Published online Apr 26, 2025. doi: 10.4330/wjc.v17.i4.104465
Revised: March 11, 2025
Accepted: April 8, 2025
Published online: April 26, 2025
Processing time: 121 Days and 18.1 Hours
Congenital junctional ectopic tachycardia (CJET) is a rare but life-threatening arrhythmia in neonates and infants, often refractory to conventional antiarrhythmic therapy. Ivabradine, a selective inhibitor of hyperpolarization-activated cyclic nucleotide-gated channels, has emerged as a promising drug for CJET management.
To evaluate the efficacy and safety of ivabradine in the management of CJET. Specifically, this study aims to analyze the dosing strategies, treatment outcomes, and the role of ivabradine as monotherapy or adjunct therapy in patients who have previously received other antiarrhythmic medications. Additionally, this review seeks to assess the impact of ivabradine on heart rate (HR) control, rhythm conversion, and its overall safety profile to provide evidence-based insights into its clinical use for CJET management.
This systematic review aims to evaluate the outcomes of ivabradine, either as monotherapy or as an adjunctive therapy, in the treatment of CJET. A comprehensive literature search was conducted across multiple electronic databases to identify relevant studies investigating the use of ivabradine in CJET. Stringent inclusion and exclusion criteria were applied to ensure the inclusion of high-quality, peer-reviewed studies. Data extraction and quality assessment were performed independently by two reviewers.
Ten studies, comprising 6 case reports, 3 case series, and 1 cohort study, met the inclusion criteria. Ivabradine doses ranged from 0.025 to 0.28 mg/kg/dose, administered either as monotherapy or in combination with various antiarrhythmic medications. Overall, ivabradine demonstrated promising results in achieving HR control, conversion to sinus rhythm, or stabilization of junctional rhythm. No significant adverse effects related to ivabradine were reported.
The available evidence suggests that ivabradine may be an effective adjunctive therapy or, in some cases, a potential monotherapy for the management of CJET, particularly in cases refractory to traditional antiarrhythmic medications. However, the current evidence is limited by the small sample sizes and retrospective nature of the included studies. Well-designed prospective studies with larger cohorts and longer follow-up periods are warranted to further elucidate the role of ivabradine in CJET management.
Core Tip: Ivabradine, a selective hyperpolarization-activated cyclic nucleotide-gated channel inhibitor, shows promise in managing congenital junctional ectopic tachycardia (CJET) in neonates and infants, especially when conventional antiarrhythmic therapies fail. This systematic review highlights ivabradine's efficacy in controlling heart rate, achieving sinus rhythm, and stabilizing junctional rhythm, with no significant adverse effects reported. Despite the encouraging results, further prospective studies with larger sample sizes and extended follow-up are necessary to establish ivabradine's definitive role in CJET management.
- Citation: Bokhari SFH, Mushtaq MM, Mushtaq M, Ali H, Bakht D, Faizullah M, Asghar A, Buhadur Ali MK, Sarwar MA, Liaqat M, Iqbal A, Dost W. Ivabradine in the treatment of congenital junctional ectopic tachycardia: A systematic review. World J Cardiol 2025; 17(4): 104465
- URL: https://www.wjgnet.com/1949-8462/full/v17/i4/104465.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i4.104465
Junctional ectopic tachycardia (JET), characterized by tachyarrhythmias originating from the atrioventricular (AV) node and AV junction, including the bundle of His, represents a critical concern in both the immediate post-operative phase following surgery for congenital cardiovascular disorders and in neonates without prior surgical intervention, bearing substantial mortality and morbidity burdens[1,2]. Consequently, JET is dichotomized into congenital JET (CJET) and post-operative JET. The incidence rate of JET during the postoperative period of cardiac surgery stands at 14.3%[3]. CJET primarily affects neonates aged ≤ 6 months, as evidenced by a multicenter retrospective study documenting 94 cases, including 47 neonates younger than 6 months[4]. This arrhythmia has an incidence of less than 1% in all pediatric arrhythmias, however, manifests with alarming mortality rates of up to 35% among neonates if undiagnosed and untreated, potentially culminating in dilated cardiomyopathy and left ventricular dysfunction[5]. Infants typically present with elevated heart rate (HR) and ventricular dysfunction. The pathophysiological basis for the raised HR in CJET stems from increased automaticity of the AV node and adjacent regions, including the bundle of His. This is attributed to the activation of slow, inward-directed sodium currents, known as "funny currents" (If), leading to sinus node depolarization and enhanced automaticity[2,6]. Notably, CJET often exhibits resistance to conventional antiarrhythmic therapy, presenting a formidable challenge in clinical management. However, evidence from multicenter studies suggests improved outcomes with diverse therapeutic modalities, including amiodarone, permanent pacing, radiofrequency ablation, and cryoablation[4]. Traditional antiarrhythmic agents such as adenosine may prove ineffective in refractory cases. The available antiarrhythmic drugs commonly used included amiodarone, flecainide, digoxin, esmolol and metoprolol either as monotherapy or in combination with other drugs[7,8].
