Published online Feb 26, 2025. doi: 10.4330/wjc.v17.i2.101851
Revised: December 24, 2024
Accepted: February 6, 2025
Published online: February 26, 2025
Processing time: 149 Days and 17.7 Hours
Cardiac metastatic tumors (CMTs) are rare yet pose significant medical concerns. Clinical studies on CMT are limited, particularly those involving multicenter data analysis.
To systematically analyze the etiology, sources, classification, treatment, and prognosis of CMT.
A total of 226 CMT patients from two centers (2013 to 2023) were reviewed, and 153 tumor patients from China Health and Retirement Longitudinal Study were used as controls. The survival rates of 96 CMT patients were tracked through medical records and telephone follow-ups. Logistic regression and survival analyses were conducted to characterize CMT.
CMTs were predominantly male (67.26% vs 39.47%, P < 0.001). Intracardiac metastasis patients had worse heart and coagulation function than pericardial metastasis patients (prothrombin time: 13.90 vs 13.30, P = 0.002), D-dimer levels (2.16 vs 0.85, P = 0.001), B-type natriuretic peptide (BNP) levels (324.00 vs 136.50, P = 0.004), and troponin levels (5.35 vs 0.03, P < 0.001)). Lung and liver cancers were the predominant primary tumor types in CMT. Patients with lung cancer (76.40% vs 30.77%) and thymoma (7.45% vs 1.54%) exhibited a higher prevalence of pericardial metastasis, while those with liver cancer (35.38% vs 0.62%) showed a higher prevalence of intracardiac metastasis. Overall survival was better for pericardial metastasis than for intracardiac metastasis patients (median survival: 419 days vs 129 days, log-rank test P = 0.0029). Cox proportional hazards model revealed that advanced age [hazard ratio (HR) = 1.034, 95% confidence interval (95%CI): 1.011-1.057] and higher BNP and troponin levels (HR = 1.011, 95%CI: 1.004-1.018) were associated with worse survival. Surgery significantly improved the survival rate of patients. The median survival time was 275 days for patients who did not undergo surgery and 708 days for those who had surgery (log-rank test P = 0.0128)
Clinicians should consider CMT in the male lung or liver cancer patients with cardiac symptoms. Abnormal coagulation, impaired heart function, tumor location, and age are key prognostic factors for CMT. Surgical intervention is the preferred treatment option, as it significantly prolongs median survival.
Core Tip: Cardiac metastatic tumor (CMT) is a rare disease often accompanied by a poor prognosis. Currently, there is a dearth of clinical studies with sufficient cases to provide comprehensive insights. The present research has thoroughly characterized CMT, including its etiology, sources, classification, treatment options, and prognosis. It was found that CMT is predominant in male patients with liver cancer (often as intracardiac metastasis) and lung cancer (primarily involving the pericardium). Moreover, abnormal coagulation, heart function, tumor location, and age are key prognostic factors for CMT.
- Citation: Luo LY, Yang TS, He Z, Lin L, Luo XL. Comprehensive understanding of a rare disease: Cardiac metastatic tumor, a double-center 10-year case review. World J Cardiol 2025; 17(2): 101851
- URL: https://www.wjgnet.com/1949-8462/full/v17/i2/101851.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i2.101851
Cardiac metastatic tumor (CMT) is a rare disease with a poor prognosis and an incidence rate of 0.7%-3.5%[1]. Theoretically, malignant neoplasms can metastasize to the heart, but the routes vary among different tumors[2]. Malignant tumors can spread to the heart via three primary pathways: Direct infiltration, as seen with mediastinal and intrathoracic tumors that result in pericardial metastasis; hematogenous dissemination, which typically leads to myocardial metastasis; and lymphatic spread[3]. The rarity and poor prognosis of cardiac metastases lead to a predominance of case reports in the literature, with a significant lack of clinical research, especially multi-center studies[4-6]. Consequently, our understanding of CMT remains limited.
The China Health and Retirement Longitudinal Study (CHARLS) encompasses 17708 participants drawn from 10257 households across 150 counties or districts and 450 villages in 28 provinces throughout China. It collects a high-quality nationally representative sample of Chinese residents ages 45 and older to assess the social, economic, and health circumstances of community residents[7].
