INTRODUCTION
The endothelium consists of a single layer of cells lining the inner wall of the vasculature. It serves as a barrier between blood and tissues and actively participates in the regulation of vascular function. Vascular homeostasis and tone are controlled by the endothelium via the synthesis and release of a number of endothelial-derived relaxing and constricting substances. Under healthy conditions, the prevailing role of the endothelium is to release vasodilators, including the endothelium-derived relaxing factor (EDRF)[1], nitric oxide (NO)[2], prostacyclin (PGI2)[3] and endothelium-derived hyperpolarizing factor (EDHF)[4,5]. However, the balance or homeostasis between endothelial relaxing and constricting factors is disrupted in diseases such as hypertension, hyperlipidemia, heart failure, ischemia-reperfusion and diabetes mellitus. In the particular case of diabetes, it has been established that vascular dysfunction caused by impairment of endothelial-dependent vasodilation is present in various vascular beds of different animal models and humans. Several signaling pathways have been reported as underlying mechanisms responsible for the endothelial dysfunction associated with diabetic complications including the protein kinase C pathway, the polyol pathway, the pathway for formation and signaling of advanced glycation end products and the hexosamine pathway[6]. Most studies on the mechanisms associated with vasodilation impairment in diabetes have focused on the role of NO and PGI2[7-9]. Recently, however, as the nature of EDHF and its ability to induce vasodilation is being revealed, EDHF is receiving increasing interest.
IDENTIFICATION AND CHARACTERIZATION OF EDHF
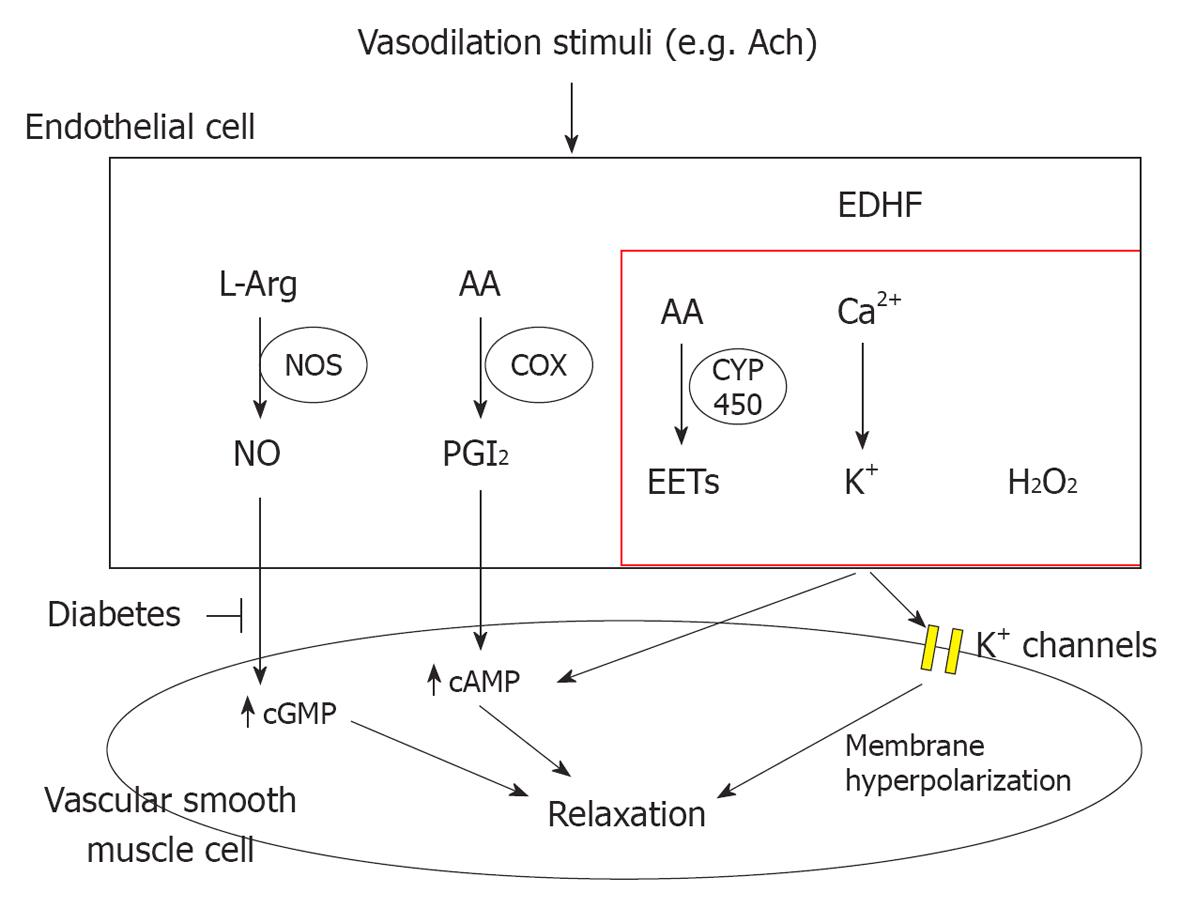
The first report concerning EDHF and its effects on smooth muscle cells can be traced back to over 20 years ago[10]. Since then, the nature of EDHF has been widely studied. However, even to this day, the very existence of EDHF remains obscure. In general, EDHF-mediated responses are considered to be endothelium-dependent relaxation responses that persist after blockade of PGI2 and NO synthesis (Figure 1)[11,12]. Although the chemical identification and functional characterization of EDHF appears to vary depending on vessel size, vascular bed and species studied, there are several major candidate molecules that, to varying extents, fulfill the criteria that would be