INTRODUCTION
Dual-chamber atrio-ventricular cardiac (DDD) pacing has produced clinical benefits with a considerable improvement in the symptoms and life expectancy of millions of patients suffering from chronic or paroxysmal disorders of cardiac electric excitation-conduction (atrio-ventricular block and/or sinus node disease).
Conventional sites for typical DDD pacing are the right atrial appendage (RAA) and the right ventricular apex (RVA), with well-known long-term safety and efficacy[1]. In the last few years some skepticism has emerged about pacing in both the RAA and RVA sites.
Papageorgiou et al[2] showed that during conventional RAA pacing, patients without a history of atrial arrhythmias but with inducible atrial fibrillation, exhibited a significant increase in conduction time of the posterior triangle of Koch and a marked broadening of the electrocardiogram recorded in this area. Saksena et al[3] reported that atrial fibrillation initiation was commonly associated with the appearance of an intra-atrial conduction delay of the initiating extra stimulus at the septal and coronary sinus ostial regions, and much less frequently at the crista terminalis. Thus, conventional RAA pacing does not seem to be able to prevent atrial fibrillation in DDD pacing for brady-tachi syndrome[4].
On the other hand, similar to the negative hemodynamic and clinical effects related to the presence of spontaneous left bundle branch block, recent data suggest a possible negative effect associated with intraventricular conduction such as left bundle branch block induced by RVA pacing[5-9].
The negative effects of RVA pacing have been evaluated by some authors and could be summarized as: electric and mechanical left ventricular asynchrony; negative remodeling of the left ventricular chamber; alterations of the myocardial histopathology; systolic and diastolic left ventricular dysfunction; heart failure; regional myocardial and kinetic perfusion defects; functional mitral regurgitation; left atrial dilation; increased risk of atrial fibrillation; induction of spontaneous ventricular arrhythmias; and hyperactivation of the sympathetic nervous system.
Several studies have shown the negative clinical consequences caused by RVA pacing, including CTOPP[10], a Danish study[11], DAVID[12], MOST[13], and a substudy of MADIT II[14]. In all these trials, when the percentage of RVA pacing, obtained from the conventional apical site, was high (> 40%), the incidence of atrial fibrillation increased, along with heart failure, hospitalization and even death.
These negative effects appear more in patients with impairment of left ventricular systolic function, but not so much in patients without left ventricular systolic dysfunction before pacing. In particular, these effects do not seem to be predictive parameters for identifying patients with a higher risk of a long-term detrimental outcome related to RVA pacing.
In our study[15], we evaluated 33 patients treated with RVA pacing frequently for 2 years and showed: (1) a significant decrease in left ventricular ejection fraction (from 56% ± 6% to 43% ± 9%, P < 0.001); (2) an increase in left the ventricular volumes (telediastolic from 98 ± 22 to 139 ± 31 mL, P < 0.001; telesystolic from 43 ± 12 to 80 ± 22 mL, P < 0.001); (3) an increase in mitral valve regurgitation (semi-quantitative index from 0.8 ± 0.68 to 1.45 ± 0.93, P < 0.001); and (4) a worsening of NYHA class (from 1.15 ± 0.36 to 1.88 ± 0.99, P < 0.05).
We also observed a reduction in quality of life (Minnesota score) between the study and control group (score 29 ± 18 vs 21 ± 13, P < 0.06) and in a 6-min walking test (338 ± 158 m vs 448 ± 110 m, P < 0.05)
In the control group of 22 patients treated with a permanent pacemaker but with a very low frequency of RVA pacing, we did not find statistically significant differences, either echocardiographically or clinically, between pre-implantation and follow-up parameters.
ALTERNATIVE SITES OF ATRIAL PACING
Inter- and intra-atrial conduction delay due to RAA pacing is associated with a high incidence of atrial fibrillation. Thus in patients affected by sick sinus syndrome alternative sites of atrial pacing have been tested. The principal methods were: (1) biatrial pacing[16] achieved by a pacing system that ensured permanent biatrial pacing using two atrial leads, one placed in the high right atrium and the other in the mid or the distal part of the coronary sinus; (2) dual right atrial pacing[17] achieved by a simultaneous pacing at the high right atrium (conventional site in the atria) and coronary sinus ostium; and (3) interatrial septum pacing at the triangle of Koch achieved by a single lead placed superiorly and posteriorly at the coronary sinus ostium[18].
The main benefits that these sites should provide are: (1) a very short interatrial conduction delay and a significant decrease in P-wave duration; (2) a reduction in dispersion of atrial refractoriness; (3) a more homogeneous recovery of excitability and atrial activation; and (4) electrical atrial remodeling, with a gradual reduction in left atrial diameters and volume.
