Published online Jul 26, 2025. doi: 10.4330/wjc.v17.i7.108745
Revised: June 4, 2025
Accepted: July 1, 2025
Published online: July 26, 2025
Processing time: 89 Days and 13.9 Hours
A key cardiac magnetic resonance (CMR) challenge is breath-holding duration, difficult for cardiac patients.
To evaluate whether artificial intelligence-assisted compressed sensing CINE (AI-CS-CINE) reduces image acquisition time of CMR compared to conventional CINE (C-CINE).
Cardio-oncology patients (n = 60) and healthy volunteers (n = 29) underwent sequential C-CINE and AI-CS-CINE with a 1.5-T scanner. Acquisition time, visual image quality assessment, and biventricular metrics (end-diastolic volume, end-systolic volume, stroke volume, ejection fraction, left ventricular mass, and wall thickness) were analyzed and compared between C-CINE and AI-CS-CINE with Bland–Altman analysis, and calculation of intraclass coefficient (ICC).
In 89 participants (58.5 ± 16.8 years, 42 males, 47 females), total AI-CS-CINE acquisition and reconstruction time (37 seconds) was 84% faster than C-CINE (238 seconds). C-CINE required repeats in 23% (20/89) of cases (approximately 8 minutes lost), while AI-CS-CINE only needed one repeat (1%; 2 seconds lost). AI-CS-CINE had slightly lower contrast but preserved structural clarity. Bland-Altman plots and ICC (0.73 ≤ r ≤ 0.98) showed strong agreement for left ventricle (LV) and right ventricle (RV) metrics, including those in the cardiac amyloidosis subgroup (n = 31). AI-CS-CINE enabled faster, easier imaging in patients with claustrophobia, dyspnea, arrhythmias, or restlessness. Motion-artifacted C-CINE images were reliably interpreted from AI-CS-CINE.
AI-CS-CINE accelerated CMR image acquisition and reconstruction, preserved anatomical detail, and diminished impact of patient-related motion. Quantitative AI-CS-CINE metrics agreed closely with C-CINE in cardio-oncology patients, including the cardiac amyloidosis cohort, as well as healthy volunteers regardless of left and right ventricular size and function. AI-CS-CINE significantly enhanced CMR workflow, particularly in challenging cases. The strong analytical concordance underscores reliability and robustness of AI-CS-CINE as a valuable tool.
Core Tip: In this prospective study of 89 patients and volunteers, we demonstrate that artificial-intelligence-assisted compressed sensing (AI-CS-CINE) significantly streamlines cardiac magnetic resonance imaging workflows, reducing acquisition time by 84% (37 seconds vs 238 seconds) compared to conventional CINE imaging. Quantitative analysis showed excellent agreement in biventricular volumes and function (intraclass correlation coefficient 0.73-0.98). AI-CS-CINE proved especially valuable in challenging cases, such as for patients with cardiac amyloidosis, enabling faster acquisition and more reliable interpretation. These findings highlight AI-CS-CINE as a robust, time-efficient alternative to conventional methods, with potential to improve clinical efficiency and image quality in diverse cardiac populations.
- Citation: Wang H, Schmieder A, Watkins M, Wang P, Mitchell J, Qamer SZ, Lanza G. Artificial intelligence-assisted compressed sensing CINE enhances the workflow of cardiac magnetic resonance in challenging patients. World J Cardiol 2025; 17(7): 108745
- URL: https://www.wjgnet.com/1949-8462/full/v17/i7/108745.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i7.108745
Cardiac magnetic resonance (CMR) is the gold standard for noninvasive evaluation of cardiovascular disease, providing robust quantitative assessments critical for diagnoses, serial monitoring, and healthcare management[1-4]. CINE magne
Conventional CINE (C-CINE) uses segmented CMR acquisition over multiple cardiac cycles to create a single image, but faces two key challenges. First, image quality relies on controlled breath holding, which many patients, particularly those with heart failure, struggle to maintain, leading to motion artifacts. Second, the prolonged acquisition time needed to combine heartbeats can cause misalignment, reducing image quality and introducing variability in quantitative mea
Compressed sensing (CS) accelerates image acquisition by combining k-space under-sampling with iterative recon
In clinical practice, one of the major challenges of CMR is the need for a comprehensive imaging protocol, with most sequences requiring patients to hold their breath to achieve high-quality images. This workflow is particularly difficult for patients with various cardiac conditions that cause inconsistent breathing or, in some cases, an inability to hold their breath. As a result, many patients struggle to complete CMR examinations. Challenging cases make up nearly a quarter of our referrals, often involving individuals with the greatest medical need.
To address these challenges, in the current study, we aimed to explore the incorporation of AI-CS-CINE into the CMR workflow on a 1.5-T scanner to enhance the process by enabling near real-time acquisition, faster reconstruction, and preserve image quality for both diagnosis and longitudinal measurements. We compared AI-CS-CINE with C-CINE for quantifying left ventricle (LV) and right ventricle (RV) structure-function metrics in both referred cardiology patients and healthy volunteers. Additionally, we assessed the feasibility and efficiency of AI-CS-CINE in complex cases, particularly in cardiac amyloidosis, which requires an extensive CMR protocol and longer acquisition times.
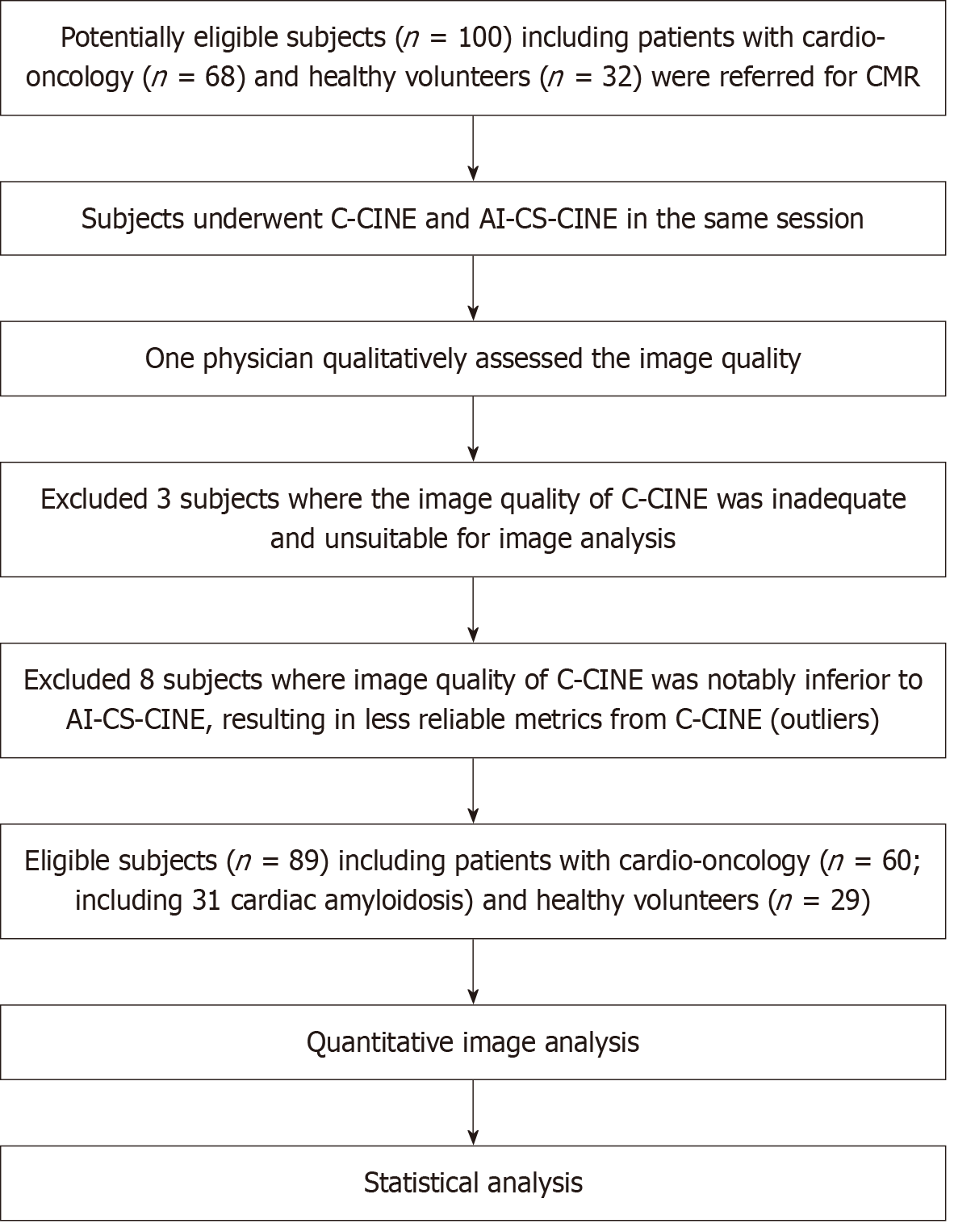
The CMR protocol was approved by the institutional ethics committee, and written informed consent was obtained from all participants. Data were prospectively acquired from 100 participants (32 healthy volunteers and 68 patients with various cardiac conditions) between May 2023 and January 2024. Including both groups allowed assessment of variations across a broad range of CMR metrics. Healthy volunteers, with no history of cardiovascular events or risk factors (e.g., hypertension or diabetes), were scanned without gadolinium contrast and had normal cardiac parameters (including normal LV and RV volumes, native myocardial T1 and T2 relaxation times in accordance with Society for Cardiovascular Magnetic Resonance, and institutional reference ranges). We excluded participants with inadequate image quality for analysis due to poor breath holding and/or ECG triggering. Out of 100 acquired cases, three cases were excluded due to poor image quality that rendered them unsuitable for analysis. Eight cases were identified as outliers because the image quality of C-CINE was substantially inferior to that of AI-CS-CINE (Results and Supplementary material).
