Published online Apr 26, 2025. doi: 10.4330/wjc.v17.i4.104168
Revised: March 14, 2025
Accepted: March 28, 2025
Published online: April 26, 2025
Processing time: 130 Days and 11.7 Hours
The management of severe symptomatic aortic stenosis has been revolutionized by transcatheter aortic valve replacement (TAVR), offering a minimally invasive alternative to surgical aortic valve replacement (SAVR). However, the compara
To compare the clinical outcomes and safety of TAVR vs SAVR in patients with severe symptomatic aortic stenosis.
A systematic review and meta-analysis were conducted according to PRISMA guidelines. Randomized controlled trials (RCTs) comparing TAVR and SAVR were identified from databases including PubMed, Scopus, and Web of Science up to May 31, 2024. Data were extracted on clinical outcomes, including mortality, procedural compli
A total of 10 RCTs were included. TAVR demonstrated a significantly lower risk of acute kidney injury (RR: 0.33; 95%CI: 0.25–0.44), major bleeding (RR: 0.37; 95%CI: 0.30–0.46), and new-onset atrial fibrillation (RR: 0.44; 95%CI: 0.34–0.57) compared to SAVR. However, TAVR was associated with higher risks of new permanent pacemaker implantation (RR: 3.49; 95%CI: 2.77–4.39), major vascular complications (RR: 2.47; 95%CI: 1.91–3.21), and paraval
TAVR offers a less invasive option with significant benefits in reducing acute kidney injury, major bleeding, and new-onset atrial fibrillation, making it particularly advantageous for high-risk surgical candidates. However, higher risks of permanent pacemaker implantation, vascular complications, and paravalvular leaks highlight the need for individualized patient selection and shared decision-making to optimize outcomes.
Core Tip: This systematic review and meta-analysis provide a comprehensive comparison of transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement in managing severe symptomatic aortic stenosis. TAVR demonstrated significant benefits, including reduced risks of acute kidney injury, major bleeding, and new-onset atrial fibrillation, particularly favoring high-risk surgical candidates. However, higher rates of permanent pacemaker implantation, vascular complications, and paravalvular leaks were observed with TAVR. Mortality and stroke rates were similar between interventions, underscoring the importance of individualized patient selection and shared decision-making to balance risks and optimize clinical outcomes.
- Citation: Moradi I, Mustafa MS, Sardar Sheikh J, Shojai Rahnama B, Fredericks M, Kumar Yennam A, Arain M, Saha U, Richard Ma A, Nagendran A, Bin Omer M, Armaghan M, Jaimes DCC, Avinash Bojanki NLSV, Shafique MA. Comparative effectiveness of transcatheter vs surgical aortic valve replacement: A systematic review and meta-analysis. World J Cardiol 2025; 17(4): 104168
- URL: https://www.wjgnet.com/1949-8462/full/v17/i4/104168.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i4.104168
The advent of transcatheter aortic valve replacement (TAVR) has transformed the treatment paradigm for patients with severe symptomatic aortic stenosis who are at a high risk for surgical complications and mortality. Initially targeted at high-risk patients, TAVR has demonstrated significant benefits, offering symptom relief and improved quality of life[1]. Although formal guidelines have not officially endorsed the use of TAVR as a first-line treatment for low-risk patients[2], several recent trials have reported promising early- and mid-term results in patients at lower risk levels. However, available evidence has shown that the incorporation of TAVR into shared decision-making (SDM) for low-risk patients with supravalvular aortic stenosis (SAS) is not very effective[3]. Decision aids have been used in SDM for patients with SAS, and evidence is available to show their importance in improving patient knowledge and satisfaction. Most of the patients, however, reported that the decision aids did not provide adequate information, which is a major problem barring the SDM process.
Recent studies have compared the outcomes of patients treated with TAVR and Surgical aortic valve replacement (SAVR). For example, Ahmad et al[4] examined death, stroke, and the composite of death or disabling stroke occurring at 1 year (early) or after 1 year (later) in patients undergoing TAVR and SAVR. They found that in lower-risk patients, there was an early reduction in mortality and the composite of death or disabling stroke with TAVR, but no differences after later follow-up[5]. These findings underline the need for a systematic review and meta-analysis that covers a broad range of outcomes and presents findings in ways that are directly applicable to SDM.
We also noted that recent systematic review and meta-analysis hardly perform extensive subgroup analyses with respect to the transcatheter aortic valve implantation (TAVI) approach itself, such as transfemoral access, regarding the level of surgical risk, and with regard to the type of valve used. These are key factors that are influenced by patient frailty, anatomic constraints that might influence the feasibility of transfemoral access, or by national or institutional policies governing valve choice. In addition, our review aimed to incorporate new evidence from studies of TAVR worldwide. The primary objective of this study was to produce user-friendly data with which patients and their healthcare providers could jointly make informed, individualized treatment decisions based on the specific benefits and risks which indi
A systematic literature search, adhering to Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines[6], was conducted to identify relevant original studies comparing transcatheter and SAVR from PubMed, ScienceDirect, Scopus, Web of Science, and Clinicaltrials.gov from Jan 1, 1990 until May 31, 2024. The systematic literature review was conducted by two independent investigators, utilizing a comprehensive search strategy that incorporated terms such as “transcatheter aortic valve”, “Surgical aortic valve”, “Aortic valve replacement”, and “Aortic Valve”. In addition to the database search, a manual review of the references cited in the included articles was performed to identify any additional relevant studies. No language restrictions were imposed in the selection of studies for inclusion in the analysis. The study followed the guidelines outlined in the PRISMA statement, ensuring accuracy and transpa
For inclusion in this study, eligible studies were restricted to randomized controlled trials (RCTs) that directly compared TAVR with SAVR. These studies were required to report effect estimates, including risk ratios (RRs) or hazard ratios, along with 95%CIs, or to provide sufficient raw data to allow for their calculation.
These studies were required to report certain comorbidities such as all-cause mortality, stroke, disabling stroke, cardiac death, myocardial infarction, new permanent pacemaker insertion, acute kidney injury, re-hospitalization, aortic valve reintervention, new atrial fibrillation, paravalvular leak, major bleeding, major vascular complications, and new-onset left bundle branch block. There were no restrictions on the size of the studies considered for inclusion.
A structured data collection form was utilized in an Excel sheet to systematically extract relevant information from each included study, ensuring comprehensive data collection. The extracted data covered multiple key aspects, including the study title, publication year, first author's name, study year, country of origin, participant count, type of intervention performed, and the outcome measures assessed. Data extraction was performed independently by four investigators to ensure accuracy. Subsequently, the lead investigator reviewed the data.