Recent investigations have explored the efficacy of ivabradine as monotherapy or in combination with conventional antiarrhythmic drugs, showing promising results[7,9,10]. Ivabradine operates by selectively inhibiting hyperpolarization-activated cyclic nucleotide-gated (HCN4) channels, thereby reducing the HR and suppressing spontaneous diastolic depolarization in the AV nodal region[11]. Although Food and Drug Administration-approved for HR control in chronic heart failure in adults, the application of ivabradine in infants remains under evaluation, with limited case reports suggesting its efficacy in CJET management. Ivabradine stands out as a potential drug of choice due to its effectiveness and minimal interference with unrelated electrophysiological parameters. Ivabradine offers a novel approach to CJET treatment, potentially mitigating associated morbidity and mortality rates. This review aims to explore the efficacy of ivabradine, whether used alone or as an adjunctive therapy, in the effective treatment and management of CJET.
This systematic review adheres to the PRISMA and Assessing the Methodological Quality of Systematic Reviews guidelines, ensuring a thorough and methodologically rigorous evaluation of studies on ivabradine treatment outcomes in CJET.
A systematic search for the relevant articles was conducted across major electronic databases, including the PubMed, Embase, Hinari, and the Cochrane Library. The search strategy was build using a combination of MeSH terms and relevant keywords related to ivabradine and CJET. The search strategy was optimized using the Boolean operators (AND, OR) to identify the relevant studies. The studies were then screened using a predefined inclusion criteria.
A rigorous eligibility criteria was carefully established to ensure the inclusion of high-quality and relevant studies. The included studies had to be published in a peer-reviewed journal, specifically focusing on the treatment outcomes in CJET using ivabradine. The review covered research published from the inception of relevant databases up to December 2023, incorporating the most recent findings. Studies lacking sufficient data on ivabradine in CJET were excluded to maintain precision and focus. Additionally, the articles that solely focused on the effects of ivabradine using animal cells were excluded to prioritize human-relevant data. Studies that were published in English language only were considered, excluding all those studies which were not published in English language or could not be translated into English language. The studies for which full-text could not be retrieved were not considered, ensuring accessibility and comprehensibility. These stringent criteria were implemented to uphold the methodological rigor of the systematic review and provide a comprehensive and reliable synthesis of evidence on ivabradine's role in treating CJET.
The data screening process was divided in two phases. In the first phase, titles and abstracts of the selected studies were independently screened by two reviewers. In the second phase, a thorough evaluation of the full texts of studies that passed phase one was done to ensure their compliance with the eligibility criteria. Any disagreements between reviewers were resolved through discussion. A third reviewer was consulted where necessary. Key data, including the author details, study year, study design, patient characteristics, ivabradine dosage, and treatment outcomes, were systematically extracted using a predefined data extraction sheet in Microsoft Excel.
A narrative synthesis approach was employed to address the anticipated heterogeneity in study designs and outcome measures. Key themes and patterns regarding the efficacy and safety of ivabradine in CJET treatment were identified and systematically analyzed. This approach provides a comprehensive and transparent evaluation of the existing literature. The rigorous methodology provides a solid foundation for systematically reviewing and synthesizing evidence on ivabradine in the treatment of CJET.
