Published online Apr 26, 2025. doi: 10.4330/wjc.v17.i4.105842
Revised: March 18, 2025
Accepted: March 31, 2025
Published online: April 26, 2025
Processing time: 72 Days and 11.1 Hours
There is widespread debate about the impact of metabolically healthy obesity (MHO) on cardiovascular outcomes. However, studies have not exclusively examined the impact of MHO on cardiovascular outcomes in the postmenopausal population.
To explore the prevalence of MHO and its relationship with hospitalization outcomes, including major adverse cardiac or cerebrovascular events (MACCE), in postmenopausal women.
We extracted data from the National Inpatient Sample 2020 database using International Classification of Disease, Tenth Revision, Clinical Modification codes for all admissions of postmenopausal women. We excluded patients with diabetes, hypertension, and hyperlipidemia to obtain metabolically healthy patients and then identified patients with obesity to create obese and non-obese cohorts. We used a 1:1 propensity score matching method to match patients with and without MHO based on age, and then we did a multivariable regression analysis for in-hospital MACCE.
In 2020, 1304185 metabolically healthy postmenopausal women were admitted; 148250 (11.4%) had MHO. After propensity score matching for age, a statistically significant difference was observed in overall MACCE [odds ratio (OR): 1.08, 95% confidence interval (CI): 1.01-1.16, P = 0.028] among MHO and non-MHO cohorts, especially in patients of African-American ethnicity (OR: 1.23, 95%CI: 1.01-1.49, P = 0.035) and the lowermost income quartile (OR: 1.24, 95%CI: 1.06-1.44, P = 0.007).
Postmenopausal patients with MHO are at risk of MACCE, especially black patients and those with lower incomes. Larger prospective studies can demystify MHO’s impact on cardiovascular outcomes among postmenopausal women.
Core Tip: In our retrospective population-based cohort study using a national inpatient sample (2020), we analyzed the impact of metabolically healthy obesity (MHO) on cardiovascular outcomes in hospitalized postmenopausal women. MHO was found to be associated with increased odds of major adverse cardiac and cerebrovascular events [adjusted odds ratio (aOR): 1.08, P = 0.028], particularly among black patients (aOR: 1.23, P = 0.035), and the lowest income quartile (aOR: 1.24, P = 0.007). Further, longitudinal studies are needed to assess the long-term cardiovascular risks in MHO patients.
- Citation: Pingili A, Desai R, Vempati R, Vemula M, Lakkimsetti M, Madhavaram H, Nanjundappa A, Singh S, Sunkara P, Gummadi J. Prevalence and impact of metabolically healthy obesity on cardiovascular outcomes in postmenopausal women and disparities: An age-matched study. World J Cardiol 2025; 17(4): 105842
- URL: https://www.wjgnet.com/1949-8462/full/v17/i4/105842.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i4.105842
The World Obesity Federation has defined obesity as a chronic, relapsing, progressive disease process requiring intervention[1,2]. Metabolically healthy obesity (MHO) can be considered a subgroup of people with obesity who do not exhibit overt cardiovascular or metabolic complications in contrast to metabolically unhealthy obesity (MUO)[1,3]. One proposed set of criteria includes body mass index (BMI) > 30 kg/m², with fasting serum triglycerides, systolic blood pressure, and blood glucose within normal limits, and the patient must also not currently be on medication for diabetes, hypertension, or dyslipidemia[1,3]. The last decade has seen a significant amount of literature published on MHO. However, there is no standardized definition yet, and the true prevalence and outcomes are yet to be defined[3].
According to existing literature, MHO confers a higher risk of developing cardiovascular disease despite the absence of overt risk factors[4]. A study performed in French hospitals with five years of follow-up identified an association between MHO and heart failure, with contrasting results when stratified for sex[5]. Few studies found a positive association between MHO and the risk of stroke[6,7]. It is established that, although the absence of metabolic abnormalities may reduce the risk of cardiovascular disease in MHO compared to the MUO population, it is still higher than in metabolically healthy lean individuals[4,8]. Furthermore, it is determined to be a dynamic and continuous process with a high risk of transition to a metabolically unhealthy phenotype over time, thus increasing the risk of cardiovascular events[1,3,4]. Therefore, there is a need for personalized decision-making following the risk stratification of patients with MHO[1,9]. The individualized intervention strategies range from lifestyle interventions to bariatric surgery, uniquely tailored to the patient and their metabolic profile[9]. Our study sought to stratify the effect of MHO in hospitalized post-menopausal women. We aim to understand the vulnerability of metabolically healthy obese postmenopausal women to major adverse cardiac or cerebrovascular events (MACCE) and identify independent predictors of MACCE. Through this avenue of study, we hope to lay the foundation for the hospitalized post-menopausal women’s population subset and boost further research to study the progression of their metabolic health.
This study used the 2020 National Inpatient Sample (NIS) database from the Healthcare Cost and Utilization Project. It is the most extensive United States all-payer inpatient healthcare dataset that is available to the public. The data includes the discharge information of 20% of hospitals from over 47 states in the United States. On average, there are 7 million unweighted discharges each year, which amounts to more than 35 million weighted discharges nationwide. A primary diagnosis and up to 39 secondary discharge diagnoses are present in every NIS inpatient admission. As the NIS has de-identified data, Institutional Review Board approval was not required. For more information about the database, please visit the Healthcare Cost and Utilization Project website, https://hcup-us.ahrq.gov/nisoverview.jsp.
