INTRODUCTION
More than a century has passed since Einthoven’s early concepts matured into the modern-day 12 lead electrocardiogram (ECG), which has become a routine test item to aid clinicians diagnose and manage various cardiovascular diseases (CVDs)[1-3]. Cardiologists have dedicated much of their training to detect ischemic heart disease (IHD) early, given its high mortality rate. Artificial intelligence (AI) has been extensively applied to the analysis and interpretation of ECGs for determining diagnosis and prognosis[4-6].
A large number of studies have explored AI-ECG models for the diagnosis and discovery of CVD, including dilated cardiomyopathy, hypertrophic cardiomyopathy, myocardial infarction (MI), coronary artery disease (CAD) and atrial fibrillation[7-9]. Recent studies support that AI model based on ECG might serve early screening of patients with IHD outside the hospital and identify patients who require further diagnostic tests[10]. The area under the receiver operating characteristic curve (AUC) of a convolutional neural networks model from China aimed to detect CAD using the 12954 ECGs from 2303 patients with CAD (> 70% stenosis) and 2090 ECGs from 1053 patients without CAD was 0.869, which showed that an AI-enable ECG algorithm can be an effective tool for detecting significant CAD[11]. New technologies such as single-lead ECG (SLECG) can now be integrated into various portable devices, thereby offering greater levels of convenience and efficiency compared to standard 12-lead ECG devices. The application of AI to both standard 12-lead ECG and SLECG represents a transformative advancement in cardiovascular diagnostics, particularly for IHD management. These advanced analytical approaches show significant potential for IHD detection, monitoring, even ischemia localization.
In this editorial, we discuss the research by Marzoog et al[12] recently published in World Journal of Cardiology. The authors presented a machine learning (ML) model for diagnosis of IHD using SLECG parameters. Combining advanced ML algorithms with clinically relevant SLECG parameters, it establishes a novel, accessible framework. While validation using larger cohorts is needed, the findings highlight the roles of AI in elevating precision cardiology and redefining diagnostic paradigms.
ENHANCED DETECTION OF IHD USING SLECG
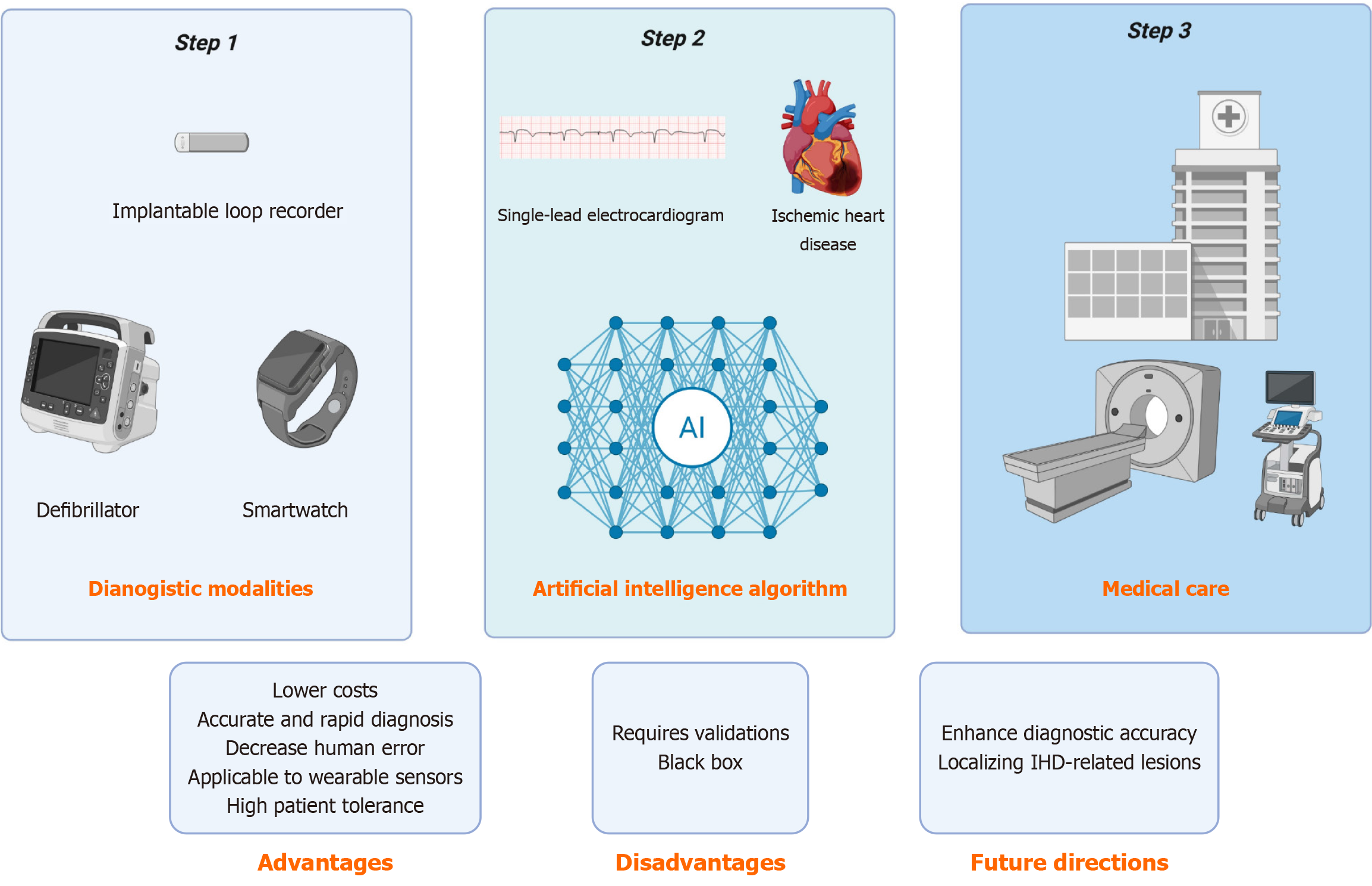
Non-invasive monitoring and screening are now increasingly convenient with the availability of mobile health technologies. Devices equipped with photoplethysmography and ECG capabilities now offer clinically acceptable performance for IHD detection, such as implantable loop recorder, electronic sphygmomanometer, smartwatches (Figure 1). Meanwhile, modern cardiac implantable electronic devices are designed to identify IHD through specialized algorithms that detect ST-segment elevation in intracardiac ECG signals[13]. Unlike implantable cardiac monitors and rhythm management devices, smartwatch-based ECG monitoring is used broadly without specific medical indications. The potential of mobile digital health solutions continues to expand rapidly, enabling millions of individuals to access continuous, patient-generated heart rate monitoring and rhythm surveillance through SLECG recordings[14].
