Published online Feb 26, 2025. doi: 10.4330/wjc.v17.i2.99074
Revised: October 8, 2024
Accepted: January 17, 2025
Published online: February 26, 2025
Processing time: 227 Days and 18.1 Hours
Primary percutaneous coronary intervention (PCI) is the preferred treatment for ST-segment elevation myocardial infarction (STEMI). However, in patients with high thrombus burden, immediate stenting during PCI can lead to poor outcomes due to the risk of thrombus migration and subsequent microvascular occlusion, resulting in no-reflow phenomena. Deferred stenting offers a potential advantage by allowing for the reduction of thrombus load, which may help to minimize the incidence of slow-flow and no-reflow complications. This study explores the effectiveness of a deferred stenting strategy in improving outcomes for STEMI patients.
To evaluate the effectiveness and safety of deferred PCI in a real-world setting in acute STEMI patients.
This study was conducted at King George’s Medical University, Lucknow, from October 1, 2018, to October 30, 2019 and included a total of 55 participants. Pa
Anterior wall myocardial infarction was the predominant type of STEMI in 62% of the selected 55 patients (mean age: 54 years; 70% males), and diabetes mellitus was the most common risk factor (18.2%), followed by hypertension (16.2%). On the second angiogram of these patients measures of thrombus grade, thrombolysis in myocardial infarction flow grade, myocardial blush grade, and severity of stenosis of culprit lesion were considerably improved compared to the first angiogram, and the average culprit artery diameter had increased by 7.8%. Most patients (60%) had an uneventful hospital stay during the second angiogram and an uneventful intraprocedural course (85.19%), with slow-flow/no-reflow occurring only in 7.4% of the patients; these patients recovered after taking vasodilator drugs. In 29.3% of patients, the culprit artery was recanalized, preventing unnecessary stent deployment.
Deferred PCI strategy is safe and reduces the thrombus burden, improves thrombolysis in myocardial infarction (TIMI) flow, improves myocardial blush grade, and prevents unwarranted stent deployment.
Core Tip: This study evaluated the effectiveness and safety of a deferred percutaneous coronary intervention (PCI) strategy in patients with ST-segment elevation myocardial infarction (STEMI). The deferred PCI approach led to significant reductions in thrombus burden, improved thrombolysis in myocardial infarction (TIMI) flow, and enhanced myocardial blush grade. It also minimized the incidence of slow-flow/no-flow events and prevented unnecessary stent deployments. These findings suggest that deferred PCI is a promising strategy to manage STEMI patients with high thrombus burden, offering better angiographic and clinical outcomes by optimizing intervention timing and reducing complications.
- Citation: Pradhan A, Uppal S, Vishwakarma P, Singh A, Bhandari M, Shukla A, Sharma A, Chaudhary G, Chandra S, Sethi R, Dwivedi SK. Outcomes of patients with acute ST-segment elevation myocardial infarction treated by a prolonged “Deferred” percutaneous coronary intervention strategy. World J Cardiol 2025; 17(2): 99074
- URL: https://www.wjgnet.com/1949-8462/full/v17/i2/99074.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i2.99074
The reperfusion strategy for those with ST-segment elevation myocardial infarction (STEMI) is percutaneous coronary intervention (PCI) and stent deployment[1,2]. However, in emergency settings, patients with STEMI have a high thrombus load in the infarct-related artery. Stenting in such a situation increases the likelihood of slow-flow/no-reflow phenomenon, malapposition, and stent thrombosis[3]. In fact, no-reflow occurs in 10% of patients after primary PCI, which is associated with an impaired prognosis[4,5].