Given the existing gaps in the literature and the growing elderly population, it is essential to develop a thorough and systematic comprehension of cardiac metastases. These conditions are more prevalent among older individuals and frequently result in significantly adverse clinical outcomes. Therefore, the current study aimed to identify factors contributing to cardiac metastases and potential clinical predictors in patients with CMT and the CHARLS cohort. We also examined two main types of cardiac metastases: intracardiac and pericardial metastasis, analyzing baseline data, clinical indicators and manifestations, primary tumor type, and prognosis.
From 2013 to 2023, the clinical data of patients with CMT were obtained from Tongji Hospital Affiliated to Huazhong University of Science and Technology and The Third Affiliated Hospital of Chongqing Medical University. Data were extracted from an electronic clinical management system and normalized. Ethical approval was obtained from the respective hospitals, and participants provided informed consent.
The control participants were sourced from 2015 CHARLS Wave 3 and 2018 CHARLS Wave 4[7].
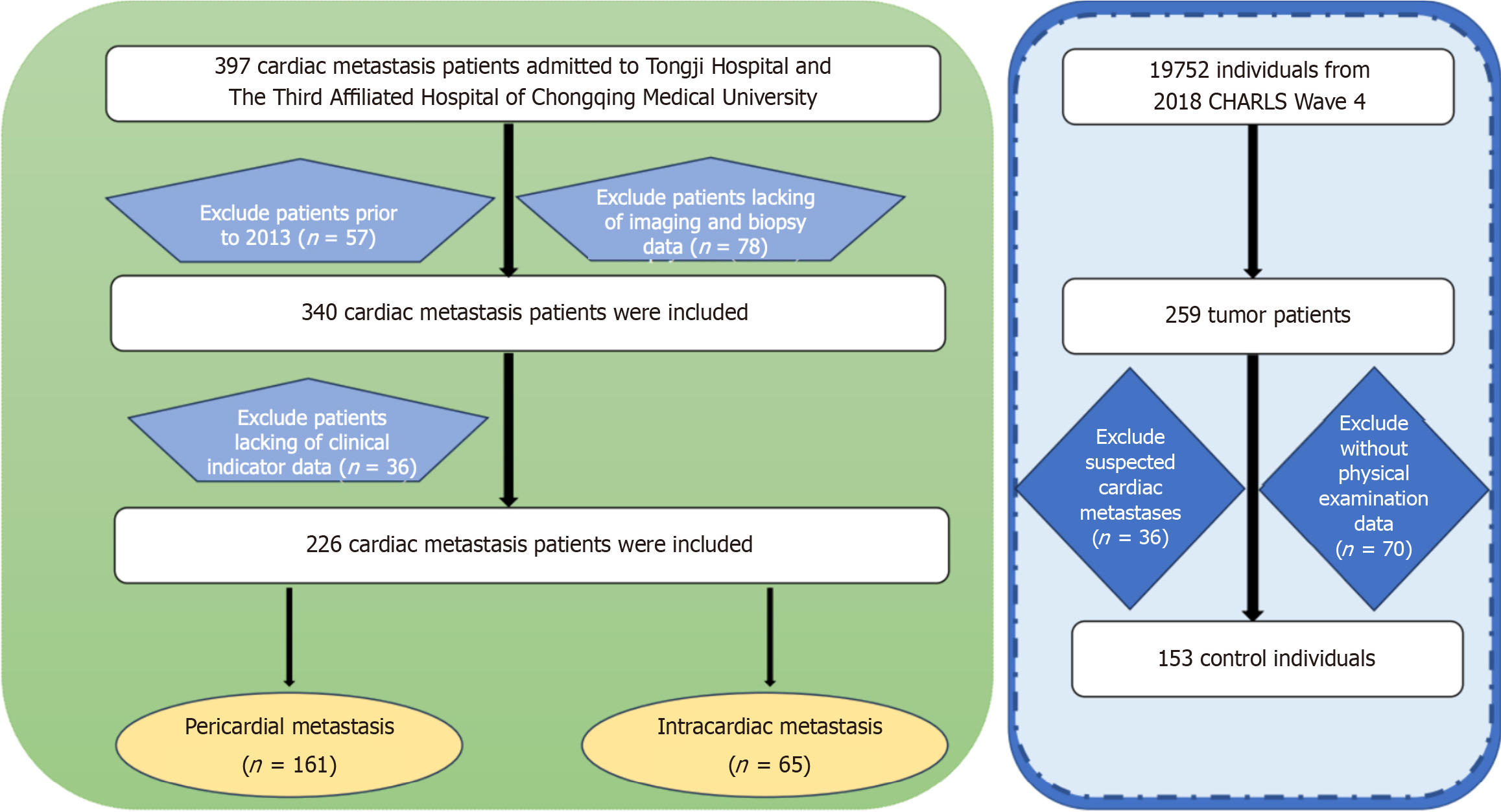
A thorough search was conducted in the medical record systems of the two hospitals to identify eligible patients. A total of 397 patients with cardiac metastases were initially identified. After excluding patients with medical records predating 2013, those lacking imaging or pathological biopsy data, and those without sufficient clinical indicators, 226 patients (161 cases of pericardial metastasis and 65 cases of intracardiac metastasis) were included in the final analysis. Imaging and pathological biopsy data are presented in Supplementary Figures 1-4.