Data on the two lead techniques (dual site atrial pacing or biatrial pacing) are still controversial[19-21]. In contrast, the single lead interatrial septum pacing seems to be easier, and more effective and feasible, compared with conventional RAA pacing, providing significant benefits in preventing paroxysmal atrial fibrillation and decreasing the progression to chronic atrial fibrillation[18,22].
Thus, in patients with sinus bradycardia and paroxysmal atrial fibrillation, interatrial single lead septal pacing should be considered as the gold standard technique for permanent atrial pacing.
Recently, in the South European and South American Select Secure Registry, interatrial septal pacing was safely achieved in 125 patients using a new catheter (Select Secure 3830, Medtronic Inc., Minneapolis, Minnesota), applied at the pacing site from the outside through a steerable introducer (Select Site, Medtronic Inc., Minneapolis, Minnesota)[23].
ALTERNATIVE SITES OF VENTRICULAR PACING
Based on the results of various clinical studies, it is clear that a physiologic pacing modality should preserve a correct atrio-ventricular activation in order to achieve physiological ventricular synchronization.
The simplest way to avoid right ventricular pacing is atrial single chamber pacing in patients with intact atrio-ventricular and intraventricular conduction[24]. This solution, however, has seldom been applied because of the unjustified fear of late atrio-ventricular block. An alternative solution could be to implant a DDD device with an atrial and a ventricular lead, using a dedicated algorithm that limits RVA pacing as much as possible[25].
When permanent ventricular pacing is necessary, physiologic pacing sites should be used in order to prevent ventricular desynchronization[26]. This can be obtained through biventricular pacing or pacing from alternative sites of the right ventricle.
In addition to the important randomized trials evaluating cardiac resynchronization therapy (COMPANION[27], CARE-HF[28], MADIT-CRT[29], REVERSE30]) that showed functional improvement and higher survival rates in patients with refractory heart failure and left bundle branch block, various studies were performed in order to compare biventricular pacing and conventional RVA pacing. These studies have shown how resynchronization therapy leads to an improvement in hemodynamic parameters and systolic functioning, a reduction in mitral regurgitation and diameter of the left ventricle, and a reduction in the activity of the sympathetic nervous system[31-34]. Recently, Yu et al[35] documented a positive effect of biventricular pacing vs conventional RVA pacing in patients with normal left ventricular function needing permanent ventricular pacing.
The right ventricular outflow tract (RVOT) has been evaluated as a potential alternative site of ventricular pacing[36]. In a metaanalysis of nine prospective, but not randomized studies, de Cock et al[37] demonstrated an improvement in hemodynamic parameters achieved by RVOT pacing compared with conventional RVA pacing. However, the ROVA study, the only randomized study comparing RVOT with RVA pacing, gave disappointing results on the quality of life[38].
A further evolution of RVOT pacing is contemporaneous bifocal pacing of the apex and RVOT[39]; even in this case, however, the results are still controversial[40].
DIRECT HIS BUNDLE PACING AND PARA-HISIAN PACING
In 2000, Deshmukh et al[41] presented a case history of patients with permanent pacing of the bundle of His, documenting the reliability and effectiveness of this type of pacing after ablation of the atrio-ventricular junction (12/18 patients with chronic atrial fibrillation, left ventricular ejection fraction < 40% and QRS < 120 ms). In 2004, the same author[42] presented a wider study in a population of 54 patients suffering from dilated cardiomyopathy, with ejection fraction 23 ± 11%, persistent atrial fibrillation and QRS < 120 ms, in which direct pacing of the His bundle was achieved in 36/54 patients (66%): after a follow-up of 42 mo, 29 patients were still alive and an improvement in the ejection fraction and clinical and hemodynamic parameters of the left ventricle was obtained.
The parameters that allow for the direct pacing of the His bundle are: (1) the morphology and the duration of the native QRS and the paced QRS must be identical on the 12 standard ECG derivations (Figure 1: a patient in our study); (2) the HV interval on the original rhythm and the spike-QRS distance in the paced signal must be equal (with a tolerance margin of 10 ms); (3) the pacing threshold must be high (> 2 V), since it must capture specific non-muscular conduction tissue; and (4) the pacing lead should be positioned with the distal pole (screw in) at the same level as one of the two electrodes of a mapping catheter on the His bundle (in X-rays in both right and left anterior oblique projections) (Figure 2: a patient in our study).