To assess the quality of the included RCTs, the Cochrane Risk of Bias tool was employed. Two independent reviewers evaluated the risk of bias for each study, considering key factors such as participant blinding, random sequence generation, outcome assessment, incomplete outcome data, and other potential sources of bias. Each study was assigned a risk of bias rating categorized as low, high, or unclear for each variable. Any disagreements between the reviewers were resolved through discussion with a third reviewer.
Data analysis was conducted using Review Manager 5.4 software, developed by the Cochrane Collaboration. For outcomes such as all-cause mortality, stroke, disabling stroke, cardiac death, myocardial infarction, new permanent pacemaker insertion, acute kidney injury, re-hospitalization, aortic valve reintervention, new atrial fibrillation, paraval
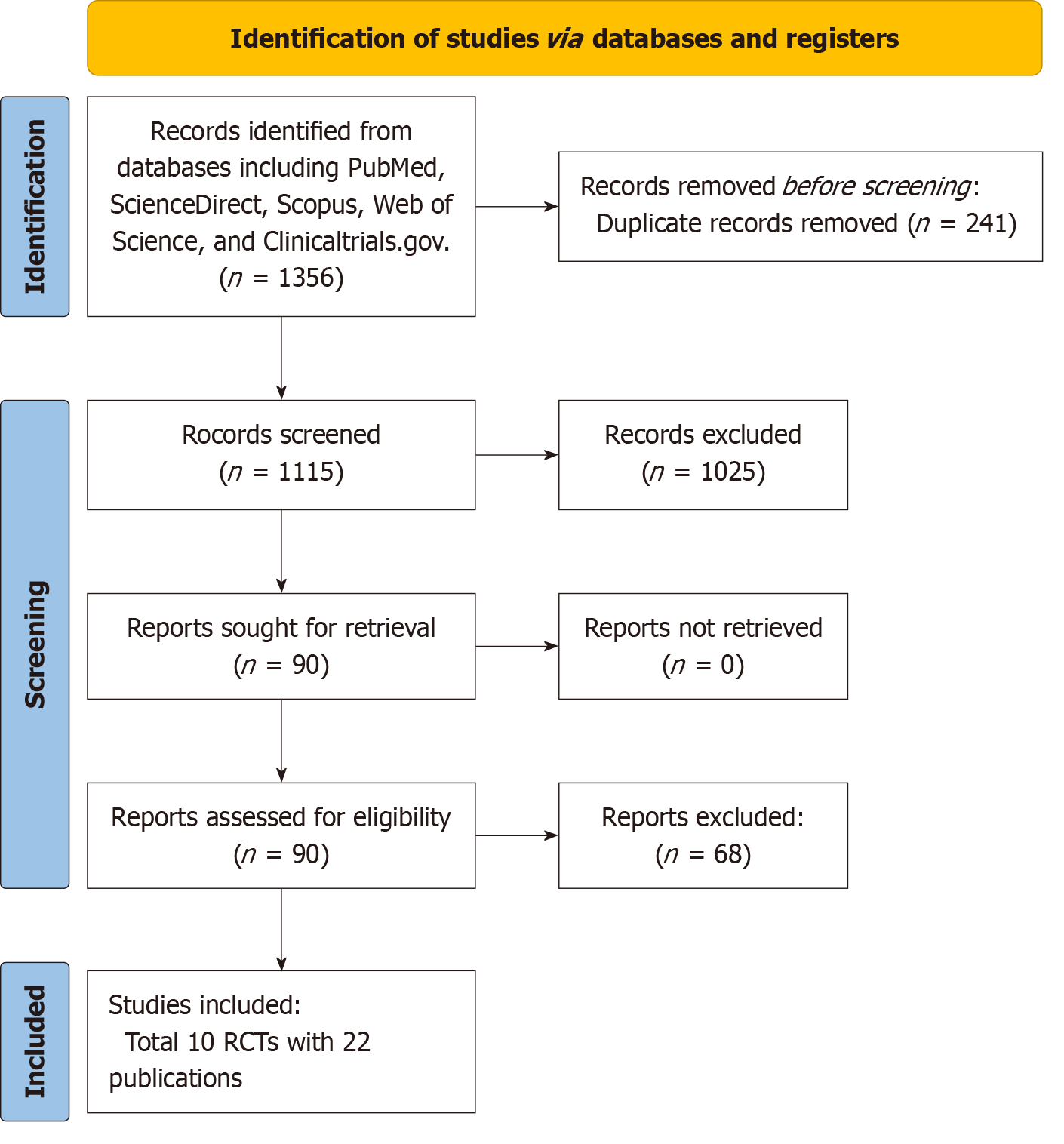
The study identification and selection process are depicted in Figure 1. Initially, 1356 records were identified from databases and registers, including PubMed, ScienceDirect, Scopus, Web of Science, and Clinicaltrials.gov. A total of 241 duplicate records were removed prior to screening. Initially, articles were excluded based on titles, which allowed for the elimination of case reports, reviews, and meta-analyses among other study types. Subsequently, articles were excluded based on their abstracts when they failed to align with the population, intervention, comparator, and outcome criteria were excluded based on their abstracts. After this initial screening, 1025 records were deemed unsuitable for this study, and 90 records were retrieved for a complete review. Of these, 68 reports were excluded because of the absence of the required population, intervention, or necessary outcomes. Ultimately, 10 RCTs among 22 publications[7-28] were in
The baseline characteristics of the studies included in this meta-analysis reveal a broad spectrum of patient populations and follow-up durations, enhancing the robustness and generalizability of the findings. The mean ages of participants ranged from 73 to 84 years, indicating that the studies primarily focused on older adults, which is typical for populations undergoing TAVR and SAVR. The total sample sizes varied widely, with the smallest study including 156 participants and the largest encompassing 2032 participants. Follow-up periods across the studies also varied, ranging from 1 year to 5 years. This variation provides a comprehensive view of both short-term and long-term outcomes, offering valuable insights into the durability and effectiveness of the treatments over time. The geographical locations of the studies were extensive, including the United States, Canada, Northern Europe, the United Kingdom, Germany, Australia, France, Japan, the Netherlands, and New Zealand. This wide geographical distribution ensures that the results are applicable to diverse healthcare settings and populations, adding to the external validity of the meta-analysis (Table 1).