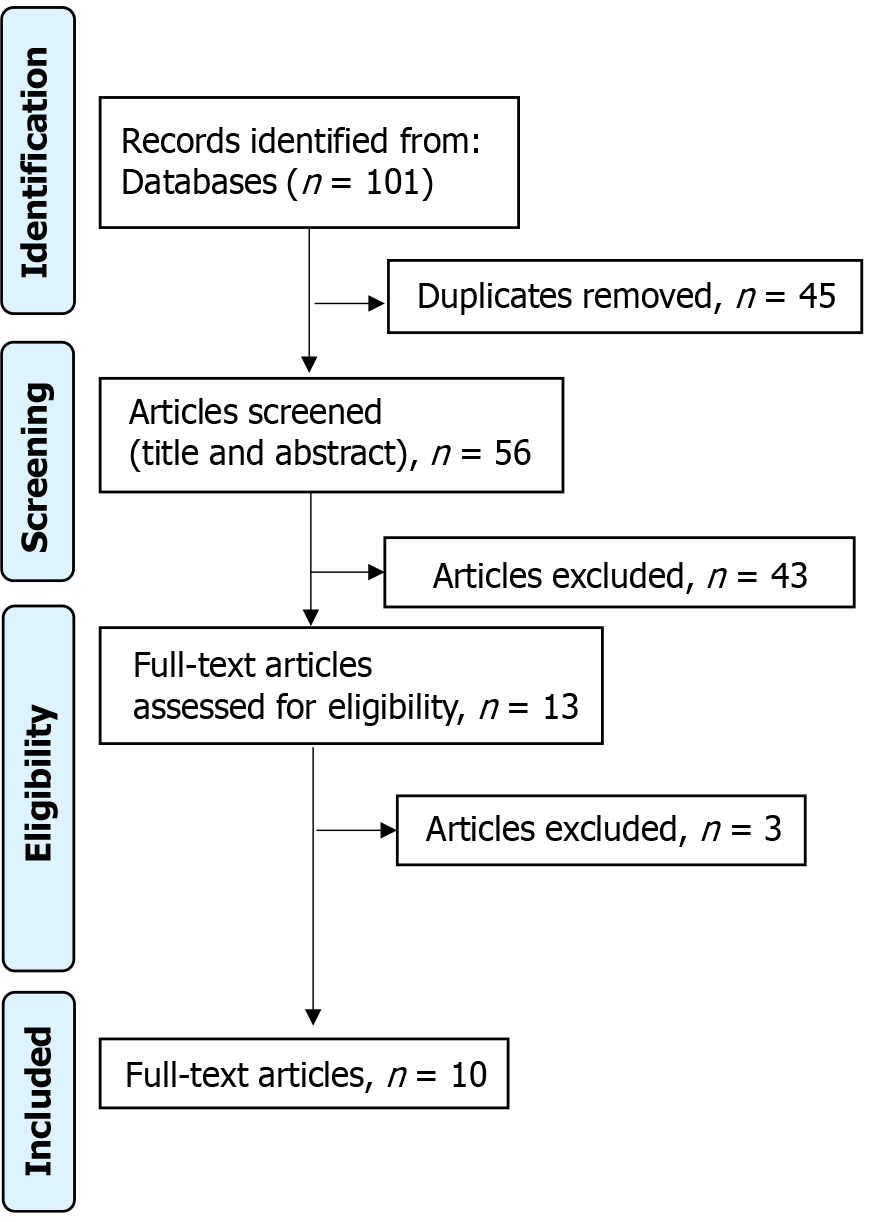
The study selection process for this review was carried out meticulously to ensure transparency following the PRISMA (2020) guidelines. A thorough search of the included databases yielded 101 studies. After removing 45 duplicates, a refined pool of 56 unique studies was obtained. Screening of titles and abstracts of these studies resulted in exclusion of 43 studies that did not meet predefined eligibility criteria. Subsequent evaluation of the full texts of the remaining 13 articles resulted in the exclusion of three studies that did not align with stringent inclusion criteria. Following this rigorous selection process, 10 studies were identified as suitable for inclusion in the systematic review. The detailed illustration of this study selection process is provided in the PRISMA flowchart (Figure 1).
