Published online Nov 26, 2025. doi: 10.4330/wjc.v17.i11.113411
Revised: September 6, 2025
Accepted: October 21, 2025
Published online: November 26, 2025
Processing time: 88 Days and 18.6 Hours
Supraventricular tachycardia (SVT) is a frequent cause of emergency presentations. Troponin elevation is common, but its clinical significance remains uncertain and may trigger unnecessary downstream testing. In this mini-review, we aimed to review the prevalence, mechanisms, prognostic relevance, and management of troponin elevation in adult paroxysmal SVT. A narrative review was conducted using PubMed and EMBASE (2000-2025) with MeSH terms related to SVT and troponin. Eligible studies included original research or registry analyses in adults with paroxysmal SVT. Pediatric and atrial fibrillation/flutter cohorts were excluded. Additional data were obtained from reference lists and expert commentaries. Troponin elevation occurs in approximately 30%-50% of adult SVT cases, primarily reflecting a tachycardia-induced supply-demand imbalance or myocardial stretch, rather than plaque rupture. Short-term registry data suggest potential prognostic associations, but long-term outcomes remain inconsistent and are largely determined by comorbidities and underlying coronary artery disease. Troponin-driven management often leads to increased admissions, consultations, and additional testing without a demonstrable benefit. Troponin elevation in SVT is frequent but usually benign. Routine measurement in all patients is not justified. A selective, risk-based approach – focused on ischemic symptoms, electrocardiogram changes, or high-risk clinical features – offers more appropriate, efficient, and patient-centered care.
Core Tip: Supraventricular tachycardia (SVT) is a frequent emergency presentation where troponin elevation is common but often misinterpreted. This narrative review highlights that in adult patients with paroxysmal SVT, elevated troponin usually reflects supply-demand imbalance or myocardial stretch rather than plaque rupture. Although troponin testing may occasionally reveal underlying coronary artery disease or acute coronary syndrome, most elevations have limited prognostic value. Overuse of troponin can drive unnecessary admissions and procedures; therefore, selective, risk-based testing represents the most appropriate and patient-centered approach.
- Citation: Özlek B, Tanık VO, Barutçu S. Troponin elevation in supraventricular tachycardia: A narrative review. World J Cardiol 2025; 17(11): 113411
- URL: https://www.wjgnet.com/1949-8462/full/v17/i11/113411.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i11.113411
Supraventricular tachycardia (SVT) is a common arrhythmia in adult emergency department (ED) presentations[1]. While SVT is often hemodynamically tolerated and responds well to acute therapies, evaluation frequently includes cardiac biomarkers, such as troponin[2]. Although classically a marker of myocardial injury in the setting of acute coronary syndrome (ACS), troponin elevation is now recognized in a variety of non-ischemic conditions[3]. Episodes of SVT may be associated with elevated troponin even when obstructive coronary artery disease (CAD) is absent[2]. This creates a clinical challenge: What does troponin elevation signify in SVT, and how should it guide management? On one hand, elevations may reflect myocardial ischemia triggered by tachycardia or concurrent ACS[4]. On the other hand, they may result from transient supply-demand imbalance or myocardial stretch, with limited long-term consequences[5]. This narrative review summarizes the prevalence, mechanisms, prognostic implications, and management considerations of troponin elevation in adult patients with paroxysmal SVT (atrioventricular nodal re-entrant tachycardia, atrioventricular re-entrant tachycardia, atrial tachycardia) presenting to the ED. The focus is on adult paroxysmal SVT and troponin elevation, while pediatric SVT and atrial fibrillation/flutter (AFL/AF) are not the primary focus. The objective is to synthesize available evidence, highlight areas of consensus and uncertainty, and provide practical clinical guidance. Evidence from observational studies, systematic reviews, and expert commentaries is synthesized, with areas of consensus and ongoing uncertainty highlighted. Overall, the aim is to clarify whether troponin elevation in SVT represents a benign epiphenomenon or a signal of underlying pathology, and to outline practical approaches to risk stratification and management.