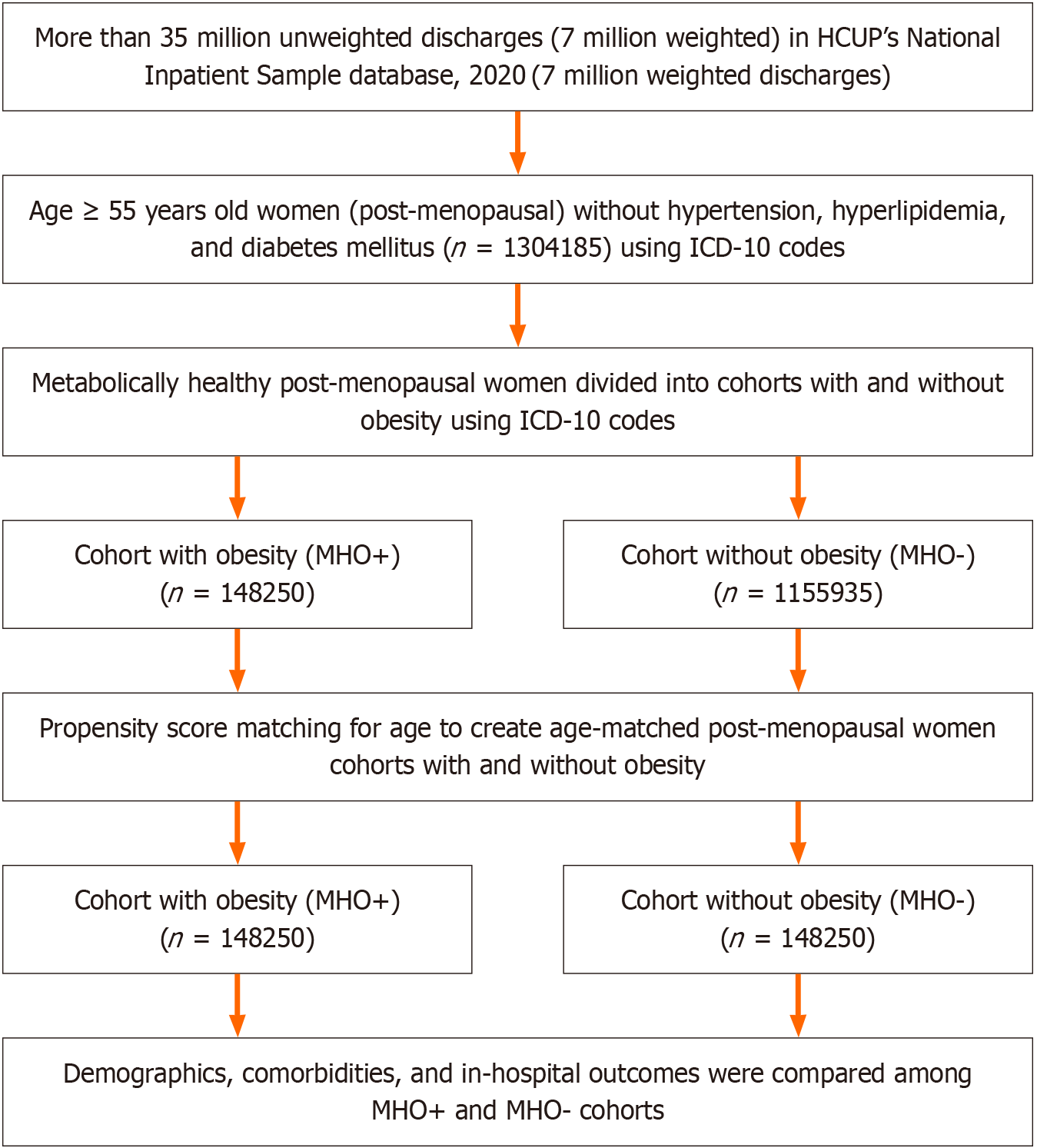
In the NIS 2020 database, we identified women of post-menopausal age (age ≥ 55 years), and we excluded patients hospitalized with diabetes, hypertension, and hyperlipidemia [comorbidities coded by Elixhauser Comorbidity Software or identified using International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10 CM) codes], allowing us to more accurately identify metabolically healthy patients. Then we identified metabolically healthy postmenopausal women with and without obesity using ICD-10 CM codes, forming two groups: One composed of metabolically healthy postmenopausal women with obesity and one composed of those without obesity. Supplementary Table 1 represents all the ICD-10 codes used in our study. To adjust for patient and hospital characteristics, we used propensity score matching for age to match patients for multivariate regression analysis. We assessed comorbidities such as acquired immune deficiency syndrome, alcohol abuse, autoimmune conditions, depression, smoking, chronic pulmonary disease, peripheral vascular disease, valvular disease, prior myocardial infarction (MI)/percutaneous coronary intervention, prior stroke/transient ischemic attack, prior venous thromboembolism, and cancer. Finally, we determined the adjusted odds ratio (aOR) for in-hospital outcomes (Figure 1).
The study aimed to assess hospitalizations in metabolically healthy postmenopausal women with and without obesity, taking into account demographics, hospital characteristics, and comorbidities. It also aimed to examine the prevalence of MHO and its relationship with hospitalization due to MACCE. The primary outcomes were to evaluate and compare mortality and MACCE, which is a composite of all-cause in-hospital mortality (discharge status), acute myocardial infarction, cardiac arrest, and acute ischemic stroke in hospitalized post-menopausal women with and without obesity. The secondary outcomes focused on evaluating and comparing patient disposition, length of stay, and hospital cost in both groups.
