Published online Apr 26, 2025. doi: 10.4330/wjc.v17.i4.102669
Revised: March 7, 2025
Accepted: March 28, 2025
Published online: April 26, 2025
Processing time: 178 Days and 16 Hours
The Perceval Sorin S (perceval valve) is a sutureless bioprosthetic designed for use in a high-risk cohort who may not be suitable for transcatheter aortic valve implantation or a conventional surgical aortic valve replacement (AVR).
To compare five-year post-operative outcomes in a cohort undergoing isolated AVR with the perceval valve to a contemporary cohort undergoing surgical AVR with a sutured bioprosthesis.
This study was a retrospective, cohort study at a single tertiary unit. Between 2017 and 2023, 982 suitable patients were identified. 174 Perceval valve replacements were matched to 174 sutured valve replacements. Cohort characteristics, intra-operative details, and post-operative outcomes were compared between the two groups.
Time under the aortic cross-clamp (P < 0.001), time on the cardiopulmonary bypass (P < 0.001) and total operative time (P < 0.001) were significantly reduced in the Perceval group. Patients in the Perceval valve group were at a lower risk of postoperative pneumonia [odds ratio (OR) = 0.53 (0.29-0.94)] and atrial fibrillation [OR = 0.58 (0.36-0.93)]. After propensity-matching, all-cause mortality did not significantly differ between the two groups in the five-year follow-up period. Larger valve sizes conferred an increased risk of mortality (P = 0.020).
Sutureless surgical AVR (SAVR) is a safe and efficient alternative to SAVR with a sutured bioprosthesis, and may confer a reduced risk of post-operative atrial fibrillation. Clinician tendency towards ‘oversizing’ sutureless aortic valves translates into adverse clinical outcomes. Less time on the cardiopulmonary bypass circuit allows for the treatment of otherwise high-risk patients.
Core Tip: The use of sutureless aortic valves has been well established in the past decade. However, its midterm outcomes are not fully established in comparison to standard surgical aortic valve replacements (AVR). With the advent of Transcatheter aortic valve insertions, the use of sutureless valves may play a major role especially post transcatheter aortic valve implantation explants. We look at the midterm outcomes compared to standard surgical AVR.
- Citation: French T, Avtaar Singh SS, Giordano V, Koutsogiannidis CP, Lim KHH, Pessotto R, Zamvar V. Sutureless aortic valve and post-operative atrial fibrillation: Five-year outcomes from a propensity matched cohort study. World J Cardiol 2025; 17(4): 102669
- URL: https://www.wjgnet.com/1949-8462/full/v17/i4/102669.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i4.102669
The prevalence of aortic valve disease is notable, particularly in patients aged over 65, in which calcific aortic valve degeneration over time results in the development of aortic stenosis – the second most common cause of valvular heart disease worldwide[1]. Developing an effective, definitive treatment for aortic valve pathologies, remains a priority in both developing and developed nations, and will only become increasingly important in a rapidly aging and increasingly wealthy global population.
One alternative to the traditional surgical method of aortic valve replacement (AVR) is the transcatheter aortic valve implantation (TAVI)[2]. Valves implanted in this manner are secured to the aortic annulus through radial force on surrounding tissues, and a TAVI approach to AVR negates the requirement for sutures and invasive thoracotomy or sternotomy[3]. Consequently, TAVI represents an appealing alternative to surgical AVR in the elderly, comorbid population[4].
There exists a population of high-risk patients with aortic valve pathology who might not be suitable for TAVI replacement, due to reasons such as tortuous coronary artery anatomy, difficult femoral or radial artery access, active endocarditis, or those requiring several concomitant cardiac surgical procedures[5].
In this select high-surgical risk group of patients for whom surgical valve replacement remains the preferred option, and the use of surgically implanted sutureless aortic valves may be a suitable option. These constitute a group of valves requiring few or no securing sutures between the aortic annulus and the valve frame. This allows for faster implantation of the prosthetic valve, and the associated benefits – reduced time on the cardiac bypass machine, reduced time spent under general anaesthetic – whilst retaining the benefits of the traditional surgical approach, including debridement of the calcified aortic annulus, and concomitant heart surgery, such as coronary artery bypass grafting (CABG)[6]. The practical benefit of this valve type, then, is that it allows for surgical valve replacement in a higher risk population, who might not have been suitable for TAVI due to tortuous coronary artery anatomy, or difficult femoral or radial artery access[5].
Unfortunately, due to the relatively recent introduction of the sutureless surgically implanted valve to commercial markets, there exists only a small body of literature investigating the medium term and long-term outcomes of sutureless valve replacement compared to sutured bioprostheses[7]. Recent multicentre randomised control trials include the CAVALIER trial[8], the PERCEVAL trial[9] and, more recently, the PERSIST-AVR trial[10], the pooled results of which suggest non-inferiority of sutureless AVR, but indicate a tendency towards postoperative thrombocytopaenia.
The objective of this study was to address this paucity of evidence by investigating immediate and mid-term outcomes in a large cohort of patients undergoing AVR with a sutureless aortic valve over a period of up to five years, and comparing these to outcomes of patients undergoing AVR with a sutured bioprosthesis.
We hypothesised that all-cause mortality would be higher in the group of patients undergoing AVR with a sutureless bioprosthesis than patients undergoing AVR with a traditional sutured bioprosthesis, due to higher rates of paravalvular leak and structural valve degeneration. The primary aim of this study was to test this hypothesis.
Secondary aims of the study were to compare the frequency of early mortality and common post-operative complications between the two groups. Post-operative complications were judged to be of interest due either to high post-operative frequency (e.g. atrial fibrillation) or perceived negative effect on patient outcomes (e.g. cardiac arrest).
The need for local NHS Research and Ethics Committee approval was evaluated with the NHS Health Research Authority toolkit, which deemed the study would be exempt from local committee review given the anonymized, retrospective nature of the study. Written consent was not sought from study participants.