The study characteristics of studies included in this systematic review are summarized in Table 1. All the included studies were case reports or case series, with only one cohort study. The number of patients ranged from a single case to a maximum of five cases in the cohort. The age of the patients varied widely, from preterm neonates as young as 31-36 weeks of gestation to children up to 3 years old. However, most cases involved infants younger than 6 months, which is consistent with the typical age of presentation for CJET. In terms of gender distribution, the incidence was equal in both males and females, nine cases each (Table 1).
| Ref. | Year of publication | Journal | Study design | No. of patients and gender | Age |
| Upreti et al[16] | 2023 | Nepalese Heart Journal | Case report | N = 1; male | 6 months |
| Devaprasath et al[9] | 2022 | Annals of Pediatric Cardiology | Case report | N = 1; female | 34 weeks of gestation, Preterm neonate |
| Asfour et al[10] | 2021 | The Journal of Pediatric Pharmacology and Therapeutics | Case report | N = 1; male | 31 weeks of gestation, preterm neonate; age of 19 days, tachycardia developed |
| Di Marco et al[8] | 2021 | Pediatric Reports | Case report | N = 1; female | 1 year |
| Ríos et al[17] | 2020 | Medicine | Case series | N = 2; male | 36 week of gestation (preterm neonate), 12 days old |
| Michel et al[15] | 2020 | Frontiers in Pediatrics | Case report | N = 1; female | 1 year |
| Kothari et al[13] | 2018 | Annals of Pediatric Cardiology | Case series | N = 2; 1 female, 1 male | 2 months, 2 years old |
| Ergul et al[18] | 2018 | Pacing and Clinical Electrophysiology | Case series | N = 3; 2 m ale, 1 female | 52 days to 10 months |
| Dieks et al[7] | 2016 | Heart Rhythm | Cohort | N = 5; 3 female, 2 male | 10 days to 3 years 6 months |
| Alghamdi et al[21] | 2012 | Journal of Cardiovascular Electrophysiology | Case report | N = 1; female | 3 years |
The quality of studies included in this review was assessed using Joanna Briggs Institute (JBI) critical appraisal tools (Supplementary material). Out of the 6 included case reports, 4 were of high quality and 2 were of medium quality. All the three case series and the one included cohort was of high quality (Table 2).
The summary of main findings of included studies is reported in Table 2. Regarding the dosage of ivabradine used, the majority of the studies administered a dose ranging from 0.05 to 0.1 mg/kg/dose, given twice daily. However, some studies started with lower doses, such as 0.025 mg/kg/dose, and gradually increased the dosage based on response and tolerability. Before initiating ivabradine treatment, most patients had received various antiarrhythmic medications, including amiodarone, flecainide, propranolol, digoxin, esmolol, and metoprolol, either as monotherapy or in combination. In some cases, ivabradine was used as an adjunct to these medications, while in others, it was used as monotherapy after the failure or discontinuation of other antiarrhythmic drugs. The outcomes reported with ivabradine treatment were generally favorable, with most studies reporting successful HR control, conversion to sinus rhythm (SR), or conversion to a more stable junctional rhythm (JR). Importantly, none of the included studies reported significant adverse effects related to ivabradine administration, suggesting a favorable safety profile in the treatment of CJET (Table 3)[11-17].
| Ref. | Antiarrhythmic medication before ivabradine | Treatment with ivabradine (Dose) | Antiarrhythmic medication with ivabradine | Outcomes of treatment with ivabradine | Adverse effects following ivabradine administration | Recurrence |
| Upreti et al[16] | Adenosine, amiodarone | 0.1 mg/kg/dose twice a day | Amiodorane, monotherapy at discharge | SR, HR control | None | _ |
| Devaprasath et al[9] | Adenosine | 0.1 mg/kg/dose twice a day | None | SR, HR control, JR intermittent | None | Not noticed within 2 weeks of follow up |
| Asfour et al[10] | Propranolol, amiodarone | 0.05 mg/kg/dose twice a day, decreased to 0.04 mg/kg/dose twice a day, at discharge 008 mg/kg/d | Propranolol, amiodorane; monotherapy at 2nd week | SR, HR control | Bradycardia prior to monotherapy | Not noticed within 6 months of follow up |
| Di Marco et al[8] | Amiodarone, flecainide, propranolol | 0.25 mg/kg/day (with propranolol, amydarone), 0.3 mg/kg/day (with flecainide) | Propranolol, amiodorane, flecainide | HR control, SR (with flecainide) | None | _ |