Deferred stenting is a cutting-edge technique that aims to delay stent implantation until a specific period has passed after stable distal flow is obtained[3]. Meta-analyses have concluded that a deferred stenting strategy might be preferable to immediate stenting in patients with STEMI[6,7]. Qiao et al[6] observed no significant differences in the prevalence of slow-flow/no-reflow between deferred stenting and immediate stenting in randomized controlled trials (RCTs; P = 0.23). However, observational studies revealed a markedly lower incidence of slow-flow/no-reflow after deferred stenting (P < 0.0001). Delaying stenting had the benefit of improving left ventricular ejection fraction (LVEF) over time, but the incidences of major adverse cardiovascular events remained the same for deferred and immediate stenting[6].
An observational study showed that deferring stenting was associated with a lower need for culprit artery stenting[8]. In the present study, we aimed to evaluate the effectiveness and safety (measured by angiographic and clinical outcomes) of deferred PCI in a real-world clinical setting in acute STEMI.
This single-center study was conducted at King George’s Medical University, Lucknow, a high-volume, tertiary care PCI center with an annual volume exceeding 1000 PCIs for the last 5 years. The study protocol was reviewed and approved by the institutional review board (Approval letter no. 503/Ethics/19). The study was performed in accordance with the ethical standards of the responsible institution and the Declaration of Helsinki. In compliance with the institutional ethics committee requirements, written informed consent was obtained from all participants or their legal guardians. The study participants included patients with acute STEMI who were treated using a selective and prolonged deferred PCI strategy.
Individuals with STEMI, aged > 18 years, who presented with chest pain consistent with acute coronary syndrome and had ST-segment elevation of > 1 mm and involved > 2 contiguous leads, and who required revascularization via primary PCI, pharmacoinvasive PCI, and routine PCI within 48 hours were included in the study. Angiographic criteria determined the decision to use deferred stenting (such as the presence of a thrombus burden of ≥ grade 3, an ectatic vessel with a higher risk of slow-flow/no-reflow, persistence of thrombus after three or more runs of manual thrombus aspiration, and failure to achieve distal TIMI 3 flow after the use of a low profile (i.e., < 2 mm) or undersized balloon dilatation. Patients aged < 18 years of age, with a block in the left bundle branch and those with contraindications to antiplatelet therapy or glycoprotein (GP) IIb/IIIa inhibitors, cardiogenic shock, cardiac arrest at presentation, persistent chest pain, clinically significant liver or renal dysfunction, and stent thrombosis were excluded. Participants with other significant systemic diseases like human immunodeficiency virus infection, malignancy with a life expectancy of less than a year, and active systemic infection/sepsis were also excluded.
Patients with STEMI underwent coronary angiography as a primary, pharmacoinvasive or routine PCI < 48 hours in accordance with the standard guideline-directed protocols. Angiographic features were considered to determine the need for a deferred stenting protocol. Fifty-five patients were selected for deferred PCI based on the discretion of the surgeon. The most common reasons for deferral were an increased probability of slow-flow/no-reflow and an increased thrombus burden. The anatomy of the coronary artery was assessed via invasive coronary angiography from the radial artery (preferred) or femoral artery route. Coronary angiography was performed in all standard views to delineate the complete anatomy of the coronary system. An intervention was performed on those having obstructive coronary disease according to the European Society of Cardiology 2018 guidelines[9].
For all cases undergoing angiography, a qualified and experienced interventional cardiologist gave his opinion on various parameters, which included the coronary dominance system; obstructive or nonobstructive disease involving the coronary artery, type, and severity of vessel involvement; presence of thrombus; an ectasia; and the type of intervention done (if any). Angiography findings were reviewed by a second interventional cardiologist separately. During the primary PCI, the use of thrombus aspiration and small-sized balloon dilation either alone or in combination to achieve distal TIMI flow was mandatory. However, during pharmacoinvasive or routine PCI, these interventions were at the discretion of the surgeon.