A retrospective case-control design was used to compare patients who had undergone AVR with a perceval valve to who had undergone valve replacement with one of several subtypes of traditional sutured bioprosthesis over a period of five years.
Demographic details, operational details and data on post-operative patient course is routinely logged before, during and after operation for all patients undergoing AVR at the hospital site for use in the Central Cardiac Audit Database. The hospital database was searched for patients undergoing tissue AVR at the Edinburgh Royal Infirmary during the period March 1, 2017 to April 3, 2022. Participant records were prospectively updated with the study endpoint fixed at March 3, 2023.
A minimum sample size of 115 participants per arm was considered sufficient to prove non-inferiority of the sutureless valve based on a statistical power of 0.8, a presumed hazard ratio of 2.5, and an estimated two-year survival of 80%.
Demographic, inter-operative and post-operative data were compared both before and after propensity score matching, which enabled evaluation of the matching process, and some insight into the patient characteristics that might determine valve-type recommendations.
The study was reported according to the STROBE guidelines for case-control studies[11,12].
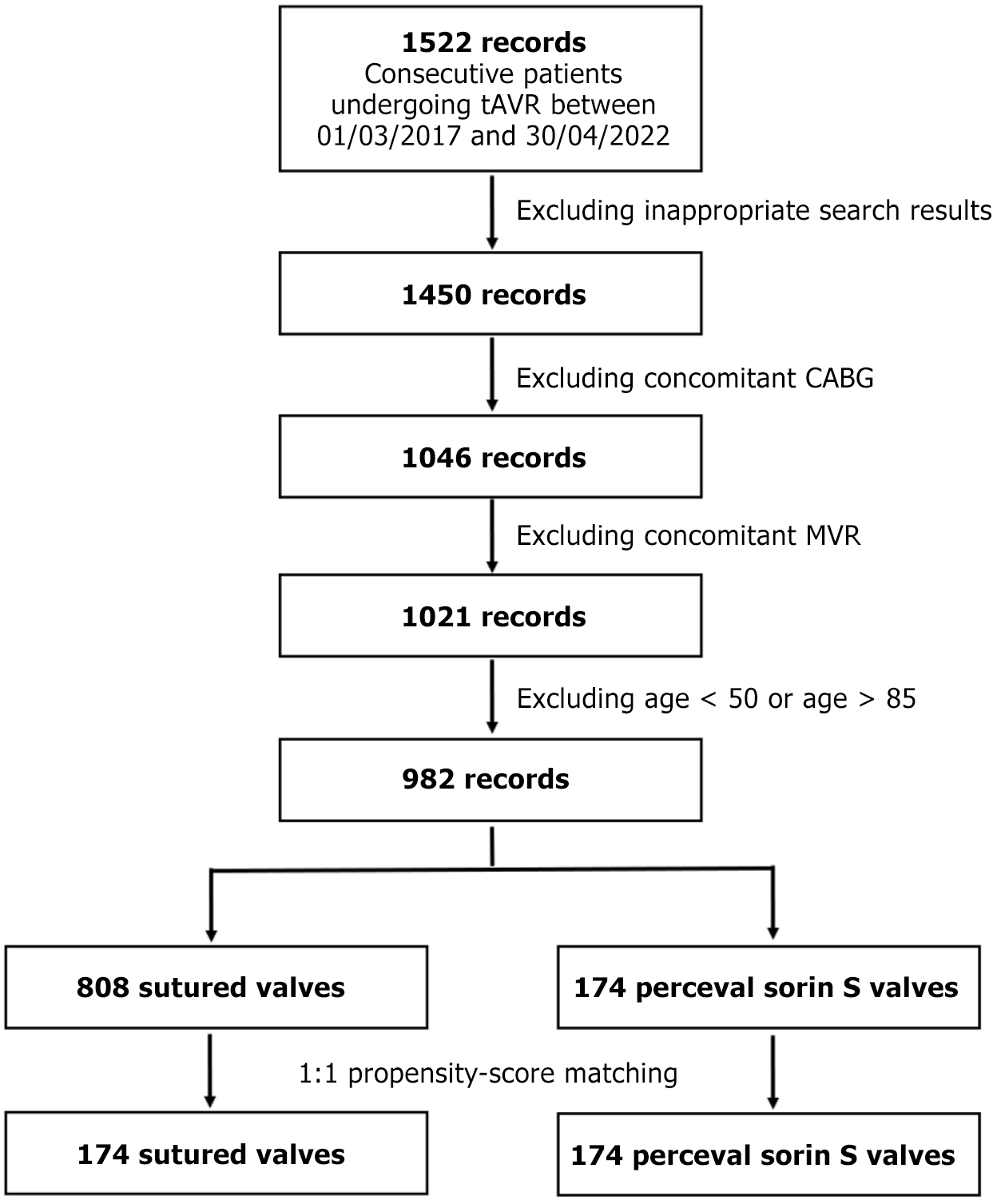
Inclusion criteria included consecutive patients from fifty to eighty-five years of age undergoing tissue AVR (tAVR)at the Edinburgh Royal Infirmary between the specified dates. Exclusion criteria included concomitant CABG and concomitant mitral valve repair (MVR). Numbers of individuals at each stage are summarized under Figure 1. Valve selection was determined by surgeon’s preference and familiarity.
The primary outcome in this study was all-cause mortality. In-hospital mortality was considered to mean patients who died before discharge home from any ward. Thirty-day mortality was defined as patients who died within thirty days of the operation, regardless of discharge status.
Secondary outcomes of interest were post-operative atrial fibrillation, cardiac arrest, pneumonia, complete heart block, delirium, myocardial infarction, permanent pace-maker (PPM) insertion, ventricular fibrillation, ventricular tachycardia, pneumothorax, stroke, time spent ventilated, time spent in the cardiac intensive care unit, total length of hospital stay, total time in theatre, total time spent ventilated, and time spent on cardiopulmonary bypass, and time spent under aortic cross-clamping. Total time in theatre was calculated from induction of anaesthesia to recorded surgery finish time. Total length of stay was calculated in hours, from the time and date of admission to the time and date of discharge to either home or bridging care.