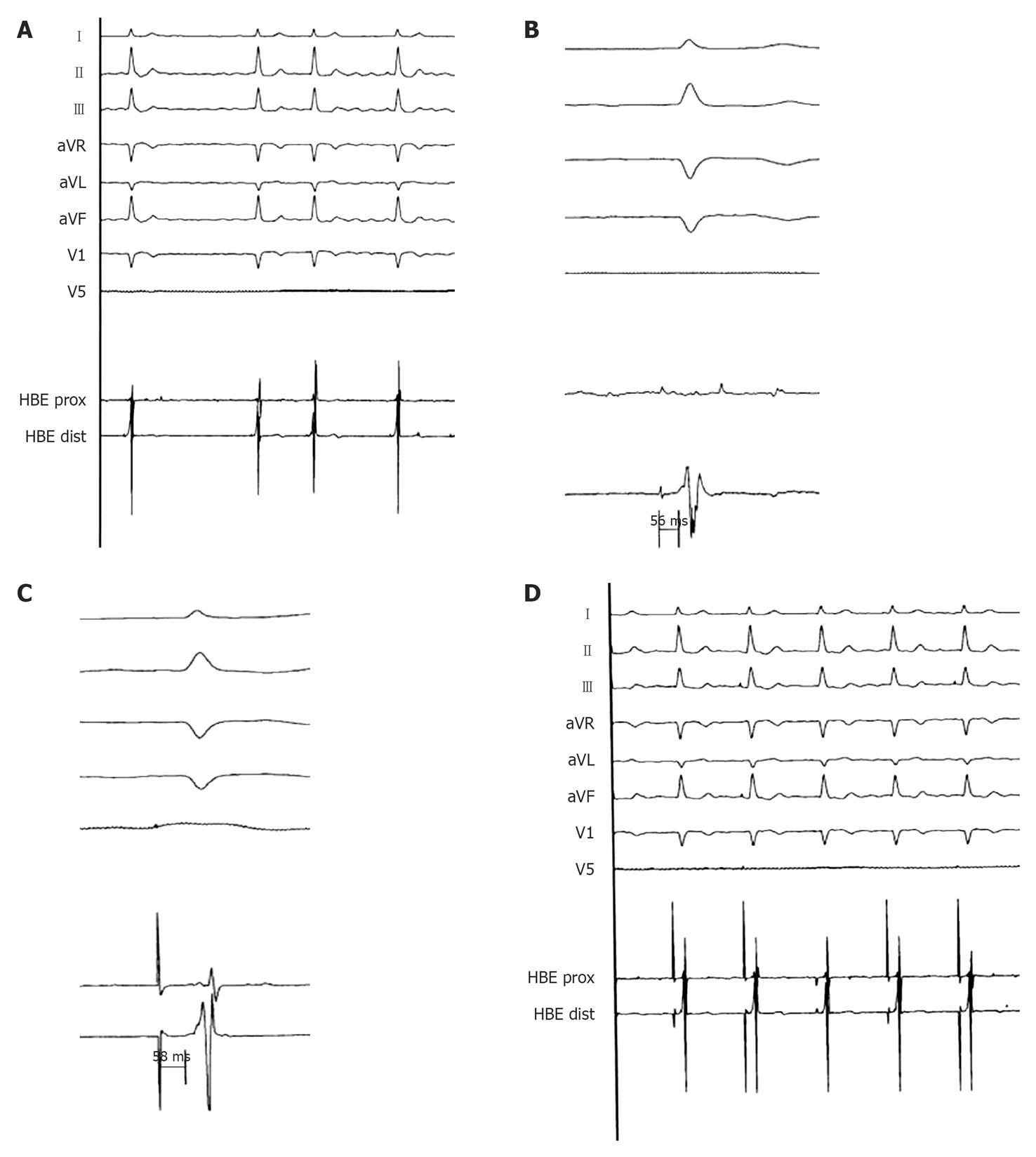
Figure 1 Spontaneous and Hisian paced ECGs.
A: Surface electrocardiogram (ECG; peripheral derivations and V1-V5) and endocardial electrogram (EGM; His bundle site) during chronic atrial fibrillation: the QRS is narrow (90 ms) (registration speed 25 mm/s); B: His bundle registration: HV 56 ms (registration speed 50 mm/s); C: Spike-V during direct Hisian pacing equal to the basal HV (58 ms) (registration speed 100 mm/s); D: Surface ECG (peripheral derivations and V1-V5) and endocardial EGM (His bundle site) during direct Hisian pacing: the QRS is equal to the native QRS (registration speed 25 mm/s).