| Trial name | Ref. | Year | Location | Total sample | Sample size (TAVR/SAVR) | Mean age (years) | Follow-up (years) | |
| PARTNER 1 | Smith et al[26] | 2011 | United States, Canada, and Germany | 699 | 348 | 351 | 84 ± 6.6 | 1 |
| Kodali et al[27] | 2012 | United States, Canada, and Germany | 699 | 348 | 351 | 84 ± 6.6 | 2 | |
| Mack et al[28] | 2015 | United States, Canada, and Germany | 699 | 348 | 351 | 84 ± 6.6 | 5 | |
| Medtronic Core Valve® United States Pivotal Trial | Adams et al[7] | 2014 | United states | 747 | 390 | 357 | 83.2 ± 6.7 | 1 |
| Reardon et al[8] | 2015 | 750 | 391 | 359 | 83.2 ± 6.7 | 2 | ||
| Deeb et al[9] | 2016 | 750 | 391 | 359 | 83.2 ± 6.7 | 3 | ||
| Arnold et al[10] | 2021 | 713 | 377 | 336 | 83.2 ± 6.7 | 5 | ||
| NOTION | Thyregod et al[18] | 2015 | Northern Europe | 280 | 145 | 135 | 79.1 ± 4.8 | 1 |
| Søndergaard et al[19] | 2016 | 280 | 142 | 134 | 79.1 ± 4.8 | 2 | ||
| Thyregod et al[20] | 2019 | 280 | 145 | 135 | 79.1 ± 4.8 | 5 | ||
| PARTNER 2 | Leon et al[11] | 2016 | United States, Canada | 2032 | 1011 | 1021 | 81.5 ± 6.7 | 2 |
| Makkar et al[12] | 2020 | 2032 | 1011 | 1021 | 81.5 ± 6.7 | 5 | ||
| Medtronic SURTAVI trial | Reardon et al[14] | 2017 | United States | 1660 | 863 | 794 | 79.8 ± 6.2 | 2 |
| PARTNER 3 | Mack et al[15] | 2019 | United States | 950 | 496 | 454 | 73 | 1 |
| Leon et al[16] | 2021 | 1,000 | 503 | 497 | 73 | 2 | ||
| Mack et al[17] | 2023 | 1000 | 503 | 497 | 73 | 5 | ||
| Evolut low-risk | Popma et al[23] | 2019 | Australia, Canada, France, Japan, the Netherlands, New Zealand, and the United States | 1403 | 725 | 678 | 73.9 ± 5.85 | 1 and 2 |
| Forrest et al[24] | 2022 | Australia, Canada, France, the Netherlands, New Zealand, Japan, and the United States | 1414 | 730 | 684 | 73.9 ± 5.85 | 2 | |
| Forrest et al[25] | 2023 | Australia, Canada, France, the Netherlands, New Zealand, Japan, and the United States | 1414 | 730 | 684 | 73.9 ± 5.85 | 3 | |
| UK TAVI | Toff et al[21] | 2022 | United Kingdom | 931 | 458 | 455 | 81 ± 3.7 | 1 |
| The VIVA trial | Rodés-Cabau et al[13] | 2024 | United States | 156 | 79 | 77 | 75.5 ± 5.1 | 1-5 |
| DEDICATE-DZHK6 | Blankenberg et al[22] | 2024 | Germany | 1414 | 701 | 713 | 74 ± 4 | 1 |
To assess the risk of bias in this meta-analysis, we adhered to rigorous methodologies and employed standardized tools to ensure the credibility and reliability of the findings. The risk of bias for each included study was evaluated using the Cochrane Collaboration Tool for assessing the risk of bias in randomized trials. This assessment scrutinized several domains, including random sequence generation, allocation concealment, blinding of participants and personnel, blin
Our analysis revealed a generally low risk of bias in the domains of random sequence generation and allocation concealment, indicating that the studies adequately randomized the participants and concealed the allocation sequence securely, thus minimizing the selection bias. However, we identified concerns regarding the blinding of participants and personnel in several studies. Given the nature of the compared interventions, complete blinding was not feasible in all trials, which could have led to performance bias.
Blinding of outcome assessment was achieved in most studies, particularly for objective outcomes such as mortality and hospital readmissions. However, for subjective outcomes, such as quality of life assessments, fewer studies have reported adequate blinding, raising concerns about detection bias.
Quantitative synthesis of the data was inspected for signs of reporting bias or data manipulation. Statistical heterogeneity was explored using I² statistics, and potential sources of heterogeneity were investigated considering the clinical and methodological differences among the studies.
This meta-analysis maintained a transparent approach for evaluating the risk of bias, ensuring that the conclusions drawn were based on evidence with a high degree of integrity. The findings should be interpreted considering the limitations of blinding and potential conflicts of interest, which could have influenced the overall risk of bias in the included studies. These assessments reinforce the importance of maintaining methodological rigor and transparency when conducting systematic reviews and meta-analyses.
Among the 10 studies included, 8 provided 1-year all-cause mortality data. A total of 3834 and 3613 patients underwent TAVR and SAVR, respectively (Figure 2A). At the one-year follow-up checkpoint, 295 and 370 individuals who received TAVR and SAVR, respectively, expired. TAVR was associated with lower 1-year all-cause mortality rates than SAVR, with moderate heterogeneity (RR: 0.74, 95%CI: 0.61-0.91, I2: 41%). Five studies provided 2-year all-cause mortality data. Rates of mortality at the two-year checkpoint were 637 out of 3421 individuals who received TAVR and 631 out of 3285 individuals who received SAVR. There were no significant all-cause mortality differences at 2-years between TAVR and SAVR with negligible heterogeneity (RR: 0.99, 95%CI: 0.89-1.10, I2: 8%). Two studies provided 3-year all-cause mortality data. There were no significant all-cause mortality differences at 3 years between TAVR and SAVR with negligible heterogeneity (RR: 0.85, 95%CI: 0.72-1.02, I2: 0%). Five studies provided 5-year all-cause mortality data. Rates of mortality at the 5-year checkpoint were 1124 out of 2377 individuals who received TAVR and 957 out of 2297 individuals who received SAVR. TAVR was associated with higher 5-year all-cause mortality rates than SAVR, with high heterogeneity (RR: 1.13, 95%CI: 1.01-1.26, I2: 57%). Eliminating any of the five studies from the analysis did not significantly change the odds ratio (OR). Most of the statistical heterogeneity was from the study by Arnold et al[10].
Nine, seven, two, and four studies provided 1-year, 2-year, 3-year, and 5-year cardiac mortality data, respectively. None of the follow-up periods demonstrated a significantly lower stroke rate using either method. The RR values are listed in Table 2. The rates of stroke in the 1-year follow-up period were largely heterogeneous (I2: 56%). Eliminating any one of the nine studies did not significantly change the heterogeneity or RR. The rates of stroke in the 3-year follow-up period were highly heterogeneous (I2: 69%), although eliminating studies did not change the analysis (Figure 2B).