The study ultimately included 89 participants with an average age of 58 years, comprising 42 males and 47 females (Figure 1). Baseline demographics and characteristics are provided in Table 1. Some patients were referred to CMR to evaluate for more than one potential diagnosis, resulting in overlapping referral categories in Table 1.
| Male | Female | Total | |||
| Participants (%) | 42 (47) | 47 (53) | 89 | ||
| Patients | 26 (43) | 34 (57) | 60 | ||
| Volunteers | 16 (55) | 13 (45) | 29 | ||
| Ethnicity (%) | White | Black | Hispanic | Asian | Total |
| Participants | 68 (76) | 15 (18) | 1 (1) | 5 (5) | 89 |
| Patients | 46 (77) | 13 (21) | 1 (2) | 0 (0) | 60 |
| Volunteers | 22 (77) | 2 (7) | 0 (0) | 5 (16) | 29 |
| Age (year) | Male | Female | Total | ||
| Participants | 57.3 (19-83) | 59.6 (18-83) | 58.4 (18-83) | ||
| Patients | 62.5 (30-88) | 63.0 (18-83) | 62.8 (18-83) | ||
| Volunteers | 48.6 (19-77) | 50.8 (24-71) | 49.6 (19-77) | ||
| BSA (m2) | Male | Female | Total | ||
| Participants | 2.04 (1.70-2.45) | 1.84 (1.31-2.87) | 1.93 (1.31-2.87) | ||
| Heart rate | Male | Female | Total | ||
| Participants | 66 (46-113) | 67 (47-108) | 67 (46-113) | ||
| Clinical referral indication | Patients | ||||
| r/o cardiotoxicity | 29 | ||||
| r/o amyloidosis | 30 | ||||
| r/o myocarditis | 3 | ||||
| r/o cardiomyopathy | 17 | ||||
| Arrhythmia | 9 | ||||
| Chest pain | 9 |
All participants underwent CMR examination on a 1.5-T scanner (uMR570; United Imaging Healthcare, Shanghai, China) with a body coil (12 channel) with ECG triggering and respiratory monitoring (Invivo Corp, Orlando, FL, United States). A short-axis stack of 11 CINE images and 2-, 3-, 4-chamber CINE images in expiratory breath hold was acquired for ventricular volume and function estimates with C-CINE and AI-CS-CINE at matched slice positions to facilitate the intra-participant comparison during the same scanning session. The shortest possible breath hold for C-CINE was 11 seconds to acquire one slice, and 2 seconds for AI-CS-CINE to acquire one slice, which appeared to be rhythmic breathing rather than breath holding. Consequently, AI-CS-CINE with 2 seconds slice acquisition was employed successfully with only rhythmic breathing in a subset of patients. AI was used for both acquisition and reconstruction during AI-CS-CINE. The imaging parameters of C-CINE and AI-CS-CINE are summarized in Table 2.
| C-CINE | AI-CS-CINE | |
| ECG mode | Retrospective | Retrospective |
| TR/TE (millisecond) | 3.57/1.75 | 2.74/1.28 |
| Image matrix | 224 × 85 | 192 × 100 |
| Reconstruction matrix | 2.0 | 1.5 |
| Spatial resolution (mm) | 1.89 × 1.61 | 1.88 × 1.88 |
| Flip angle (°) | 80 | 60 |
| Bandwidth (Hz/pixel) | 1500 | 1200 |
| Temporal resolution (millisecond) | 54 | 41 |
| Reconstructed cardiac phases | 25 | 25 |
| Field of view (mm) | 360 × 320 | 360 × 320 |
| Slice thickness (mm) | 8 | 8 |
| Gap (mm) | 0 | 0 |
| Number of slices | 11 | 11 |
| Longest breath-hold for acquisition (second) | 11 | 11 |
| Number of slices acquired per 11-sec breath hold | 1 | 6 |
| Number of breath-holds | 11 | 2 |
| Shortest breath-hold time to acquire one slice (second) | 11 | 2 |
| Total acquisition time including breath-holds (second) | 238 | 37 |
The acquisition time was recorded retrospectively for both C-CINE and AI-CS-CINE. Image reconstruction for both techniques was automatically completed in real-time after the acquisition. Consequently, the primary improvement in the workflow stemmed from the faster acquisition process and real-time reconstruction of AI-CS-CINE. Upon reconstruction, the technologist quickly reviewed image quality to determine if a repeat scan was necessary. In most cases, additional time was required to repeat C-CINE scans for patients with unsatisfactory breath-holds. The extra time spent on repeat scans was also documented. Additionally, participants received a standard-of-care protocol which included T1 and T2 mapping, strain, perfusion, and late gadolinium enhancement for diagnostic determinations.
The AI model used for image reconstruction is detailed in a previous study[14]. AI-CS-CINE images were reconstructed using a neural network called Res-CRNN, which unrolls over five iterative stages. Each stage refined the output from the previous one and included an independent subnetwork with three bidirectional convolutional gated recurrent unit (GRU) layers, 2D convolutional layers, data consistency layers, and two levels of residual connections. GRU layers captured both forward and backward temporal dependencies, while 2D convolutions offered efficient spatial processing with reduced memory requirements. Residual connections enhanced high-frequency detail recovery, and data consistency layers enforced fidelity to the originally acquired k-space data.