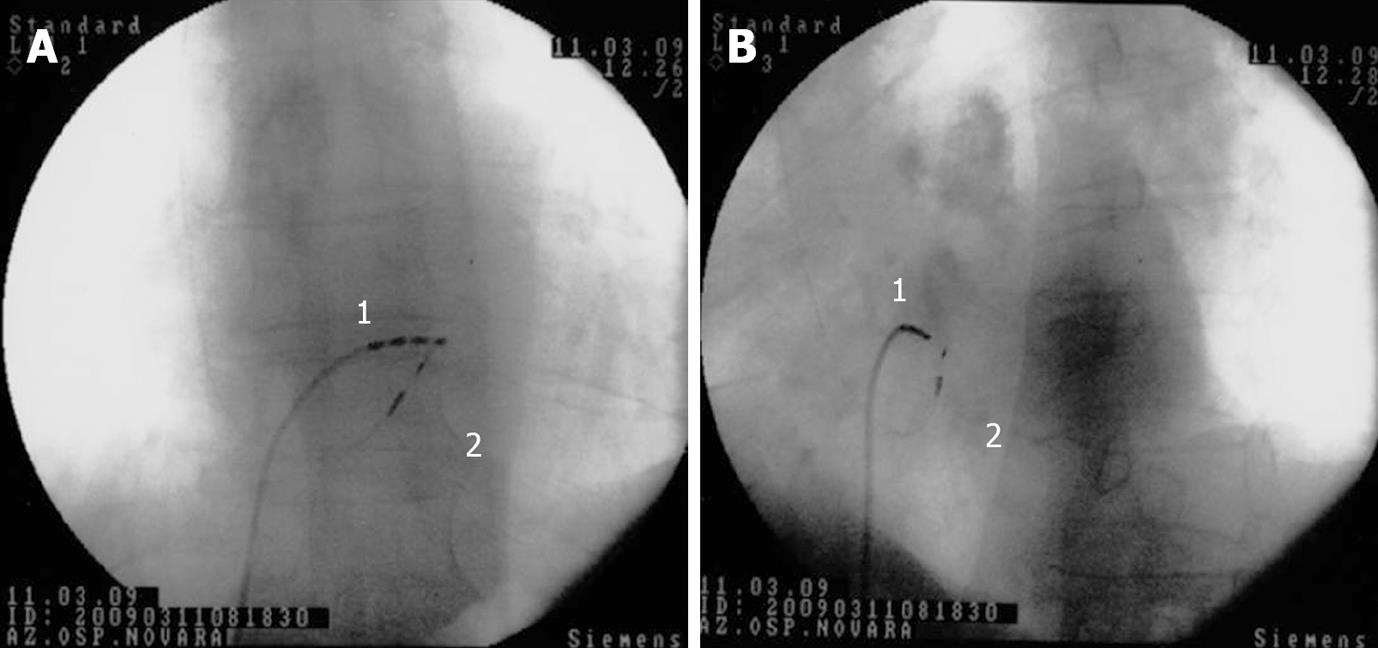
Figure 2 His bundle pacing.
Antero-posterior (A) and left anterior oblique (B) fluoroscopic projections showing the screw-in lead (Select Secure, Medtronic) position during the procedure for a direct His bundle pacing; 1 = quadripolar Hisian mapping catheter; 2 = screw-in bipolar lead positioned in close proximity to the His bundle.
Other authors have also assessed the feasibility and effectiveness of permanent Hisian pacing. Recently, Zanon et al[43] published a study showing how direct His bundle pacing can be obtained in a more reliable way using a new catheter (Select Secure 3830, Medtronic Inc., Minneapolis, Minnesota) applied at the pacing site from the outside through a steerable introducer (Select Site, Medtronic Inc., Minneapolis, Minnesota). The results showed that in 24 of 26 patients a direct stable pacing of the His bundle was obtained. The time needed to reach the His bundle with the permanent catheter varied from 2 to 60 min, with 3.8 ± 2.5 attempts required. The acute pacing threshold was 2.3 ± 1 V (0.5 ms) and the endocardial detected potential was 2.9 ± 2 mV.
These studies have shown that permanent pacing of the His bundle is a reliable and effective method preventing the desynchronization and negative effects of RVA pacing. It remains, however, a complex methodology requiring longer average implant times and presenting high pacing thresholds. Hisian pacing is also affected by the theoretical risk of His bundle block induced by the trauma and injury caused by the catheter screw-in lead[44]. In addition, due to the possible progression of conduction system disease, a right apical back-up pacing lead, avoiding future problems related to a high pacing threshold and/or a conduction block below the Hisian pacing site, could be necessary.
Para-Hisian pacing (PHP), which is simpler and more reliable, seems to guarantee physiological ventricular activation of the high muscular part of the intraventricular septum, and also early invasion of the His-Purkinje conduction system, very similar to the activation that can be achieved by direct His bundle pacing[45].