Baseline characteristics of interest for patients undergoing elective surgery were reported in a pre-operative clinic, and included age, sex, gender, BMI, smoking status, diabetic status, pre-operative heart rhythm, angina status grouped according to the Canadian Cardiovascular Society guidelines[12], breathlessness status (defined by New York Heart Association of Heart Failure classification), hypertension status, left ventricular ejection fraction (LVEF) [measured through pre-operative echocardiography and classified into either good (LVEF > 50%), moderate (LVEF 31%-50%), poor (LVEF 21%-30%) or very poor (LVEF < 21%)], previous myocardial infarction, re-operative status, and Additive Euro
It was predicted that patients undergoing Perceval valve replacement would be older and more co-morbid than their tissue valve counterparts, and in order to address this treatment bias, propensity score matching was conducted on the cohort.
Continuous variables were assessed for normality visually by means of histograms. Normally distributed continuous variables between the two groups were represented as the group mean and 95%CI, and were compared with two-tailed, unpaired t-tests. Non-normally distributed continuous variables were represented as the group median and accompanying interquartile ranges, and were compared using the Wilcoxon rank-sum test. Categorical variables were reported as absolute frequencies, along with percentage frequency, and were compared with the χ² test.
Univariable logistic regression was used to identify seven patient characteristics (age, gender, additive Euroscore, NYHA status, diabetic status, hypertension and smoking status) potentially confounding valve choice (alpha value ≤ 0.25)[14]. These characteristics were included as confounders in the multivariable logistic regression equation used to generate propensity scores. Participants were matched using nearest neighbour-matching with calipers of width 0.2 of the standard deviation of the logit of the propensity score[15]. Participants were matched with 1:1 matching based on propensity score, resulting in 174 patients in the perceval valve being matched to 174 patients in the sutured group. Acceptable standardised mean difference between the two groups for all measured characteristics was considered to be < 0.1 following matching.
Propensity score matching was chosen to simulate randomisation of participants over alternative methods of adjusting for treatment bias such as propensity score stratification, inverse probability weighting, or traditional statistical controlling. Propensity score matching is frequently shown to achieve more accurate estimations of covariate balance than the aforementioned methods, and has been recommended for matching covariates in observational studies with a high proportion of ‘untreated’ participants[16,17].
A summary of demographic details prior to matching is visible in Supplementary Table 1, immediate post-operative outcomes (adjusted and unadjusted odds ratios) in Supplementary Table 2, and a summary of standardized mean differences and density plots for continuous variables are available in Supplementary Table 3, Supplementary Figures 1 and 2. Propensity score distributions before and after matching are visible in Supplementary Figure 3.
Unadjusted odds ratios for the rates of complication in the two groups were obtained using univariate logistic regression.
Survival analysis was performed, examining all-cause mortality in both patient groups. Records were considered censored in the event of either patient death or loss to follow-up. The results of time-to-event analysis were plotted in Kaplan-Meier survival charts, and the Log-rank test was used to compare all-cause mortality between the two groups. For all statistical tests except the specific example given above, an alpha value of 0.05 or less was considered statistically significant. All statistical testing was carried out using R Statistical Software (v4.3.2; R Core team 2023). The package ‘MatchIt’ was used for propensity score matching.
Missing data was treated as missing completely at random. Mode imputation for categorical variables and median imputation for continuous variables was used in propensity score generation. A sensitivity analysis was conducted, comparing results to those obtained after analysis on a ‘complete case’ basis. The detailed analysis can be seen in Supplementary Tables 4 and 5.
A search for patients undergoing tAVR for the specified dates yielded 1522 records. Excluding inappropriate valve types, concomitant CABG, concomitant MVR and individuals outside of the specified range resulted in 982 suitable records, of which 174 were in the Perceval group, and 808 were in the sutured group. 1:1 propensity score matching resulted in 174 records in both groups, resulting in a matched cohort of 348 patients. Patient selection is summarised in the flow diagram below (Figure 1).
The pre-operative characteristics of both treatment groups are presented in Table 1. Patients undergoing Perceval valve replacement were on average older than their sutured tissue valve counterparts [77 (74-80) vs 70 (64-76), P < 0.001] and had a higher median Additive Euroscore [5.00 (1.88-7.00) vs 6.00 (2.22-7.00), P = 0.003] suggestive of an increased pre-operative risk. A higher proportion of patients in the perceval group were hypertensive [126 (72.4) vs 511 (63.2), P = 0.022] and a higher proportion in the Perceval group were diabetic (P = 0.002). No significant difference in angina status, LVEF status or NYHA grade was measured between the two groups.