expected of an EDHF. These molecules include: (1) The epoxyeicosatrienoic acids (EETs), which are metabolites of the arachidonic P450 epoxygenase pathway, which seem to account for a substantial portion of EDHF effects in a number of vascular beds[13,14], notably in the coronary circulation[15,16]; (2) The K+ ions that are released from the endothelium through endothelial K-Ca channels[17,18]; (3) The electrical communications occurring through myoendothelial gap junctions that provide a pathway for the passage between endothelial and smooth muscle cells of small water-soluble molecules (< 1000 Da), such as cAMP, cGMP and Ca2+, but not of proteins[19]; and (4) The H2O2 produced in the endothelium; as a catalase, a specific inhibitor of H2O2, it is able to inhibit mesenteric EDHF-mediated vascular relaxations and hyperpolarization in normal and more significantly in endothelial nitric oxide synthase (eNOS)-/- mice[20-23].
Figure 1 Endothelium-dependent vasodilation occurs in response to stimuli that induce the endothelial production and/or release of factors that ultimately cause the relaxation of the adjacent vascular smooth muscle.
These factors include nitric oxide (NO), prostacyclin (PGI2), and endothelium-derived hyperpolarizing factor (EDHF). Depending on the species and vascular bed studied, the nature of the latter has been narrowed to include epoxyeicosatrienoic acids, K+ ions, H2O2, and the myoendothelial junctions. In diabetes the presence of endothelial dysfunction is mostly characterized by a reduced bioavailability of NO. The effect of diabetes on EDHF is controversial, but a number of studies suggest that EDHF-dependent signaling may be increased therapeutically to ameliorate endothelial dysfunction. ACh: Acetylcholine; AA: Arachidonic acid; NOS: Nitric oxide synthase; COX: Cyclooxygenase; CYP 450: Cytochrome P450.
In relatively large vessels, such as the aorta and epicardial coronary arteries, NO has generally been considered the principal mediator of endothelial dependent relaxations[24-26]. This is supported by observations indicating there is no evidence for a contribution of EDHF to the endothelium-dependent relaxation observed in isolated rat aortic vessels[27]. In comparison, in small resistance vessels, including the human forearm microcirculation, it has become clear that EDHF is an important vasodilator and regulator of vascular tone and reactivity[11,26,28].