A control group of 19752 participants from the 2018 CHARLS Wave 4 was established, supplemented by 259 tumor patients. After excluding 36 patients with cardiac metastases and 70 without physical exam data, the final control group included 153 individuals (Figure 1).
Data normality was tested using the Kolmogorov-Smirnov test or the Shapiro-Wilk test. Normally distributed data were analyzed utilizing an independent sample t-test, whereas non-normally distributed data were analyzed using the Mann-Whitney U test. Categorical variables were compared using χ2 tests. Data were analyzed using Stata (version 18.0), IBM SPSS Statistics 27, and R studio (version 4.3.2). P < 0.05 was considered statistically significant.
Survival analysis, also known as time-to-event analysis, is a statistical method for assessing the time until a predefined endpoint of interest occurs[8]. Herein, the date of confirmed CMT served as the starting point and the date of death as the endpoint. The Kaplan-Meier estimator, log-rank test, and Cox proportional hazards (PH) model were employed to evaluate patient survival.
Data were analyzed using Stata (version 18.0), IBM SPSS Statistics 27, and R studio (version 4.3.2).
A total of 153 control individuals and 226 CMT cases were included in the final analysis. The baseline characteristics of patients in the three groups are summarized in Table 1. Patients with CMT were predominantly male compared with those with primary tumors (67.26% vs 39.47%, P < 0.001)
| All (n = 379) | Primary tumors (n = 153) | Cardiac metastasis (n = 226) | P value | |
| Age (year) | 64.0 (55.0, 71.0) | 69.0 (61.0, 74.0) | 59.5 (50.0, 68) | < 0.001 |
| Male | 212 (55.94%) | 60 (39.47%) | 152 (67.26%) | < 0.001 |
| BMI, kg/m2 | 23.32 (21.20, 25.66) | 23.96 (21.63, 26.59) | 22.74 (20.57, 24.88) | < 0.001 |
| Basic vital signs | ||||
| HR | 80.00 (73.00, 88.00) | 73.00 (67.00, 81.00) | 80.50 (78.00, 96.00) | < 0.001 |
| SBP (mmHg) | 122.00 (111.00, 132.00) | 122.00 (111.50, 135.50) | 120.00 (110.00, 130.00) | 0.151 |
| DBP (mmHg) | 75.00 (69.00, 82.00) | 74.00 (68.00, 81.00) | 76.00 (70.00, 82.00) | 0.061 |
| Past history | ||||
| Smoking | 155 (40.90) | 67 (43.79) | 88 (38.94) | 0.346 |
| Drinking | 102 (26.91) | 51 (33.33) | 51 (22.57) | 0.020 |
| Diabetes | 28 (7.71) | 15 (10.95) | 13 (5.75) | 0.072 |
| Hyperlipidemia | 25 (6.96) | 15 (11.28) | 10 (4.42) | 0.014 |
| Hypertension | 63 (18.98) | 17 (16.04) | 46 (20.35) | 0.350 |
A total of 226 CMT patients with detailed imaging and laboratory examination data were recruited. Patients were divided into two groups based on metastatic sites: the pericardial and intracardiac metastasis groups. The baseline characteristics of patients in the three groups are presented in Table 2.