The study used two statistical tests, the Pearson χ2-test and the Mann-Whitney U test, to compare different variables between hospitalized post-menopausal women with and without obesity. Categorical variables were compared using the χ2-test, while continuous variables that did not follow a normal distribution were compared using the Mann-Whitney U test. We conducted a normality test using the Kolmogorov-Smirnov method and determined that the continuous data was not normally distributed. Consequently, we report the median values for continuous data. We used percentage and median along with the interquartile range to classify both categorical and continuous variables. We used the Database Discharge Weight to generate national estimates. A propensity score matching analysis for age was conducted with a caliper width of 0.001 and a near neighbor match to obtain a 1:1 matched cohort with and without obesity after excluding missing data. The pre-matched and post-matched cohorts had absolute standardized differences that were less than 10% after the matching process. Multivariable regression models were created to evaluate the risk of in-hospital outcomes for post-menopausal patients with obesity. We have also identified the various predictors of MACCE and assessed the predictive performance using C-statistics > 0.7, which indicates good model discrimination. Factors considered in the models included race, admission type, median household income, length of stay, insurance provider, bed size, ownership, and hospital location or teaching status. The results of logistic regression were presented in terms of an aOR, a 95% confidence interval, and P values. Trend analyses were performed using linear-by-linear associated tests. A two-tailed P value below 0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics 25.0 (IBM Corp., Armonk, NY, United States).
In our large population-based analysis, 1304185 post-menopausal women who were metabolically healthy and hospitalized in 2020 were identified, out of which 148250 were obese and 1155935 were non-obese; after propensity score matching for age, cohorts of MHO and non-MHO with 148250 patients in each were created (Figure 1).
The median age for both groups was 65 years. Most of the patients were of Caucasian descent, with 114615 in non-MHO and 111095 in MHO (81.5% vs 78.6%). Black patients represented 11865 in non-MHO and 16855 in MHO (8.4% vs 11.9%). Hispanics represented 3255 in non-MHO and 1040 in MHO (2.3% vs 0.7%). Asians represented 3255 in non-MHO and 1040 in MHO (2.3% vs 0.7%). Native Americans represented 815 in non-MHO and 970 in MHO (0.6% vs 0.7%). We found the median household income for the patient’s zip code and found that the 0-25th quartile for MHO vs non-MHO was 28.2% vs 25.9%, the 26th-50th quartile was 28.25 vs 26.9%, the 51th-75th quartile was 24.8% vs 24%, and the 76th-100th quartile was 18.8% vs 23.2% (Table 1).
| Baseline characteristics | With-out MHO, 148250 (50) | With MHO, 148250 (50) | Total | P value |
| Age in years at admission, median | 65 | 65 | 65 | 1.000 |
| Race | < 0.001 | |||
| White | 114615 (81.5) | 111095 (78.6) | 225710 (80.1) | |
| Black | 11865 (8.4) | 16855 (11.9) | 28720 (10.2) | |
| Hispanic | 10030 (7.1) | 11415 (8.1) | 21445 (7.6) | |
| Asian/PI | 3255 (2.3) | 1040 (0.7) | 4295 (1.5) | |
| Native Americans | 815 (0.6) | 970 (0.7) | 1785 (0.6) | |
| Median household income national quartile for patient zip code | < 0.001 | |||
| 0-25 | 37800 (25.9) | 41190 (28.2) | 78990 (27.1) | |
| 26-50 | 39165 (26.9) | 41120 (28.2) | 80285 (27.5) | |
| 51-75 | 34980 (24) | 36260 (24.8) | 61190 (21) | |
| 76-100 | 33800 (23.2) | 27390 (18.8) | 61190 (21) | |
| Payer type | < 0.001 | |||
| Medicare | 80025 (57.6) | 83045 (59) | 163070 (58.3) | |
| Medicaid | 15185 (10.9) | 14130 (10) | 29315 (10.5) | |
| Private | 43640 (31.4) | 43555 (30.9) | 87195 (31.2) | |
| Elective | < 0.001 | |||
| Non-elective | 113055 (76.4) | 110505 (74.6) | 223560 (75.5) | |
| Elective | 35015 (23.6) | 37610 (25.4) | 72625 (24.5) | |
| Weekend admission | 0.021 | |||
| Monday-Friday | 119490 (80.6) | 119990 (80.9) | 239480 (80.8) | |