EDHF AND DIABETES
Diabetes-associated vascular complications constitute the most common clinical problem and the major cause of mortality in humans. Endothelial dysfunction is a key vascular complication in type 1 and type 2 diabetes, and is considered a major risk factor for life threatening cardiovascular events. Several mechanisms have been proposed to account for endothelial dysfunction and the increased risk of vascular disease in diabetes. These mechanisms include a reduced availability of substrate for the enzyme eNOS, an altered signal transduction pathway for the stimulation of vascular relaxation, an enhanced destruction of NO due to an increase in oxidative stress, and an increased release of endothelium-derived contracting factors that decrease the ability of smooth muscle to respond to EDRFs[29-33]. As mentioned above, EDHF is characterized by its leading to endothelial-dependent vasodilation via NO- and prostaglandin-independent mechanisms, particularly in resistance arteries where the contribution of NO appears to be less important than in conduit vessels, and where EDHF appears to play a major role in regulating tissue blood flow[4].
Insulino-dependent diabetes
Streptozotocin causes necrosis of β cells in the pancreas and is commonly used to induce type 1 diabetes in experimental animals. Type 1 diabetes is characterized by insulin deficiency compared to the insulin-resistance associated with type 2 diabetes. The exact role of EDHF in type 1 diabetes vascular dysfunction is not clear, but evidence suggests that there is both decreased and increased EDHF-mediated responses. A decrease in EDHF-mediated responses has been reported in mesenteric and carotid arteries as well as in the renal circulation of streptozotocin-treated diabetic rats[31,34-39]. The reduced EDHF-mediated response could be attributed to an increase in phosphodiesterase-3 activity inducing a reduction in the action of cAMP[40]. This suggests that a selective phosphodiesterase inhibitor may improve EDHF-mediated responses in diabetes and that cAMP is partially involved in the EDHF-mediated relaxation, likely through the enhancement of electronic conduction via gap junctions[41,42]. In streptozotocin-treated diabetic rat models, there is a selective impairment of endothelium-dependent relaxation to acetylcholine in mesenteric arteries, but not in femoral arteries and this impairment is attributable to reduced EDHF-dependent rather than NO-dependent responses[43]. This again suggests that the EDHF-mediated response may be associated with vascular size and/or vascular beds. In streptozotocin-treated diabetic mice, EDHF-mediated relaxation is also weaker than in controls. However, the mRNA expression levels of putative EDHF components are unexpectedly increased in diabetic mesenteric arteries. Therefore, the EDHF-relaxation impairment may be, at least, in part, due to an increase in plasma low-density lipoprotein and/or lysophosphatidylcholine[44]. In apoE-deficient mice, streptozotocin also induces endothelial dysfunction associated with a reduced contribution of the EDHF component in acetylcholine-induced endothelial-dependent responses in small mesenteric arteries suggesting that an impairment in EDHF-dependent signaling may be conserved across species in Type 1 diabetes[45].
In contrast to the above reported results, an augmentation in EDHF-mediated relaxation was reported in femoral and mesenteric arteries of streptozotocin-treated animals[46]. This increase in EDHF-mediated vasodilation is believed to compensate for a reduced bioavailability of NO and to counteract the augmented endothelium-dependent vasoconstriction observed in these animals. This augmented relaxation mediated by EDHF, however, was not found in carotid arteries further illustrating the marked heterogeneity of endothelium-dependent responses in peripheral arteries of healthy rats and their deferential adaptation in the course of type 1 diabetes.