After the coronary angiogram, the patients were administered intravenous GP IIb/IIIa (eptifibatide or tirofiban) for 18 to 24 hours. Following this, subcutaneous low-molecular-weight heparin was initiated until the next angiogram along with all guideline-directed medical therapy (aspirin, P2Y12 inhibitor, beta-blocker, and renin-angiotensin-aldosterone system blockers). All patients selected for deferred PCI strategy were evaluated for in-hospital major adverse cardiovascular events (a composite of left ventricular failure, bradyarrhythmia, and tachyarrhythmia requiring intervention, reinfarction, cardiogenic shock, and death). If any patient showed signs of recurring ischemia, such as chest pain, new electrocardiographic changes, or hemodynamic deterioration, a rescue PCI was planned. Patients were scheduled for a second angiography after 5–7 days, and angiographic parameters such as vessel size, thrombus burden, TIMI flow grade, and myocardial blush grade were compared to the index angiogram. The decision to perform a PCI was taken for residual stenosis and vessel size of > 2.25 mm. PCI was performed with contemporary techniques, and a third-generation drug-eluting stent was implanted. Any slow-flow/no-reflow occurrence was noted during deferred PCI.
Categorical variables are presented as percentages, whereas continuous variables are presented as median (range) or mean ± SD. Student’s t-test was used to compare pre- and post-treatment values, with P values < 0.05 considered as statistically significant. All statistical analyses were performed using SPSS (v 29.0).
A total of 55 adult patients with acute STEMI (mean age: 54 years; 70.9% males) were treated with selective and prolonged deferred PCI strategy in this study. Baseline characteristics are presented in Table 1.
| Characteristics | Patients with STEMI (n = 55) |
| Mean age, years | 54 |
| Gender | |
| Male | 39 (70.9) |
| Female | 16 (29.1) |
| Diagnosis | |
| AWMI | 34 (61.8) |
| IWMI + PWMI | 12 (21.8) |
| LWMI | 1 (1.8) |
| IWMI + RVMI | 8 (14.5) |
| Risk factor | |
| DM | 10 (18.2) |
| HTN | 9 (16.4) |
| Tobacco chewers | 13 (23.6) |
| LVEF at admission | |
| < 40 | 9 (16.4) |
| 40-50 | 32 (58.2) |
| ≥ 50 | 14 (25.5) |
| Mean LVEF, % | 44.2 |
| LV systolic dysfunction at the time of presentation, % | 74.6 |
| Time of presentation to hospital in hours | |
| 0-12 | 21 (38.2) |
| 12.1-24 | 21 (38.2) |
| 24.1-48 | 9 (16.4) |
| Above 48 | 4 (7.3) |
| Thrombolysis | |
| Yes | 21 (38.2) |
| No | 34 (61.8) |
| Killip class | |
| I | 31 (60) |
| II | 14 (28) |
| III | 5 (10) |
| IV | 1 (2) |
| History | |
| Prior MI | 8 (16) |
| CABG | 4 (8) |
| Mean heart rate, beats/minute | 90 |
| Mean SBP, mm Hg | 106 |
| Mean DBP, mm Hg | 70 |
| Mean eGFR, mL/min | 60.2 |
In the deferred PCI strategy, after the first angiography, all patients received low-molecular-weight heparin for approximately 1 week and GP IIb/IIIa inhibitors were administered to only 38.2% of patients.
