Jahnke T. [Endovascular treatment of atherosclerotic arterial stenoses and occlusions of the supraaortic arteries: mid-term results from a single center analysis]. Rontgenpraxis. 2007;56:119-128. [PubMed] |
| 7. | Finsterer J, Dossenbach-Glaninger A, Krugluger W, Stöllberger C, Hopmeier P. Risk-factor profile in severe, generalized, obliterating vascular disease. South Med J. 2004;97:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Insall RL, Lambert D, Chamberlain J, Proud G, Murthy LN, Loose HW. Percutaneous transluminal angioplasty of the innominate, subclavian, and axillary arteries. Eur J Vasc Surg. 1990;4:591-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Iannone LA, Rayl KL. Takayasu's disease with axillary, right coronary artery, and right internal mammary stenosis treated with angioplasty. Cathet Cardiovasc Diagn. 1991;22:42-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Romanowski CA, Fairlie NC, Procter AE, Cumberland DC. Percutaneous transluminal angioplasty of the subclavian and axillary arteries: initial results and long term follow-up. Clin Radiol. 1992;46:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Boyer L, Carrié D, Ribal JP, Glanddier G, Viallet JF. [Percutaneous transluminal angioplasty of subclavian, axillary and brachiocephalic trunk arteries. Apropos of 18 patients]. Arch Mal Coeur Vaiss. 1994;87:371-378. [PubMed] |
| 12. | McBride KD, Beard JD, Gaines PA. Percutaneous intervention for radiation damage to axillary arteries. Clin Radiol. 1994;49:630-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Wholey MH, Wholey MH, Tan WA, Eles G, Jarmolowski C, Cho S. A comparison of balloon-mounted and self-expanding stents in the carotid arteries: immediate and long-term results of more than 500 patients. J Endovasc Ther. 2003;10:171-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Ponec D, Jaff MR, Swischuk J, Feiring A, Laird J, Mehra M, Popma JJ, Donohoe D, Firth B, Keim E. The Nitinol SMART stent vs Wallstent for suboptimal iliac artery angioplasty: CRISP-US trial results. J Vasc Interv Radiol. 2004;15:911-918. [PubMed] |
| 15. | Singh M, Lennon RJ, Darbar D, Gersh BJ, Holmes DR, Rihal CS. Effect of peripheral arterial disease in patients undergoing percutaneous coronary intervention with intracoronary stents. Mayo Clin Proc. 2004;79:1113-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Peer reviewer: Maurizio Turiel, Professor, Cardiology Unit, IRCCS Galeazzi Orthopedic Institute, Università di Milano, Via R. Galeazzi 4 - 20161 Milan, Italy
S- Editor Cheng JX L- Editor O’Neill M E- Editor Zheng XM