Non-insulino-dependent diabetes
Type 2 diabetes is characterized by insulin-resistance, hyperinsulinemia, moderate hyperglycemia and is often associated with hypertension. In animal models of type 2 diabetes, including the fructose-fed rat, the leptin-deficient, genetically obese and mildly hypertensive Zucker rat, and the Otsuka Long-Evans Tokushima Fatty (OLETF) rat, EDHF-mediated responses are inhibited with no or minor alterations in NO-dependent responses[47-51]. For example, in Zucker diabetic fatty rats relaxation to acetylcholine of sciatic nerve epineurial arterioles is impaired and this impairment is caused by a reduced acetylcholine-evoked relaxation mediated by the EDHF pathway[52,53]. There is also a report indicating that both EDHF-dependent hyperpolarization and relaxation and endothelium-independent relaxation are impaired in mesenteric arteries of Goto-Kakizaki rats (type 2 diabetic rat model), and that treatment with an AT1 receptor blocker failed to ameliorate the impaired EDHF-mediated responses[54].
In contrast, in db/db-/- mice, which have a mutation on the leptin receptor, the NO-mediated relaxation of mesenteric arteries is reduced, while the EDHF-mediated responses are preserved[55]. Whether these characteristics change when this animal model develops diabetes is controversial. However, a number of reports indicate that, while there is no change in the acetylcholine-induced contribution of EDHF to the relaxation responses of small mesenteric arteries from type 2 diabetic db/db mice, these vessels have a severe impairment in NO contribution to endothelium-dependent relaxation caused by a decrease bioavailability of NO without changes in eNOS protein levels[55-57]. Similarly in rabbits, where EDHF appears to act mainly through Ca2+-activated K+ channels, the EDHF-dependent contribution to endothelial dependent vasodilation in isolated renal arteries is not affected under diabetic conditions[58]. Cumulative evidence, thus, supports a role for an unchanged or augmented contribution of EDHF as a mechanism for maintaining endothelial dependent relaxation in type 2 diabetes and hyperlipidemic states when endothelial production of NO and prostaglandins are compromised, in particularly during the early stages of disease progression[59-62]. As for the mechanisms responsible for maintaining the EDHF response, there are multiple options. There are reports indicating that acetylcholine-induced EDHF-mediated relaxations in small mesenteric arteries in db/db mice involve Ca2+-activated and inward rectifying K+ channels as well as Na/K ATPase exchangers, but not EETs; conversely bradykinin-induced EDHF-dependent relaxations occur via cytochrome p450 products that activate large conductance Ca2+-activated K+ channels[45,55-57,63]. This indicates that the relative contribution of mediators/cellular pathways to the EDHF-mediated vasodilation to acetylcholine and bradykinin are both disease and agonist dependent.
Our own results indicate that endothelium-mediated vasodilation is primarily NO-dependent in coronary arterioles in wild-type mice[64]. However, we found that a portion of the NO-, endothelium-dependent vasodilation is significantly reduced in db/db mice, supporting the view that EDHF plays a pivotal role in type 2 diabetes-induced endothelial dysfunction[64]. We also found that in the coronary arterioles of wild-type mice three EDHF candidates, namely H2O2, K+ and EETs, may play important roles in acetylcholine-induced endothelial-dependent vasodilation[64]. The impairment of the H2O2 response and/or the abnormalities in K+ channels in advanced diabetes may be potential mechanisms for the reduction in EDHF-mediated vascular function in db/db mice. However, our studies suggest that the EETs are not involved in the endothelium-dependent, EDHF-mediated vasodilation in db/db mice. Furthermore, our findings support the concept that interleukin (IL)-6 plays a pivotal role in the EDHF-dependent endothelial dysfunction in type 2 diabetes, based on the observation that the presence of anti-IL-6-neutralizing antibody normalized coronary vascular function in diabetic db/db mice. Addition of IL-6 to the bath of wild type coronary vessels produced a similar degree of dysfunction as that in the db/db mice, corroborating that IL-6 is an important contributor to vascular dysfunction in this animal model. At the molecular level we found that the expression of IL-6 was significantly increased in db/db mice[64]. Administration of an anti-IL-6 antibody attenuated IL-6 expression in db/db mice compared with wild type mice, while the expression of IL-6 