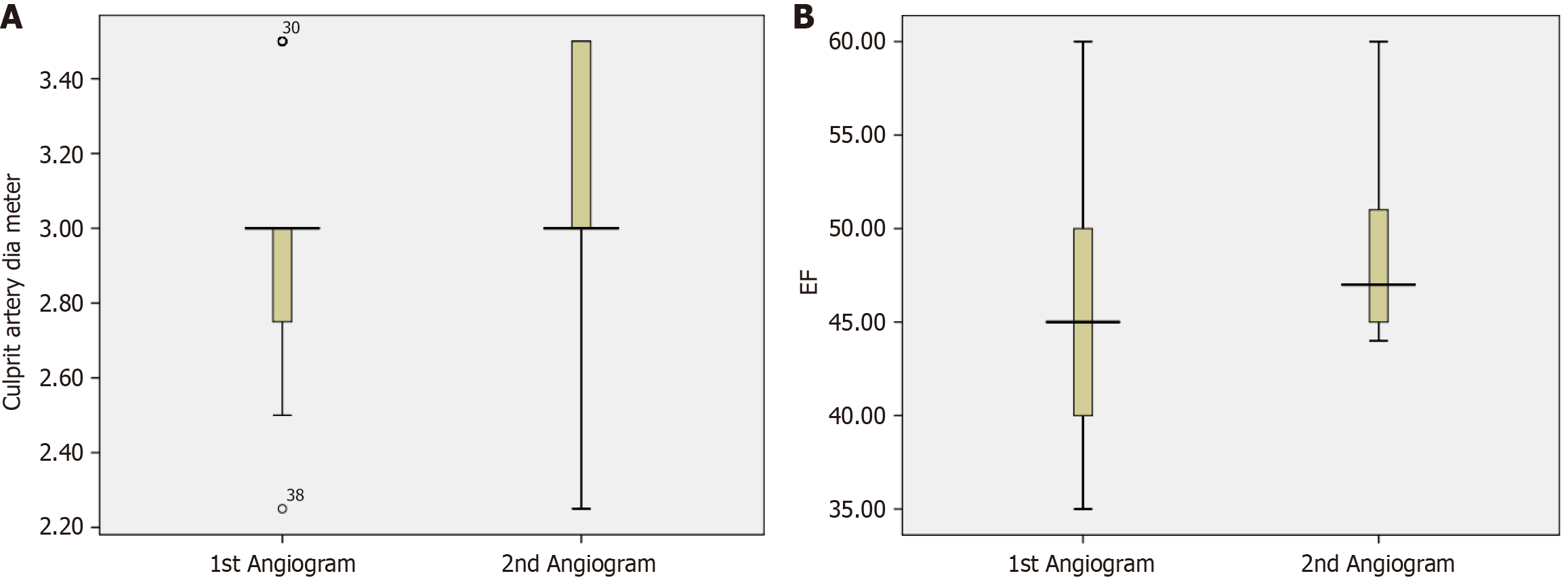
On the first angiogram, the culprit artery was the left anterior descending artery in 61.8% of patients, right coronary artery in 25.5% of patients, and left circumflex artery in 12.7% of patients. Ten patients (18.2%) had thrombus grade 2, whereas 45 patients (81.8%) had thrombus grade 3 or higher on the first angiogram. This was markedly improved on the second angiogram, with 55.6% of patients having grade 0, 24.1% with grade 1, 16.7% with grade 2, and 3.7% with grade 3 (Table 2). On the first angiogram, 34.5% of patients had grade 3 TIMI flow, 58.2% had grade 2, 5.5% had grade 1, and 1.8% had grade 0. On the second angiogram, this was significantly improved, with grade 3 in 96.3% of patients and grade 2 in 3.7% of patients (Table 2). On the first angiogram, 54.5% of patients had a grade 2 myocardial blush, whereas 38.2% had a grade 1. However, myocardial blush was notably improved on the second angiogram, with 63% of patients with grade 3 and 37% with grade 2 (Table 2). On the first angiogram, 12.7% of patients had culprit artery stenosis severity of < 70%, 54.5% of patients had stenosis severity of 70%-95%, and 32.7% of patients had culprit artery with 95%-99% stenosis. Many patients (42.59%) had < 70% stenosis on the second angiogram compared to the first angiogram, indicating a marked reduction in the severity of stenosis in the culprit artery due to thrombus resolution (Table 2). Between the two angiograms, the mean ± SD culprit artery diameter increased by 7.8% (2.94 ± 0.29 mm to 3.19 ± 0.31 mm). On the second angiogram, recanalized culprit arteries were present in 29.6% of patients (Table 2, Figure 1A).