The model simultaneously reconstructed multi-coil images, treating each coil as a separate input channel and com
Image analysis was qualitatively evaluated by a physician according to four criteria: overall image quality (including contrast and signal-to-noise ratio), ability to assess wall motion and suitability for segmentation, visualization of valve morphology and function, and the presence of imaging artifacts. Each aspect was rated on a 5-point scale, where a score of 5 indicated excellent quality or performance or no artifacts; 4, very good quality or minimal artifacts; 3, adequate quality or some artifacts, though still diagnostic; 2, poor quality or obvious artifacts that impaired interpretation; and 1, non-diagnostic images or severe artifacts.
Image analysis was performed by a technologist with 25 years of experience in CMR. LV and RV volumes and functions were evaluated with built-in scanner software (Cardiac Analysis). Short-axis stacks of 11 CINE images were loaded into the software and the software automatically delineated the contours of endo-myocardium and epi-myocardium. Papillary muscles were included in the blood pool. The technologist reviewed the correctness of the automatic segmentation and could manually fine-tune the bounds on a slice-by-slice basis, if necessary. The EDV, ESV, stroke volume (SV), EF of LV and RV, and LV mass (LVM) and wall thickness were obtained and normalized to body surface area and indexed metrics were reported. EF was calculated as follows: EF = (EDV - ESV)/EDV × 100.
Statistical analysis was performed using R software version 4.3.2 (R Foundation for Statistical Computing). The mean and SD for each CMR measurement was calculated and further compared between male and female with unpaired t-test. The Shapiro–Wilk Test was used to confirm normal distribution of measurement differences for intra-participant comparison and correlation evaluation.
The time of image acquisition for C-CINE and AI-CS-CINE was compared with a paired Student t-test. Qualitative image quality scores of C-CINE and AI-CS-CINE were compared with the Wilcoxon Rank test. Agreement between quan
The study ultimately included 89 participants including 60 patients (31 of 60 had confirmed cardiac amyloidosis) and 29 healthy volunteers (Table 1). Notable quantitative differences between the C-CINE and AI-CS-CINE scans related to image quality were observed in eight cases (designated as outliers). Detailed results for comparisons are presented, both for the dataset excluding outliers (n = 89) and the complete dataset including outliers (n = 97; Supplementary material).