| Saturday/Sunday | 28755 (19.4) | 28260 (19.1) | 57015 (19.2) | |
| Hospital location and teaching status | < 0.001 | |||
| Rural | 15075 (10.2) | 13180 (8.9) | 28255 (9.5) | |
| Urban non-teaching | 26875 (18.1) | 28575 (19.3) | 55450 (18.7) | |
| Urban teaching | 106300 (71.7) | 106495 (71.8) | 212795 (71.8) | |
| Hospital region | < 0.001 | |||
| Northeast | 28845 (19.5) | 28470 (19.2%) | 57315 (19.3) | |
| Midwest | 30105 (20.3) | 35610 (24) | 65715 (22.2) | |
| South | 56840 (38.3) | 54185 (36.5) | 111025 (37.4) | |
| West | 32460 (21.9) | 29985 (20.2) | 62445 (21.1) | |
| Comorbidities | ||||
| Peripheral vascular disease | 5340 (3.6) | 6150 (4.1) | 11490 (3.9) | < 0.001 |
| Tobacco use disorder | 32915 (22.2) | 35455 (23.9) | 68370 (23.1) | < 0.001 |
| Alcohol abuse | 7630 (5.1) | 3625 (2.4) | 11255 (3.8) | < 0.001 |
| Drug abuse | 5335 (3.6) | 4065 (2.7) | 9400 (3.2) | < 0.001 |
| Cancer | 22550 (15.2) | 14095 (9.5) | 36645 (12.4) | < 0.001 |
| Chronic kidney disease | 9315 (6.3) | 15920 (10.7) | 25235 (8.5) | < 0.001 |
| Prior MI | 2505 (1.7) | 2990 (2) | 5495 (1.9) | < 0.001 |
| Prior PCI | 170 (0.1) | 210 (0.1) | 380 (0.1) | 0.040 |
| Prior CABG | 855 (0.6) | 1090 (0.7) | 1945 (0.7) | < 0.001 |
| Heart failure | 55 (0) | 65 (0) | 120 (0) | 0.361 |
| Prior venous thrombo-embolism | 7670 (5.2) | 11955 (8.1) | 19625 (6.6) | < 0.001 |
| Prior stroke/TIA | 5305 (3.6) | 4870 (3.3) | 10175 (3.4) | < 0.001 |
| Acquired immunodeficiency syndrome | 570 (0.4) | 415(0.3) | 985 (0.3) | < 0.001 |
| Depression | 20870 (14.1) | 25535 (17.2) | 46405 (15.7) | < 0.001 |
| Chronic pulmonary disease | 32495 (21.9) | 43225 (29.2) | 75720 (25.5) | < 0.001 |
| Hypothyroidism | 24360 (16.4) | 30655 (20.7) | 55015 (18.6) | < 0.001 |
| Other thyroid disorders | 2705 (1.8) | 2770 (1.9) | 5475 (1.8) | 0.375 |
| Valvular disease | 1235 (0.8) | 1410 (1) | 2645 (0.9) | 0.001 |
| Autoimmune conditions | 7985 (5.4) | 9150 (6.2) | 17135 (5.8) | < 0.001 |
| Obstructive sleep apnea | 3570 (2.4) | 20640 (13.9) | 24210 (8.2) | < 0.001 |
| COVID-19 | 7670 (5.2) | 11810 (8) | 19480 (6.6) | < 0.001 |
| Bariatric surgery status | 2515 (1.7) | 7835 (5.3) | 10350 (3.5) | < 0.001 |
| Prior breast cancer | 7690 (5.2) | 6315 (4.3) | 14005 (4.7) | < 0.001 |
| Anxiety disorders | 27420 (18.5) | 27700 (18.7) | 55120 (18.6) | 0.186 |
| Psychoses | 4275 (2.9) | 3420 (2.3) | 7695 (2.6) | < 0.001 |
| Dementia | 8690 (5.9) | 5425 (3.7) | 14115 (4.8) | < 0.001 |
| Prior cancer | 19465 (13.1) | 16140 (10.9) | 35605 (12) | < 0.001 |
| Outcome | ||||
| MACCE | 12385 (8.4) | 11695 (7.9) | 24080 (8.1) | < 0.001 |
| Death during hospitalization | 7385 (5) | 6295 (4.2) | 13680 (4.6) | < 0.001 |
| Acute myocardial infarction | 3750 (2.5) | 4400 (3) | 8150 (2.7) | < 0.001 |
| Cardiac arrest | 1595 (1.1) | 1835 (1.2) | 3430 (1.2) | < 0.001 |
| Acute ischemic stroke | 2035 (1.4) | 1600 (1.1) | 3635 (1.2) | < 0.001 |
| Disposition of the patient | < 0.001 | |||
| Routine | 84060 (60.5) | 77015 (54.6) | 161075 (57.5) | |
| Transfer to the short-term hospital | 3270 (2.4) | 3115 (2.2) | 6385 (2.3) | |
| Transfer other: SNF, ICF | 22650 (16.3) | 27575 (19.5) | 50225 (17.9) | |
| Home health care | 28970 (20.8) | 33345 (23.6) | 62315 (22.3) | |
| Length of the stay, days, median | 3 | 4 | 3 | < 0.001 |
| Cost, United States dollar, median | 44874.81 | 51497.44 | 48134.09 | < 0.001 |
The geographic location of the hospitals among MHO vs non-MHO was distributed in the northeast (19.2% vs 19.5%), Midwest (24% vs 20.3%), south (36.5% vs 38.3%), and west (20.2% vs 21.9%). The setting of the hospitals among MHO vs non-MHO was rural (8.9% vs 10.2%), urban non-teaching (19.3% vs 18.1%), and urban teaching (71.8% vs 71.7%). The weekend admission rates among MHO vs non-MHO were distributed as follows: Monday-Friday (80.9% vs 80.6%), Saturday/Sunday (19.1% vs 19.4%), and elective admission rates among MHO vs non-MHO (25.4% vs 23.6%) and non-elective admission rates (74.6% vs 76.4%). The payer types among MHO vs non-MHO were as follows: Medicare (59% vs 57.6%), Medicaid (10% vs 10.9%), and private (30.9% vs 31.4%) (Table 1).