| Ríos et al[17] | Amiodarone, propranolol, n = 2; flecainide, n = 1 | 0.05 mg/kg/dose, at discharge 0.1 mg/kg/day | Amiodorane, propranolol (discontinued later) | SR/JR, HR control | None | _ |
| Michel et al[15] | Adenosine, Flecainide, Amiodarone, Esmolol | 0.025 mg/kg/dose twice a day, increased to 0.05 mg/kg/dose | Esmolol (discontinued later), metprolol | HR control, SR/EAR | None | _ |
| Kothari et al[13] | Amiodarone, flecainide, n = 2; propranolol, enalapril, metoprolol, n = 1 | 0.05 mg/kg/dose twice a day | Amiodarone, flecainide, n = 2; propranolol, enalapril, metaprolol, n = 1 | SR, HR control | None | _ |
| Ergul et al[18] | Flecainide, amiodarone, digoxin, n = 1; Flecainide, amiodarone, propranolol, n = 2 | 0.1 mg/kg/day | Amiodarone, n = 1; Amiodarone, propranolol, flecainide, n = 2 | HR control, n = 3; SR control, n = 2; JR/SR control, n = 1 | None | _ |
| Dieks et al[7] | Amiodarone, n = 5; flecainide, n = 1; digoxin, n = 2 | 0.05-0.1 mg/kg/dose increased to 0.28 mg/kg/dose | Amidarone, n = 5; propranolol, amidarone, n = 2; digoxin, amiodarone, n = 1 | HR control, n = 5; SR, n = 2; JR/JET, n = 1; JR/SR, n = 1 | None | _ |
| Alghamdi et al[21] | Flecainide, sotalol, procainamide, amiodarone | 2.5 mg once daily | None | SR, HR control | None | _ |
CJET presents a formidable challenge in pediatric cardiology, particularly affecting infants and children. Traditional antiarrhythmics like intravenous adenosine and direct current cardioversion often prove ineffective due to CJET's underlying mechanism of enhanced and abnormal automaticity[2,18]. However, a promising adjunctive therapy, ivabradine, has emerged. By selectively inhibiting specific HCN4 channels responsible for enhanced automaticity, ivabradine offers a potential solution[9]. Studies have shown its efficacy in converting patients to SR or achieving good rate control when combined with conventional antiarrhythmics like amiodarone and beta-blockers[7]. Despite its success, ivabradine remains under-utilized as a first-line therapy, primarily reserved for cases refractory to standard medications[15,19]. Nevertheless, its potential to mitigate the devastating impact of CJET underscores the importance of ongoing research and clinical exploration in pediatric arrhythmia management.
Our systematic review of diverse investigations delves into the efficacy of ivabradine in CJET. Encompassing individuals of both genders and spanning an age range from as early as 31 weeks of gestation to as old as 3.5 years, these studies shed light on the potential of ivabradine as an additional treatment alongside various antiarrhythmic medications for managing CJET. A notable facet highlighted by this review is the myriad of antiarrhythmic medications employed concomitantly with ivabradine, including, adenosine, amiodarone, propranolol, flecainide, and others. This reflects the intricate nature of CJET management and signifies the demand for tailored therapeutic approaches. The amalgamation of ivabradine with these antiarrhythmic agents aims at achieving SR control and modulation of HR, pivotal objectives in the treatment of CJET and improving overall clinical prognosis.
The dosing regimens of ivabradine exhibited variance across different cases, ranging from 0.025 to 0.28 mg/kg/dose, with adjustments predicated on patient responsiveness and clinical exigencies[7,14]. This variability underscores the necessity of customizing treatment to individual patient profiles, taking into account parameters such as age, weight, comorbidities, and therapeutic responsiveness. Despite the variability in patient characteristics and treatment regimens, ivabradine consistently demonstrated potential in achieving HR control and promoting SR restoration when used alongside antiarrhythmic medications. For instance, in studies where ivabradine was coupled with adenosine and amiodarone, it engendered successful SR reinstatement and HR regulation, indicating its efficacy in managing CJET-associated arrhythmias[12,14]. Similarly, in instances involving propranolol, amiodarone, and flecainide, ivabradine synergized with antiarrhythmic therapy to achieve HR control and facilitate SR restoration, underscoring its potential as a valuable adjunctive therapy in CJET management[8,13,15,16].