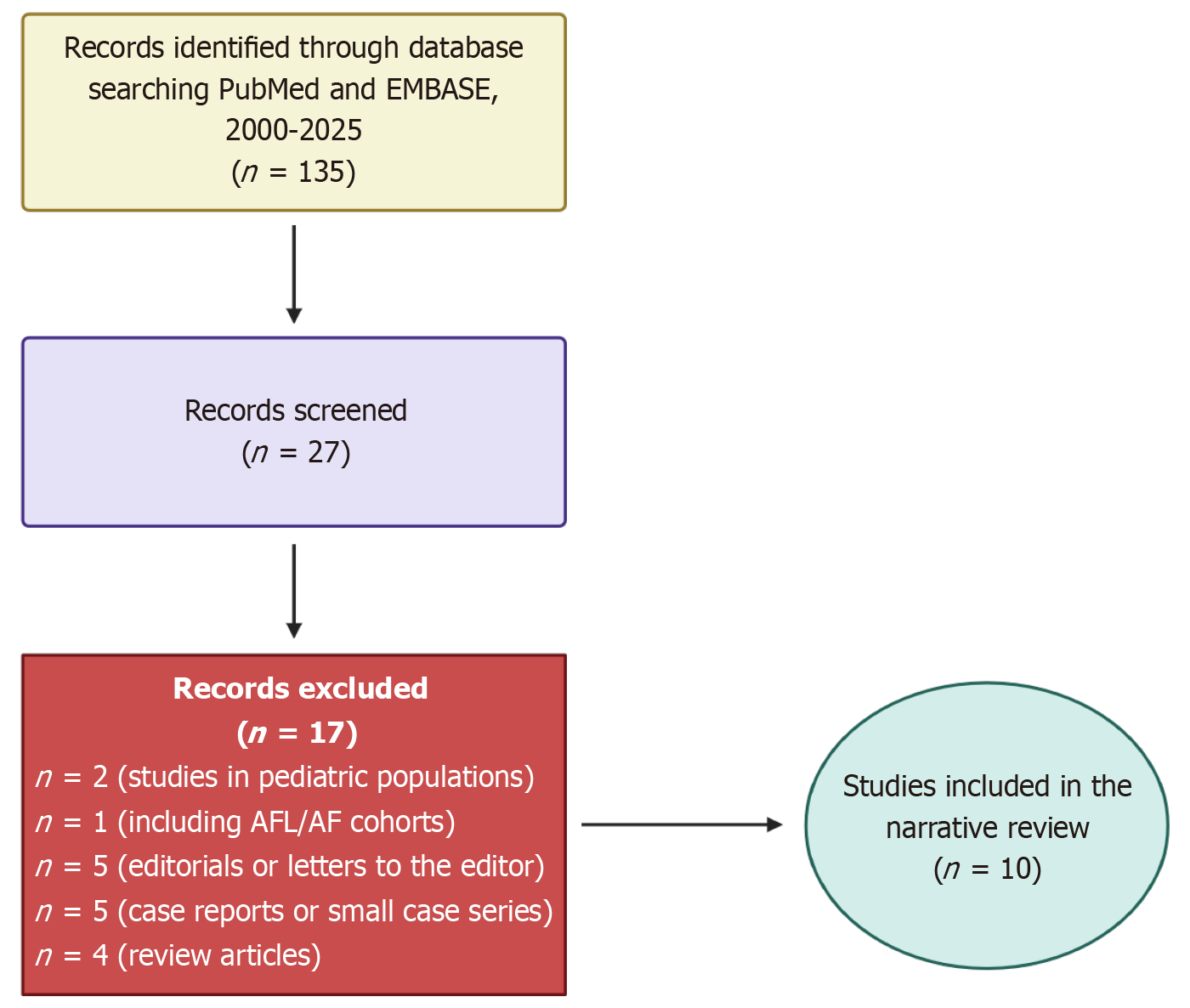
We conducted a focused literature search in PubMed and EMBASE from January 2000 through September 2025. Keywords and MeSH terms included “supraventricular tachycardia”, “paroxysmal supraventricular tachycardia”, “atrioventricular nodal reentrant tachycardia”, “atrioventricular reentrant tachycardia”, “atrial tachycardia”, and “troponin”. Eligible studies were original articles or registry analyses reporting troponin measurements in adult patients with paroxysmal SVT. We excluded case reports, non-English language publications without available translations, and studies restricted to pediatric or AFL/AF populations. Reference lists of included articles and relevant reviews were also screened for additional studies. Evidence from expert commentaries was considered to contextualize findings and highlight areas of uncertainty. The overall search and selection process is summarized in Figure 1.
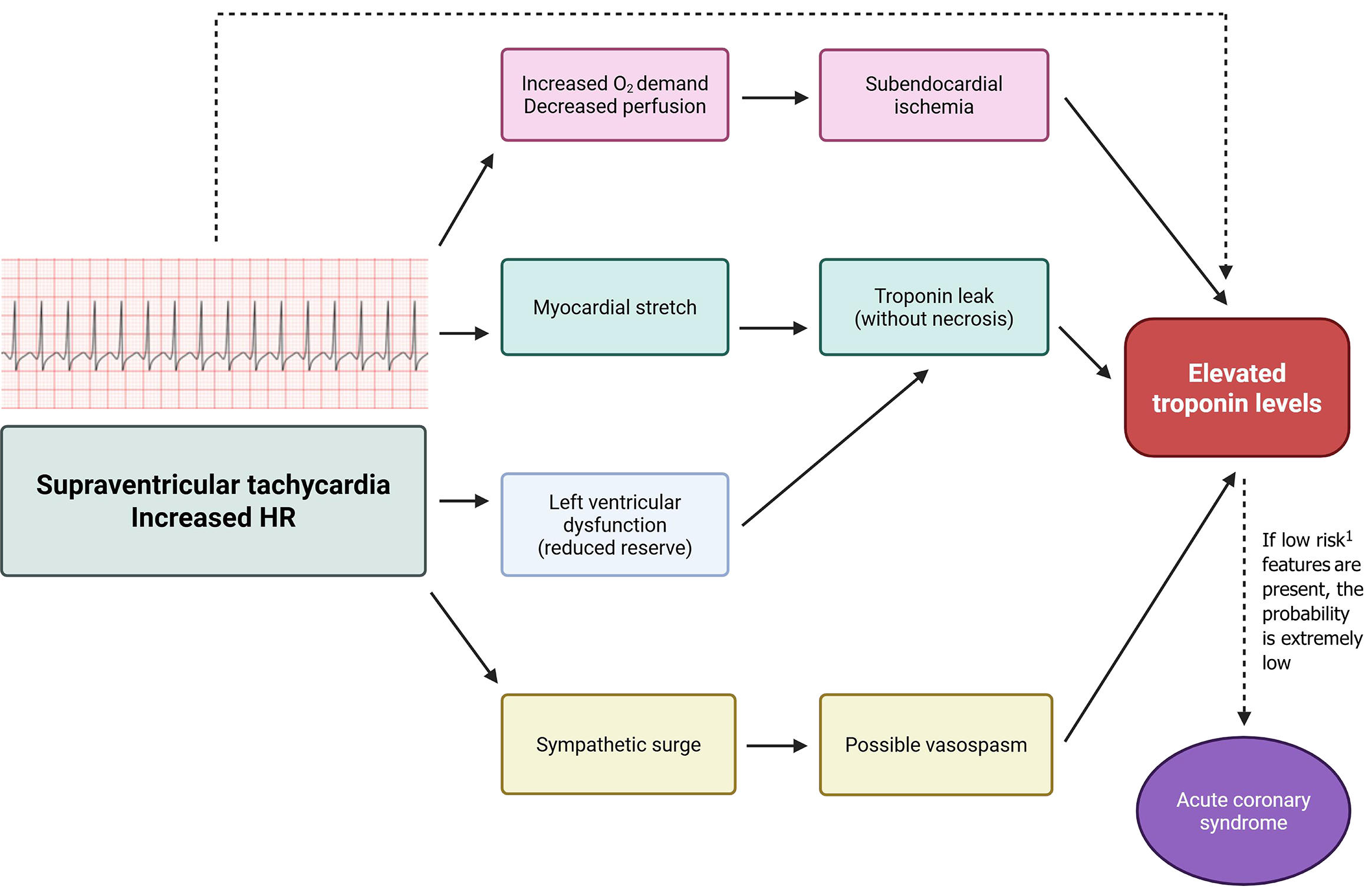
Several mechanisms have been proposed to explain troponin release during adult SVT in the absence of acute plaque rupture, and are summarized in Figure 2. The most widely accepted cause is a supply-demand mismatch [type II myocardial infarction (MI)] due to extreme heart rates[6]. Rapid tachycardia increases myocardial oxygen demand while shortening diastole and coronary perfusion, which can lead to subendocardial ischemia[6]. Supporting this, troponin-positive SVT patients frequently demonstrate transient ST-segment depression on electrocardiogram (ECG), consistent with demand ischemia[7]. In a Canadian study, patients with affected cases had significantly higher mean heart rates compared with troponin-negative cases, and the degree of elevation correlated with the peak rate[8]. These findings suggest tachycardia-induced myocardial strain as a major contributor. It is hypothesized that myocyte stretch and transient ischemia allow troponin leakage without resulting in irreversible necrosis[3].
Pre-existing cardiac dysfunction represents another factor. Patients with reduced left ventricular (LV) ejection fraction or structural heart disease may have limited reserve, making troponin elevation more likely during SVT[9]. In one cohort, impaired LV systolic function independently predicted biomarker rise[9].
Coronary vasospasm has also been reported in small series, with up to 74% prevalence despite normal coronary angiograms, although its causal link with SVT remains speculative and likely represents a rare, alternative mechanism[10]. Direct evidence that SVT itself induces spasm is lacking; however, elevations are more commonly attributed to sympathetic surge and oxygen debt during arrhythmia[11].