| Characteristics | Patients with STEMI | P value | |
| Culprit artery | |||
| LAD | 34 (61.8) | ||
| LCX | 7 (12.7) | ||
| RCA | 14 (25.5) | ||
| First angiogram | Second angiogram | ||
| Thrombus grade | 0.127 | ||
| Grade 0 | 0 (0) | 30 (55.6) | |
| Grade 1 | 0 (0) | 13 (24.1) | |
| Grade 2 | 10 (18.2) | 9 (16.7) | |
| Grade 3 | 18 (32.7) | 2 (3.7) | |
| Grade 4 | 18 (32.7) | 0 (0) | |
| Grade 5 | 9 (16.4) | 0 (0) | |
| Myocardial blush grade | 0.179 | ||
| Grade 0 | 0 (0) | 0 (0) | |
| Grade 1 | 21 (38.2) | 0 (0) | |
| Grade 2 | 30 (54.5) | 20 (37) | |
| Grade 3 | 4 (7.3) | 34 (63) | |
| TIMI flow grade | 0.699 | ||
| 0 | 1 (1.8) | 0 (0) | |
| 1 | 3 (5.5) | 0 (0) | |
| 2 | 32 (58.2) | 2 (3.7) | |
| 3 | 19 (34.5) | 52 (96.3) | |
| Culprit artery stenosis | |||
| < 70% | 7 (12.73) | 23 (42.59) | |
| 70%-95% | 30 (54.55) | 21 (38.89) | |
| 95%-99% | 18 (32.73) | 10 (18.52) | |
| Culprit artery diameter, mm | |||
| mean ± SD | 2.94 ± 0.29 | 3.13 ± 0.31 | |
| Median (range) | 3.00 (2.25-3.50) | 3.00 (2.25-3.50) | |
| POBA/thrombosuction | |||
| Yes | 4 (7.3) | ||
| No | 51 (92.7) | ||
| LMWH | |||
| Yes | 55 (100) | ||
| No | 0 (0) | ||
| GP IIb/IIIa inhibitors | |||
| Yes | 21 (38.2) | ||
| No | 34 (61.8) | ||
The hospital course was uneventful in most patients (60%). Left ventricular failure occurred in 12.6% of patients, hypotension in 14.5% of patients, atrial fibrillation in 5.4% of patients, complete heart block in 5.4% of patients, and ventricular tachycardia in 3.6% of patients. Eight hours after the first angiography, 1 patient passed away with bradycardia and asystole (Table 3). Most patients who were chosen for the deferred PCI method had no intraprocedural complications (85.1%), with only 7.41% of patients experiencing slow-flow/no-reflow. Additionally, 1.85% of patients experienced left ventricular failure, ventricular tachycardia, complete heart block, or bradycardia during the procedure, whereas 7.41% of patients experienced intraprocedural hypotension (Table 3). Percutaneous old balloon angioplasty or thrombosuction was performed in 4 (7.3%) patients (Table 3).