| Outcome | Follow up period | Events/totals | Risk ratio (95%CI) | P value | |
| TAVR | SAVR | ||||
| All-cause mortality | 1 year | 295/3834 | 370/3613 | 0.74 (0.61-0.91) | 0.004 |
| 2 years | 637/3421 | 631/3285 | 0.99 (0.89-1.10) | 0.87 | |
| 3 years | 170/1121 | 185/1043 | 0.85 (0.72-1.02) | 0.08 | |
| 5 years | 1124/2377 | 951/2297 | 1.13 (1.01-1.26) | 0.03 | |
| Stroke | 1 year | 253/4845 | 274/4634 | 0.89 (0.67-1.19) | 0.44 |
| 2 years | 259/3566 | 266/3420 | 0.93 (0.75-1.16) | 0.54 | |
| 3 years | 98/1121 | 101/1043 | 0.90 (0.75-1.16) | 0.67 | |
| 5 years | 109/1380 | 115/1299 | 0.89 (0.69-1.14) | 0.43 | |
| Disabling stroke | 1 year | 127/3814 | 157/3688 | 0.77 (0.53-1.13) | 0.05 |
| 2 years | 91/2692 | 115/2575 | 0.72 (0.48-1.08) | 0.11 | |
| 5 years | 98/1507 | 86/1475 | 1.11 (0.84-1.47) | 0.45 | |
| Cardiac mortality | 1 year | 225/3914 | 248/3804 | 0.87 (0.70-1.10) | 0.24 |
| 2 years | 320/3566 | 331/3420 | 0.94 (0.81-1.08) | 0.38 | |
| 3 years | 112/1121 | 121/1043 | 0.86 (0.68-1.09) | 0.21 | |
| 5 years | 448/2000 | 398/1961 | 1.13 (1.01-1.27) | 0.04 | |
| Myocardial infarction | 1 year | 74/4346 | 81/4156 | 0.88 (0.64-1.21) | 0.42 |
| 2 years | 89/3566 | 87/3420 | 1.00 (0.75-1.35) | 0.98 | |
| 3 years | 33/1121 | 23/1043 | 1.33 (0.79-2.26) | 0.28 | |
| 5 years | 110/2000 | 99/1961 | 0.87 (0.48-1.59) | 0.04 | |
| New permanent pacemaker insertion | 1 year | 483/3482 | 245/3360 | 2.03 (1.41-2.94) | 0.0002 |
| 2 years | 681/3566 | 274/3420 | 2.45 (1.54-3.90) | 0.0002 | |
| 3 years | 264/1121 | 104/1043 | |||
| 2.33 (1.83-2.98) | < 0.00001 | ||||
| 5 years | 287/2000 | 189/1961 | 1.72 (1.03-2.88) | 0.04 | |
| Acute kidney injury | 1 year | 98/3027 | 165/2929 | 0.54 (0.34-0.86) | 0.009 |
| 2 years | 95/2614 | 470/2527 | 0.35 (0.09-1.39) | < 0.00001 | |
| 5 years | 24/348 | 24/351 | 1.01 (0.58-1.74) | 0.98 | |
| Hospitalization | 1 year | 297/2492 | 293/2437 | 0.98 (0.76-1.27) | 0.91 |
| 2 years | 424/3030 | 356/2926 | 1.14 (0.95-1.37) | 0.15 | |
| 5 years | 454/1855 | 364/1825 | 1.16 (0.87-1.55) | 0.31 | |
| Aortic valve re-intervention | 1 year | 53/3856 | 17/3694 | 2.68 (1.54-4.64) | 0.0005 |
| 2 years | 53/3073 | 18/2934 | 2.58 (1.40-4.73) | 0.002 | |
| 3 years | 16/1121 | 7/1043 | 2.50 (0.33-18.84) | 0.37 | |
| 5 years | 36/1652 | 19/1610 | 1.89 (0.66-5.36) | 0.23 | |
| New atrial fibrillation | 1 year | 299/3444 | 686/3298 | 0.53 (0.31-0.89) | 0.02 |
| 2 years | 332/2488 | 846/2385 | 0.39 (0.30-0.49) | < 0.00001 | |
| 5 years | 196/1507 | 446/1475 | 0.40 (0.27-0.60) | < 0.00001 | |
| Paravalvular leak | 2 years | 23/1431 | 6/1397 | 3.62 (1.46-8.95) | 0.005 |
| Major bleeding | 1 year | 406/3485 | 945/3384 | 0.43 (0.30-0.62) | < 0.00001 |
| 2 years | 330/2410 | 385/2264 | 0.74 (0.52-1.04) | 0.08 | |
| Major vascular complication | 1 year | 296/3485 | 107/3384 | 3.07 (1.75-5.40) | < 0.0001 |
| 2 years | 232/3344 | 107/3211 | 2.39 (1.39-4.11) | 0.002 | |
Six, four, and two studies provided 1-year, 2-year, and 5-year data, respectively, on disabling stroke. None of the follow-up periods demonstrated a significant superiority of either method. Insignificant heterogeneity was observed in the 2-year and 5-year follow-up groups. The 1-year follow-up analysis showed large heterogeneity (I2: 55%). Elimination of the study by Smith et al[26] resulted in a statistically significant decrease in the rates of debilitating stroke in 1-year post-TAVR individuals. (RR: 0.71, 95%CI: 0.53-0.95, I2: 24%) (Figure 2C).
Seven, seven, two, and four studies provided 1-year, 2-year, 3-year, and 5-year cardiac mortality data, respectively. None of the follow-up periods demonstrated a significant superiority for either method, while all follow-up periods were highly statistically non-heterogeneous (Figure 2D). The RRs are listed in Table 2.
Eight, seven, two, and four studies provided 1-year, 2-year, 3-year, and 5-year myocardial infarct data, respectively. No statistically significant differences were noted for any of the follow-up periods. The RRs are listed in Table 2. There was insignificant statistical heterogeneity for the 1-year, 2-year, and 3-year follow-up periods, while a large statistical heterogeneity was noted for 5-year follow-up (I2: 65%). Elimination of either of the four studies did not alter the analysis (Figure 2E).
Among the 10 studies, 7 provided 1-year rates of new permanent pacemaker insertion. TAVR was associated with higher rates of new permanent pacemaker insertion than SAVR, with high heterogeneity (RR: 2.03, 95%CI: 1.41-2.94, I2: 81%) (Figure 2F). Elimination of each study did not alter the results of this analysis. Seven studies reported 2-year rates of new permanent pacemaker insertion. TAVR was associated with higher rates of new permanent pacemaker insertion than SAVR, with high heterogeneity (RR: 2.45, 95%CI: 1.54-3.90, I2: 89%). Elimination of each study did not alter the results of this analysis. Four studies provided 3-year rates of new permanent pacemaker insertion. TAVR was associated with higher rates of new permanent pacemaker insertion than SAVR, with high heterogeneity (RR: 1.72, 95%CI: 1.03-2.88, I2: 85%). The elimination of Mack et al[28], Mack et al[17], and Makkar et al[12] leads to the results of the analysis becoming statistically insignificant.
Six studies provided 1-year follow-up data on acute kidney injury. TAVR has significantly lower rates of acute kidney injury at 1-year. (RR: 0.54, 95%CI: 0.34-0.86, I2: 63%) We observed high heterogeneity in the data. Eliminating any of the six studies did not change the final analysis. The 2-year follow-up revealed significantly lower rates of acute kidney injury associated with TAVR (Figure 2G).