Importantly, troponin elevation in SVT does not necessarily indicate acute thrombotic ACS. Evidence suggests that significant CAD is seldom the underlying cause. Healey, for example, found that 95% of patients with troponin T > 0.03 μg/L had no critical coronary lesions on follow-up; only one, with known CAD, required intervention[12]. Thus, in most otherwise healthy patients, troponin release reflects type II injury rather than type I infarction. This pattern mirrors that of other non-ACS conditions (e.g., heart failure, pulmonary embolism, sepsis), where troponin conveys prognostic information without indicating an acute occlusion[13-15].
In summary, SVT can cause mild myocardial injury through demand ischemia and myocardial stretch, particularly at high heart rates or in patients with reduced reserve. These mechanistic insights explain why elevations are observed without ACS and highlight the importance of contextual interpretation – investigating underlying disease when appropriate, but avoiding automatic classification of all troponin rises as acute MI.
Research over the past two decades has consistently shown that troponin elevation is common in SVT[16]. The reported prevalence varies according to the study population, assay sensitivity, and whether testing was performed routinely or selectively[17]. Overall, about one-third of adults presenting with SVT have an elevated troponin during their ED visit. For example, a Canadian series reported troponin T levels ≥ 0.03 μg/L in 33% of hospitalized patients with SVT[9], while a United States study found troponin I levels ≥ 0.06 ng/mL in 37% of patients across two hospitals[9]. A 2019 systematic review estimated the overall prevalence to be 30%-40%[18], and a 2024 meta-analysis reported a pooled prevalence of approximately 46% among ED patients with SVT, although heterogeneity was high[2]. Thus, up to half of SVT presentations may be associated with a troponin rise of uncertain significance, indicating that this is a frequent rather than exceptional finding.
Assay type and cut-off strongly influence prevalence estimates. Earlier studies[19] using conventional conventional cardiac troponin I (cTnI) assays with higher thresholds (e.g., > 0.05 ng/mL or 0.06 ng/mL) likely underestimated the frequency. With high-sensitivity assays [high-sensitivity troponin T (hs-TnT)], more patients are identified as “positive” due to the detection of smaller injuries. Ghersin et al[20] reported a 43% prevalence using a sensitive cTnI assay. In dialysis patients with SVT, cTnI elevation was present in 82.5%, reflecting both baseline troponin abnormalities in renal disease and the effect of tachycardia[21]. These findings highlight how assay characteristics and comorbidities influence interpretation.