| All (n = 226) | Pericardial metastasis (n = 161) | Intracardiac metastasis (n = 65) | P value | |
| Age (year) | 58.89 ± 12.86 | 60.01 ± 12.51 | 56.14 ± 13.40 | 0.040 |
| Male | 152 (67.26) | 107 (66.46) | 45 (69.23) | 0.687 |
| BMI (kg/m2) | 22.56 ± 3.00 | 22.45 ± 2.97 | 22.96 ± 3.14 | 0.380 |
| Basic vital signs | ||||
| RR | 20.00 (20.00, 20.00) | 20.00 (20.00, 20.00) | 20.00 (19.50, 20.00) | 0.038 |
| HR | 80.50 (78.00, 96.00) | 81.00 (78.00, 96.00) | 80.00 (78.00, 95.00) | 0.683 |
| SBP (mmHg) | 120.00 (110.00, 130.00) | 120.00 (110.00, 130.00) | 120.00 (112.00, 126.00) | 0.775 |
| DBP (mmHg) | 76.00 (70.00, 82.00) | 76.00 (69.00, 81.00) | 78.00 (70.00, 85.00) | 0.175 |
| Temperature (°C) | 36.50 (36.40, 36.60) | 36.50 (36.40, 36.60) | 36.50 (36.40, 36.60) | 0.981 |
| SpO2 | 99.00 (99.00, 99.00) | 99.00 (99.00, 99.00) | 99.00 (99.00, 99.00) | 0.975 |
| Past history | ||||
| Smoking | 88 (38.94%) | 71 (44.10%) | 17 (26.15%) | 0.010 |
| Drinking | 51 (22.57%) | 45 (27.95%) | 6 (9.23%) | 0.001 |
| Diabetes | 13 (5.75%) | 11 (6.83%) | 2 (3.08%) | 0.245 |
| Hyperlipidemia | 10 (4.42%) | 9 (5.59%) | 1 (1.54%) | 0.140 |
| Hypertension | 46 (20.35%) | 33 (20.55%) | 13 (20.00%) | 0.933 |
| Clinical indicators | ||||
| EF (%) | 66.00 (63.00, 69.00) | 66.00 (62.00, 69.00) | 67.00 (63.00, 71.00) | 0.183 |
| D-Dimer (mg/L) | 1.06 (0.40, 2.95) | 0.84 (0.31, 2.36) | 2.16 (1.00, 3.67) | < 0.001 |
| PT (second) | 13.30 (12.20, 14.30) | 13.00 (12.00, 14.00) | 13.90 (12.95, 15.10) | < 0.001 |
| Fibrinogen (g/L) | 3.79 (2.65, 4.98) | 3.70 (1.83, 4.83) | 4.15 (3.23, 5.53) | 0.003 |
| INR | 1.11 (1.03, 1.35) | 1.11 (1.04, 2.20) | 1.10 (1.03, 1.22) | 0.203 |
| BNP (pg/mL) | 137.50 (56.00, 456.00) | 120.00 (54.00, 292.00) | 324.00 (110.00, 903.00) | 0.001 |
| Troponin (ug/L) | 1.00 (0.010, 7.75) | 0.022 (0.0060, 4.40) | 5.35 (2.43, 36.08) | < 0.001 |
| Treatment | ||||
| Operation | 104 (46.02%) | 73 (45.34%) | 31 (47.69%) | < 0.001 |
Significant differences in several key parameters were observed between the intracardiac and pericardial metastasis groups. Specifically, prothrombin time [13.90 (12.95, 15.10) vs 13.30 (12.15, 14.05), P = 0.002], D-dimer levels [2.16 (1.00, 3.67) vs 0.85 (0.30, 3.02), P = 0.001], B-type natriuretic peptide (BNP) levels [324.00 (110.00, 903.00) vs 136.50 (53.5, 289.00), P = 0.004], and troponin levels [5.35 (2.43, 36.08) vs 0.03 (0.00, 2.70), P < 0.001] were significantly higher in the intracardiac, indicating worse coagulation and cardiac function than the pericardial metastasis group. Approximately 46.02% of patients underwent surgical treatment, with pericardial metastasis at 45.34% and intracardiac metastasis at 47.69%.