| Sutured (n = 808) | Perceval (n = 174) | P value | |
| Demographics | |||
| Age (n = 982) | 70 (64-76) | 77 (74-80) | < 0.001 |
| Gender (n = 982) | < 0.001 | ||
| Male | 520 (64.4) | 70 (40.2) | |
| Female | 288 (35.6) | 104 (59.8) | |
| BMI (n = 982) | 28.4 (25.0-32.4) | 29.0 (25.6-32.8) | 0.165 |
| Smoking status (n = 848) | 0.024 | ||
| Non-smoker | 334 (41.3) | 65 (37.4) | |
| Ex-smoker | 279 (34.5) | 76 (43.7) | |
| Current smoker | 86 (10.6) | 8 (4.6) | |
| Diabetes (n = 982) | 0.002 | ||
| No | 659 (81.6) | 124 (71.3) | |
| Diet | 38 (4.7) | 7 (4.0) | |
| Oral therapy | 79 (9.8) | 27 (15.5) | |
| Insulin | 32 (4.0) | 16 (9.2) | |
| NYHA grade (n = 879) | 0.148 | ||
| Grade I | 144 (17.8) | 21 (12.1) | |
| Grade II | 208 (25.7) | 37 (21.3) | |
| Grade III | 282 (34.9) | 69 (39.7) | |
| Grade IV | 93 (11.5) | 25 (14.4) | |
| Cardiovascular risk factors | |||
| Heart rhythm (n = 914) | 0.54 | ||
| Sinus rhythm | 607 (80.8) | 129 (79.1) | |
| Atrial fibrillation/flutter | 123 (16.4) | 32 (19.6) | |
| Complete heart block/pacing | 11 (1.5) | 2 (1.2) | |
| Other dysrhythmia | 9 (1.2) | 0 (0.0) | |
| Hypertension (n = 882) | 511 (63.2) | 126 (72.4) | 0.022 |
| CCS angina status (n = 858) | 0.863 | ||
| CCS0 | 422 (59.1) | 79 (54.9) | |
| CCS1 | 83 (11.6) | 19 (13.2) | |
| CCS2 | 95 (13.3) | 23 (16.0) | |
| CCS3 | 82 (11.5) | 16 (11.1) | |
| CCS4 | 32 (4.5) | 7 (4.9) | |
| LVEF (n = 681) | 0.578 | ||
| Good (> 50%) | 424 (75.7) | 92 (76.0) | |
| Moderate (21%-30%) | 109 (19.5) | 22 (18.2) | |
| Poor (31%-50%) | 22 (3.9) | 7 (5.8) | |
| Very poor (< 25%) | 5 (0.9) | 0 (0.0) | |
| Previous MI (n = 918) | 62 (8.2) | 12 (7.5) | 0.794 |
| Reoperation procedure (n = 982) | 42 (5.2) | 7 (4.0) | 0.518 |
| Risk scores | |||
| Additive Euroscore (n = 980) | 5.00 (1.88-7.00) | 6.00 (2.22-7.00) | 0.003 |
| Logistic Euroscore (n = 600) | 5.28 (3.29-8.56) | 7.01 (5.48-10.27) | < 0.001 |
Intra-operative details are presented in Table 2. After propensity score matching, patients in the Perceval group spent, on average, less time on the cardiopulmonary bypass circuit [60 (49-78) minutes vs 82 (69-110) minutes, P < 0.001], less time under aortic cross-clamping [46 (36-57) minutes vs 71 (56-91) minutes, P < 0.001], less time ventilated [9.9 (7.3-18) hours vs 14 hours (9.4-21), P = 0.005], and less total time in theatre [140 (120-170) minutes vs 170 (140-200) minutes, P < 0.001] (Supplementary Figures 4 and 5).
| Before propensity score matching | After propensity score matching | |||||
| Sutured | Perceval | P value | Sutured | Perceval | P value | |
| Bypass time (minutes) (n = 925) | 93.0 (72.0-123) | 60.0 (49.0-78.0) | < 0.001 | 87.0 (69.0-112) | 60.0 (49.0-78.0) | < 0.001 |
| Cross-clamp time (minutes) (n = 924) | 75.0 (58.0-99.0) | 45.5 (36.0-57.0) | < 0.001 | 71.0 (56.0-90.6) | 45.5 (36.0-57.0) | < 0.001 |
| Time Ventilated (hours) (n = 873) | 11.5 (7.6-19.0) | 9.9 (7.3-17.9) | 0.383 | 13.6 (9.35-20.5) | 9.88 (7.33-17.9) | 0.005 |
| Time in theatre (minutes) (n = 981) | 180 (153-215) | 139 (119-165) | < 0.001 | 171 (143-201) | 139 (119-165) | < 0.001 |
Immediate post-operative outcomes before and after matching are present in Table 3 and Supplementary Figure 6. There were no cases of intra-operative mortality in either treatment group. Rate of in-hospital mortality [1 (0.6) vs 2 (1.1), P = 0.562] and thirty-day mortality [4 (2.3) vs 3 (1.7), P = 0.703] did not significantly differ across the two groups, both before and after matching.
| Before propensity score matching | After propensity score matching | Odds ratio1 | |||||
| Sutured valves | Perceval valves | P value | Sutured valves | Perceval valves | P value | ||
| Intra-operative mortality | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) | 0 (0.0) | NA | NA |
| In-hospital mortality | 16 (2.0) | 1 (0.6) | 0.197 | 2 (1.1) | 1 (0.6) | 0.562 | 0.50 (0.04-5.55) |
| < 30 days mortality | 23 (2.8) | 3 (1.7) | 0.403 | 4 (2.3) | 3 (1.7) | 0.703 | 0.73 (0.16-3.35) |
| Length of CICU stay (days) (n = 917) | 1.14 (0.97-2.11) | 1.13 (0.98-1.91) | 0.543 | 1.25 (1.00-2.50) | 1.13 (0.96-2.00) | 0.132 | NA |
| Total length of stay (days) (n = 917) | 6.90 (5.31-9.99) | 7.08 (5.93-9.90) | 0.248 | 8 (6.00-11.0) | 7 (6.00-10.00) | 0.315 | NA |
| Post-operative complications | |||||||
| Atrial fibrillation | 229 (28.3) | 39 (22.4) | 0.111 | 58 (33.3) | 39 (22.4) | 0.023 | 0.57 (0.35-0.91) |
| Pneumonia | 152 (18.8) | 21 (12.1) | 0.030 | 36 (20.7) | 21 (12.1) | 0.030 | 0.49 (0.27-0.90) |
| Delirium | 100 (12.4) | 28 (16.1) | 0.187 | 27 (15.5) | 28 (16.1) | 0.883 | 1.03 (0.58-1.83) |
| MI | 1 (0.1) | 0 (0.0) | 0.642 | 0 (0.0) | 0 (0.0) | NA | NA |
| PPM | 19 (2.4) | 6 (3.4) | 0.405 | 4 (3.10) | 6 (3.4) | 0.521 | 1.50 (0.42-5.44) |
| VT/VF | 9 (1.1) | 0 (0.0) | 0.162 | 0 (0.0) | 0 (0.0) | NA | 0.94 (0.06-15.9) |
| Pneumothorax | 8 (1.0) | 1 (0.6) | 0.602 | 1 (0.6) | 1 (0.60) | 1.000 | 0.95 (0.06-15.89) |
| Stroke | 25 (3.1) | 6 (3.4) | 0.808 | 4 (2.3) | 6 (3.4) | 0.521 | 1.51 (0.42-5.47) |
After propensity score matching, rates of atrial fibrillation [39 (22.4) vs 58 (33.3), P = 0.023] and pneumonia [21 (12.1) vs 36 (20.7), P = 0.030] were significantly lower in the Perceval group compared to the sutured tissue valve group.