TAVR had lower hospitalization rates at the 1-year follow up (RR: 0.77, 95%CI: 0.53-1.13, I2: 55%), and 2- year follow up (OR: 0.72, 95%CI: 0.48-1.08, I2: 38%), though neither reached statistical significance (Figure 2H).
Six studies were analyzed for the 1-year follow-up period, and a significantly higher rate of aortic valve reintervention was required with SAVR (RR: 2.68, 95%CI: 1.54-4.64, I2: 0%). The 2-year follow-up period also showed a significantly higher need for aortic valve reintervention (RR: 2.58, 95%CI: 1.4-4.73, I2: 12%). Three-year and 5-year follow-up periods also showed a statistically insignificant association between SAVR and increased rates of aortic reintervention (Figure 2I).
The 1-year (RR: 0.53, 95%CI: 0.31-0.89, I2: 92%), 2-year (RR: 0.39, 95%CI: 0.30-0.49, I2: 73%), and 5-year (RR: 0.40, 95%CI: 0.27-0.60, I2: 83%) follow-up periods showed a decreased rate of new atrial fibrillation associated with TAVR, despite high heterogeneity at the 1-year, 2-year, and 5-year follow-ups. Elimination of Adam et al[7], Thyregod et al[18], and Leon et al[11] in the 1-year period led to the result of the analysis becoming statistically insignificant, whereas eliminating any of the studies included in the 2-year follow-up period did not change the results of the analysis (Figure 2J).
Forrest et al[24] and Blankenberg et al[22] were the two studies analyzed for 1–2-year follow-up. An increase in paraval
TAVR was associated with significantly lower rates of major bleeding in the 1-year follow-up period (RR: 0.43, 95%CI: 0.30-0.62, I2: 90%). Eliminating any studies did not alter the results of the analysis. TAVR was also associated with statistically insignificant lower rates of major bleeding during the 2-year follow-up period (RR: 0.74, 95%CI: 0.52-1.04, I2: 82%) (Figure 2L).
TAVR was associated with a statistically significant increased risk of major vascular complications for the 1-year (OR: 3.07, 95%CI: 1.75-5.40, I2: 80%) and 2-year follow-up periods (RR: 2.39, 95%CI: 1.39-4.1, I2: 78%). Seven studies were analyzed for 1-year follow-up, and five studies were analyzed for 2-year follow-up periods. Eliminating any of the studies did not change the 1-year or 2-year analysis results (Figure 2M).
This study is an updated, comprehensive comparison between the role of TAVI and SAVR in the treatment of severe aortic stenosis in high-, moderate-, and low-risk surgical patient populations. We incorporated new data from the VIVA and DEDICATE trials studying clinical outcomes in low-intermediate-risk populations, with pre-existing information, to provide more extensive insights into clinical outcome comparisons between TAVI and SAVR across different patient-risk populations. Overall, TAVR was associated with lower 1-year all-cause mortality rates than SAVR but showed higher 5-year mortality rates. Additionally, TAVR has been linked to a higher incidence of new permanent pacemaker insertions and major vascular complications. Conversely, TAVR was associated with lower rates of acute kidney injury, major bleeding, and new atrial fibrillation. Stroke and cardiac mortality rates were similar between TAVR and SAVR across various follow-up periods. Disabling stroke rates did not show significant differences between the two methods at the 1-year, 2-year, and 5-year follow-ups. Myocardial infarction rates were also comparable between the TAVR and SAVR groups at all follow-up periods. TAVR patients have a lower risk of acute kidney injury and major bleeding than SAVR patients, highlighting the potential benefits in these areas. Rehospitalization rates were slightly lower with TAVR, and there was a decreased incidence of new atrial fibrillation. However, TAVR is associated with higher rates of major vascular complications and greater need for permanent pacemaker insertion.
Previous studies, such as Ahmad et al[4], did not identify any statistically significant differences in the main post-procedure outcomes, such as all-cause mortality, stroke, death, or debilitating stroke, when comparing TAVI and SAVR in low-risk populations owing to insufficient data. Our study bridges this knowledge gap. Compared with other mana
There was high heterogeneity in multiple studies across various follow-up periods. Sensitivity analysis revealed that Arnold et al[10] was the primary source for high heterogeneity in the 5-year follow-up for all-cause mortality. The high heterogeneity in other results likely arises from clinical and procedural variations across studies, as well as differences in study design and quality. Except for the aforementioned study, no single study significantly influenced the outcomes, suggesting a robust yet diverse dataset.
TAVI has been shown to be superior to medical management in patients with severe AS who are poor surgical candidates and non-inferior to SAVR in high-, intermediate-, and low-risk patient populations at the 5-, 5 and 2 years follow up, respectively[37]. Acute complications such as acute kidney injury, rehospitalization, new-onset atrial fibrillation, and valve reintervention were more commonly observed in SAVR, leading to lower short-term all-cause mortality benefits. TAVI due to procedure-related conduction disturbances (new-onset left bundle-branch block and advanced atrioventricular block) requiring pacemaker insertion and a high risk of paravalvular regurgitation may lead to poorer long-term mortality outcomes[38]. Further development of newer generations of prosthetic valves may result in a lower risk of paravalvular leaks[39].
With TAVI being an economically comparable and increasingly cost-effective alternative to SAVR, physicians must carefully consider treatment approaches considering patient-centered characteristics[40,41]. Treatment decisions should be made based on a number of factors, including patient age, life expectancy, multimorbidity, aortic valve architecture and dimensions, degree of valve calcification, prosthetic valve durability, practicality of vascular access (transfemoral vs transapical), disability, frailty, and cognition. The deciding factor between the two should not solely focus on long-term survival, as life expectancy is a labile parameter in the geriatric population, even in the absence of disease. These factors should also be discussed with patients to promote informed and transparent decision-making.
This study has several limitations that should be acknowledged. First, the analysis did not account for several patient-specific factors that may influence the choice and outcomes of TAVR vs SAVR. These include comorbidities such as smoking, hypertension, hyperlipidemia, and diabetes, as well as demographic factors such as age and gender. These variables are known to impact surgical decision-making and postoperative outcomes, and their exclusion may limit the generalizability of our findings. For instance, smoking has been associated with worse long-term health status after TAVR, although its impact on short-term outcomes is less clear[42]. Diabetes has been linked to increased mortality after SAVR but not TAVR[43]. Similarly, older age and female sex have been associated with different outcomes after TAVR, with women generally experiencing better long-term survival but higher rates of bleeding complications[44]. Future studies should incorporate these factors into subgroup analyses to provide more personalized insights into the optimal treatment strategy for severe aortic stenosis.