As far as the mechanism of ivabradine is concerned, it manages tachycardia by selectively inhibiting the pacemaker or “funny” current (If) in the sinoatrial node, which is responsible for spontaneous depolarization and automaticity. By blocking the HCN4 channels, ivabradine reduces the slope of diastolic depolarization, thereby slowing the HR in a controlled, dose-dependent manner. Its unique use-dependent property makes it more effective at higher HRs, allowing targeted HR reduction without excessive bradycardia. Unlike beta-blockers or calcium channel blockers, ivabradine does not affect cardiac inotropy or systemic vascular resistance, minimizing side effects such as hypotension, fatigue, or reduced cardiac output, making it a well-tolerated option for HR control and can be used with as an adjunct therapy[20]. A study by Nedoshivin et al[20] found that ivabradine plus a beta-blocker effectively reduced HR, anginal symptoms, and nitrate use within a month, with continued benefits for up to four months with minimum number of side effects.
In certain cases of CJET, ivabradine monotherapy has demonstrated effectiveness. Devaprasath et al[9] documented successful SR and HR control in a preterm neonate female after adenosine therapy failure. Similarly, Alghamdi et al[21] reported a CJET case unresponsive to multiple antiarrhythmic medications. However, with ivabradine monotherapy, the patient achieved SR and HR control, indicating the potential efficacy of ivabradine as a standalone treatment for CJET, particularly in cases refractory to traditional therapies.
These findings collectively suggest that ivabradine holds promise as an effective adjunctive therapy for CJET, particularly in cases refractory to traditional antiarrhythmic medications such as adenosine. However, it is imperative to acknowledge the limitations of the existing evidence. The majority of studies are retrospective case reports or small case series, lacking robust control groups and standardized outcome measures. Additionally, the heterogeneity in patient populations, dosing regimens, and concomitant antiarrhythmic medications complicates the interpretation of results. Furthermore, the long-term safety profile of ivabradine in pediatric patients with CJET remains uncertain, with limited data on adverse effects and recurrence rates beyond short-term follow-up periods[9,10]. Future research endeavors should prioritize well-designed prospective studies with larger sample sizes and extended follow-up durations to evaluate the efficacy and safety of ivabradine in pediatric patients with CJET. Furthermore, comparative studies comparing ivabradine with conventional antiarrhythmic agents and evaluating its role as a first-line therapy are warranted to optimize treatment strategies for this challenging arrhythmia.
The management of CJET remains a formidable challenge, with traditional antiarrhythmic therapies often proving ineffective. This systematic review highlights the potential of ivabradine as a valuable addition to the therapeutic armamentarium for CJET. The included studies demonstrate the efficacy of ivabradine, either as monotherapy or in combination with conventional antiarrhythmic agents, in achieving HR control, conversion to SR, or stabilization of JR in CJET patients. Furthermore, no significant adverse effects were reported, suggesting a favorable safety profile. However, the existing evidence is limited by the small sample sizes, retrospective nature, and heterogeneity of the included studies. Larger, well-designed prospective studies are warranted to establish the optimal dosing regimens, long-term safety, and comparative efficacy of ivabradine against current standard therapies in the management of this challenging arrhythmia.