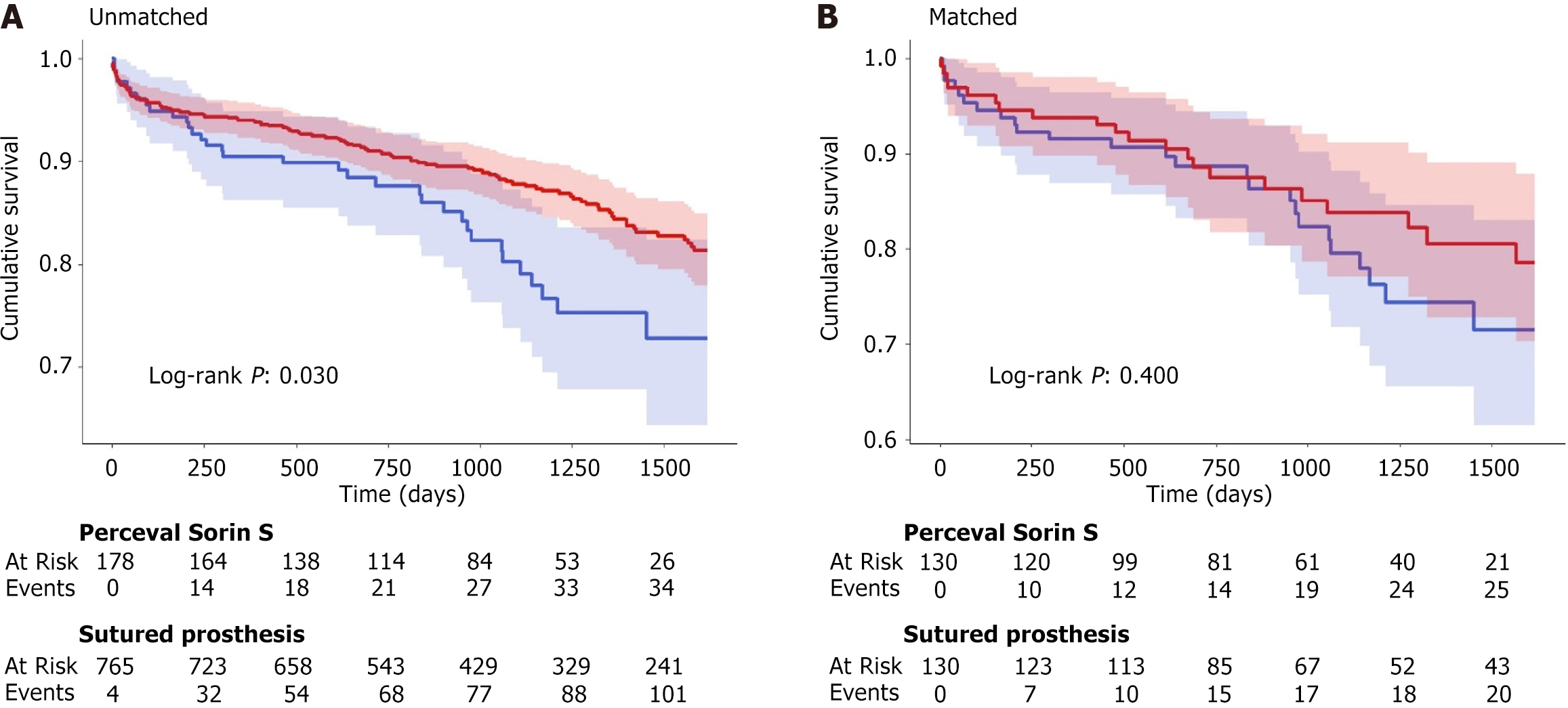
Survival curves in relation to all-cause mortality are shown in Figure 2. Survival curves with event tables are visible in Supplementary Figures 7 and 8.
Median follow-up time for the entire cohort was 3.05 years. Cohort survival at 1 year was 91.6% (95%CI: 88.7-94.5), 2 years was 88% (95%CI: 84.6-91.5) and 4 years was 73.8% (95%CI: 68.2-79.8).
Perceval group survival at 1 year was 90.8% (95%CI: 86.6-95.2). This dropped to 87.9% (95%CI: 83.1-93.1) at 2 years, and 72.3% (95%CI: 63.4-82.5) at four years.
After propensity matching, in the sutured valve group, 1-year, 2-year and 4-year survival rates were 95.4% (95%CI: 92.3-98.6), 91.6% (95%CI: 87.4-95.9) and 77.4% (95%CI: 69.6-86.2) respectively.
Prior to propensity score matching, post-operative survival rates were significantly lower in the Perceval valve group
The most common valve size implanted was medium [59 (34.1%)], followed by extra-large [48 (27.7%)], small [45 (26.0%)], and large [21 (12.1%)]. All-cause mortality was higher across the follow-up period in patients treated with large or extra-large valves compared to those treated with medium or small valves (log-rank P = 0.020) (Supplementary Figures 9 and 10).
Patients in the cohort treated with a sutureless aortic valve were significantly less likely to suffer from new onset post-operative atrial fibrillation. This is a new finding: Similar literature on this topic has shown comparable rates of atrial fibrillation on both perceval and stented groups[18,19], but not significantly reduced rates of atrial fibrillation in cohorts treated with a sutureless valve. Post-operative atrial fibrillation is associated with higher rates of rehospitalisation, mortality stroke, prolonged hospital stays and higher per-patient costs[20].