Additionally, the study was limited by non-uniformity in the data provided by different trials. Variations in methodo
In conclusion, TAVR represents a transformative advancement in the management of severe symptomatic aortic stenosis, offering a less invasive alternative that is particularly beneficial for patients deemed at high surgical risk. Our comprehensive meta-analysis has highlighted TAVR's robust efficacy in reducing short-term mortality and improving various clinical outcomes compared to SAVR. While TAVR shows clear advantages in terms of lower rates of acute kidney injury, major bleeding, and new atrial fibrillation, it also presents challenges such as higher 5-year mortality rates and increased procedural complexities such as new permanent pacemaker insertion and major vascular complications. These findings underscore the critical importance of SDM that considers individual patient characteristics and preferences, thereby facilitating informed choices and optimizing clinical outcomes in the complex landscape of treating severe aortic stenosis.
| 1. | Wang D, Huang L, Zhang Y, Cheng Z, Zhang X, Ren P, Hong Q, Kang D. Transcatheter aortic valve implantation versus surgical aortic valve replacement for treatment of severe aortic stenosis: comparison of results from randomized controlled trials and real-world data. Braz J Cardiovasc Surg. 2020;35:346-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Bonow RO, Brown AS, Gillam LD, Kapadia SR, Kavinsky CJ, Lindman BR, Mack MJ, Thourani VH; Aortic Stenosis Rating Panel, Dehmer GJ, Bonow RO, Lindman BR, Beaver TM, Bradley SM, Carabello BA, Desai MY, George I, Green P, Holmes DR Jr, Johnston D, Leipsic J, Mick SL, Passeri JJ, Piana RN, Reichek N, Ruiz CE, Taub CC, Thomas JD, Turi ZG; Appropriate Use Criteria Task Force, Doherty JU, Dehmer GJ, Bailey SR, Bhave NM, Brown AS, Daugherty SL, Dean LS, Desai MY, Duvernoy CS, Gillam LD, Hendel RC, Kramer CM, Lindsay BD, Manning WJ, Mehrotra P, Patel MR, Sachdeva R, Wann LS, Winchester DE, Allen JM, Aortic Stenosis Writing Group. ACC/AATS/AHA/ASE/EACTS/HVS/SCA/SCAI/SCCT/SCMR/STS 2017 Appropriate Use Criteria for the Treatment of Patients With Severe Aortic Stenosis: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, European Association for Cardio-Thoracic Surgery, Heart Valve Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Soc Echocardiogr. 2018;31:117-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Coylewright M, O'Neill E, Sherman A, Gerling M, Adam K, Xu K, Grande SW, Dauerman HL, Dodge SE, Sobti NK, Saunders CH, Schott SL, Elwyn G, Durand MA. The Learning Curve for Shared Decision-making in Symptomatic Aortic Stenosis. JAMA Cardiol. 2020;5:442-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Ahmad Y, Howard JP, Arnold AD, Madhavan MV, Cook CM, Alu M, Mack MJ, Reardon MJ, Thourani VH, Kapadia S, Thyregod HGH, Sondergaard L, Jørgensen TH, Toff WD, Van Mieghem NM, Makkar RR, Forrest JK, Leon MB. Transcatheter versus surgical aortic valve replacement in lower-risk and higher-risk patients: a meta-analysis of randomized trials. Eur Heart J. 2023;44:836-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 74] [Reference Citation Analysis (0)] |
| 5. | Swift SL, Puehler T, Misso K, Lang SH, Forbes C, Kleijnen J, Danner M, Kuhn C, Haneya A, Seoudy H, Cremer J, Frey N, Lutter G, Wolff R, Scheibler F, Wehkamp K, Frank D. Transcatheter aortic valve implantation versus surgical aortic valve replacement in patients with severe aortic stenosis: a systematic review and meta-analysis. BMJ Open. 2021;11:e054222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51217] [Article Influence: 10243.4] [Reference Citation Analysis (2)] |
| 7. | Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J Jr, Kleiman NS, Chetcuti S, Heiser J, Merhi W, Zorn G, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte J, Maini B, Mumtaz M, Chenoweth S, Oh JK; U. S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1971] [Cited by in RCA: 2215] [Article Influence: 184.6] [Reference Citation Analysis (0)] |
| 8. | Reardon MJ, Adams DH, Kleiman NS, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Lee JS, Hermiller JB Jr, Chetcuti S, Heiser J, Merhi W, Zorn GL 3rd, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Maini B, Mumtaz M, Conte JV, Resar JR, Aharonian V, Pfeffer T, Oh JK, Qiao H, Popma JJ. 2-Year Outcomes in Patients Undergoing Surgical or Self-Expanding Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2015;66:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 345] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 9. | Deeb GM, Reardon MJ, Chetcuti S, Patel HJ, Grossman PM, Yakubov SJ, Kleiman NS, Coselli JS, Gleason TG, Lee JS, Hermiller JB Jr, Heiser J, Merhi W, Zorn GL 3rd, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Maini B, Mumtaz M, Conte J, Resar J, Aharonian V, Pfeffer T, Oh JK, Qiao H, Adams DH, Popma JJ; CoreValve US Clinical Investigators. 3-Year Outcomes in High-Risk Patients Who Underwent Surgical or Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2016;67:2565-2574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 287] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 10. | Arnold SV, Chinnakondepalli KM, Magnuson EA, Reardon MJ, Deeb GM, Gleason T, Yakubov SJ, Cohen DJ; CoreValve US Pivotal Trial Investigators. Five-Year Health Status After Self-expanding Transcatheter or Surgical Aortic Valve Replacement in High-risk Patients With Severe Aortic Stenosis. JAMA Cardiol. 2021;6:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (1)] |
| 11. | Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG; PARTNER 2 Investigators. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374:1609-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3232] [Cited by in RCA: 3972] [Article Influence: 397.2] [Reference Citation Analysis (3)] |