Recently, Laske et al[46] assessed left ventricular activation in pigs during pacing from various zones of the interventricular septum. During intrinsic activation with pacing from the right atrium, the activation spread along the septum and rapidly reached the left apical ventricular region, continued along the lateral wall and finally reached the postero-lateral region. Even during pacing from the para-Hisian region, the activation was the same as the intrinsic activation: it originated from the high septum and from the posterior region of the left ventricle, and then activated the anterior wall, the septum and the left ventricular apex.
From September 2000 to December 2009, at the Cardiology Clinic of the “Maggiore della Carità” Hospital, University of Eastern Piedmont, Novara, Italy, 135 patients underwent permanent right ventricular pacing in the Hisian/para-Hisian region (85 males, 50 females; 74 ± 8 years aged). In 92 patients we used a conventional screw-in lead, and in 43 patients the fixed screw was a 4 French lead (Select Secure 3830, Medtronic Inc., Minneapolis, Minnesota).
The correct criteria for the realization of para-Hisian pacing were[47]: (1) the distal pole of the catheter (screw-in) must be positioned as much as possible next to the mapping dipole of the electrophysiological catheter of reference (within 1 cm in the right and left oblique projections) (Figure 3: a patient in our study); (2) the duration of the paced QRS can be larger than the spontaneous QRS, but the duration must be at least 50 ms shorter than the QRS obtained with RVA pacing and, in any case, not more than 120-130 ms (Figure 4: a patient in our study); (3) the electrical axis of the paced QRS must be concordant with the electrical axis of the spontaneous QRS; (4) the interval between the spike and start of the paced QRS is less than the HV interval of the original rhythm; and (5) the pacing threshold must be less than 1 V, since the muscular portion of the interventricular septum is paced.
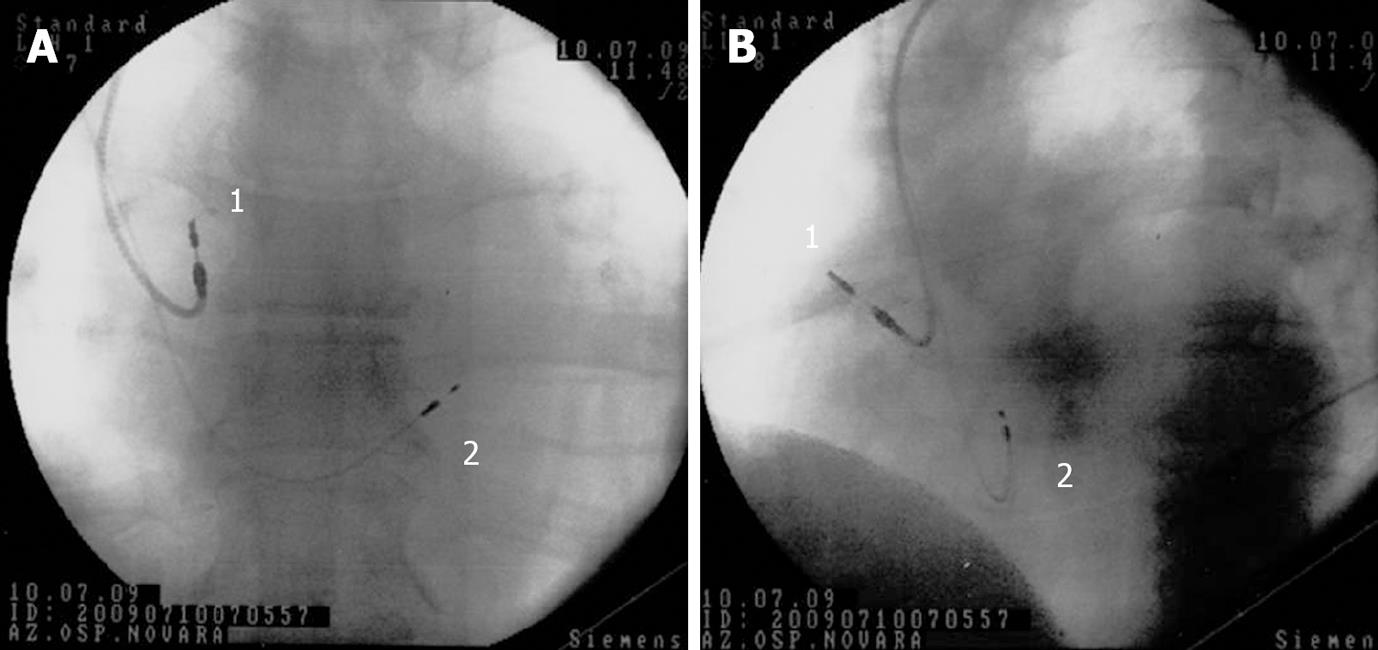
Figure 3 Para-Hisian pacing.