One potential explanation for this finding is the observed difference in times under cardiopulmonary bypass. Increased time on the cardiopulmonary bypass circuit confers a higher likelihood of increased oxidative stress, bypass-triggered inflammation and electrolyte imbalances – all factors conferring a higher likelihood of post-operative atrial fibrillation[21]. Use of a minimally invasive approach in implanting a sutureless aortic valve may result in reduced levels of key pro-inflammatory cytokines such as tumor necrosis factor alpha, interleukin (IL) -6 and IL-2 compared to a traditional surgical approach[20]. Plasma concentration of inflammatory cytokines has been suggested as a potential mediator of atrial fibrillation through direct action on the cardiac myocardium[22]. The incidence and effect on outcomes of postoperative atrial fibrillation is also dependent to some degree on treatment strategy – rapid correction with amiodarone and prophylactic postoperative beta-blockers may result in a lessened impact on outcomes.
The same mechanisms might account for the differences observed in rates of post-operative pneumonia between the two cohorts[23]. Prolonged time on cardiopulmonary bypass confers an increased risk of pulmonary compromise as a result of alveocapillary membrane injury, surfactant impairment, and mucociliary dysfunction[24,25]. Additionally, a thoracotomy or mini-sternotomy might minimise changes to the topology of the chest wall compared to a sternotomy, thus reducing the impact on ventilation of chest wall trauma from surgical incision[25].
The observed lower rates of post-operative atrial fibrillation, however, did not translate into reduced post-operative mortality or a significantly reduced hospital stay across the post-operative period in this study.
No significant difference in early mortality (in-hospital, intra-operative and less-than-thirty-day mortality) was elicited between the two groups both before and after matching, in-keeping with a 2023 meta-analysis by Colarossi et al[23] comparing sutureless and sutured valve replacement across 20 retrospective case-control studies, finding no significant difference in the aforementioned outcomes between the two groups.
As expected, the average patient undergoing Perceval valve replacement was older and had a higher surgical risk-score compared to their counterparts undergoing sutured valve replacement. This could account for the higher observed death rates in the Perceval group prior to matching.
Mortality rates in this study were comparable to the current literature on the topic. A 2023 meta-analysis by Jolliffe et al[24] examining mid to long-term outcomes across seven studies investigating long term outcomes for the Perceval valve alone showed comparable average 1 year (93.4%), 2 year (89.4%) and 4 year (82%) mortality rates[26,27].
Patients in the Perceval group spent a lower average time both on the cardiac bypass circuit and under aortic cross-clamping without a corresponding increased risk of mortality both in the immediate and long term. This is important from a patient safety perspective, as both cardiopulmonary bypass and aortic cross-clamping time are independent predictors of short-term and long-term mortality[28,29].
The reduction in time operational time and time on the bypass in particular, then, in patients treated with rapid deployment valves, is of particular benefit to older, more co-morbid patients that might not have been otherwise suitable for surgery, a fact of particular relevance to the United Kingdom population where the absolute numbers of people aged 65 years or older in England will increase by 48.6% between 2015-2035[30].
Reduced time spent on the bypass without a corresponding increase in mortality or side-effect profile is beneficial also from a cost-benefit perspective. AVR is an expensive and time-consuming operation[31]. Median operation times in both groups were well above three hours, but patients in the Perceval group spent significantly less time in theatre [210, interquartile range (IQR): 190-235] vs 248 (IQR: 220-290), P < 0.001]. Faster operations may help to reduce costs associated with performing the procedure, enable more operations in a given time period and reduce patient waiting lists for elective operations.
The observed difference in mortality rates by valve size is likely indicative of the tendency for clinicians to insert the largest possible valve that fits in the aortic annulus, as a means of avoiding patient-prosthesis mismatch. Currently, the Perceval valve is available in four sizes: Small (referring to an aortic annulus diameter of 19-21 mm), medium (21-23 mm), large (23-25 mm) and extra-large (aortic annulus diameter 25-27 mm). In contrast to TAVI–in which valve size is determined by non-invasive imaging such as transthoracic echocardiogram or ECG-gated multidetector computerized tomographic imaging, valve sizing in surgical AVR with a sutureless prosthetic is somewhat subjective, with a surgeon measuring the aortic annulus with a sizing device. Patient’s with a particularly small aortic annulus (i.e. less than or equal to 19mm), or a particular large aortic annulus (greater than 27 mm) may benefit from a sutured bioprosthesis instead of a sutureless device.
The expandable nature of the Perceval valve introduces the potential to ‘oversize’ a valve. A nitinol frame implanted into small an annulus may fail to expand suitably, resulting in recoil, crimped leaflets and a lower effective orifice area. Oversizing of the perceval valve results in higher post-operative transvalvular gradients and earlier structural degeneration of the valve[32]. Resulting pressure on the atrio-ventricular node may disrupt normal conductive function, and lead to higher rates of AV-block in patients treated with poorly sized sutureless aortic valve[33].
Clinician education as to the appropriate use of sizing obturators may mitigate this risk and result in further improved post-operative outcomes in patients treated with a sutureless valve. Further analysis investigating the immediate and long-term outcomes of patients treated with different sizes of valve stratified by patient body surface area and estimated aortic annulus size may suggest an optimal treatment strategy for those treated with this particular valve type – for example, the avoidance of sutureless AVR in patients with a particularly small body surface area.
Higher rates of heart block requiring PPM implantation and higher rates of stroke have been observed in patients undergoing SAVR with a perceval valve compared to a sutured valve, along with a propensity for thrombocytopaenia in the Perceval valve group[34].
However, no significant difference in the rate of PPM insertion or stroke was elicited between the two groups observed in this study.