| 12. | Makkar RR, Thourani VH, Mack MJ, Kodali SK, Kapadia S, Webb JG, Yoon SH, Trento A, Svensson LG, Herrmann HC, Szeto WY, Miller DC, Satler L, Cohen DJ, Dewey TM, Babaliaros V, Williams MR, Kereiakes DJ, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Brown DL, Fearon WF, Russo MJ, Pibarot P, Hahn RT, Jaber WA, Rogers E, Xu K, Wheeler J, Alu MC, Smith CR, Leon MB; PARTNER 2 Investigators. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N Engl J Med. 2020;382:799-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 606] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 13. | Rodés-Cabau J, Ribeiro HB, Mohammadi S, Serra V, Al-Atassi T, Iñiguez A, Vilalta V, Nombela-Franco L, Sáez de Ibarra Sánchez JI, Auffret V, Forcillo J, Conradi L, Urena M, Moris C, Muñoz-Garcia A, Paradis JM, Dumont E, Kalavrouziotis D, Maria Pomerantzeff P, Rosa VEE, Pezzute Lopes M, Sureda C, Diaz VAJ, Giuliani C, Avvedimento M, Pelletier-Beaumont E, Pibarot P; VIVA (Transcatheter Aortic Valve Replacement Versus Surgical Aortic Valve Replacement for Treating Elderly Patients With Severe Aortic Stenosis and Small Aortic Annuli) Trial Investigators. Transcatheter or Surgical Aortic Valve Replacement in Patients With Severe Aortic Stenosis and Small Aortic Annulus: A Randomized Clinical Trial. Circulation. 2024;149:644-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 50] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 14. | Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, Chetcuti S, Gleason T, Heiser J, Lange R, Merhi W, Oh JK, Olsen PS, Piazza N, Williams M, Windecker S, Yakubov SJ, Grube E, Makkar R, Lee JS, Conte J, Vang E, Nguyen H, Chang Y, Mugglin AS, Serruys PW, Kappetein AP; SURTAVI Investigators. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2017;376:1321-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1824] [Cited by in RCA: 2301] [Article Influence: 255.7] [Reference Citation Analysis (0)] |
| 15. | Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG, Smith CR; PARTNER 3 Investigators. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019;380:1695-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3668] [Cited by in RCA: 3719] [Article Influence: 531.3] [Reference Citation Analysis (0)] |
| 16. | Leon MB, Mack MJ, Hahn RT, Thourani VH, Makkar R, Kodali SK, Alu MC, Madhavan MV, Chau KH, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Blanke P, Leipsic JA, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Herrmann HC, Szeto WY, Genereux P, Pershad A, Lu M, Webb JG, Smith CR, Pibarot P; PARTNER 3 Investigators. Outcomes 2 Years After Transcatheter Aortic Valve Replacement in Patients at Low Surgical Risk. J Am Coll Cardiol. 2021;77:1149-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 243] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 17. | Mack MJ, Leon MB, Thourani VH, Pibarot P, Hahn RT, Genereux P, Kodali SK, Kapadia SR, Cohen DJ, Pocock SJ, Lu M, White R, Szerlip M, Ternacle J, Malaisrie SC, Herrmann HC, Szeto WY, Russo MJ, Babaliaros V, Smith CR, Blanke P, Webb JG, Makkar R; PARTNER 3 Investigators. Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. N Engl J Med. 2023;389:1949-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 404] [Article Influence: 134.7] [Reference Citation Analysis (0)] |
| 18. | Thyregod HG, Steinbrüchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, Chang Y, Franzen OW, Engstrøm T, Clemmensen P, Hansen PB, Andersen LW, Olsen PS, Søndergaard L. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Valve Stenosis: 1-Year Results From the All-Comers NOTION Randomized Clinical Trial. J Am Coll Cardiol. 2015;65:2184-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 728] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 19. | Søndergaard L, Steinbrüchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, Ngo AT, Olsen NT, Chang Y, Franzen OW, Engstrøm T, Clemmensen P, Olsen PS, Thyregod HG. Two-Year Outcomes in Patients With Severe Aortic Valve Stenosis Randomized to Transcatheter Versus Surgical Aortic Valve Replacement: The All-Comers Nordic Aortic Valve Intervention Randomized Clinical Trial. Circ Cardiovasc Interv. 2016;9:e003665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 20. | Thyregod HGH, Ihlemann N, Jørgensen TH, Nissen H, Kjeldsen BJ, Petursson P, Chang Y, Franzen OW, Engstrøm T, Clemmensen P, Hansen PB, Andersen LW, Steinbruüchel DA, Olsen PS, Søndergaard L. Five-Year Clinical and Echocardiographic Outcomes From the NOTION Randomized Clinical Trial in Patients at Lower Surgical Risk. Circulation. 2019;139:2714-2723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 258] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 21. | Toff WD, Hildick-Smith D, Kovac J, Mullen MJ, Wendler O, Mansouri A, Rombach I, Abrams KR, Conroy SP, Flather MD, Gray AM, MacCarthy P, Monaghan MJ, Prendergast B, Ray S, Young CP, Crossman DC, Cleland JGF, de Belder MA, Ludman PF, Jones S, Densem CG, Tsui S, Kuduvalli M, Mills JD, Banning AP, Sayeed R, Hasan R, Fraser DGW, Trivedi U, Davies SW, Duncan A, Curzen N, Ohri SK, Malkin CJ, Kaul P, Muir DF, Owens WA, Uren NG, Pessotto R, Kennon S, Awad WI, Khogali SS, Matuszewski M, Edwards RJ, Ramesh BC, Dalby M, Raja SG, Mariscalco G, Lloyd C, Cox ID, Redwood SR, Gunning MG, Ridley PD. Effect of Transcatheter Aortic Valve Implantation vs Surgical Aortic Valve Replacement on All-Cause Mortality in Patients With Aortic Stenosis: A Randomized Clinical Trial. JAMA. 2022;327:1875-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 132] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 22. | Blankenberg S, Seiffert M, Vonthein R, Baumgartner H, Bleiziffer S, Borger MA, Choi YH, Clemmensen P, Cremer J, Czerny M, Diercks N, Eitel I, Ensminger S, Frank D, Frey N, Hagendorff A, Hagl C, Hamm C, Kappert U, Karck M, Kim WK, König IR, Krane M, Landmesser U, Linke A, Maier LS, Massberg S, Neumann FJ, Reichenspurner H, Rudolph TK, Schmid C, Thiele H, Twerenbold R, Walther T, Westermann D, Xhepa E, Ziegler A, Falk V; DEDICATE-DZHK6 Trial Investigators. Transcatheter or Surgical Treatment of Aortic-Valve Stenosis. N Engl J Med. 2024;390:1572-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 143] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 23. | Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, Askew J, Sorajja P, Rovin J, Chetcuti SJ, Adams DH, Teirstein PS, Zorn GL 3rd, Forrest JK, Tchétché D, Resar J, Walton A, Piazza N, Ramlawi B, Robinson N, Petrossian G, Gleason TG, Oh JK, Boulware MJ, Qiao H, Mugglin AS, Reardon MJ; Evolut Low Risk Trial Investigators. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med. 2019;380:1706-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2802] [Cited by in RCA: 2831] [Article Influence: 404.4] [Reference Citation Analysis (0)] |