Antero-posterior (A) and left anterior oblique (B) fluoroscopic projections showing lead positions after dual-chamber atrio-ventricular cardiac para-Hisian pacing. 1 = conventional screw-in atrial lead, placed into the right atrium; 2 = screw-in bipolar lead (Select Secure, Medtronic) positioned near the His bundle.
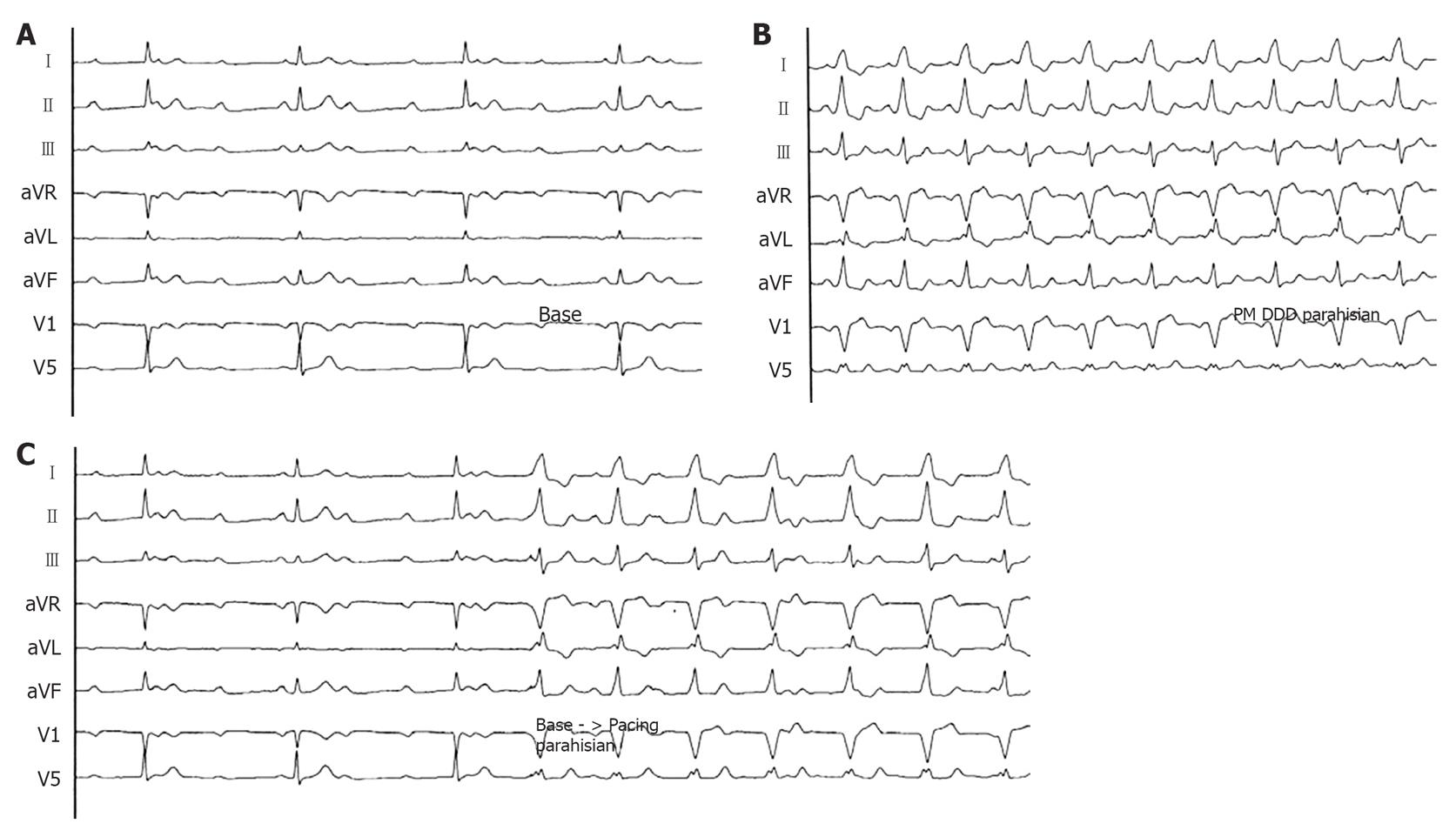
Figure 4 Spontaneous and para-Hisian paced ECGs.