Clinical factors have also been linked to troponin release. A higher heart rate during the arrhythmia is the most consistent association[4]. Episode duration, in contrast, often shows no significant difference between patients with troponin-positive and troponin-negative results[4]. LV dysfunction has been identified as a predictor in some cohorts[9] and a history of CAD in at least one study[22]. Interestingly, in the Sherbrooke study[8], troponin-positive patients did not have more traditional risk factors or CAD compared with troponin-negative patients, suggesting that even low-risk individuals may exhibit troponin elevations due to the hemodynamic stress of SVT.
Ultimately, it is crucial to recognize the influence of selection bias. In clinical practice, troponin is often ordered when concern exists (e.g., chest pain, older age, risk factors)[23]. Reported prevalence, therefore, depends on whether testing is routine or selective. Studies applying troponin universally may find a lower prevalence than those restricted to higher-risk or symptomatic patients, where physicians are more inclined to order testing.
Interpretation of troponin elevation in patients with paroxysmal SVT requires awareness of the universal definition of MI, which distinguishes between acute and chronic myocardial injury, and between type 1 MI and type 2 MI[24]. Additionally, clinical application relies on assay-specific 99th percentile cut-offs and dynamic changes (delta criteria) derived from rapid diagnostic algorithms[25-27]. To provide clarity, these key definitions and thresholds are summarized in Table 1[24-28]. The prognostic significance of troponin elevation in the setting of SVT has been evaluated in multiple original studies, with heterogeneous results (Table 2)[5,9,16,20,21,29-33]. Overall, while troponin release during SVT episodes is common, its independent predictive value for adverse outcomes remains inconsistent and appears to be influenced by patient population, comorbidities, and study design.
| Category | Details |
| Universal definition of myocardial infarction categories | Acute myocardial injury: Hs-cTn ≥ 99th percentile + significant rise/fall. Chronic myocardial injury: Persistent elevation without dynamic change. Type 1 MI: Acute injury + ischemia evidence due to coronary thrombosis/plaque rupture. Type 2 MI: Acute injury + imbalance in supply-demand (e.g., tachyarrhythmia, hypotension) with supportive clinical/electrocardiogram/echo evidence |
| 99th percentile cut-offs (assay-specific examples)1 | Roche hs-cTnT (gen 5): 14 ng/L (general); some United States reports: 14 ng/L women, 22 ng/L men. Abbott hs-cTnI: 17 ng/L (women), 35 ng/L (men). Siemens hs-cTnI: Approximately 18 ng/L (women), approximately 27 ng/L (men) |
| Delta thresholds (European Society of Cardiology 0/1 hour and 0/2 hours algorithms; assay-specific)1 | Roche hs-cTnT 0/1 hour: Rule-out: < 5 ng/L or < 12 ng/L with Δ < 3 ng/L; rule-in: ≥ 52 ng/L or Δ ≥ 5 ng/L. Roche hs-cTnT 0/2 hours: Rule-out: Δ ≤ 3 ng/L below 99th percentile; rule-in: Δ ≥ 10 ng/L. Abbott hs-cTnI 0/1 hour (examples): Rule-out: Baseline < 4 ng/L + Δ < 2 ng/L; rule-in: ≥ approximately 64 ng/L or Δ ≥ approximately 6 ng/L. Abbott hs-cTnI 0/2 hours: Rule-out Δ < 2 ng/L; rule-in Δ ≥ 15 ng/L |
| Ref. | Country/setting | Study design | Number (analyzed) | Population and SVT subtype(s) | Troponin assay and cut-off | Sampling timing | Prevalence of elevated troponin (%) | Outcomes and follow-up | Main result (direction) | Effect size | Adjusted covariates | Risk of bias | Key limitations/notes |
| Aletras et al[5], 2025 | Greece, Venizelio General Hospital of Heraklion | Retrospective, single-center observational study | 120 | Adults ≥ 18 years, PSVT (AVNRT, AVRT, AT); excluded AFL/AF and structural heart disease | Siemens hs-cTnI, > 99th percentile (sex-specific; 53.5 pg/mL men, 38.6 pg/mL women) | ED presentation; repeat at 3 hours in 80% | 48.3% | 1-year SVT recurrence, rehospitalization, ablation, mortality | Troponin elevation common but not prognostic; independent predictors were chest pain, absence of prior SVT, higher HR, lower SBP | HR cut-off 165 bpm predicted conventional cardiac troponin + (area under the curve = 0.697, sensitivity = 62%, specificity = 73%); CAD found in only 4% | Multivariable logistic regression (HR, SBP, prior SVT, chest pain) | Moderate (retrospective, single-center, limited generalizability) | No routine advanced imaging; incomplete CAD evaluation; predominantly female cohort; retrospective data collection |
| Camp et al[29], 2025 | United States, TriNetX database | Retrospective, propensity-matched cohort | 62582 (31k vs 31k) | ED, first-time SVT (International Classification of Diseases, 10th Revision I47.1) | Mixed assays, within 24 hours | ED arrival | 21.6% | 30-day MACE (MI, heart failure, stroke, death) | Increased 30-day MACE in troponin + group | Risk difference = 11.1%, P < 0.001 | Age, sex, comorbidities | Moderate | Confounding by indication, registry data |
| Laursen et al[16], 2025 | Denmark, national registry | Retrospective registry cohort | 1203 | De novo PSVT, no cardiovascular disease | High-sensitivity troponin T (roche ≥ 14 ng/L) | First 24 hours hospitals | 65.8% | 30-day and 1-year mortality; composite CV events | Increased 30-day mortality only | HR significant at 30 days; NS at 1 year | Age, comorbidities, laboratories | Low-moderate | No electrocardiogram data, registry limits |