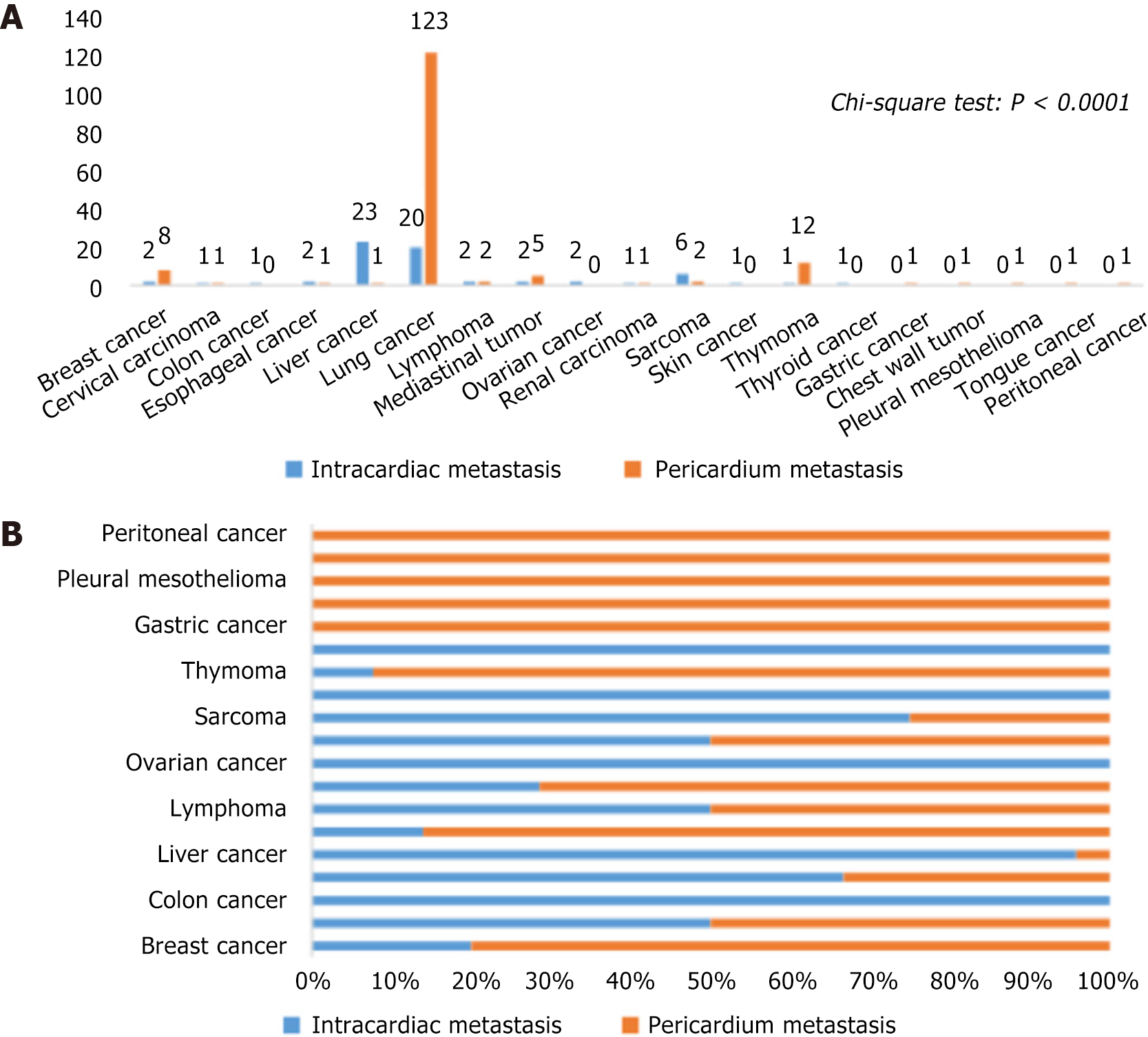
The primary tumor types linked to CMT are depicted in Figure 2. Lung and liver cancers were the most common primary tumor types in CMT, collectively accounting for 73.89%. Other cancer types included breast cancer, cervical carcinoma, chest wall tumor, colon cancer, esophageal cancer, lymphoma, mediastinal tumor, ovarian cancer, pleural mesothelioma, renal carcinoma, sarcoma, skin cancer, thymoma, thyroid cancer, and tongue cancer. Intriguingly, after excluding primary tumor types with < 5 cases, further χ2 tests showed a significant difference in primary tumor types between pericardial and intracardiac metastasis (P < 0.0001). Notably, patients with lung cancer (76.40% vs 30.77%) and thymoma (7.45% vs 1.54%) exhibited a higher prevalence of pericardial metastasis, while those with liver cancer (35.38% vs 0.62%) showed a higher prevalence of intracardiac metastasis.