A: Surface ECG (peripheral derivations and V1-V5) during complete AV block with narrow QRS (90 ms) (registration speed 25 mm/s); B: Surface ECG (peripheral derivations and V1-V5) during para-Hisian pacing: the QRS is larger respect to the native QRS (registration speed 25 mm/s); C: Passage from to the native QRS to the para-Hisian paced QRS: the electrical axis is exactly the same (registration speed 25 mm/s). DDD: Dual-chamber atrio-ventricular cardiac pacing.
Before the pacing (basal conditions) and during the follow-up all patients underwent: (1) NYHA class evaluation; (2) a quality of life questionnaire (Minnesota Living Heart Failure score); (3) a 6-min walking test; and (4) echocardiographic transthoracic standard evaluation (left ventricular end diastolic and end systolic volumes with consequent ejection fraction) and mitral and tricuspidal regurgitation semiquantitative scoring.
The pacing threshold from the Hisian site varied from 3.8 V in case of direct Hisian pacing (obtained in 20/135 patients: 15%), to values of always < 1 V in case of para-Hisian pacing (in 115/135 patients: 85%). The average duration of the basal QRS was 97.9 ± 12.4 ms, of QRS by para-Hisian pacing 123.9 ± 10.6 ms.
The mean follow-up of our patients is currently 27 mo, ranging from 103 mo for the first patient enrolled to 3 mo for the last patient. In the medium to long-term follow-up, patients with para-Hisian pacing showed the same QRS duration as the value recorded at the implant. The pacing threshold in Hisian/para-Hisian region did not have any significant variations, with values remaining within acceptable safety margins.
A significant improvement in clinical outcomes was obtained with Hisian/para-Hisian pacing: NYHA functional class from 2.2 ± 0.5 to 1.5 ± 0.6; exercise tolerance (6 min walk) from 349 ± 100 to 400 ± 88 m; quality of life score from 28 ± 18 to 19 ± 16; mitral regurgitation score from 1.6 ± 0.8 to 1.1 ± 0.8 and tricuspid regurgitation score from 1.4 ± 0.9 to 1.2 ± 0.8. The mean ejection fraction showed a slight impairment (from 52% ± 11% before pacemaker implantation to 48% ± 12% in the follow-up), but with absolute values always above 40%-45%; this fact confirms that the Hisian/para-Hisian pacing can prevent deterioration of left ventricular function.
Our data showed that a selective right ventricular pacing, which is more physiological that RVA pacing, could prevent some detrimental effects on left ventricular function achieving a better clinical outcome. Other authors documented a preservation of coronary perfusion[48] and ventricular synchronization[49] with Hisian pacing compared with RVA pacing. Obviously, at present, we do not know if the Hisian/para-Hisian pacing could prevent all the negative effects of RVA pacing, discussed in the Introduction. Dedicated and larger studies should evaluate and confirm the physiopathological (dyssynchronia, atrial and ventricular remodeling, myocardial histopathology, regional myocardial perfusion, hyperactivation of the sympathetic nervous system) and clinical (heart failure, increased risk of atrial fibrillation, spontaneous ventricular arrhythmias, etc.) effects.
FINAL CONSIDERATIONS
The main purpose of permanent cardiac electrostimulation is to maintain an adequate cardiac rhythm, and restore the physiology of the normal excitatory-conductive physiology of the heart as much as possible. Until now, importance had been given to two elements considered crucial for physiological pacing: maintenance of the atrioventricular sequence and the rate-responsive function. Pacemakers, therefore, were considered “physiological”.
Today and in the future, a truly physiological pacing, must: (1) maintain the correct stimulation-contraction sequence in the right and left atria; (2) maintain the synchrony between right and left ventricles; (3) maintain the sequence between the atria and ventricles; and (4) help increase the cardiac rate according to metabolic need.
We can identify three categories of patient requiring permanent cardiac pacing: (1) patients with paroxysmal excitation and/or conduction diseases: in this kind of patient, atrial and ventricular leads could be placed at conventional sites (RAA and RVA), because of the need for a very low rate of pacing. Current algorithms that decrease the frequency of ventricular pacing (e.g. SafeR, Sorin Group, Italy; MVP, Medtronic, USA; AV search hysteresis) could also be used in these situations; (2) patients with left ventricular dysfunction and electro-mechanical desynchronization indexes (QRS > 120 ms and/or echocardiographic asynchrony): these patients should have resynchronization therapy (CRT) with biventricular pacing (atrial, right and left ventricular leads); and (3) patients needing permanent atrial and/or ventricular pacing, with electric intraventricular conduction preserved (QRS < 120 ms): in these patients the optimal physiological pacing should be performed with: (1) an atrial lead actively fixed on the inter atrial septum (at the triangle of Koch site); and (2) a ventricular lead actively fixed at the para-Hisian region (Figure 5). The recently proposed biventricular pacing also in these patients[35] could be too invasive and expensive, and not even indicated in all patients.