| Chen et al[30], 2023 | Taiwan, 4 hospitals | Retrospective, multicenter | 124 | Elderly ≥ 65, SVT (exclude AFL/AF) | The cTnI (Beckman ≥ 0.04) | ED, peak | 31.5% | 5-year MACE and recurrence | No prognostic effect; CAD history predicted | CAD is transformed into MACE HR = 4.30, P = 0.01 | Age, smoking, prior SVT | Moderate | Small, Asian elderly pop, conventional assay |
| Noorvash et al[31], 2018 | United States, Texas academic ED | Retrospective chart review | 46 | Adults with SVT, HEART 1-6 | The cTnI > 0.05 ng/mL | ED | Some positive | Admissions, 3-months MACE | Increased admissions, no MACE impact | Admissions 86% vs 21%, P = 0.006 | HEART score | High risk | Tiny cohort, resource utilization focus |
| Ghersin et al[20], 2020 | Israel, Rambam Medical Center | Retrospective, single center | 165 (131 with cTnI) | Acute PSVT episodes | The cTnI > 0.028 ng/dL | ED admission | 43% | 23 ± 7 months follow-up, composite death/MI/percutaneous coronary intervention | Adverse outcomes only if CAD present | HR = 3.3, P = 0.05 (CAD subgroup) | HR > 150, CAD | Moderate | Limited sample, few events |
| Carlberg et al[32], 2011 | United States (Virginia ED) | Retrospective chart review | 51 | Adults with PSVT | The cTnI, Abbott, cut-off 0.02 ng/dL | ED | 29% | 30-day outcomes | No prognostic effect; 2 non-ST-elevation MI identified | Descriptive | None | Moderate | Small, variable sampling |
| Wang et al[21], 2022 | Taiwan, 5 hospitals (dialysis) | Multicenter retrospective | 62 | Adult ESKD on dialysis with SVT | The cTnI Beckman ≥ 0.04 | ED | 82.5% | 3-year and 6-week MACE | No prognostic value; CAD predicted MACE | HR CAD 2.73 (1.01–7.41) | Hypertension, LVEF | Moderate | ESKD-specific, high baseline risk |
| Chow et al[9], 2010 | United States (Johns Hopkins) | Retrospective cohort | 78 | Hospitalized SVT (AVNRT/AVRT/AT) | The cTnI Beckman ≥ 0.06 | 0.5-8 hours after SVT | 37% | ≥ 1 year, death/MI/CV rehospitalization | Troponin + predicted adverse outcomes | HR = 3.67 (1.22-11.1), P = 0.02 | Peak HR, LVEF | Moderate | Retrospective, limited centers |
| Bukkapatnam et al[33], 2010 | United States (University of California Davis) | Retrospective cohort | 104 (80 tested) | Adults with SVT, ED | The cTnI > 0.07 ng/mL | ED | 48% | CAD by testing | Troponin elevation not linked to CAD | NS | CAD risk factors | Moderate | Referral bias, CAD not prognosis |
Large registry-based analyses provide some evidence for short-term prognostic utility. In the largest study to date, Camp et al[29] analyzed more than 62000 patients from a United States claims database and demonstrated that patients with SVT and elevated troponin had significantly higher rates of 30-day major adverse cardiovascular events (MACE), although confounding by indication could not be fully excluded. Similarly, a Danish nationwide registry by Laursen et al[16] found that among 1203 patients with de novo paroxysmal SVT, hs-TnT elevation was strongly associated with increased 30-day mortality; however, this association did not persist at one year. These large-scale data suggest that troponin release may reflect acute hemodynamic stress during SVT with short-term prognostic implications.
In contrast, smaller observational cohorts have yielded more nuanced findings. Aletras et al[5], in a retrospective single-center study of 120 adults with paroxysmal SVT, found that nearly half of patients (48%) had elevated hs-cTnI, yet troponin positivity was not predictive of recurrence, rehospitalization, ablation, or mortality at one year; instead, chest pain, absence of prior SVT, higher heart rate, and lower systolic blood pressure were independent predictors of troponin elevation. Chen et al[30], in a Taiwanese multicenter study of 124 elderly patients, reported that conventional troponin I had no prognostic role for long-term outcomes, with a history of CAD – not troponin – emerging as the key predictor of MACE. Noorvash et al[31] studied 46 patients in a United States ED and observed that elevated troponin mainly increased hospitalization and resource utilization without improving risk stratification for MACE. Similarly, Ghersin et al[20] in Israel (n = 165) found that troponin elevation predicted adverse outcomes only in the subgroup with underlying CAD.
Earlier single-center studies also failed to demonstrate consistent prognostic value. Carlberg et al[32] reviewed 51 United States patients with paroxysmal SVT and found no adverse 30-day outcomes attributable to troponin positivity, aside from two cases of concomitant non–ST-elevation MI. In a special population of dialysis patients, Wang et al[21] reported an extremely high prevalence (83%) of troponin elevation among 62 patients; however, prognosis was driven by a history of CAD rather than troponin status itself. Conversely, Chow et al[9] provided evidence from 78 hospitalized patients that elevated troponin independently predicted adverse cardiovascular outcomes during follow-up. In contrast, Bukkapatnam et al[33] found no relationship between troponin elevation and underlying CAD among 104 admissions, emphasizing the nonspecificity of troponin in this context.