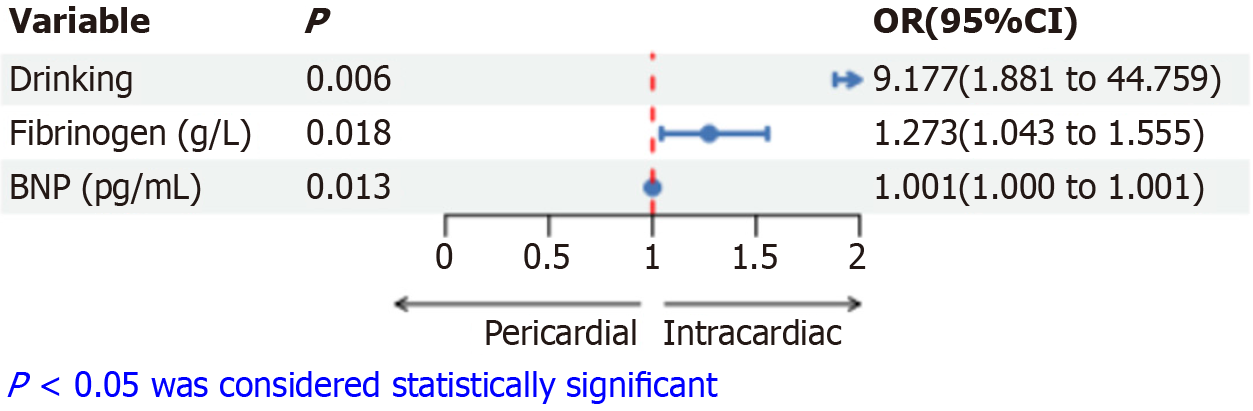
Binary logistic regression showed that drinking [odds ratio (OR) = 9.177, 95% confidence interval (95%CI): 1.881-44.759, P = 0.006], fibrinogen (OR = 1.273, 95%CI: 1.043-1.555, P = 0.018), and BNP (OR = 1.001, 95%CI: 1.000-1.001, P = 0.013) were associated with the CMT location (Figure 3). This suggests that CMT patients with a history of drinking and higher levels of fibrinogen and BNP are more likely to experience intracardiac metastasis rather than pericardium metastasis.
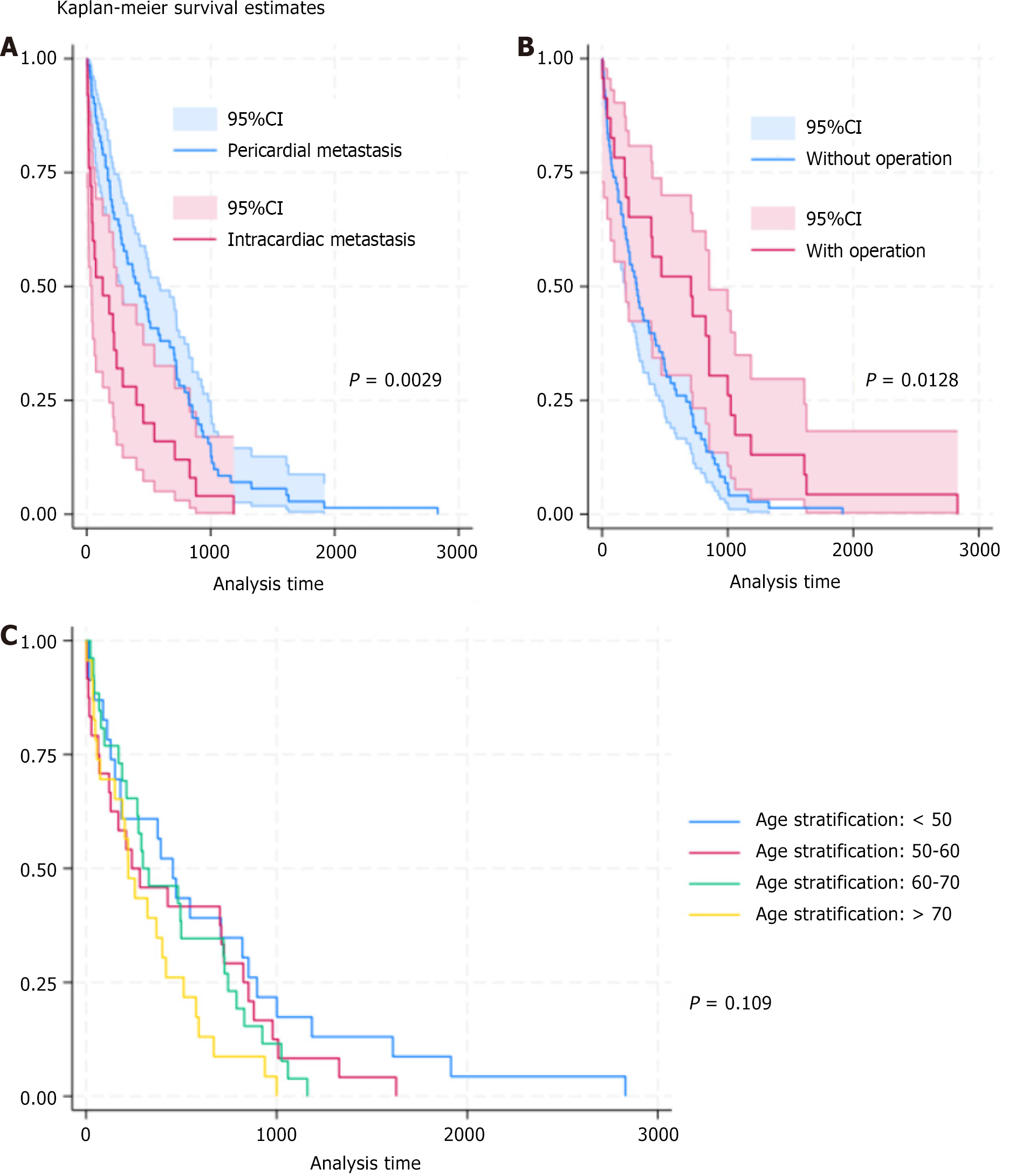
Survival analyses were conducted using age stratification and types of CMT as comparative factors (Figure 4). The results showed that the type of CMT significantly influenced survival outcomes. Patients in the pericardial metastasis group exhibited better overall survival than those in the intracardiac metastasis group (median survival time: 419 vs 129 days, log-rank test P = 0.0029). Surgery significantly improved the survival rate of patients. The median survival time was 275 days for patients who did not undergo surgery and 708 days for those who had surgery (log-rank test P = 0.0128). The survival curve with age stratification showed an inverse relationship between age and survival time. The Cox PH model revealed that advanced age and higher levels of BNP and troponin were associated with adverse survival periods (Table 3).
| Variable | Hazard ratio | Standard error | z | P > |z| | 95% confidence interval | |
| Metastasis | 2.046 | 0.775 | 1.89 | 0.059 | 0.975 | 4.297 |
| Age | 1.034 | 0.012 | 2.89 | 0.004 | 1.011 | 1.057 |
| BNP (pg/mL) | 1 | 0 | 2.3 | 0.022 | 1 | 1.001 |
| Troponin (ug/L) | 1.011 | 0.004 | 2.98 | 0.003 | 1.004 | 1.018 |