The self-expanding design of surgically implanted sutureless valves such as the perceval valve has been suggested as a cause for the high observed rate of post-operative PPM. Notably, high rates of PPM implantation are observed in TAVI-treated patients. High calcium load on the left coronary cusps may shift a sutureless prosthetic towards the right coronary cusp (and therefore the bundle of His), predisposing to post-procedural complete heart block[33]. Implantation of a sutureless valve through a surgical technique allows for debridement of the aortic annulus, and this study results suggest that high grade AV block may not be an inevitable outcome of the expanding prosthesis model, but rather the implantation technique used.
With regards to stroke, it is important to mention the potential link between post-operative thrombocytopaenia and haemorrhagic stroke in perceval valve treated patients. Thrombocytopaenia is commonly observed in patients treated with the perceval valve[32], which may go someway towards explaining previously observed high rates of stroke.
Examining this relationship was felt to be outside the bounds of this study, as post-operative platelet count was not recorded routinely for the study population, but the link between the two post-operative complications is still poorly understood, and merits further exploration.
This study was limited by the fact that it was a retrospective study, and thus whilst able to identify correlations, was not able to conclusively identify causative links between variables. The propensity score matching method used to account for bias is in itself prone to bias – selecting inappropriate confounders or simply omitting important confounders can result in a poorly matched patient cohort. Although a systematic approach to choosing confounders for propensity score matching has been applied here, the loss of a significant difference in long-term survival between the two groups after propensity score matching could be an artefact of the matching process, and this result will need confirmation from a prospective trial involving true randomisation of participants. One example would be the choice of anticoagulation and antiplatelet agent was primarily driven by the presence of post-operative atrial fibrillation lasting for more than 48 hours. However, the patients who may have been on an anticoagulant pre-operatively would be resumed on this rather than be commenced on an antiplatelet agent.
Due to a lack of routinely recorded echocardiographic data, the authors were not able to comment on the estimated effective orifice area for individual patients, nor rates of post-operative paravalvular leak or valve embolism. We were also unable to control for echocardiographic data in the propensity-matching process. Reoperation was not considered as a competing risk for mortality.
Finally, this was a single-center study, and the findings observed here may not be generalisable to a wider population. Future prospective studies with a multi-centre design will be necessary to confirm the generalisability of the findings observed in this study.
This retrospective, single-center cohort study investigated all-cause mortality across five years following isolated AVR with either a surgically implanted sutureless valve or a sutured stented bioprosthesis. We note higher rates of all-cause mortality in those treated with larger valve sizes, highlighting the risk of over-sizing this particular valve design. We note also, in contrast to previously published literature on the topic, that use of a sutureless valve was associated with a lower rate of post-operative atrial fibrillation and post-operative pneumonia, without a correspondingly increased risk of PPM implantation or stroke.
We wish to thank Dr Timothy Cannings and Dr Michael Allerhand from the School of Mathematics at the University of Edinburgh, who kindly reviewed the statistical methodology and provided their valuable feedback.
| 1. | Strange GA, Stewart S, Curzen N, Ray S, Kendall S, Braidley P, Pearce K, Pessotto R, Playford D, Gray HH. Uncovering the treatable burden of severe aortic stenosis in the UK. Open Heart. 2022;9:e001783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Salis S, Mazzanti VV, Merli G, Salvi L, Tedesco CC, Veglia F, Sisillo E. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Anesth. 2008;22:814-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 301] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 3. | Postolache A, Sperlongano S, Lancellotti P. TAVI after More Than 20 Years. J Clin Med. 2023;12:5645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 4. | Rotman OM, Bianchi M, Ghosh RP, Kovarovic B, Bluestein D. Principles of TAVR valve design, modelling, and testing. Expert Rev Med Devices. 2018;15:771-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Park DY, An S, Kassab K, Jolly N, Attanasio S, Sawaqed R, Malhotra S, Doukky R, Vij A. Chronological comparison of TAVI and SAVR stratified to surgical risk: a systematic review, meta-analysis, and meta-regression. Acta Cardiol. 2023;78:778-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Nabil N. Transcatheter Aortic Valve Implantation Two Decades of Evolution - TAVI From Current Perspective. Acta Inform Med. 2023;31:312-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Barnhart GR, Shrestha ML. Current Clinical Evidence on Rapid Deployment Aortic Valve Replacement: Sutureless Aortic Bioprostheses. Innovations (Phila). 2016;11:7-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Dokollari A, Ramlawi B, Torregrossa G, Sá MP, Sicouri S, Prifti E, Gelsomino S, Bonacchi M. Benefits and Pitfalls of the Perceval Sutureless Bioprosthesis. Front Cardiovasc Med. 2021;8:789392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Fischlein T, Caporali E, Asch FM, Vogt F, Pollari F, Folliguet T, Kappert U, Meuris B, Shrestha ML, Roselli EE, Bonaros N, Fabre O, Corbi P, Troise G, Andreas M, Pinaud F, Pfeiffer S, Kueri S, Tan E, Voisine P, Girdauskas E, Rega F, García-Puente J, De Kerchove L, Lorusso R. Hemodynamic Performance of Sutureless vs. Conventional Bioprostheses for Aortic Valve Replacement: The 1-Year Core-Lab Results of the Randomized PERSIST-AVR Trial. Front Cardiovasc Med. 2022;9:844876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 10. | Shrestha M, Folliguet T, Meuris B, Dibie A, Bara C, Herregods MC, Khaladj N, Hagl C, Flameng W, Laborde F, Haverich A. Sutureless Perceval S aortic valve replacement: a multicenter, prospective pilot trial. J Heart Valve Dis. 2009;18:698-702. [PubMed] |