Taken together, these 10 studies highlight several recurring themes. First, troponin elevation during SVT is frequent, with a prevalence ranging from approximately 20% to over 60%, depending on the assay type, cut-off, and patient population. Second, while large registry-based analyses suggest possible short-term prognostic utility, smaller cohorts generally demonstrate limited or no independent predictive value, especially in the absence of structural heart disease or CAD. Third, troponin appears to serve more as a marker of tachycardia severity, myocardial oxygen demand, or comorbidity burden rather than as a direct prognostic biomarker in isolated SVT. Finally, across nearly all studies, methodological limitations – such as small sample sizes, retrospective design, heterogeneity of assays, and low event rates – limit the generalizability of the findings.
In summary, current evidence suggests that troponin elevation in SVT is common but not consistently predictive of prognosis. Its clinical utility appears context-dependent and should be interpreted cautiously, particularly in pediatric patients and those without significant comorbidities.
Troponin testing in SVT can lead to a cascade of admissions, consultations, and downstream diagnostics, resulting in a significant impact on the health system. Multiple studies consistently show that biomarker-driven management increases utilization without improving patient outcomes.
In one representative series, troponin-positive patients were several times more likely to be admitted, receive a cardiology consultation, and remain hospitalized longer compared with troponin-negative patients – yet no excess of MACE or deaths was observed on follow-up[31]. These findings illustrate how a single biomarker result can disproportionately influence decision-making, independent of symptoms or ECG findings.
Other cohorts support this observation. An Australian review found that troponin testing in SVT frequently prompted stress testing or invasive angiography, but the diagnostic yield was minimal[18]. Similarly, in a small series, the majority of troponin-positive patients underwent advanced imaging, yet clinically significant CAD was detected in only one case, already known to have coronary disease[8]. Troponin-negative patients also rarely showed abnormalities when tested, underscoring the low predictive value of indiscriminate measurement. In line with these observations, Ashok et al[34] reported that although troponin testing was performed in nearly half of SVT presentations, elevations were frequent but typically rate-related rather than ischemic, and acute myocardial ischemia was ultimately confirmed in only a very small minority. The authors concluded that troponin has poor predictive value in this context and should be guided by clinical suspicion rather than used indiscriminately.
Beyond the low diagnostic yield, there are system-level consequences. Increased admissions contribute to ED crowding, longer lengths of stay, and higher costs[35]. Misinterpretation of troponin as ACS can also expose patients to antiplatelet or anticoagulant therapy unnecessarily, with potential bleeding risk. These patterns highlight the importance of selective testing. While a minority of patients with true ACS clearly benefit from troponin measurement, routine testing in every SVT presentation primarily generates additional utilization without tangible clinical benefit.
Overall, the available data highlight that troponin-driven management often increases hospital utilization without measurable prognostic benefit, underscoring the need for more evidence-based testing strategies in SVT.
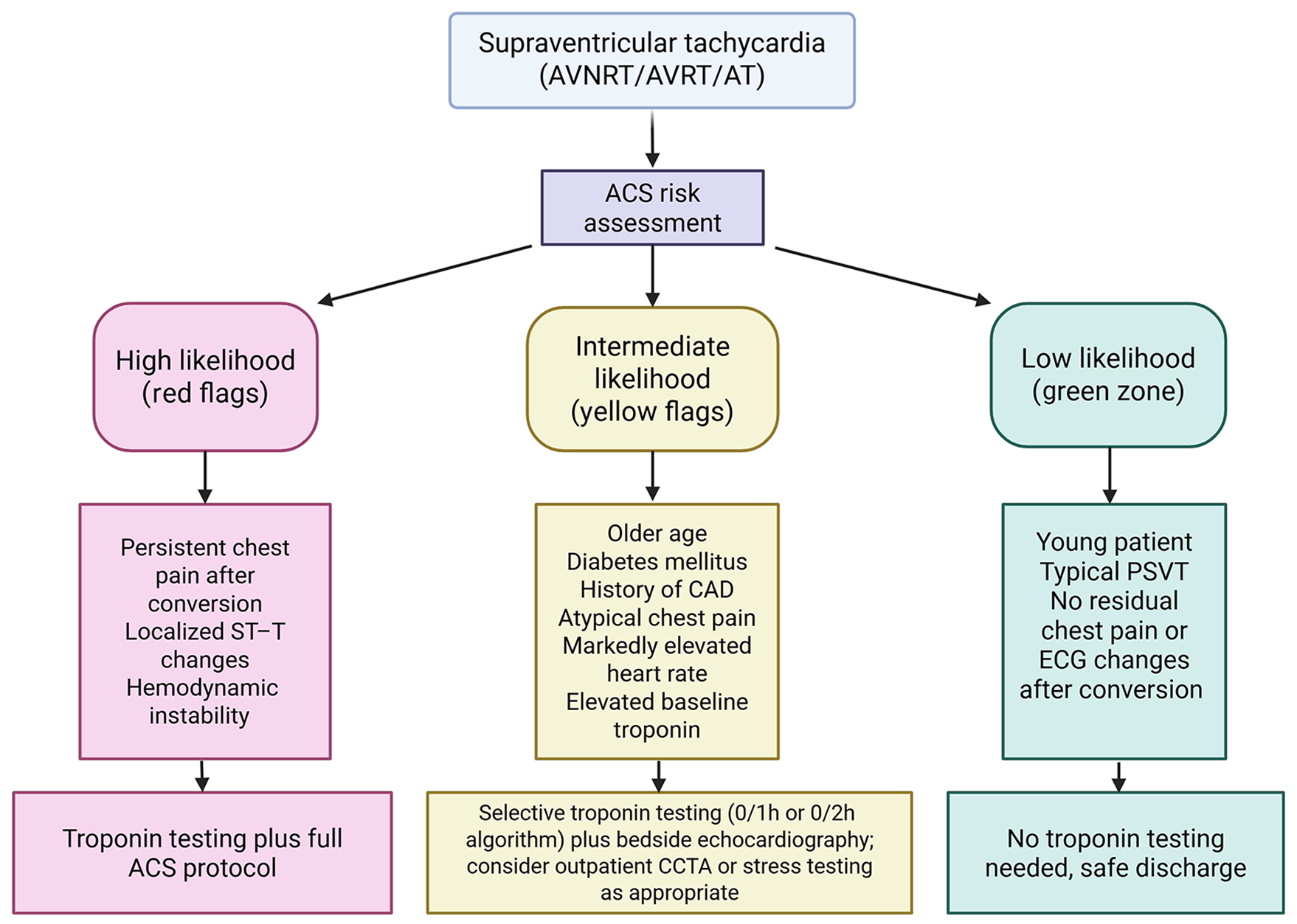
Given the above, an important clinical task is risk stratification, which involves distinguishing between SVT patients with possible ischemia or underlying CAD and those with a benign troponin rise. This distinction helps determine when troponin testing and subsequent interventions are appropriate. Current evidence supports a tailored approach guided by the patient’s risk profile and presentation, rather than routine testing for all[5,35].