The average total acquisition-reconstruction time for the short-axis stack views was 238 seconds with C-CINE and 37 seconds with AI-CS-CINE sequence, resulting in 84% time saving with AI-CS-CINE. The acquisition time included only the actual scans, excluding pauses between breath-holds. During CMR, C-CINE sequences often needed to be repeated due to unsatisfactory image quality. The time loss associated with evaluating and repeating C-CINE sequences during the scan was compared with AI-CS-CINE. Across all 89 participants, time loss for C-CINE ranged from 0 to 8 minutes, and C-CINE had to be repeated in 20 cases [23%; 12 male (approximately 8 minutes) vs 8 female (approximately 5 minutes)], whereas AI-CS-CINE required only one repeat scan (female) out of 89 cases (1%) with only 2 second loss. This highlighted the efficiency and reliability of AI-CS-CINE in the workflow of CMR.
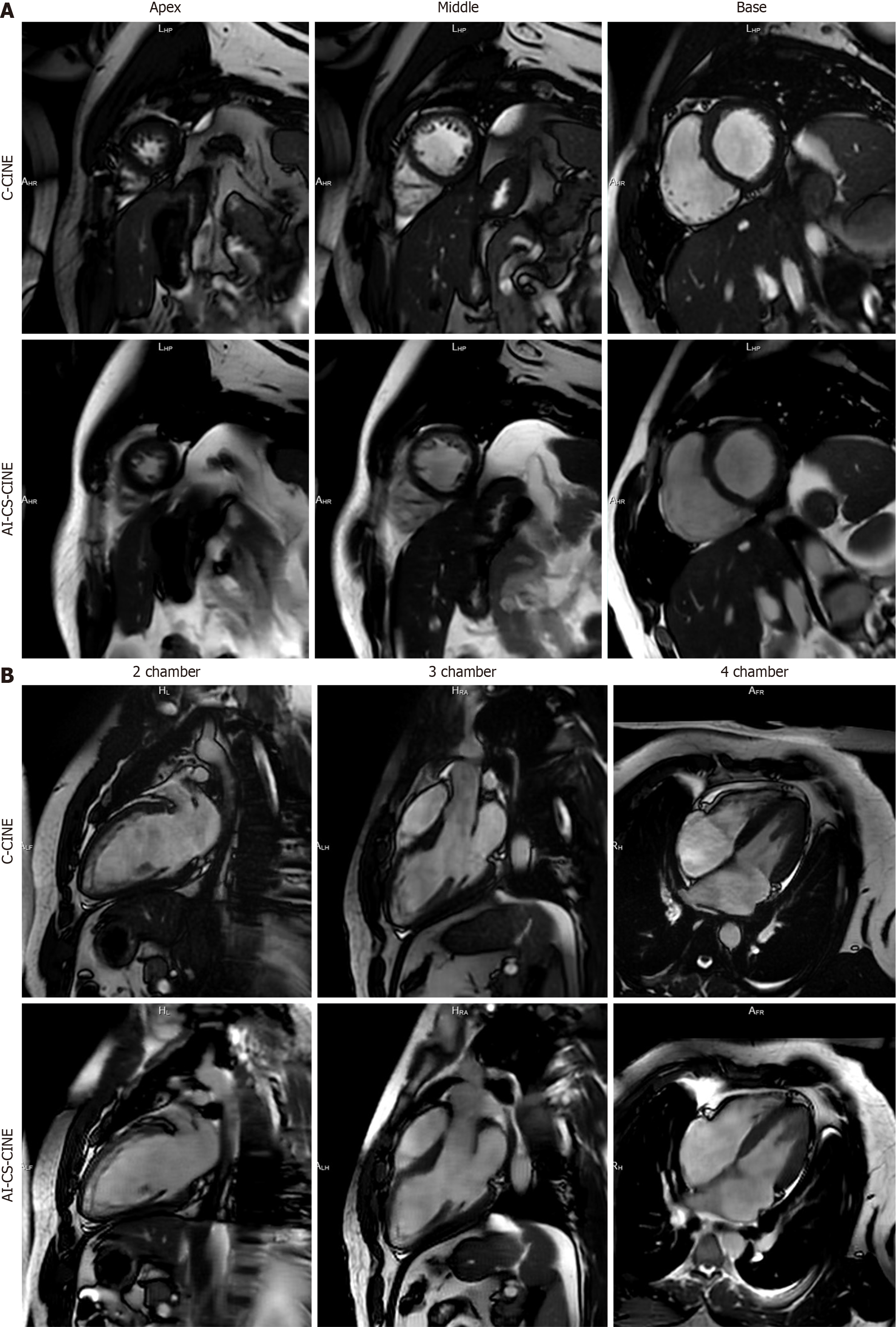
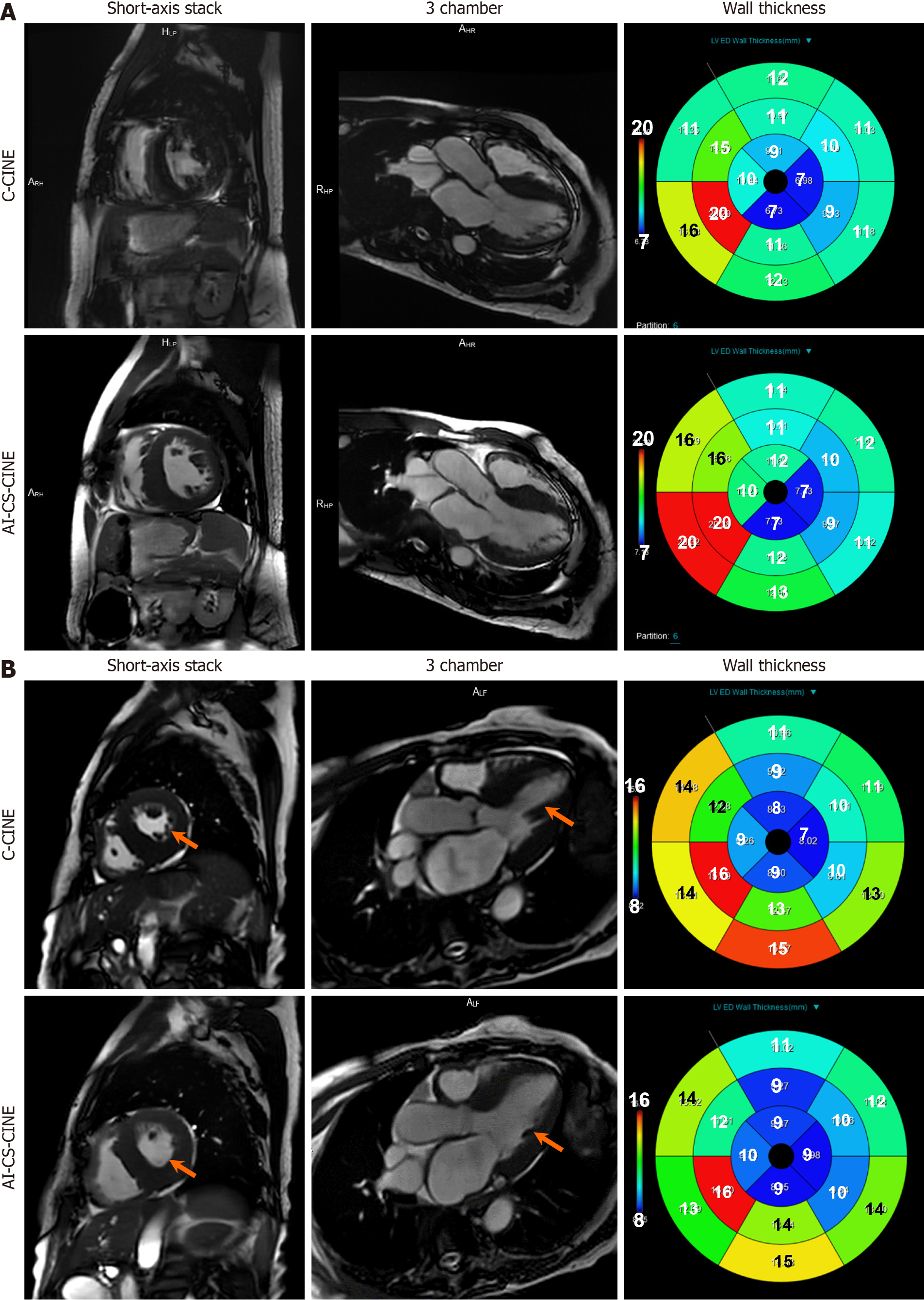
In general, AI-CS-CINE images had a slightly lower contrast but smoother appearance than C-CINE. All anatomical structures and functions were well preserved by AI-CS-CINE (Figures 2 and 3). The use of blood volume dephasing as a qualitative marker of valve regurgitation is flow-rate dependent with high velocity regurgitant jets, regardless of valve, clearly seen as sharp dark phase artifacts in some cases. Mild regurgitant jets (1+ to 2+) were less clearly defined com
The image quality scores were as follows: (1) General image quality: 5.0 ± 0.0 for both C-CINE and AI-CS-CINE (P > 0.99); (2) Wall motion and segmentation: 5.0 ± 0.0 for both C-CINE and AI-CS-CINE (P > 0.99); (3) Valve morphology and function: 5.0 ± 0.0 for C-CINE and 3.9 ± 0.6 for AI-CS-CINE (P < 0.001); and (4) Artifacts: 5.0 ± 0.0 for both C-CINE and AI-CS-CINE (P > 0.99). No significant differences were observed between C-CINE and AI-CS-CINE in any category except valve morphology and function. Despite the lower score for valve evaluation, AI-CS-CINE provided diagnostically sufficient image quality for assessing valve morphology and function (all scores ranging from 3-5).