| Characteristics | Patients with STEMI |
| Procedural outcomes for all patients | |
| Hospital course | |
| Uneventful | 33 (60) |
| AF | 1 (1.8) |
| CHB | 3 (5.4) |
| Death | 1 (1.8) |
| Hypotension | 8 (14.5) |
| LVF | 5 (9) |
| LVF, AF | 2 (3.6) |
| VT | 2 (3.6) |
| Intraprocedural complications in patients with deferred stenting | |
| No complications | 46 (85.19) |
| Slow/no-reflow after PCI | |
| Slow-reflow | 4 (7.41) |
| No-reflow | 4 (7.41) |
| No | 46 (85.19) |
| MACE | |
| Bradycardia | 1 (1.85) |
| CHB | 1 (1.85) |
| Hypotension | 4 (7.41) |
| LVF | 1 (1.85) |
| VT | 1 (1.85) |
The preferred course of treatment for STEMI is primary PCI[10]. In individuals with STEMI with high thrombus burden, poor outcomes are seen if the stent is placed during PCI[10]. Stenting increases the likelihood of the thrombus moving within the microvasculature, occluding it, and causing the no-reflow phenomena[3]. In such cases, deferred stenting has the advantage of minimizing the slow-flow/no-reflow process by reducing the thrombus load[3]. This study emphasizes the effectiveness and safety of deferred stenting in a select group of patients after the first angiogram. Due to their delayed presentation, most individuals in this study did not receive thrombolytic therapy. Individuals with a significant thrombus load and a high chance of slow-flow/no-reflow were selected for deferred PCI at the discretion of the surgeon. Following the first angiogram, most patients had uneventful hospital stays. In comparison with the first angiogram, most patients displayed markedly reduced thrombus burden, TIMI flow, and myocardial blush in the second angiogram. Similar results have been shown in previously published reports investigating the effectiveness of deferred stenting following primary PCI in STEMI patients[5,7,11]. A meta-analysis of seven RCTs and three prospective cohort studies showed significantly higher grade 3 TIMI flow and myocardial blush in individuals managed with deferred stenting[7]. In a randomized controlled prospective trial, the proportion of patients with deferred stenting showed significantly lower angiographic grades and signs of thrombus in the second angiograph as compared to the first (62.7% vs 98.1%; P < 0.0001). Following stenting, the group with deferred stenting had a greater number of patients with TIMI flow grade 3 and higher myocardial blush grades[11]. Similar results were found in another study that included a group of individuals with STEMI and a substantial thrombus load[5]. Attar et al[12] evaluated deferred stenting after intense antiplatelet and antithrombotic therapy instead of PCI and immediate stenting, and showed that despite 82% of patients having TIMI thrombus grades 4 or 5 in the initial angiogram, 33% of patients did not require further coronary intervention[12]. Similarly, deferred stenting resulted in TIMI flow improvement and reduced thrombus burdens in individuals with STEMI in the SUPER-minimalist immediate mechanical intervention (MIMI) study[13].
Furthermore, in our study, the culprit artery stenosis was markedly reduced with deferred stenting. This is consistent with the outcomes of a prospective observational study (SUPER-MIMI), which showed that individuals with STEMI had less severe stenosis. At the completion of the first PCI, 84.0% of patients had stenosis > 50%; this number dropped to 50.0% at the start of the repeat PCI (P < 0.001)[13]. On the contrary, a single-center observational study found no changes in the degree of stenosis in patients treated with delayed stenting between the two angiograms (53.2% vs 57.3%, P = 0.69)[8]. In this study, on the second angiogram, there was a trend toward a larger culprit artery diameter than on the first. This matches the DEFER-STEMI study wherein the maximum stent diameter increased significantly (P < 0.0001) with deferred stenting at the end of the first procedure, from 3.0 mm [interquartile range (IQR): 3.0-3.5] to 3.5 mm (IQR: 3.0-4.0); in addition, a higher proportion of patients had a larger diameter stent in this group[11].
Patients selected for deferred PCI had post-discharge LVEF values that were higher than those at admission (Figure 1B). This was consistent with a meta-analysis (Qiao et al[6]) that demonstrated enhanced LVEF over a longer time period in individuals with STEMI who underwent deferred stenting (P = 0.001)[6]. In the present study, PCI was completed in 66.6% of patients after the second angiogram, whereas in 3.7% of patients, it was attempted[12]. In this study, the duration between the first angiogram and deferred angiogram was determined by the surgeon with a minimum duration of 5 days. The time interval between the reference angiography and the delayed angiogram ranges from 4-16 hours to 7 days across several RCTs and observational studies[11,13-15]. The DEFER-STEMI and MIMI studies demonstrated that deferred stenting was beneficial in individuals with STEMI at median intervals of 9 hours (IQR: 6-12 hours) and 36 hours (IQR: 29-46 hours), respectively[11,14]. The INNOVATION trial found that patients who had stenting 3-7 days after their initial intervention had less microvasculature occlusion than those who received immediate stenting[15]. Pascal et al[8] reported that a mean delay of 4.3 days between the first and second angiograms resulted in a substantial decrease in the thrombus burden in the delayed stenting group. A longer deferral interval of 3-7 days or ≥ 7 days after initial intervention resulted in reduced microvasculature occlusion and improved angiographic outcomes in the INNOVATION trial and SUPER-MIMI study, respectively[13,15]. Magdy et al[5] showed that when comparing two alternative deferral time intervals, a delay of 1 week had significantly superior thrombus resolution than a 4-16-hour deferral at the second coronary angiography (P < 0.001). This illustrates that deferring stenting for a longer period of time provides more time for drug action and also aids in thrombus clearance[5].