The most common comorbidities in MHO vs non-MHO cohorts were chronic obstructive pulmonary disease (29.2% vs 21.95%), hypothyroidism (20.7% vs 16.4%), other thyroid disorders (1.9% vs 1.8%), cancer (9.5% vs 15.2%), prior breast cancer (4.3% vs 5.2%), prior venous thromboembolism (8.1% vs 5.2%), tobacco use disorders (23.9% vs 22.2%), anxiety disorders (18.7% vs 18.5%), depression (17.2% vs 14.1%), psychoses (2.3% vs 2.9%), peripheral vascular disease (4.1% vs 3.6%), prior MI (2% vs 1.7%), prior percutaneous coronary intervention (0.1% vs 0.1%), prior coronary artery bypass grafting (0.7% vs 0.6%), prior transient ischemic attack/stroke (3.3% vs 3.6%), valvular heart disease (1% vs 0.8%), acquired immunodeficiency syndrome (0.3% vs 0.4%), chronic kidney disease (10.7% vs 6.5%), auto-immune conditions (6.2% vs 5.4%), obstructive sleep apnea (13.9% vs 2.4%), coronavirus disease 2019 (COVID-19) (8% vs 5.2%), bariatric surgery status (5.3% vs 1.7%), alcohol abuse (2.4% vs 5.1%), drug abuse (2.7% vs 3.6%), dementia (3.7% vs 5.9%), and prior cancer (10.9% vs 13.1%) (Table 1).
In hospitalized postmenopausal women, the mortality rate is 4.2% among those with MHO, compared to 5% in the non-MHO cohort. The incidence of MACCE in MHO was 7.9%, while it was 8.4% in non-MHO patients. 3% of patients among the MHO cohort and 2.5% among the non-MHO cohort had MACCE, while 1.1% among the MHO and 1.4% among the non-MHO cohort had an acute ischemic stroke. 1.2% of the MHO cohort and 1.1% of the non-MHO cohort had a cardiac arrest. The multivariable logistic regression analysis revealed statistically significant odds of MACCE (aOR: 1.08, 95%CI: 1.01-1.16, P = 0.028), especially MACCE significant in the black population (aOR: 1.23, 95%CI: 1.01-1.49, P = 0.035), and in the lowest-income quartile (aOR: 1.24, 95%CI: 1.06-1.44, P = 0.007), while it revealed non-statistically significant odds for MACCE in the white population (aOR: 1.05, 95%CI: 0.97-1.13, P = 0.243), MACCE in the Hispanic population (aOR: 1.19, 95%CI: 0.93-1.53, P = 0.175), MACCE in Asian/pacific islander (aOR: 1.28, 95%CI: 0.65-2.51, P = 0.482), MACCE in native American (aOR: 1.83, 95%CI: 0.70-4.76, P = 0.216), and MACCE in highest-income quartile (aOR: 1.10, 95%CI: 0.97-1.25, P = 0.149). Additionally, the all-cause mortality showed no statistically significant association (aOR: 1.23, 95%CI: 0.94-1.12, P = 0.621) (Table 2).
| Outcome | Odds ratio | 95%CI | P value |
| All-cause mortality | 1.02 | 0.94-1.12 | 0.621 |
| MACCE | 1.08 | 1.01-1.16 | 0.028 |
| MACCE (white) | 1.05 | 0.97-1.13 | 0.243 |
| MACCE (black) | 1.23 | 1.01-1.49 | 0.035 |
| MACCE (Hispanic) | 1.19 | 0.93-1.53 | 0.175 |
| MACCE (Asian/PI) | 1.28 | 0.65-2.51 | 0.482 |
| MACCE (Native American) | 1.83 | 0.70-4.76 | 0.216 |
| MACCE (median household income national quartile for patient zip code = 1) | 1.10 | 0.97-1.25 | 0.149 |
| MACCE (median household income national quartile for patient zip code = 4) | 1.24 | 1.06-1.44 | 0.007 |
| Effect modification by comorbidities and prior surgeries | |||
| MACCE (COVID-19 negative) | 1.02 | 0.94-1.09 | 0.675 |
| MACCE (COVID-19 positive) | 1.59 | 1.29-1.96 | < 0.001 |
| MACCE (no history of bariatric surgery) | 1.07 | 0.041 | |
| MACCE (history of bariatric surgery) | 2.11 | 0.049 | |
| MACCE (no tobacco use disorder) | 1.14 | 1.06-1.23 | 0.001 |
| MACCE (tobacco use disorder) | 0.85 | 0.72-0.99 | 0.039 |
| MACCE (no depression) | 1.07 | 0.99-1.15 | 0.080 |
| MACCE (depression) | 1.20 | 0.98-1.47 | 0.072 |
In addition, we have studied the modification of effect on MACCE by various comorbidities and prior bariatric surgery status, which revealed statistically significant odds among patients who were COVID-19 positive (OR: 1.59, 95%CI: 1.29-1.96, P < 0.001), both with (OR: 2.11, 95%CI: 1.00-4.46, P = 0.049) and without (OR: 1.07, 95%CI: 1.00-1.15, P = 0.041) history of bariatric surgery, both with (OR: 0.85, 95%CI: 0.72-0.99, P = 0.039) and without (OR: 1.14, 95%CI: 1.06-1.23, P = 0.039) tobacco use disorder, while it showed statistically non-significant odds among patients who were negative for COVID-19 (OR: 1.02, 95%CI: 0.94-1.09, P = 0.675), with (OR: 1.20, 95%CI: 0.98-1.47, P = 0.072) and without (OR: 1.07, 95%CI: 0.99-1.15) depression (Table 2).
The majority of patients from both MHO and non-MHO cohorts were discharged routinely (54.6% vs 60.5%), followed by home health care (23.6% vs 20.8%), transfer to other centers (skilled nursing facility, intermediate care facility, etc.) (19.5% vs 16.3%), and transfer to short-term hospital (2.2% vs 2.4%). The median length of the hospital stay among the MHO patients was four days, while it was three days among non-MHO patients. The median hospital costs among the MHO cohort were 51497.44 United States dollar, while they were 44874.81 United States dollar among the non-MHO cohort (Table 1).