was similar in diabetic mice null for tumor necrosis factor α (TNF-α) compared to wild type mice. Our findings, therefore, provide for a better understanding of the mechanisms that contribute to the role of EDHF in endothelial dysfunction at the level of the coronary microcirculation in type 2 diabetes. These findings also provide important new insights into the identity and mechanisms of EDHF-mediated vasodilation in the coronary circulation and may help identify novel therapeutic targets for the treatment of cardiovascular diseases associated with elevated levels of IL-6. EDHF contributes to endothelial-dependent vasodilation in maintaining coronary blood flow when the bioavailability of NO is substantially reduced in type 2 diabetes. Three EDHF candidates, K+, EETs, and/or H2O2, are involved in the EDHF-mediated vasodilation in normal coronary circulation, but the EETs appear not to be involved in the diabetic condition. Studies investigating endothelial function in animal models of type 2 diabetes are still scarce and have yielded conflicting results. For each of the candidates for major contributors to EDHF-dependent signaling mentioned above there are both positive and negative reports on their involvement in diabetes-dependent endothelial dysfunction. While differences in diabetes model and in duration or severity of diabetes undoubtedly play a role in some of the discrepancies, difference in vascular bed, size of the vessel and conditions of the studies may be a much more important source of disparity.
EDHF AS A POTENTIAL TARGET IN THE TREATMENT OF DIABETES-ASSOCIATED ENDOTHELIAL DYSFUNCTION
Endothelial dysfunction in diabetes is primarily associated with a reduced bioavailability of endothelium-derived NO. However, as mentioned above, evidence indicates that EDHF, in a number of vascular beds, may act as a compensatory vasodilator in response to the reduced bioavailability of NO (Figure 1)[59,60]. This suggests that EDHF may be up-regulated despite the presence of the endothelial damage that reduces NO bioavailability. Due to the putative nature of EDHF, up-regulation of pathways that augment the conductivity of myoendothelial junctions support the hyperpolarization of endothelial cells, produce EETs, or maintain the vasodilatory effects of H2O2, may potentially be used to increase the production or effects of EDHF. However, only a small number of studies reporting significant therapeutically-induced changes in EDHF-related signaling in diabetes were originally aimed at manipulating EDHF; most were initially designed to affect NO-associated pathways, glucose, or lipid metabolism, but ultimately affected EDHF. Treatment for 4 wk with Metformin improved EDHF-mediated relaxation in mesenteric arteries isolated from the OLETF rat model of diabetes[65]. Metformin, is a biguanide used to treat diabetes primarily due to its antihyperglycemic properties, but its effects include cardiovascular protection via mechanisms independent of its glycemic effects[66,67]. Treatment with metformin also improved NO-dependent vascular relaxation in the OLEFT mesenteric arteries, and reduced the increased production of vasoconstrictor prostanoids that is associated with the diabetic state in that animal model[65]. Metformin, thus, appears to increase EDHF-mediated signaling through processes that improve overall endothelial function, and are likely related to a reduced production of derivatives of the cyclooxygenase pathway. In the same animal model (the OLETF rat), treatment with the selective phosphodiesterase 3 inhibitor, cilostazol, also increased EDHF-mediated vasodilation in mesenteric arteries without an improvement in endothelium derived NO-dependent responses[68]. In the OLETF rat model of diabetes, cAMP-dependent signaling is impaired, and in streptozotocin-treated animals, cilostazol improves cAMP-dependent signaling. Therefore, it has been suggested that cilostazol improves EDHF-dependent vasodilation in the OLETF rat by increasing cAMP-dependent signaling[68]. Matsumoto et al[69] also treated OLETF rats with eicosapentaenoic acid, an omega-3 polyunsaturated fatty acid, with the goal of ameliorating the endothelial dysfunction present in this animal model of diabetes. Treatment with eicosapentaenoic acid for 4 wk improved overall endothelial function in OLETF rats including an augmentation of EDHF- and NO-dependent vasodilations in mesenteric arteries. Importantly, eicosapentaenoic acid reduced nuclear factor κB activation and COX-2 expression, indicating that a reduced level of inflammation played a significant role in improving endothelial function.