Expected significant size and function differences were determined between male and female groups for LV and RV EF and body-mass-indexed LVEDV (LVEDVi), LVESVi, LVMi, RVEDVi, and RVESVi, consistent with recent prior reports (all P < 0.05) (Supplementary Table 1). The LVEF of females was marginally increased compared to males (P = 0.08), and female LVEDVi and LVESVi were smaller than males. Relative wall thickness, a metric used with muscle mass to differentiate LV size as normal, concentric remodeling, concentric LV hypertrophy, or eccentric hypertrophy did not differ with sex[22]. RVEF was greater in females, but RVEDVi and RVESVi were smaller than males. No sex difference was noted for LVSVi or RVSVi. Collectively, clinically critical determinants of cardiac structure and function known to vary by sex were consistent with published reports when derived from AI-CS-CINE images[23].
Correlation of LV and RV volume and function metrics between C-CINE and AI-CS-CINE: In this study, CMR mea
| mean ± SD | Bland–Altman | Intraclass coefficient | ||||
| C-CINE | AI-CS-CINE | Bias ± SD | LoA | r | 95%CI | |
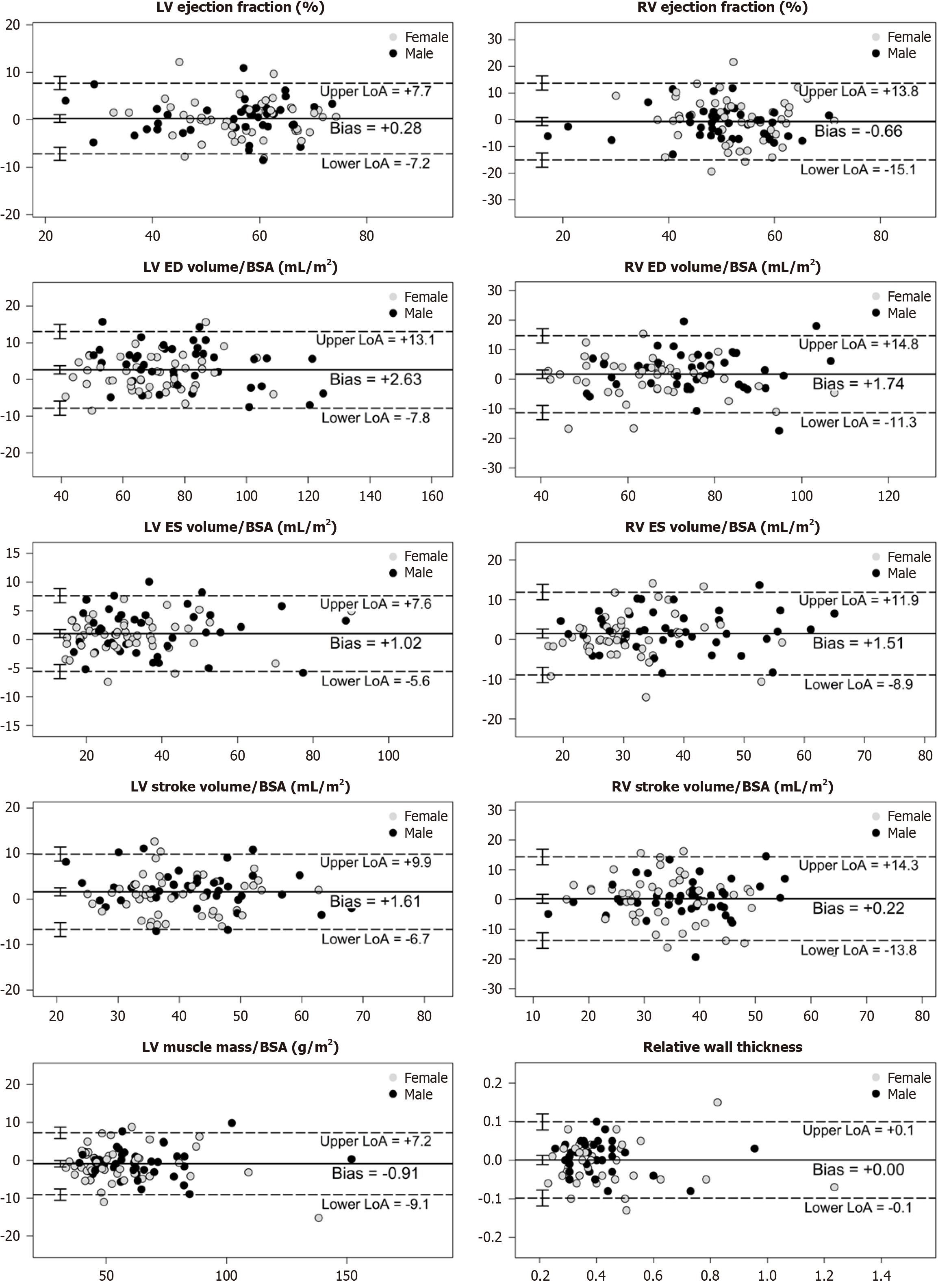
| LVEF (%) | 56.2 ± 11.0 | 55.9 ± 11.1 | 0.28 ± 3.78 | -7.13, 7.69 | 0.94 | 0.91, 0.96 |
| LVEDVi (mL/m2) | 75.9 ± 19.0 | 73.3 ± 19.0 | 2.63 ± 5.30 | -7.76, 13.01 | 0.95 | 0.93, 0.97 |
| LVESVi (mL/m2) | 34.4 ± 16.0 | 33.4 ± 15.7 | 1.02 ± 3.35 | -5.55, 7.59 | 0.98 | 0.96, 0.98 |
| LVSVi (mL/m2) | 41.5 ± 9.3 | 39.9 ± 9.6 | 1.61 ± 4.21 | -6.63, 9.85 | 0.89 | 0.83, 0.92 |
| LVMi (mL/m2) | 58.4 ± 20.3 | 59.3 ± 20.6 | 0.91 ± 4.14 | -9.01, 7.20 | 0.98 | 0.97, 0.99 |
| Relative wall thickness | 0.4 ± 0.2 | 0.4 ± 0.2 | 0.00 ± 0.05 | -0.10, 0.10 | 0.95 | 0.93, 0.97 |
| RVEF (%) | 50.8 ± 10.0 | 51.5 ± 10.3 | 0.66 ± 7.32 | -15.01, 13.69 | 0.73 | 0.62, 0.82 |
| RVEDVi (mL/m2) | 71.0 ± 14.8 | 69.3 ± 14.7 | 1.74 ± 6.60 | -11.21, 14.68 | 0.89 | 0.84, 0.93 |
| RVESVi (mL/m2) | 35.1 ± 11.0 | 33.6 ± 10.4 | 1.51 ± 5.28 | -8.84, 11.87 | 0.87 | 0.81, 0.91 |
| RVSVi (mL/m2) | 35.9 ± 9.7 | 35.7 ± 10.5 | 0.22 ± 7.12 | -13.74, 14.18 | 0.75 | 0.64, 0.83 |
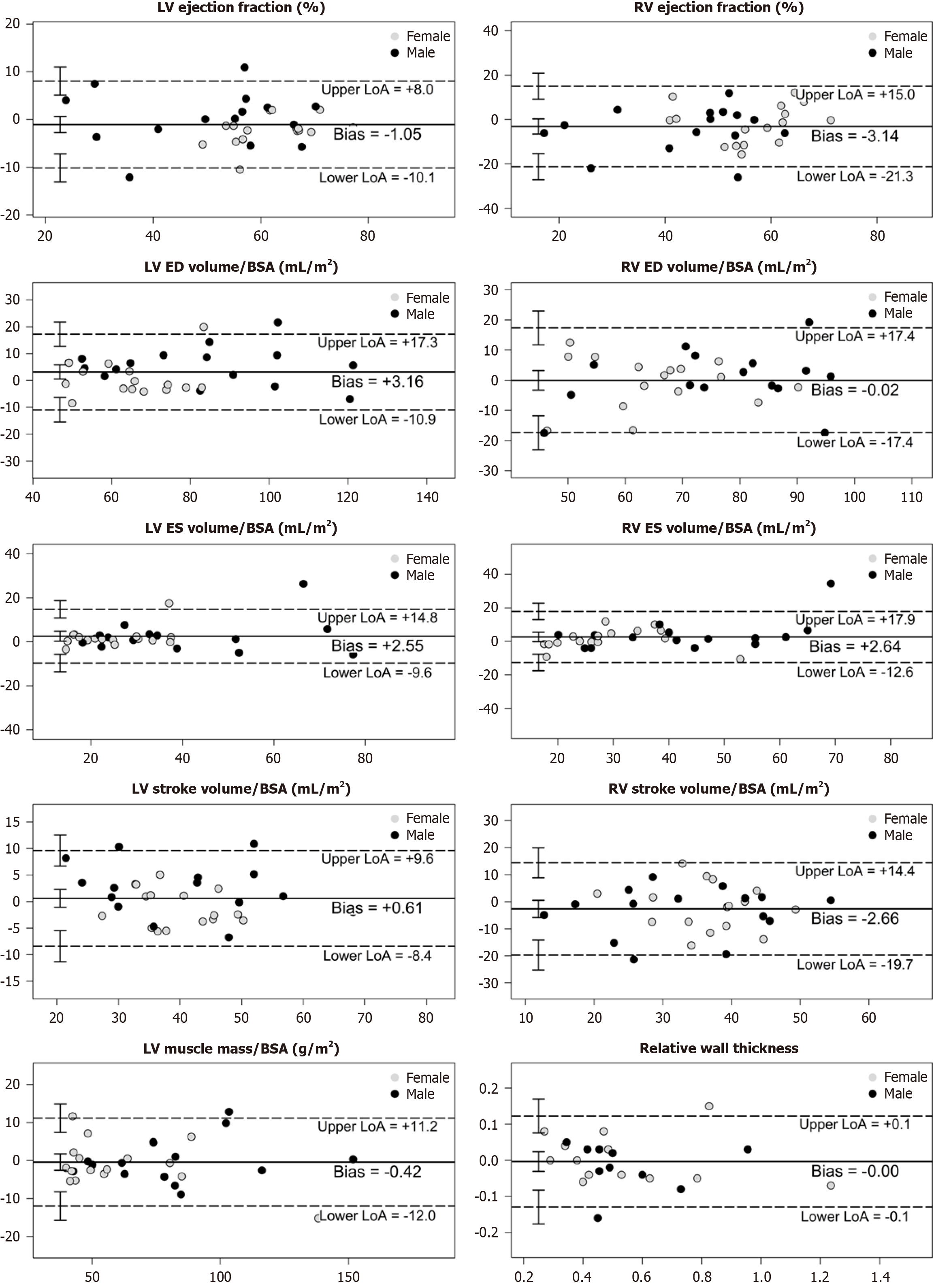
Bland–Altman analysis: Visual inspection of the Bland–Altman plots revealed no relationship between the differences and the magnitude of measurements, nor any systematic bias. For each variable, C-CINE and AI-CS-CINE means were very close and the mean difference was acceptable. Nearly all measurement pairs lie within the limits of agreement, indicating strong comparability between methods (Figure 4). A comprehensive summary of Bland–Altman results is provided in Table 3. While some variables showed statistically significant bias, this did not affect clinical interpretation. The limits of agreement supported that the observed differences were acceptable for clinical use.