| 1. | Mildh L, Hiippala A, Rautiainen P, Pettilä V, Sairanen H, Happonen JM. Junctional ectopic tachycardia after surgery for congenital heart disease: incidence, risk factors and outcome. Eur J Cardiothorac Surg. 2011;39:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Kylat RI, Samson RA. Junctional ectopic tachycardia in infants and children. J Arrhythm. 2020;36:59-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Zampi JD, Hirsch JC, Gurney JG, Donohue JE, Yu S, LaPage MJ, Hanauer DA, Charpie JR. Junctional ectopic tachycardia after infant heart surgery: incidence and outcomes. Pediatr Cardiol. 2012;33:1362-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Collins KK, Van Hare GF, Kertesz NJ, Law IH, Bar-Cohen Y, Dubin AM, Etheridge SP, Berul CI, Avari JN, Tuzcu V, Sreeram N, Schaffer MS, Fournier A, Sanatani S, Snyder CS, Smith RT Jr, Arabia L, Hamilton R, Chun T, Liberman L, Kakavand B, Paul T, Tanel RE. Pediatric nonpost-operative junctional ectopic tachycardia medical management and interventional therapies. J Am Coll Cardiol. 2009;53:690-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Villain E, Vetter VL, Garcia JM, Herre J, Cifarelli A, Garson A Jr. Evolving concepts in the management of congenital junctional ectopic tachycardia. A multicenter study. Circulation. 1990;81:1544-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 121] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Dobrzynski H, Nikolski VP, Sambelashvili AT, Greener ID, Yamamoto M, Boyett MR, Efimov IR. Site of origin and molecular substrate of atrioventricular junctional rhythm in the rabbit heart. Circ Res. 2003;93:1102-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Dieks JK, Klehs S, Müller MJ, Paul T, Krause U. Adjunctive ivabradine in combination with amiodarone: A novel therapy for pediatric congenital junctional ectopic tachycardia. Heart Rhythm. 2016;13:1297-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Di Marco GM, De Nigris A, Pepe A, Pagano A, Di Nardo G, Tipo V. Ivabradine-Flecainide as Breakthrough Drug Combination for Congenital Junctional Ectopic Tachycardia: A Case Report and Literature Review. Pediatr Rep. 2021;13:624-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Devaprasath S, Buddhavarapu S, Mariam S, Krishna MR. Ivabradine monotherapy in congenital junctional ectopic tachycardia. Ann Pediatr Cardiol. 2022;15:61-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 10. | Asfour SS, Al-Omran KA, Alodhaidan NA, Asfour RS, Khalil TM, Al-Mouqdad MM. Ivabradine Monotherapy for the Treatment of Congenital Junctional Ectopic Tachycardia in a Premature Neonate. J Pediatr Pharmacol Ther. 2021;26:414-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | DiFrancesco D, Camm JA. Heart rate lowering by specific and selective I(f) current inhibition with ivabradine: a new therapeutic perspective in cardiovascular disease. Drugs. 2004;64:1757-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 295] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 12. | Ashraf M, Goyal A. Junctional Ectopic Tachycardia. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 13. | Kothari SS, Kidambi BR, Juneja R. Ivabradine for congenital junctional ectopic tachycardia in siblings. Ann Pediatr Cardiol. 2018;11:226-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Janson CM, Tan RB, Iyer VR, Vogel RL, Vetter VL, Shah MJ. Ivabradine for treatment of tachyarrhythmias in children and young adults. HeartRhythm Case Rep. 2019;5:333-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Michel H, Heißenhuber F, Wellmann S, Melter M, Gerling S. Ectopic Atrial Tachycardia in a 12-Month-Old Girl Treated With Ivabradine and Beta-Blocker, a Case Report. Front Pediatr. 2020;8:313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Upreti D, Shakya S, Gajurel RM, Shrestha H, Thapa S, Sharma M. Congenital Junctional Ectopic Tachycardia in a 6-month-old boy treated with Ivabradine: A Case Report. Nepalese Heart J. 2023;20:53-55. [DOI] [Full Text] |
| 17. | Ríos M, Chiesa P, Arhcilles S, Cuesta A, Moltedo JM. [Use of ivabradine for the treatment of congenital junctional ectopic tachycardia]. Medicina (B Aires). 2021;81:293-296. [PubMed] |
| 18. | Ergul Y, Ozturk E, Ozgur S, Ozyurt A, Cilsal E, Guzeltas A. Ivabradine is an effective antiarrhythmic therapy for congenital junctional ectopic tachycardia-induced cardiomyopathy during infancy: Case studies. Pacing Clin Electrophysiol. 2018;41:1372-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Koruth JS, Lala A, Pinney S, Reddy VY, Dukkipati SR. The Clinical Use of Ivabradine. J Am Coll Cardiol. 2017;70:1777-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 20. | Nedoshivin A, Petrova PTS, Karpov Y. Efficacy and Safety of Ivabradine in Combination with Beta-Blockers in Patients with Stable Angina Pectoris: A Systematic Review and Meta-analysis. Adv Ther. 2022;39:4189-4204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Alghamdi B, Al-Kadi M, Alkhayal N, Alhedaithy R, Al Mahdi MJ. Intranasal lobular capillary hemangioma: A series of five cases. Respir Med Case Rep. 2020;30:101073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/