Thrombus aspiration and small-size balloon dilatation are used in the MIMI technique performed during primary PCI in individuals with substantial thrombus load to create distal flow in the artery associated with the infarct[16]. In our study, thrombosuction or percutaneous old balloon angioplasty was performed during the first PCI in only 4 patients. After the initial angiogram, 29.9% of patients had a recanalized culprit artery, making it evident that stenting would have been completely unnecessary in them. If PCI had been performed after the initial angiogram, these individuals would also have received stents with a smaller diameter. Additionally, in these individuals, the chance of slow-flow/no-reflow, intraprocedural, and postprocedural complications would have been significantly higher.
The deferred stent approach requires a second intervention; therefore, costs associated with that procedure could be greater. However, the procedure has the benefit of reducing complications and obviating the necessity for stenting[3]. It was noted that the incidences of slow-flow/no-reflow, intraprocedural, and postprocedural complications were few with deferred PCI. This indicates that this strategy is safe for the management of STEMI and can be applied to individuals who have an increased thrombus burden and a high chance of slow-flow/no-reflow.
In our experience from the real-world clinical setting, deferred stenting in selected individuals with STEMI having risk factors for slow-flow/no-reflow represents a promising strategy that entails striking a balance between competing benefits and risks. However, there were a few limitations to this study that may affect the interpretation and generalizability of the findings. The small sample size may limit statistical power and reduce the representativeness of the results. The absence of a control group limits the ability to isolate the effects of the intervention from other influencing factors. The short follow-up period may not capture long-term outcomes or the sustained impact of the intervention. Future research with larger sample sizes, inclusion of control groups, and extended follow-up periods is recommended to validate and enhance the generalizability of these results.
A deferred PCI approach can safely reduce slow-flow/no-reflow and prevent unwarranted stent deployment in cases with high thrombus burden. This approach reduces thrombus burden, improves TIMI flow and myocardial perfusion grade, minimizes the chances of slow-flow/no-reflow, and reduces intraprocedural complications when patients are scheduled for PCI at a later stage.
| 1. | Thomas MP, Bates ER. Update on primary PCI for patients with STEMI. Trends Cardiovasc Med. 2017;27:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Kalra S, Bhatt H, Kirtane AJ. Stenting in Primary Percutaneous Coronary Intervention for Acute ST-Segment Elevation Myocardial Infarction. Methodist Debakey Cardiovasc J. 2018;14:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Pradhan A, Bhandari M, Vishwakarma P, Sethi R. Deferred Stenting for Heavy Thrombus Burden During Percutaneous Coronary Intervention for ST-Elevation MI. Eur Cardiol. 2021;16:e08. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Gupta S, Gupta MM. No reflow phenomenon in percutaneous coronary interventions in ST-segment elevation myocardial infarction. Indian Heart J. 2016;68:539-551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Magdy AM, Demitry SR, Hasan-Ali H, Zaky M, Abd El-Hady M, Abdel Ghany M. Stenting deferral in primary percutaneous coronary intervention: exploring benefits and suitable interval in heavy thrombus burden. Egypt Heart J. 2021;73:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Qiao J, Pan L, Zhang B, Wang J, Zhao Y, Yang R, Du H, Jiang J, Jin C, Xiong E. Deferred Versus Immediate Stenting in Patients With ST-Segment Elevation Myocardial Infarction: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2017;6:28275065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Hu X, Yang X, Li X, Dong H, Zhou Y. Evaluating the role of deferred stenting in ST-segment elevation myocardial infarction patients presented with high thrombus burden: A systematic review and meta-analysis. Ann Cardiol Vasc Med. 2021;4:1039-1046. |