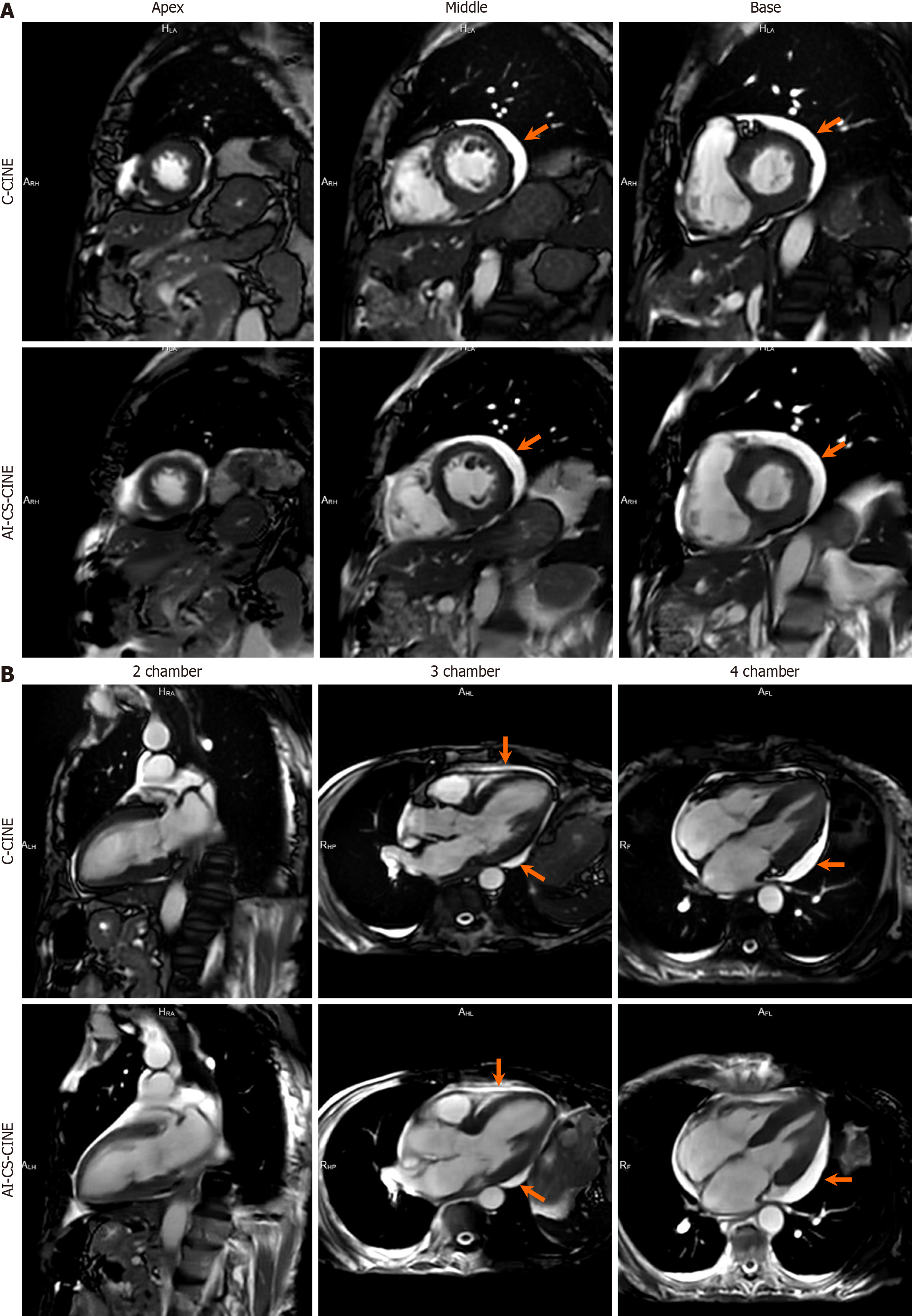
Among the 100 participants, three encountered challenges with both breath holds and ECG triggering during C-CINE imaging, rendering their scans unsuitable for diagnosis or comparison. Specifically, C-CINE scans were repeated multiple times. However, the image quality still failed to reach diagnostic standards. To avoid exhausting the patients and to preserve their ability to complete the remaining sequences necessary for diagnosis, further attempts at acquiring C-CINE were discontinued. In these cases, AI-CS-CINE successfully achieved diagnostic image quality, allowing for the extraction of volumetric and functional metrics needed for clinical evaluation (Videos 1 and 2). Other disparities between the two methods were observed in eight cases, where data points fell beyond the upper and lower limits of agreement for each metric in the Bland–Altman plot. A thorough review of these eight cases revealed that the image quality of C-CINE was significantly inferior to AI-CS-CINE, with notable issues such as blurriness and artifacts caused by inconsistent breath-holds. These shortcomings resulted in less reliable metrics derived from the C-CINE images. Thus these eight instances were categorized as outliers. Nonetheless, our results, inclusive of outliers (n = 97) are presented in Supplementary material, and showcasing a strong alignment between C-CINE and AI-CS-CINE across all metrics (Figure 5; Supplementary Table 2; Supplementary Figure 1). Overall, AI-CS-CINE produced reliable images for qualitative and quantita
Some patients and volunteers encountered difficulties maintaining expiratory breath holds during both C-CINE and AI-CS-CINE image acquisition, leading to blurring of images. Given the longer acquisition time of C-CINE compared to AI-CS-CINE, C-CINE image quality was more adversely affected. Additionally, some patients experienced intermittent arrhythmias, resulting in improper ECG triggering image acquisition. Similar to issues with breath holding, poor ECG triggering had a more pronounced impact on image quality with C-CINE compared to AI-CS-CINE due to the longer acquisition time (Figure 5).
Patients with significant cardiac amyloidosis often experience more symptoms than other cardiac patients due to the unique pathophysiology of the disease; they require a more comprehensive CMR protocol, making CMR challenging for some in this subgroup. Key metrics, including EF, EDVi, ESVi, SVi (for both LV and RV), LVMi, and relative wall thick
| mean ± SD | Bland–Altman | Intraclass coefficient | ||||
| C-CINE | AI-CS-CINE | Bias ± SD | LoA | r | 95%CI | |
| LVEF (%) | 55.9 ± 13.2 | 57.0 ± 13.2 | -1.05 ± 4.56 | -10.00, 7.89 | 0.94 | 0.87, 0.97 |
| LVEDVi (mL/m2) | 75.1 ± 21.1 | 72.0 ± 20.2 | 3.16 ± 7.07 | 10.70, 17.02 | 0.93 | 0.85, 0.97 |
| LVESVi (mL/m2) | 34.8 ± 19.0 | 32.2 ± 17.2 | 2.55 ± 6.12 | -9.45, 14.55 | 0.93 | 0.86, 0.97 |
| LVSVi (mL/m2) | 40.4 ± 10.2 | 39.8 ± 11.1 | 0.61 ± 4.52 | -8.26, 9.47 | 0.91 | 0.82, 0.95 |
| LVMi (mL/m2) | 69.9 ± 29.0 | 70.4 ± 29.5 | -0.42 ± 5.81 | 11.81, 10.97 | 0.98 | 0.96, 0.99 |
| Relative wall thickness | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.00 ± 0.06 | -0.13, 0.12 | 0.96 | 0.91, 0.98 |
| RVEF (%) | 48.9 ± 14.6 | 52.1 ± 13.0 | -3.14 ± 9.09 | 20.96, 14.68 | 0.76 | 0.56, 0.88 |
| RVEDVi (mL/m2) | 70.8 ± 16.2 | 70.9 ± 15.1 | -0.02 ± 8.72 | 17.12, 17.07 | 0.84 | 0.70, 0.92 |
| RVESVi (mL/m2) | 37.1 ± 16.5 | 34.5 ± 13.5 | 2.64 ± 7.65 | 12.36, 17.64 | 0.86 | 0.72, 0.93 |
| RVSVi (mL/m2) | 33.7 ± 10.7 | 36.4 ± 10.5 | -2.66 ± 8.57 | 19.46, 14.13 | 0.65 | 0.40, 0.81 |
Cardiovascular diseases are the leading global cause of death, with rising trends across all income levels[24]. CMR plays a crucial role in assessing ventricular volume, function, and myocardial health, as well as monitoring disease progression and treatment responses. However, a major barrier to expanding CMR access worldwide is the complexity of CMR workflows and the shortage of experienced technologists. AI-CS-CINE represents a significant step forward in over
Cardiovascular disease patients often experience mild dyspnea, tachypnea, arrhythmia, and restlessness, making it difficult for technologists to shorten study duration and minimize breath holds. Significant efforts have been made to address these challenges, including the use of accelerated MR sequences and advanced reconstruction algorithms[13,14,25]. In this study, AI-CS-CINE consistently reduced image acquisition and reconstruction time, while minimizing the need for repeat scans due to poor image quality. This improvement enhanced CMR workflow efficiency, improved image quality, and increased diagnostic reliability. This was notably beneficial for patients with breath-hold instability, often eliminating the need for repeat acquisitions and ensuring more efficient, high-quality imaging.