Expert opinion suggests that troponin should not be ordered reflexively in young, otherwise healthy SVT patients without ischemic symptoms[36]. Low-risk features include the absence of chest pain or concerning symptoms (e.g., severe dyspnea), no significant ischemic changes on the ECG beyond rate-related alterations, and minimal cardiovascular risk factors[28]. In such cases, the probability of ACS is extremely low, and troponin testing is more likely to complicate rather than clarify management. By contrast, higher-risk presentations warrant a more thorough evaluation, including measurement of troponin. High-risk features include ongoing chest pain suggestive of angina, marked ischemic ECG abnormalities (beyond rate-related ST depression, such as deep or localized depressions or T-wave inversions), hemodynamic instability, known CAD, or significant comorbidity burden (e.g., older age, diabetes)[28]. In these settings, troponin elevation should not be dismissed as “just SVT” but may represent a clue to concomitant ACS or severe underlying CAD[37]. If clinical suspicion of ACS persists after SVT termination, troponin testing and appropriate management, including anti-ischemic therapy, are indicated[23]. Ultimately, interpretation must remain contextual: A rise accompanied by chest pain should be treated as ACS, whereas an isolated elevation in a stable patient with normal post-conversion ECG may justify conservative management[2]. Coronary computed tomography angiography (CCTA) has also been proposed as a rapid means of excluding CAD in troponin-positive SVT, with small studies reporting few obstructive lesions[4]. This strategy may help avoid unnecessary invasive angiography in select patients[38].
Regarding therapy, when no ACS evidence exists aside from troponin, empirical antiplatelet or anticoagulation treatment is not recommended[28]. Management should focus on the arrhythmia itself and controlling long-term risk factors. Definitive treatment of recurrent SVT, such as elective ablation, may be considered[39]. While troponin elevation alone does not mandate earlier ablation, referral for cardiology or electrophysiology follow-up in recurrent cases is reasonable.
Based on current evidence, several practical considerations may help guide the management of SVT patients in the ED with respect to troponin. These are synthesized into a possible framework illustrated in Figure 3.
Routine troponin testing is generally not warranted in all SVT presentations. Instead, it may be most appropriate to reserve troponin measurement for patients with clinical features suggestive of myocardial ischemia or with significant cardiovascular risk factors. In low-risk individuals (e.g., younger patients without CAD history or concerning symptoms), cardiac enzymes seldom alter management and may contribute to unnecessary downstream testing and interventions[35].
When troponin is obtained and found to be elevated, interpretation should be guided by the overall clinical context. Careful assessment of chest pain characteristics, dynamic ECG changes, and, where appropriate, repeat troponin measurements may help distinguish an ACS-related rise from a transient SVT-associated elevation. In the presence of features suggestive of ACS, such as ongoing chest pain, a rising troponin trend, or localized ischemic ECG changes, management consistent with ACS protocols (including antiplatelet therapy, hospital admission, and cardiology consultation) is advisable[28]. Conversely, in patients who are pain-free after SVT termination and lack other ischemic features, initiating full ACS therapy solely on the basis of an isolated troponin elevation may not be necessary.
For SVT patients with elevated troponin but no evidence of acute MI, further evaluation for underlying heart disease may be considered before discharge or in the early outpatient setting. Depending on the patient's risk profile and symptoms, this may include non-invasive testing, such as an outpatient stress test, myocardial perfusion imaging, or CCTA, once the acute episode has resolved. Allowing time for any transient tachycardia-related myocardial stunning to normalize can help avoid misinterpretation. If these investigations are normal, clinicians can be reassured that the troponin rise was likely benign; if abnormalities are detected, referral for appropriate cardiac interventions is warranted. In selected patients, echocardiography may also be useful for evaluating ventricular function and excluding structural cardiomyopathy[4,9].