| 11. | Laborde F, Fischlein T, Hakim-Meibodi K, Misfeld M, Carrel T, Zembala M, Madonna F, Meuris B, Haverich A, Shrestha M; Cavalier Trial Investigators. Clinical and haemodynamic outcomes in 658 patients receiving the Perceval sutureless aortic valve: early results from a prospective European multicentre study (the Cavalier Trial)†. Eur J Cardiothorac Surg. 2016;49:978-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4:e297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2871] [Cited by in RCA: 3810] [Article Influence: 200.5] [Reference Citation Analysis (0)] |
| 13. | Smith ER. The angina grading system of the Canadian Cardiovascular Society. Can J Cardiol. 2002;18:439, 442. [PubMed] |
| 14. | Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg. 1999;16:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2144] [Cited by in RCA: 2213] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 15. | Villa E, Messina A, Laborde F, Shrestha M, Troise G, Zannis K, Haverich A, Elfarra M, Folliguet T. Challenge for perceval: aortic valve replacement with small sutureless valves--a multicenter study. Ann Thorac Surg. 2015;99:1248-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Gilmanov D, Miceli A, Bevilacqua S, Farneti P, Solinas M, Ferrarini M, Glauber M. Sutureless implantation of the perceval s aortic valve prosthesis through right anterior minithoracotomy. Ann Thorac Surg. 2013;96:2101-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Lopes LA, Agrawal DK. Post-Operative Atrial Fibrillation: Current Treatments and Etiologies for a Persistent Surgical Complication. J Surg Res (Houst). 2022;5:159-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 18. | Melby SJ, George JF, Picone DJ, Wallace JP, Davies JE, George DJ, Kirklin JK. A time-related parametric risk factor analysis for postoperative atrial fibrillation after heart surgery. J Thorac Cardiovasc Surg. 2015;149:886-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Dobrev D, Aguilar M, Heijman J, Guichard JB, Nattel S. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat Rev Cardiol. 2019;16:417-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 398] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 20. | Wang D, Lu Y, Sun M, Huang X, Du X, Jiao Z, Sun F, Xie F. Pneumonia After Cardiovascular Surgery: Incidence, Risk Factors and Interventions. Front Cardiovasc Med. 2022;9:911878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Sánchez-Véliz R, Carmona MJ, Otsuki DA, Freitas C, Benício A, Negri EM, Malbouisson LM. Impact of Cardiopulmonary Bypass on Respiratory Mucociliary Function in an Experimental Porcine Model. PLoS One. 2015;10:e0135564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Lagier D, Zeng C, Fernandez-Bustamante A, Vidal Melo MF. Perioperative Pulmonary Atelectasis: Part II. Clinical Implications. Anesthesiology. 2022;136:206-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 115] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 23. | Colarossi G, Migliorini F, Becker M, Arias JP, Autschbach R, Moza A, Aljalloud A. Conventional Prostheses versus Sutureless Perceval for Aortic Valve Replacement: A Meta-Analysis. Ann Thorac Cardiovasc Surg. 2023;29:107-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 24. | Jolliffe J, Moten S, Tripathy A, Skillington P, Tatoulis J, Muneretto C, Di Bacco L, Galvao HBF, Goldblatt J. Perceval valve intermediate outcomes: a systematic review and meta-analysis at 5-year follow-up. J Cardiothorac Surg. 2023;18:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Mehew JD, Hogg R, Clark S, Santhanakrishnan K, Catarino P, Mascaro J, Stock U, Dark J. Risk of prolonged ischemic time linked to use of cardiopulmonary bypass during implantation for lung transplantation in the United Kingdom. J Heart Lung Transplant. 2023;42:1378-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Mukharyamov M, Kirov H, Caldonazo T, Doenst T. Impact of Age on the Relationship between Cross-Clamp Time and Mortality in Cardiac Surgery. Thorac Cardiovasc Surg. 2024;72:539-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Al-Sarraf N, Thalib L, Hughes A, Houlihan M, Tolan M, Young V, McGovern E. Cross-clamp time is an independent predictor of mortality and morbidity in low- and high-risk cardiac patients. Int J Surg. 2011;9:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 28. | Kingston A, Comas-Herrera A, Jagger C; MODEM project. Forecasting the care needs of the older population in England over the next 20 years: estimates from the Population Ageing and Care Simulation (PACSim) modelling study. Lancet Public Health. 2018;3:e447-e455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 29. | Galper BZ, Chinnakondepalli KM, Wang K, Magnuson EA, Lu M, Thourani VH, Kodali S, Makkar R, Herrmann HC, Kapadia S, Williams M, Webb J, Smith CR, Mack MJ, Leon MB, Cohen DJ; PARTNER Investigators. Economic Outcomes of Transcatheter Versus Surgical Aortic Valve Replacement in Patients with Severe Aortic Stenosis and Low Surgical Risk: Results from the PARTNER 3 Trial. Circulation. 2023;147:1594-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Cerillo AG, Amoretti F, Mariani M, Cigala E, Murzi M, Gasbarri T, Solinas M, Chiappino D. Increased Gradients After Aortic Valve Replacement With the Perceval Valve: The Role of Oversizing. Ann Thorac Surg. 2018;106:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | González Barbeito M, Estévez-Cid F, Pardo Martínez P, Velasco García de Sierra C, Iglesias Gil C, Quiñones Laguillo C, Cuenca Castillo JJ. Surgical technique modifies the postoperative atrioventricular block rate in sutureless prostheses. J Thorac Dis. 2019;11:2945-2954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Powell R, Pelletier MP, Chu MWA, Bouchard D, Melvin KN, Adams C. The Perceval Sutureless Aortic Valve: Review of Outcomes, Complications, and Future Direction. Innovations (Phila). 2017;12:155-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Halapas A, Koliastasis L, Doundoulakis I, Antoniou CK, Stefanadis C, Tsiachris D. Transcatheter Aortic Valve Implantation and Conduction Disturbances: Focus on Clinical Implications. J Cardiovasc Dev Dis. 2023;10:469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 34. | Lorusso R, Jiritano F, Roselli E, Shrestha M, Folliguet T, Meuris B, Pollari F, Fischlein T; PERSIST-AVR Investigators. Perioperative platelet reduction after sutureless or stented valve implantation: results from the PERSIST-AVR controlled randomized trial. Eur J Cardiothorac Surg. 2021;60:1359-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/