Figure 1 Artificial intelligence-enabled single-lead electrocardiogram in detection of ischemic heart disease.
Wearable devices equipped with electrocardiogram (ECG) capabilities offer single-lead ECG data (step 1) and clinically acceptable performance for ischemic heart disease detection by artificial intelligence algorithms (step 2), so as to guide patients to seek further medical treatment (step 3). AI: Artificial intelligence; IHD: Ischemic heart disease.
Stark et al[15] reported a case of a 61-year-old male patient who sought medical attention solely based on morphological changes observed in a SLECG recording from Apple Watch 5 with his right index finger. Subsequent coronary angiography revealed an occlusion of the left anterior descending artery, which was then treated with revascularization. The SLECG collected thus has the potential to disrupt traditional health care delivery in cardiovascular medicine, especially for detection of IHD[16].
AI-ENABLED SLECG IN DETECTION OF IHD
Patients with severe IHD often exhibit significant ECG abnormalities, including ST-segment elevation or depression and T-wave inversion, which serve as key diagnostic markers of MI. With the widespread adoption of wearable devices, SLECG have improved the detection rate of IHD. When trained using large annotated datasets, AI algorithms can be optimized to automate IHD diagnosis using SLECG methodologies.
Multiple research groups have developed ML, including deep learning (DL), algorithms for IHD detection. Yahyaie et al[17] implemented an Internet of Things-enhanced, three lead model, reporting 89.5% accuracy in distinguishing between 207 healthy controls and 64 STEMI patients. A subsequent two-lead ML system achieved 98.2% diagnostic accuracy across 200 subjects [including both ST-segment elevation MI (STEMI) cases and normal ECGs][18]. Gibson's team conducted a large cohort study (training set: 8511 ECGs; validation set: 2542 ECGs) demonstrating that SLECG analysis could detect STEMI in lead V2 with 90.5% accuracy. More significantly, the localization model showed promising results for anterior and inferior wall STEMI identification, though its performance remained suboptimal for lateral wall STEMI detection[19]. A key innovation of Marzoog et al[12] is its comprehensive assessment of SLECG performance in detecting IHD during resting conditions and stress test. The study represents a groundbreaking application of ML to SLECG signals. By leveraging gradient boosting (XGBoost) and least absolute shrinkage and selection operator regression, the model achieves a notable AUC of 67%, significantly outperforming traditional stress tests (AUC = 50.7%)[12]. This demonstrates ML’s capacity to extract nuanced patterns from SLECG data, even in resource-constrained settings. However, the reliance on traditional ML algorithms rather than DL (e.g., convoluted neural networks or transformers) may limit its ability to capture intricate temporal patterns in SLECG signals. Future work could explore hybrid models combining handcrafted features with end-to-end DL architectures. These suggest that AI-enabled SLECG analysis can be utilized not only for STEMI detection but also for identifying silent or occult IHD, particularly when applied during stress test[12].
FUTURE DIRECTIONS
With the growing adoption of wearable devices, SLECG datasets are rapidly expanding, which will serve as a crucial foundation for developing IHD identification models using AI. While existing AI models show promise in localizing IHD-related lesions, their diagnostic accuracy needs enhancement[19]. Notably, Marzoog et al[12] highlighted the potential of combining resting and stress test SLECG analysis for IHD screening. The focus on portable, single-lead devices aligns with global health priorities, particularly in underserved regions. By integrating ML into wearable technology, this research paves the way for scalable, cost-effective IHD screening, democratizing access to early detection[20]. Additionally, future research could explore leveraging AI to synthesize 12-lead ECG representations from single-lead data, potentially combining the portability of SLECG with the diagnostic richness of multi-lead systems[21].
CONCLUSION
AI-enabled SLECG represents a promising modality for screening and early diagnosis of IHD. When integrated into wearable devices, it can be potentially accessible to a wide population of individuals who may not have routine or regular access to physicians.
ACKNOWLEDGEMENTS
The authors thank Professor Shenda Hong from National Institute of Health Data Science at Peking University and Institute of Medical Technology, Health Science Center of Peking University, Beijing, China for the helpful comments.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade A
Creativity or Innovation: Grade B
Scientific Significance: Grade B
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Arsalan HM S-Editor: Luo ML L-Editor: A P-Editor: Wang WB