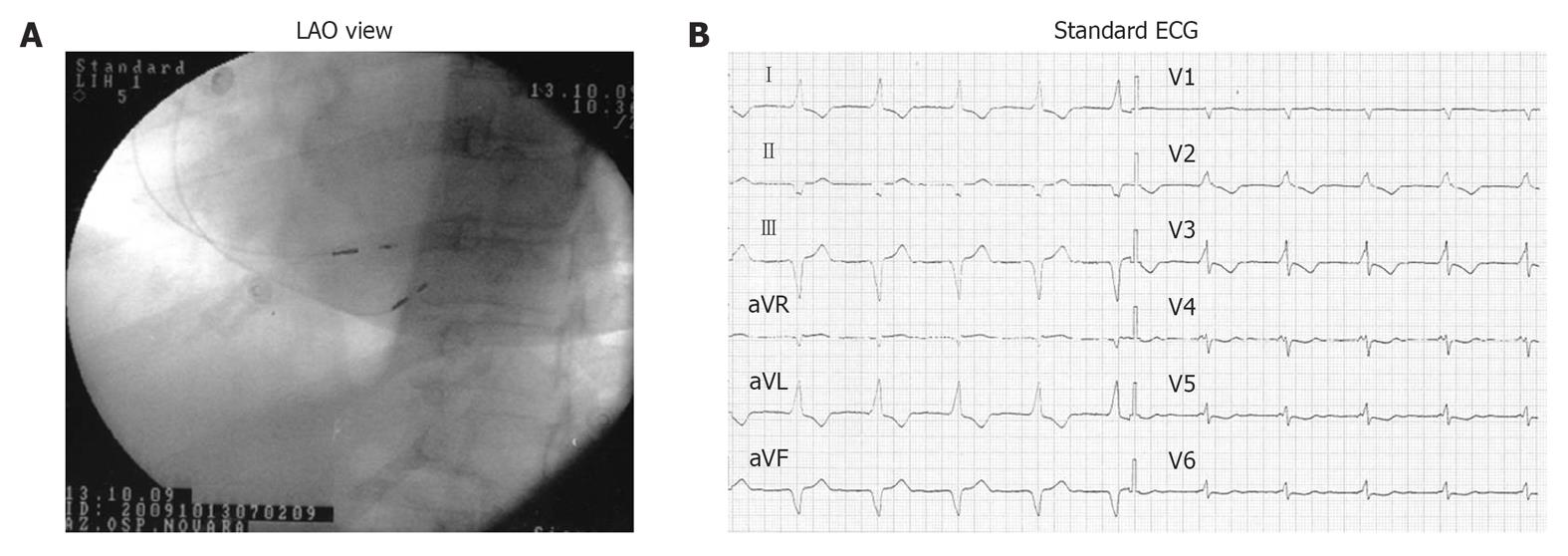
Figure 5 Atrial septal and para-Hisian dual-chamber atrio-ventricular cardiac pacing.
A: left anterior oblique (LAO) fluoroscopic projections showing atrial septal lead and para-Hisian ventricular lead positions; B: 12-lead surface ECG during dual-chamber atrio-ventricular cardiac septal pacing (atrial septal lead and para-Hisian ventricular lead).
The septal atrial lead permits a shorter and more homogeneous atrial activation, allowing better prevention of paroxysmal atrial fibrillation. Para-Hisian pacing can be obtained in a simpler and more reliable way, and is easier to perform compared with biventricular pacing and also the direct Hisian pacing. In this case, the high muscular part of the intraventricular septum is activated, while at the same time the Hisian conduction axis is penetrated, with a rather narrow QRS (120-130 ms) and with the electric axis concordant with the non-paced spontaneous QRS.
We await larger trials to consider this method of “easy and physiological pacing” as a first approach in patients who require a high frequency of pacing.
Peer reviewers: Panagiotis Korantzopoulos, MD, PhD, Lecturer in Cardiology, Department of Cardiology, University of Ioannina Medical School, 45110 Ioannina, Greece; Federico Lombardi, MD, FESC, Professor of Cardiology, University of Milan, Director of Cardiology Division, DMCO, San Paolo Hospital, Via A. di Rudinì 8, 20147, Milan, Italy
S- Editor Cheng JX L- Editor Cant MR E- Editor Zheng XM