The present study analyzed patients with CMT from two medical centers, alongside control individuals from the CHARLS cohort, yielding the following key findings: (1) Male cancer patients were highly susceptible to CMT; (2) Among CMT patients, those with intracardiac metastases displayed worse coagulation function than those with pericardial metastases; (3) Lung and liver cancers were the predominant primary tumor types that resulted in cardiac metastases. Lung cancer predominantly metastasized to the pericardium, while liver cancer was more frequently associated with intracardiac metastasis; (4) Poor cardiac function, intracardiac metastasis, and advanced age were the main predictors for poor prognosis of CMT; and (5) Surgical treatment significantly prolonged patient survival. Overall, these findings highlight the intricate dynamics and prognostic indicators underlying cardiac metastases, potentially paving the way for the creation of more targeted and effective management strategies for affected patients.
Most published autopsy series have reported that tumors with the highest rate of cardiac metastasis are pleural mesothelioma and melanoma, followed by lung adenocarcinoma[9,10]. However, our research has demonstrated that lung cancer is the predominant primary tumor for cardiac metastases. A 27-year single-center study also corroborated this finding, showing that lung carcinomas accounted for the highest proportion of cardiac metastases (38%)[1]. This discrepancy may be because patients with pericardial metastasis often exhibit milder symptoms and have a better prognosis, while autopsies typically concentrate on intracardiac metastases, which have a worse prognosis.
Regarding the underlying mechanisms that lead to the frequent metastasis of lung cancer to the pericardium and liver cancer to the myocardium, we propose that liver cancer spreads to the heart through the bloodstream, whereas lung cancer directly infiltrates the pericardium. The “seed and soil” hypothesis may help explain cardiac metastases, suggesting that some tumors (the seeds) thrive better in the cardiac microenvironment (the soil). Additionally, factors like epithelial-mesenchymal transition, extracellular matrix remodeling, and cancer stem cells may also play key roles in the development of cardiac metastases[11].