In addition, we have identified various predictors of MACCE among the study population. Lower income (OR: 1.29, 95%CI: 1.11-1.50, P < 0.001 for the lowest income quartile; OR: 1.21, 95%CI: 1.04-1.40, P < 0.001 for the low-income quartile), non-elective admission (OR: 7.38, 95%CI: 5.91-9.22, P < 0.001), urban teaching hospitals (OR: 1.44, 95%CI: 1.20-1.72, P < 0.001), and age at admission (OR: 1.02, 95%CI: 1.02-1.03, P < 0.001) predicted the increased odds of MACCE among the study population. Hospitals in the Midwest, prior transient ischemic attack, prior venous thromboembolism, hypothyroidism, obstructive sleep apnea, prior breast cancer, anxiety disorders, psychoses, prior bariatric surgery, tobacco use disorder, and depression predicted lower MACCE, which were considered paradoxical findings (Table 3).
| Predictors | Odds ratio | 95%CI | P value |
| Median household income national quartile for zip code | < 0.001 | ||
| 1 vs 4 | 1.29 | 1.11-1.50 | |
| 2 vs 4 | 1.21 | 1.04-1.40 | |
| 3 vs 4 | 0.96 | 0.83-1.12 | |
| Race | 0.116 | ||
| Black vs white | 1.14 | 0.99-1.31 | |
| Hispanic vs white | 0.90 | 0.75-1.08 | |
| Asian/PI vs white | 0.96 | 0.57-1.59 | |
| Native American vs white | 1.37 | 0.84-2.23 | |
| Payer type | 0.074 | ||
| Medicaid vs Medicare | 0.93 | 0.78-1.10 | |
| Private vs Medicare | 0.87 | 0.76-0.99 | |
| Self-pay vs Medicare | 0.70 | 0.52-0.95 | |
| No charge vs Medicare | 1.22 | 0.48-3.11 | |
| Elective admission, non-elective vs elective | 7.38 | 5.91-9.22 | < 0.001 |
| Weekend admission, Saturday/Sunday vs Monday-Friday | 1.05 | 0.94-1.17 | 0.392 |
| Hospital location and teaching status | < 0.001 | ||
| Urban non-teaching vs rural | 1.12 | 0.91-1.38 | |
| Urban teaching vs rural | 1.44 | 1.20-1.72 | |
| Hospital region | < 0.001 | ||
| Midwest vs northeast | 0.68 | 0.58-0.81 | |
| South vs northeast | 0.94 | 0.80-1.09 | |
| West vs northeast | 0.99 | 0.84-1.17 | |
| Peripheral vascular disease | 1.16 | 0.95-1.42 | 0.156 |
| Prior MI | 1.39 | 1.05-1.85 | 0.021 |
| Prior PCI | 1.39 | 0.52-3.74 | 0.509 |
| Prior CABG | 1.34 | 0.86-2.06 | 0.193 |
| Prior TIA | 0.52 | 0.39-0.71 | < 0.001 |
| Prior VTE | 0.65 | 0.53-0.79 | < 0.001 |
| Chronic kidney disease | 0.95 | 0.83-1.09 | 0.489 |
| Acquired immunodeficiency syndrome | 0.80 | 0.29-2.24 | 0.673 |
| Alcohol abuse | 0.95 | 0.70-1.29 | 0.737 |
| Drug abuse | 1.09 | 0.83-1.42 | 0.548 |
| Chronic pulmonary disease | 1.09 | 0.98-1.20 | 0.121 |
| Hypothyroidism | 0.72 | 0.64-0.81 | < 0.001 |
| Other thyroid disorders | 1.02 | 0.72-1.44 | 0.904 |
| Valvular disease | 1.27 | 0.84-1.91 | 0.258 |
| Autoimmune condition | 0.95 | 0.78-1.15 | 0.574 |
| Dementia | 1.19 | 0.97-1.46 | 0.092 |
| Obstructive sleep apnea | 0.72 | 0.62-0.85 | < 0.001 |
| Prior breast cancer | 0.70 | 0.54-0.90 | 0.006 |
| Anxiety disorders | 0.78 | 0.68-0.90 | 0.001 |
| Psychoses | 0.50 | 0.34-0.73 | < 0.001 |
| Bariatric surgery status | 0.54 | 0.40-0.72 | < 0.001 |
| Tobacco use disorders | 0.63 | 0.56-0.71 | < 0.001 |
| Depression | 0.81 | 0.70-0.94 | 0.004 |
| Age (at admission) | 1.02 | 1.02-1.03 | < 0.001 |
In this overview of a large population-based analysis using a nationally representative dataset, we studied hospitalized postmenopausal women with MHO and identified several key findings. First, the prevalence of MHO in postmenopausal women was 11.4% among inpatient admissions. Second, we found that the median age of patients in our study is 65. More than 75% of patients belong to white ethnicity, and chronic pulmonary disease was the most common comorbidity. Third, we found that postmenopausal women with MHO have increased odds of MACCE events, especially among patients from Black ethnicities and lower-most income quartiles. Finally, we found that non-elective admission, hospital admission in teaching service in an urban area, prior MI, and the lowermost income tertile were the important predictors of MACCE outcomes in postmenopausal women with MHO. To our knowledge, no previous studies about MHO discussed MACCE outcomes in this specific population subset.