AI-CS-CINE affords several practical advantages over C-CINE regarding improving the workflow of CMR procedures. First, AI-CS-CINE enables the efficient completion of functional CMR imaging within 5 minutes, using either breath-hold or rhythmic-breathing techniques. This expedited approach allows for seamless progression to subsequent tasks, such as strain analysis and native T1/T2 mapping. As a result, the entire CMR acquisition, including functional imaging, strain analysis, T1/T2 mapping, and late gadolinium enhancement, can be completed within 30 minutes. Second, multiple breath holds can lead to premature exhaustion of the patient in the early half of the study that contributes to suboptimal breath holds later during T1/T2 mapping and late gadolinium enhancement. Sometimes, patient tolerance is exceeded and premature termination ensues. AI-CS-CINE efficiently reduces the number and duration of breath holds and recently, images acquired with a continuous steady rhythmic breathing approach have been well tolerated and very successful. Third, AI-CS-CINE reduces the dependency on technologists’ experience in performing CMR. Whereas C-CINE requires technologists to adjust patient acquisition parameters based on breath-hold challenges and to continuously review image quality, AI-CS-CINE simplifies the scanning process, enabling technologists to easily complete the required scans with satisfactory image quality. Finally, AI-CINE reduces arrhythmia interference with the reduction of scan time to 1-2 seconds. The optimized implementation of AI-CS-CINE promises significant enhancement to CMR workflow, leading to improved image quality, increased study throughput, and easier patient compliance.
AI-CS-CINE reconstruction uses information from adjacent phases, which could introduce temporal blurring[26,27]. However, AI-CS-CINE images showed only slight reduction in sharpness compared to C-CINE, while maintaining overall image quality. The current analysis software, which automatically quantifies cardiac volume and function, was optimized for C-CINE. It is expected that other advanced models trained with AI-CS-CINE data will further improve the reliability of quantitative metrics.
AI-CS-CINE did not always outperform C-CINE on certain metrics, potentially due to variations in field strength, imaging sequences, technologist expertise, reconstruction algorithms, and image analysis software in previous studies[14,25,28]. In the present study, AI-CS-CINE provided superior image quality with very reliable results. When C-CINE image quality was compromised, AI-CS-CINE images salvaged otherwise non-diagnostic studies. A recent report using an AI tool to reconstruct low-resolution C-CINE images reduced acquisition time by 42%. However, that estimate did not include AI-enhancement during image acquisition[29]. In contrast, our study used AI for both acquisition and reconstruc
Although multipurpose 3T scanners have been increasingly used for CMR, the 1.5-T scanner still remains the preferred choice for CMR due to its established performance and practical considerations[30,31]. Many hospitals worldwide have limited access to 3T scanners or only 1.5T scanners. CMR at 1.5T provides higher field homogeneity, fewer air-tissue interface artifacts, lower radiofrequency power deposition, broader availability, and comparable signal-to-noise ratios vs 3T. 1.5T MRI allows for more flexible imaging protocols with fewer SAR-related constraints. Additionally, SSFP sequences, which are widely used in CMR, suffer from increased banding artifacts at 3T, making 1.5T more reliable for CINE imaging. Furthermore, MRI-compatible medical devices, such as pacemakers and implants, are more commonly tested and approved for 1.5T, reinforcing its role as the clinical standard for CMR[32]. In comparison with two recent studies[14,29], the current study is more representative in terms of using 1.5T to better reflect CMR workflow worldwide, enabling AI to facilitate both image acquisition and inline reconstruction and encompassing clinical referrals of a broader age range, diverse body habitus, and a wider racial and disease spectrum.
LV volumes and functions with AI-CS-CINE agreed with C-CINE more closely than for the RV, which in part reflected the use of LV short-axis image stacks and system auto-calculation analysis software developed with C-CINE images. Specifically, the anatomy of the RV is more complicated than the LV with thinner walls, prominent trabeculations, and a complex LV wrap-around shape. These RV anatomical features complicate the software endocardial delineation, leading to potential errors. In addition, RV is more effected by diaphragm-mediated respiratory motion. Finally, the RV is more sensitive to field inhomogeneities arising from air-tissue interfaces and variations in tissue susceptibility. These inhomogeneities can distort the magnetic field, affecting image quality and contrast[33,34].
This study design has generalization limitations. The generalizability of our findings is limited by both the study population and imaging setup. The cohort included a high proportion of healthy volunteers and cardio-oncology pa
AI-CS-CINE significantly enhances the CMR workflow by accelerating image acquisition and reconstruction, reducing motion artifacts, and minimizing the need for repeat scans. It preserves anatomical details while improving efficiency, particularly in challenging patient populations. The quantitative cardiac metrics from AI-CS-CINE closely align with C-CINE across diverse ventricular sizes and functions, including cardio-oncology referrals and healthy volunteers. By streamlining the imaging process without compromising diagnostic accuracy, AI-CS-CINE represents a major advance
We would like to thank Elena Deych, MS, for reviewing the statistical data and offering valuable suggestions.