A common treatment for patients with hypertension and diabetes consists of reducing the renin-angiotensin signaling pathway. In diabetic mice and rats, treatment with the angiotensin-II receptor blockers, olmesartan or losartan, respectively, resulted in an overall amelioration of the endothelial dysfunction that included an increased relaxation response of mesenteric arteries to EDHF-dependent signaling[70-72]. In the Goto-Kakizaki rat model of diabetes, Losartan also improved EDHF-signaling, and this improvement was abolished by inhibition of the large conductance Ca2+-activated K+ channels (BKCa) with iberiotoxin[70]. Losartan also normalized the relaxation of mesenteric arteries occurring in response to activation of the small- (SKCa) and intermediate-conductance (IKCa) Ca2+-activated K+ channels, which are reduced in a number of rat models of diabetes[70,73]. It appears, however, that under “normal” conditions SKCa and IKCa, but not BKCa channels, are essential components of EDHF-signaling[11]. Thus, it remains to be fully determined if the roles of these channels in EDHF-dependent signaling change in disease states such as diabetes.
Overall, evidence indicates that treatments that ameliorate the endothelial dysfunction present in diabetes and reduce inflammatory signals in the endothelium improve EDHF dependent signaling. Recently, a study indicated that 1 mo of IL-6 treatment in rats with streptozotocin-induced diabetes resulted in an improved response to EDHF-dependent relaxation of isolated renal artery rings[74]. Treatment with IL-6 also improved a number the nerve functional parameters that are usually impaired in diabetes. These results appear divergent from our findings indicating that direct incubation of isolated coronary microvessels with IL-6 impairs EDHF-dependent relaxation in wild type mice, while blockade of IL-6 restores it in db/db diabetic mice[64]. This discrepancy may be related to the different models of diabetes used in the studies, the different vascular beds proved for the effects of IL-6 on EDHF-dependent signaling, and/or the time frame and characteristics of IL-6 treatment. Overall, however, evidence clearly indicates that inflammation and TNF-α dependent signaling are involved in the development of endothelial dysfunction in diabetes[75]. As TNF-α has been reported to augment the expression of IL-6[76,77], the role of IL-6 and its potential use as a target for reducing diabetes related vasculopathies needs further investigation.
CONCLUSION
Diabetes has become one of the most important risk factors for vascular disease and other syndromes associated with hyperglycemia that lead to increased mortality in humans. The endothelium plays an important role in the regulation of vascular tone, helping to maintain normal vascular function through the synthesis and release of several kinds of vasoactive factors, including NO, PGI2 and EDHF. Although diabetes is clearly associated with endothelial dysfunction characterized by a reduced bioavailability of NO, evidence for the role of EDHF in the vascular dysfunction present in diabetes is not clear. While EDHF-dependent signaling appears to compensate for the reduced bioavailability of NO in some vascular beds, in others it appears to be reduced. Part of this controversy is due to the variable nature of EDHF. Nonetheless evidence indicates that EDHF may be therapeutically manipulated in the diabetic state to ameliorate endothelial dysfunction and improve vascular performance. A better understanding of the relationship that exists between diabetes and EDHF should provide new insights for novel therapeutic targets to resolve the vascular diseases associated with diabetes.
Peer reviewer: Cristina Vassalle, PhD, G. Monasterio Foundation and Institute of Clinical Physiology, Via Moruzzi 1, I-56124, Pisa, Italy
S- Editor Cheng JX L- Editor Lutze M E- Editor Zheng XM