The prevalence of MHO across the literature is highly variable because of the lack of a standard definition of it. As per a recent study on the prevalence of MHO among all adults in the United States using data from the National Health and Nutrition Examination Survey, the prevalence is estimated to be up to 7%[10]. Per existing literature, MHO is more common in women than in men and in young adults than in older adults[11,12]. Interestingly, in our study, which was exclusively about hospitalized postmenopausal women in 2020, the prevalence of MHO was 11.4%. The observed higher prevalence in postmenopausal women when compared to the overall is likely because of the higher number of baseline comorbidities in this population subset than in younger women, leading to hospitalization. Another reason for this observation could be the different inclusion criteria for MHO across existing studies.
The median age of MHO patients in our study was 65. Of all the individuals with MHO, 78.6% belonged to White ethnicity, 11.9% were black patients, and 9.5% were Asians, Hispanics, and Native Americans. These findings suggest that age and race might also be significant risk factors for MACCE in postmenopausal women with MHO. We also found the five most common comorbidities in the MHO cohort in the descending order of their prevalence: Chronic pulmonary disease, hypothyroidism, tobacco use disorder, mental health disorders, and obstructive sleep apnea, and when comparing these comorbidities in the MHO vs non-MHO cohort, all of them were more prevalent in the MHO cohort. In a nationwide cohort study in France involving 89414 individuals with MHO, which discussed MHO and major adverse cardiovascular events, these comorbidities were found to be most common as well[5]. The high prevalence of these comorbidities among individuals with MHO raises the possibility that these underlying comorbidities likely play a role in the development of MACCE. This subset of patients with MHO might benefit from individualized care plans like adequate monitoring and aggressive treatment of underlying conditions to reduce the risk of MACCE.
While some studies suggest no association between MHO and cardiovascular disease, some suggest a negative association. As per a systematic review and meta-analysis by Eckel et al[13], which included 22 articles, even though the odds of MACCE in individuals with MHO might be less than in individuals with MUO, it was also found that the overall odds of MACCE in individuals with MHO are 1.45 times higher than in metabolically healthy individuals without obesity (relative risk = 1.45, 95%CI: 1.20-1.7, P = 0.004). Similarly, in a study by Caleyachetty et al[14] of 3.5 million individuals, there is an increased risk of coronary heart disease, cerebrovascular disease, and heart failure. However, in a prospective population study of the elderly, the presence of MHO didn’t confer a higher risk of cardiovascular disease[15]. Interestingly, in a study of a total of 19412 postmenopausal women, including metabolically healthy and unhealthy, overweight and obese, there was no significant association between MHO and incident heart failure[16]. In our study, we found that postmenopausal women with MHO were at 1.08 times higher odds of MACCE compared to metabolically healthy patients with no obesity. Studies have indicated that there is an increased level of inflammation, increased visceral adipose tissue content, and low cardiorespiratory fitness level in individuals with MUO when compared to individuals with MHO; this might be true in the case of individuals with MHO than metabolically healthy individuals with no obesity, and this could be the reason for MACCE events in patients with MHO[1-4,10-15]. Furthermore, it is important to note that there is always an increased risk of individuals with MHO naturally progressing to MUO[5,7,8,15,17,18]. Unfortunately, as there are no current studies to date determining the long-term cardio-metabolic (hypertension, type 2 diabetes mellitus, and hyperlipidemia) outcomes of MHO patients, it is important to initiate treatment early in high-risk individuals with MHO to prevent the natural progression to MUO with age. Our study also found that postmenopausal women with MHO from African-American ethnicity and the lowest income quartile have higher MACCE outcomes. Increased MACCE among African-American ethnicity could be a result of differences in baseline inflammation status, impaired endothelial function, cardiopulmonary fitness levels, and alterations in lipid metabolism, as these can increase the risk of cardiovascular disease even when traditional risk factors like hypertension, type 2 diabetes mellitus, and hyperlipidemia are absent. Increased MACCE in the lowermost income quartile is likely due to limited access to healthcare, health disparities, and behavioral and lifestyle factors. Furthermore, postmenopausal women with MHO from these population subsets should be considered vulnerable, and early intervention should be considered.
Our study identified several predictors, with non-elective admission emerging as the most significant predictor of MACCE events in postmenopausal women with MHO. This could be because of the presentation severity and multiple baseline comorbidities, which can increase MACCE events. We found that postmenopausal women from the lowermost income quartile are likely to have MACCE, probably because of limited access to health care, more baseline comorbi
It’s important to consider the limitations of our study when interpreting. Since the NIS only includes data from inpatient admissions, we don’t have data on lifestyle factors and their effects on MACCE, as greater cardiopulmonary fitness is one of the characteristics of individuals with MHO. Furthermore, we didn’t consider patients’ inflammatory status or visceral adipose tissue content, which could have provided some additional information on MACCE events in MHO postmenopausal women. The retrospective design and lack of assessment of the long-term risk of MACCE are other important limitations. Next, given we used ICD-10-CM codes and an administrative dataset for this study, there is always a possibility of under-coding and over-coding. It is also important to note that we used ICD-10-CM codes to identify obese patients with a BMI of more than 30. As a consequence, the exact data on BMI distribution was not available in either group. Additionally, the NIS lacks data on detailed clinical variables like laboratory values, imaging reports, and lifestyle factors such as physical activity and diet. Importantly, this study could not establish causation, and further controlled studies are warranted to validate these findings. Despite these limitations, our study is strengthened by using a vast NIS, which offers an inclusive examination of patients from diverse demographic backgrounds and geographic locations. This facilitates the application of our findings to various patient populations and healthcare environments. Furthermore, we utilized multiple statistical tests for analysis, enhancing our study outcome’s accuracy, validity, and reliability.