| 1. | Reiter U, Reiter C, Kräuter C, Nizhnikava V, Fuchsjäger MH, Reiter G. Quantitative Clinical Cardiac Magnetic Resonance Imaging. Rofo. 2020;192:246-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff-Brenkenhoff F, Kramer CM, Pennell DJ, Plein S, Nagel E. Standardized image interpretation and post-processing in cardiovascular magnetic resonance - 2020 update : Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J Cardiovasc Magn Reson. 2020;22:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 715] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 3. | Lee E, Ibrahim EH, Parwani P, Bhave N, Stojanovska J. Practical Guide to Evaluating Myocardial Disease by Cardiac MRI. AJR Am J Roentgenol. 2020;214:546-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Thomas KE, Fotaki A, Botnar RM, Ferreira VM. Imaging Methods: Magnetic Resonance Imaging. Circ Cardiovasc Imaging. 2023;16:e014068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 5. | Wang X, Uecker M, Feng L. Fast Real-Time Cardiac MRI: a Review of Current Techniques and Future Directions. Investig Magn Reson Imaging. 2021;25:252. [DOI] [Full Text] |
| 6. | Curione D, Ciliberti P, Monti CB, Capra D, Bordonaro V, Ciancarella P, Santangelo TP, Napolitano C, Ferrara D, Perrone MA, Secchi F, Secinaro A. Compressed Sensing Cardiac Cine Imaging Compared with Standard Balanced Steady-State Free Precession Cine Imaging in a Pediatric Population. Radiol Cardiothorac Imaging. 2022;4:e210109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 7. | Rajiah PS, François CJ, Leiner T. Cardiac MRI: State of the Art. Radiology. 2023;307:e223008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 99] [Reference Citation Analysis (0)] |
| 8. | Curtis AD, Cheng HM. Primer and Historical Review on Rapid Cardiac CINE MRI. J Magn Reson Imaging. 2022;55:373-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Gamper U, Boesiger P, Kozerke S. Compressed sensing in dynamic MRI. Magn Reson Med. 2008;59:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 306] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 10. | Jaspan ON, Fleysher R, Lipton ML. Compressed sensing MRI: a review of the clinical literature. Br J Radiol. 2015;88:20150487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 238] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 11. | Altmann S, Halfmann MC, Abidoye I, Yacoub B, Schmidt M, Wenzel P, Forman C, Schoepf UJ, Xiong F, Dueber C, Kreitner KF, Varga-Szemes A, Emrich T. Compressed sensing acceleration of cardiac cine imaging allows reliable and reproducible assessment of volumetric and functional parameters of the left and right atrium. Eur Radiol. 2021;31:7219-7230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58:1182-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4286] [Cited by in RCA: 3286] [Article Influence: 182.6] [Reference Citation Analysis (0)] |
| 13. | Masutani EM, Bahrami N, Hsiao A. Deep Learning Single-Frame and Multiframe Super-Resolution for Cardiac MRI. Radiology. 2020;295:552-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 14. | Yan X, Luo Y, Chen X, Chen EZ, Liu Q, Zou L, Bao Y, Huang L, Xia L. From Compressed-Sensing to Deep Learning MR: Comparative Biventricular Cardiac Function Analysis in a Patient Cohort. J Magn Reson Imaging. 2024;59:1231-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Mazurowski MA, Buda M, Saha A, Bashir MR. Deep learning in radiology: An overview of the concepts and a survey of the state of the art with focus on MRI. J Magn Reson Imaging. 2019;49:939-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 281] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 16. | Hauptmann A, Arridge S, Lucka F, Muthurangu V, Steeden JA. Real-time cardiovascular MR with spatio-temporal artifact suppression using deep learning-proof of concept in congenital heart disease. Magn Reson Med. 2019;81:1143-1156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 17. | Sandino CM, Lai P, Vasanawala SS, Cheng JY. Accelerating cardiac cine MRI using a deep learning-based ESPIRiT reconstruction. Magn Reson Med. 2021;85:152-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 18. | Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3857] [Cited by in RCA: 5284] [Article Influence: 195.7] [Reference Citation Analysis (0)] |
| 19. | Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307-310. [PubMed] |
| 20. | Gerke O. Reporting Standards for a Bland-Altman Agreement Analysis: A Review of Methodological Reviews. Diagnostics (Basel). 2020;10:334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 21. | Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9979] [Cited by in RCA: 18115] [Article Influence: 1811.5] [Reference Citation Analysis (1)] |
| 22. | Bang CN, Gerdts E, Aurigemma GP, Boman K, de Simone G, Dahlöf B, Køber L, Wachtell K, Devereux RB. Four-group classification of left ventricular hypertrophy based on ventricular concentricity and dilatation identifies a low-risk subset of eccentric hypertrophy in hypertensive patients. Circ Cardiovasc Imaging. 2014;7:422-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Meloni A, Righi R, Missere M, Renne S, Schicchi N, Gamberini MR, Cuccia L, Lisi R, Spasiano A, Roberti MG, Zuccarelli A, Ait-Ali L, Festa P, Aquaro GD, Mangione M, Barra V, Positano V, Pepe A. Biventricular Reference Values by Body Surface Area, Age, and Gender in a Large Cohort of Well-Treated Thalassemia Major Patients Without Heart Damage Using a Multiparametric CMR Approach. J Magn Reson Imaging. 2021;53:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis J, Catapano AL, Chugh SS, Cooper LT, Coresh J, Criqui M, DeCleene N, Eagle KA, Emmons-Bell S, Feigin VL, Fernández-Solà J, Fowkes G, Gakidou E, Grundy SM, He FJ, Howard G, Hu F, Inker L, Karthikeyan G, Kassebaum N, Koroshetz W, Lavie C, Lloyd-Jones D, Lu HS, Mirijello A, Temesgen AM, Mokdad A, Moran AE, Muntner P, Narula J, Neal B, Ntsekhe M, Moraes de Oliveira G, Otto C, Owolabi M, Pratt M, Rajagopalan S, Reitsma M, Ribeiro ALP, Rigotti N, Rodgers A, Sable C, Shakil S, Sliwa-Hahnle K, Stark B, Sundström J, Timpel P, Tleyjeh IM, Valgimigli M, Vos T, Whelton PK, Yacoub M, Zuhlke L, Murray C, Fuster V; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76:2982-3021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6994] [Cited by in RCA: 7574] [Article Influence: 1262.3] [Reference Citation Analysis (0)] |
| 25. | Zucker EJ, Sandino CM, Kino A, Lai P, Vasanawala SS. Free-breathing Accelerated Cardiac MRI Using Deep Learning: Validation in Children and Young Adults. Radiology. 2021;300:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 26. | Bustin A, Fuin N, Botnar RM, Prieto C. From Compressed-Sensing to Artificial Intelligence-Based Cardiac MRI Reconstruction. Front Cardiovasc Med. 2020;7:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 27. | Küstner T, Fuin N, Hammernik K, Bustin A, Qi H, Hajhosseiny R, Masci PG, Neji R, Rueckert D, Botnar RM, Prieto C. CINENet: deep learning-based 3D cardiac CINE MRI reconstruction with multi-coil complex-valued 4D spatio-temporal convolutions. Sci Rep. 2020;10:13710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 28. | Orii M, Sone M, Osaki T, Kikuchi K, Sugawara T, Zhu X, Janich MA, Nozaki A, Yoshioka K. Reliability of respiratory-gated real-time two-dimensional cine incorporating deep learning reconstruction for the assessment of ventricular function in an adult population. Int J Cardiovasc Imaging. 2023;39:1001-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 29. | Kravchenko D, Isaak A, Mesropyan N, Peeters JM, Kuetting D, Pieper CC, Katemann C, Attenberger U, Emrich T, Varga-Szemes A, Luetkens JA. Deep learning super-resolution reconstruction for fast and high-quality cine cardiovascular magnetic resonance. Eur Radiol. 2025;35:2877-2887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 30. | Auti OB, Bandekar K, Kamat N, Raj V. Cardiac magnetic resonance techniques: Our experience on wide bore 3 tesla magnetic resonance system. Indian J Radiol Imaging. 2017;27:404-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Min JY, Ko SM, Song IY, Yi JG, Hwang HK, Shin JK. Comparison of the Diagnostic Accuracies of 1.5T and 3T Stress Myocardial Perfusion Cardiovascular Magnetic Resonance for Detecting Significant Coronary Artery Disease. Korean J Radiol. 2018;19:1007-1020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Graves MJ. 3 T: the good, the bad and the ugly. Br J Radiol. 2022;95:20210708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 33. | Bonnemains L, Mandry D, Marie PY, Micard E, Chen B, Vuissoz PA. Assessment of right ventricle volumes and function by cardiac MRI: quantification of the regional and global interobserver variability. Magn Reson Med. 2012;67:1740-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Mertens LL, Friedberg MK. Imaging the right ventricle--current state of the art. Nat Rev Cardiol. 2010;7:551-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/