Patients with intracardiac metastasis often have a worse prognosis and higher mortality risk during tumor resection surgery[12]. Overall, the predominant treatment strategy focuses on targeting the primary tumor in patients with advanced tumors that have metastasized to the heart. Palliative surgical intervention may be considered when a cardiac tumor is large and poses a risk of sudden death, or when the patient's overall health is stable. Generally, surgery does not lead to adverse clinical outcomes[13,14]. Our study indicates that surgical intervention can significantly extend median survival, offering new treatment options. Additionally, clinical practitioners should closely monitor cardiac function and age, as they significantly impact the prognosis of CMT.
To improve the diagnosis of pericardial and intracardiac metastases for better intervention and treatment, we recommend the following essential examinations: Echocardiography for identifying cardiac masses and assessing their size, cardiac magnetic resonance imaging for its diagnostic value, and routine blood biochemical tests.
In summary, clinicians should be vigilant for the potential of CMT in male patients with lung or liver cancer who exhibit cardiac symptoms. Negative prognostic factors for CMT include abnormal coagulation, impaired cardiac function, tumor invasion site, and advanced age. Surgical intervention is recommended as the primary treatment.
| 1. | Garcia Brás P, Branco LM, Galrinho A, Timóteo AT, Branco Mano T, Ferreira V, Cardoso I, Castelo A, Pinto E, Coelho P, Rodrigues R, Leal A, Bravio I, Fragata J, Cruz Ferreira R. Malignant Primary and Metastatic Cardiac Tumors: A Single-Center 27-Year Case Review. Oncology. 2023;101:292-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 2. | Bussani R, De-Giorgio F, Abbate A, Silvestri F. Cardiac metastases. J Clin Pathol. 2007;60:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 495] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 3. | Oliveira SM, Gonçalves A, Cruz C, Almeida J, Madureira AJ, Amendoeira I, Maciel MJ. Cardiac metastasis from epidermoid esophageal cancer mimicking anterior myocardial infarction. Rev Port Cardiol. 2012;31:163-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Hilal T, Anthony LB, Sorrell VL. Unexpected Cardiac Masses. JAMA Oncol. 2015;1:1343-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 5. | Uetani T, Inaba S, Nishiyama H, Yamaguchi O. Metastatic Cardiac Tumor-Induced Acute Coronary Syndrome. JACC Cardiovasc Interv. 2020;13:e179-e180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Stefàno PL, Barletta G, Andrei V, Cerillo AG, Nesi G, Pilato G, Pradella S, Santi R, Del Bene MR. Cardiac Metastasis of Sacral Chordoma. Ann Thorac Surg. 2021;111:e319-e321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43:61-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3155] [Cited by in RCA: 3375] [Article Influence: 281.3] [Reference Citation Analysis (0)] |
| 8. | Vetter TR, Schober P. Regression: The Apple Does Not Fall Far From the Tree. Anesth Analg. 2018;127:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Schober P, Vetter TR. Logistic Regression in Medical Research. Anesth Analg. 2021;132:365-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 174] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 10. | Schober P, Vetter TR. Survival Analysis and Interpretation of Time-to-Event Data: The Tortoise and the Hare. Anesth Analg. 2018;127:792-798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 166] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 11. | Katalinic D, Stern-Padovan R, Ivanac I, Aleric I, Tentor D, Nikolac N, Santek F, Juretic A, Plestina S. Symptomatic cardiac metastases of breast cancer 27 years after mastectomy: a case report with literature review--pathophysiology of molecular mechanisms and metastatic pathways, clinical aspects, diagnostic procedures and treatment modalities. World J Surg Oncol. 2013;11:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Luo L, Luo X. Diagnosis and treatment of right ventricular metastasis from esophageal squamous cell carcinoma: An unusual case report and a literature review. Medicine (Baltimore). 2023;102:e36404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Maebayashi A, Nagaishi M, Nakajima T, Hata M, Xiaoyan T, Kawana K. Successful surgical treatment of cardiac metastasis from uterine leiomyosarcoma: A case report and literature review. J Obstet Gynaecol Res. 2020;46:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (3)] |
| 14. | Mkalaluh S, Szczechowicz M, Torabi S, Sabashnikov A, Mashhour A, Karck M, Weymann A. Surgical Treatment of Cardiac Metastases: Analysis of a 13-Year Single-Center Experience. Thorac Cardiovasc Surg. 2019;67:659-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/