| 8. | Pascal J, Veugeois A, Slama M, Rahal S, Belle L, Caussin C, Amabile N. Delayed Stenting for ST-Elevation Acute Myocardial Infarction in Daily Practice: A Single-Centre Experience. Can J Cardiol. 2016;32:988-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7073] [Cited by in RCA: 6925] [Article Influence: 865.6] [Reference Citation Analysis (1)] |
| 10. | Vecchio S, Varani E, Chechi T, Balducelli M, Vecchi G, Aquilina M, Ricci Lucchi G, Dal Monte A, Margheri M. Coronary thrombus in patients undergoing primary PCI for STEMI: Prognostic significance and management. World J Cardiol. 2014;6:381-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 11. | Carrick D, Oldroyd KG, McEntegart M, Haig C, Petrie MC, Eteiba H, Hood S, Owens C, Watkins S, Layland J, Lindsay M, Peat E, Rae A, Behan M, Sood A, Hillis WS, Mordi I, Mahrous A, Ahmed N, Wilson R, Lasalle L, Généreux P, Ford I, Berry C. A randomized trial of deferred stenting versus immediate stenting to prevent no- or slow-reflow in acute ST-segment elevation myocardial infarction (DEFER-STEMI). J Am Coll Cardiol. 2014;63:2088-2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 307] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 12. | Attar M, Bader I, Dorsch M, Tyrrell B, Leung R, Brass N, Hui W, Cheung P. Deferred Stenting as an Alternative Strategy for Management of ST-Elevation Myocardial Infarction with Significant Thrombus Burden. J Adv Med Med Res. 2015;10:1-6. [DOI] [Full Text] |
| 13. | Mester P, Bouvaist H, Delarche N, Bouisset F, Abdellaoui M, Petiteau PY, Dubreuil O, Boueri Z, Chettibi M, Souteyrand G, Madiot H, Belle L. At least seven days delayed stenting using minimalist immediate mechanical intervention (MIMI) in ST-segment elevation myocardial infarction: the SUPER-MIMI study. EuroIntervention. 2017;13:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 14. | Belle L, Motreff P, Mangin L, Rangé G, Marcaggi X, Marie A, Ferrier N, Dubreuil O, Zemour G, Souteyrand G, Caussin C, Amabile N, Isaaz K, Dauphin R, Koning R, Robin C, Faurie B, Bonello L, Champin S, Delhaye C, Cuilleret F, Mewton N, Genty C, Viallon M, Bosson JL, Croisille P; MIMI Investigators*. Comparison of Immediate With Delayed Stenting Using the Minimalist Immediate Mechanical Intervention Approach in Acute ST-Segment-Elevation Myocardial Infarction: The MIMI Study. Circ Cardiovasc Interv. 2016;9:e003388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Kim JS, Lee HJ, Woong Yu C, Kim YM, Hong SJ, Park JH, Choi RK, Choi YJ, Park JS, Kim TH, Jang HJ, Joo HJ, Cho SA, Ro YM, Lim DS. INNOVATION Study (Impact of Immediate Stent Implantation Versus Deferred Stent Implantation on Infarct Size and Microvascular Perfusion in Patients With ST-Segment-Elevation Myocardial Infarction). Circ Cardiovasc Interv. 2016;9:e004101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Chacko P, Jayaprakash K, Misiriya KJR, Madhavan S, Kumary VS, Jayaprasad N, Jayaprakash VL, George R. Effect of thrombus aspiration on angiography and outcome in patients undergoing primary coronary angioplasty. Proc (Bayl Univ Med Cent). 2017;30:273-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/