| 24. | Forrest JK, Deeb GM, Yakubov SJ, Rovin JD, Mumtaz M, Gada H, O'Hair D, Bajwa T, Sorajja P, Heiser JC, Merhi W, Mangi A, Spriggs DJ, Kleiman NS, Chetcuti SJ, Teirstein PS, Zorn GL 3rd, Tadros P, Tchétché D, Resar JR, Walton A, Gleason TG, Ramlawi B, Iskander A, Caputo R, Oh JK, Huang J, Reardon MJ. 2-Year Outcomes After Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients. J Am Coll Cardiol. 2022;79:882-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 25. | Forrest JK, Deeb GM, Yakubov SJ, Gada H, Mumtaz MA, Ramlawi B, Bajwa T, Teirstein PS, DeFrain M, Muppala M, Rutkin BJ, Chawla A, Jenson B, Chetcuti SJ, Stoler RC, Poulin MF, Khabbaz K, Levack M, Goel K, Tchétché D, Lam KY, Tonino PAL, Ito S, Oh JK, Huang J, Popma JJ, Kleiman N, Reardon MJ; Low Risk Trial Investigators. 3-Year Outcomes After Transcatheter or Surgical Aortic Valve Replacement in Low-Risk Patients With Aortic Stenosis. J Am Coll Cardiol. 2023;81:1663-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 135] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 26. | Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187-2198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4547] [Cited by in RCA: 5041] [Article Influence: 336.1] [Reference Citation Analysis (1)] |
| 27. | Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, Fontana GP, Dewey TM, Thourani VH, Pichard AD, Fischbein M, Szeto WY, Lim S, Greason KL, Teirstein PS, Malaisrie SC, Douglas PS, Hahn RT, Whisenant B, Zajarias A, Wang D, Akin JJ, Anderson WN, Leon MB; PARTNER Trial Investigators. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366:1686-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1741] [Cited by in RCA: 1789] [Article Influence: 127.8] [Reference Citation Analysis (13)] |
| 28. | Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, Webb JG, Douglas PS, Anderson WN, Blackstone EH, Kodali SK, Makkar RR, Fontana GP, Kapadia S, Bavaria J, Hahn RT, Thourani VH, Babaliaros V, Pichard A, Herrmann HC, Brown DL, Williams M, Akin J, Davidson MJ, Svensson LG; PARTNER 1 trial investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477-2484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1392] [Article Influence: 126.5] [Reference Citation Analysis (0)] |
| 29. | Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 3847] [Article Influence: 769.4] [Reference Citation Analysis (0)] |
| 30. | Morselli F, Mcnally R, Nesti L, Liu B, Khan H, Thomson RJ, Stevenson A, Banerjee A, Ahmad M, Hanif M, Steeds R, Khan M. Pharmacological interventions for the treatment of aortic root and heart valve disease. Cochrane Database Syst Rev. 2021;2021. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 31. | Bain ER, George B, Jafri SH, Rao RA, Sinha AK, Guglin ME. Outcomes in patients with aortic stenosis and severely reduced ejection fraction following surgical aortic valve replacement and transcatheter aortic valve replacement. J Cardiothorac Surg. 2024;19:258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 32. | Jilaihawi H, Williams M. Surgical Versus First-Generation Self-Expanding Transcatheter Aortic Valve Replacement: Is TAVR More Durable? J Am Coll Cardiol. 2018;72:2697-2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 33. | Gleason TG, Reardon MJ, Popma JJ, Deeb GM, Yakubov SJ, Lee JS, Kleiman NS, Chetcuti S, Hermiller JB Jr, Heiser J, Merhi W, Zorn GL 3rd, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte JV, Mumtaz M, Oh JK, Huang J, Adams DH; CoreValve U. S. Pivotal High Risk Trial Clinical Investigators. 5-Year Outcomes of Self-Expanding Transcatheter Versus Surgical Aortic Valve Replacement in High-Risk Patients. J Am Coll Cardiol. 2018;72:2687-2696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 337] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 34. | Kim M, Kang DY, Ahn JM, Kim JB, Yeung AC, Nishi T, Fearon WF, Cantey EP, Flaherty JD, Davidson CJ, Malaisrie SC, Kim HJ, Lee J, Park J, Kim H, Cho S, Choi Y, Park SJ, Park DW. Sex-Specific Disparities in Clinical Outcomes After Transcatheter Aortic Valve Replacement Among Different Racial Populations. JACC Asia. 2024;4:292-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 35. | Grigorios T, Stefanos D, Athanasios M, Ioanna K, Stylianos A, Periklis D, George H. Transcatheter versus surgical aortic valve replacement in severe, symptomatic aortic stenosis. J Geriatr Cardiol. 2018;15:76-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 36. | Lindman BR, Alexander KP, O'Gara PT, Afilalo J. Futility, benefit, and transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2014;7:707-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 37. | Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5086] [Cited by in RCA: 5632] [Article Influence: 352.0] [Reference Citation Analysis (1)] |
| 38. | Auffret V, Puri R, Urena M, Chamandi C, Rodriguez-Gabella T, Philippon F, Rodés-Cabau J. Conduction Disturbances After Transcatheter Aortic Valve Replacement: Current Status and Future Perspectives. Circulation. 2017;136:1049-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 445] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 39. | Forrest JK, Mangi AA, Popma JJ, Khabbaz K, Reardon MJ, Kleiman NS, Yakubov SJ, Watson D, Kodali S, George I, Tadros P, Zorn GL 3rd, Brown J, Kipperman R, Saul S, Qiao H, Oh JK, Williams MR. Early Outcomes With the Evolut PRO Repositionable Self-Expanding Transcatheter Aortic Valve With Pericardial Wrap. JACC Cardiovasc Interv. 2018;11:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 40. | Minutello RM, Wong SC, Swaminathan RV, Feldman DN, Kaple RK, Horn EM, Devereux RB, Salemi A, Sun X, Singh H, Bergman G, Kim LK. Costs and in-hospital outcomes of transcatheter aortic valve implantation versus surgical aortic valve replacement in commercial cases using a propensity score matched model. Am J Cardiol. 2015;115:1443-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Kermanshahchi J, Thind B, Davoodpour G, Hirsch M, Chen J, Reddy AJ, Chan E, Yu Z, Javidi D. A Review of the Cost Effectiveness of Transcatheter Aortic Valve Replacement (TAVR) Versus Surgical Aortic Valve Replacement (SAVR). Cureus. 2023;15:e46535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 42. | Qintar M, Li Z, Vemulapalli S, Chhatriwalla AK, Baron SJ, Kosinski AS, Saxon JT, Spertus JA, Cohen DJ, Arnold SV. Association of Smoking Status With Long-Term Mortality and Health Status After Transcatheter Aortic Valve Replacement: Insights From the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. J Am Heart Assoc. 2019;8:e011766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | D Souza A, Bsheish K, Dargham S, Jayyousi A, Al Suwaidi J, Abi Khalil C. Diabetes is associated with a higher incidence of short-term mortality risk and readmission in patients who undergo surgical but not transcatheter aortic valve replacement. Open Heart. 2025;12:e003019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Itchhaporia D. Transcatheter aortic valve replacement in women. Clin Cardiol. 2018;41:228-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/