In conclusion, MHO in post-menopausal women is associated with 1.08 times increased odds of MACCE events when compared to metabolically healthy patients with no obesity. As MHO is a dynamic state, for a better understanding of the progression of cardio-metabolic outcomes, especially in vulnerable populations like postmenopausal women, it is essential to conduct longitudinal studies with long-term follow-ups. Apart from that, looking beyond BMI when assessing obesity could lead to a better understanding of its relationship with MACCE outcomes.
| 1. | Blüher M. Metabolically Healthy Obesity. Endocr Rev. 2020;41:bnaa004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 678] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 2. | Bray GA, Kim KK, Wilding JPH; World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18:715-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 997] [Article Influence: 110.8] [Reference Citation Analysis (1)] |
| 3. | Elías-López D, Vargas-Vázquez A, Mehta R, Cruz Bautista I, Del Razo Olvera F, Gómez-Velasco D, Almeda Valdes P, Aguilar-Salinas CA; Metabolic Syndrome Study Group. Natural course of metabolically healthy phenotype and risk of developing Cardiometabolic diseases: a three years follow-up study. BMC Endocr Disord. 2021;21:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Echouffo-Tcheugui JB, Short MI, Xanthakis V, Field P, Sponholtz TR, Larson MG, Vasan RS. Natural History of Obesity Subphenotypes: Dynamic Changes Over Two Decades and Prognosis in the Framingham Heart Study. J Clin Endocrinol Metab. 2019;104:738-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Fauchier G, Bisson A, Bodin A, Herbert J, Semaan C, Angoulvant D, Ducluzeau PH, Lip GYH, Fauchier L. Metabolically healthy obesity and cardiovascular events: A nationwide cohort study. Diabetes Obes Metab. 2021;23:2492-2501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Ma LZ, Sun FR, Wang ZT, Tan L, Hou XH, Ou YN, Dong Q, Yu JT, Tan L. Metabolically healthy obesity and risk of stroke: a meta-analysis of prospective cohort studies. Ann Transl Med. 2021;9:197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Meng M, Guo Y, Kuang Z, Liu L, Cai Y, Ni X. Risk of Stroke Among Different Metabolic Obesity Phenotypes: A Systematic Review and Meta-Analysis. Front Cardiovasc Med. 2022;9:844550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | Fingeret M, Marques-Vidal P, Vollenweider P. Incidence of type 2 diabetes, hypertension, and dyslipidemia in metabolically healthy obese and non-obese. Nutr Metab Cardiovasc Dis. 2018;28:1036-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Stefan N, Häring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1:152-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 642] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 10. | Wang JS, Xia PF, Ma MN, Li Y, Geng TT, Zhang YB, Tu ZZ, Jiang L, Zhou LR, Zhang BF, Tong WW, Shan Z, Liu G, Yang K, Pan A. Trends in the Prevalence of Metabolically Healthy Obesity Among US Adults, 1999-2018. JAMA Netw Open. 2023;6:e232145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 60] [Reference Citation Analysis (0)] |
| 11. | Smith GI, Mittendorfer B, Klein S. Metabolically healthy obesity: facts and fantasies. J Clin Invest. 2019;129:3978-3989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 452] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 12. | Rey-López JP, de Rezende LF, Pastor-Valero M, Tess BH. The prevalence of metabolically healthy obesity: a systematic review and critical evaluation of the definitions used. Obes Rev. 2014;15:781-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 242] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 13. | Eckel N, Meidtner K, Kalle-Uhlmann T, Stefan N, Schulze MB. Metabolically healthy obesity and cardiovascular events: A systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23:956-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 288] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 14. | Caleyachetty R, Thomas GN, Toulis KA, Mohammed N, Gokhale KM, Balachandran K, Nirantharakumar K. Metabolically Healthy Obese and Incident Cardiovascular Disease Events Among 3.5 Million Men and Women. J Am Coll Cardiol. 2017;70:1429-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 406] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 15. | Dhana K, Koolhaas CM, van Rossum EF, Ikram MA, Hofman A, Kavousi M, Franco OH. Metabolically Healthy Obesity and the Risk of Cardiovascular Disease in the Elderly Population. PLoS One. 2016;11:e0154273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Cordola Hsu AR, Xie B, Peterson DV, LaMonte MJ, Garcia L, Eaton CB, Going SB, Phillips LS, Manson JE, Anton-Culver H, Wong ND; WHI Investigators. Metabolically Healthy/Unhealthy Overweight/Obesity Associations With Incident Heart Failure in Postmenopausal Women: The Women's Health Initiative. Circ Heart Fail. 2021;14:e007297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Soriguer F, Gutiérrez-Repiso C, Rubio-Martín E, García-Fuentes E, Almaraz MC, Colomo N, Esteva de Antonio I, de Adana MS, Chaves FJ, Morcillo S, Valdés S, Rojo-Martínez G. Metabolically healthy but obese, a matter of time? Findings from the prospective Pizarra study. J Clin Endocrinol Metab. 2013;98:2318-2325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 18. | Magkos F. Metabolically healthy obesity: what's in a name? Am J Clin Nutr. 2019;110:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 19. | Pelascini E, Disse E, Pasquer A, Poncet G, Gouillat C, Robert M. Should we wait for metabolic complications before operating on obese patients? Gastric bypass outcomes in metabolically healthy obese individuals. Surg Obes Relat Dis. 2016;12:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/