Follow-up after an SVT episode is advisable, particularly for patients with recurrent or symptomatic episodes. Referral to an electrophysiologist for consideration of catheter ablation or medical therapy may be appropriate in selected cases, especially when episodes are frequent or when troponin-positive presentations raise concern about hemodynamic impact. While troponin elevation alone is not an established indication for ablation, ensuring ongoing cardiology or electrophysiology follow-up can help guide long-term management, reduce recurrence, and potentially prevent unnecessary future emergency visits[39].
Clear communication with patients is essential when discussing troponin results. Elevations may cause significant anxiety if patients are simply told that their “troponin was positive”. When appropriate, clinicians should explain that in the context of SVT, a mild troponin rise can often reflect the rapid heart rate rather than an acute MI. If additional testing is arranged, it can be framed as a precautionary step to ensure thorough evaluation. Providing this context can help prevent misunderstanding and reduce unnecessary worry associated with the term “troponin”.
The relationship between SVT and troponin measurement would benefit from further investigation to address unresolved questions. Prospective studies, and ideally randomized trials, could help clarify whether a selective testing strategy differs in outcomes compared with routine measurement. Such work may determine whether omitting troponin in clearly low-risk cases leads to missed adverse events or if routine testing meaningfully improves the detection of important pathology. Large multicenter cohorts may be required to adequately evaluate these questions.
Additional data on long-term outcomes according to troponin status could refine our understanding of prognosis. It remains uncertain whether an SVT-related troponin rise predicts the later development of cardiomyopathy, arrhythmia recurrence, or vascular events. Some evidence has linked paroxysmal SVT to higher stroke risk[40], but whether troponin contributes additional prognostic value is unclear.
The role of hs-cTnT also warrants closer study. Defining thresholds, patterns, or delta changes that distinguish benign tachycardia-related release from ischemic injury could provide important clinical guidance. Likewise, adjunctive tools such as bedside echocardiography or advanced ECG analysis may help identify which patients merit more urgent evaluation.
Finally, therapeutic strategies could be explored. For example, it is not yet known whether early ablation in patients who develop troponin elevations leads to better outcomes. If troponin reflects more severe episodes or increased myocardial vulnerability, reducing recurrence through ablation might influence long-term prognosis.
For practical purposes, key take-home messages on troponin testing and interpretation in adult SVT are summarized in Table 3. Troponin elevation in adult SVT is a common and well-documented finding, usually reflecting demand ischemia or myocardial stretch rather than plaque rupture. While it may unmask underlying CAD or occasionally signal concomitant ACS, its prognostic implications should be interpreted with caution. In the short term, elevated troponin conveys a risk signal in selected higher-risk populations. In contrast, long-term outcomes remain inconsistent and appear heavily dependent on comorbidities and pre-existing CAD. The key clinical task is to distinguish between benign, self-limited myocardial injury and events requiring a comprehensive cardiology work-up. Best practice is to tailor troponin testing and subsequent management to the individual’s risk profile: In low-risk presentations, routine testing offers little benefit and may drive unnecessary hospitalizations or procedures, while in higher-risk cases, it can serve as a helpful adjunct that justifies careful evaluation for ACS. Regardless of troponin status, the immediate priority remains treating the arrhythmia itself – restoring a sinus rhythm, relieving symptoms, and then considering further assessment as indicated. A balanced approach, avoiding reflexive intervention for every troponin rise yet remaining alert to true ischemic events, offers the most appropriate and patient-centered care in this context. In summary, routine troponin testing in SVT should be selective rather than universal.
| Practical tips and tricks |
| Do not test routinely in young, low-risk patients with typical paroxysmal supraventricular tachycardia and no ischemic symptoms or electrocardiogram changes after conversion |
| Remember that most troponin elevations reflect demand ischemia or myocardial stretch, not plaque rupture |
| Short-term risk is modest and confined to selected populations (older age, coronary artery disease, diabetes, significant comorbidities) |
| Long-term outcomes are inconsistent, and elevations are often prognostically neutral once comorbidities are accounted for |
| Always prioritize arrhythmia management first – terminate supraventricular tachycardia, relieve symptoms, and then decide whether troponin adds value |
| Interpret troponin within the universal definition of myocardial infarction framework, using sex-specific 99th percentiles and delta algorithms, and avoid overdiagnosis of ACS |
| Positive troponin in the absence of ACS features should prompt tailored outpatient evaluation (coronary computed tomography angiography, stress test, echocardiography) rather than reflexive invasive testing |
| Balance is key: Avoid unnecessary admissions and procedures, but remain vigilant for true ischemic events |
| 1. | Ku K, Healy J, Lee CA, Khan M, Chao KD, Hassan S, Tzeng CT, Hsieh YL, Shedd A, Bhakta T, Hassani D, Chou EH. Exploring Treatment Protocol Adherence and Variations in Paroxysmal Supraventricular Tachycardia in the Emergency Department: A Multi-Center Cohort Study. Med Sci (Basel). 2025;13:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Pourmand A, Checkeye H, Varghese B, Solomon AJ, Tran QK. The Role of Troponin Testing in Patients with Supraventricular Tachycardia, Systematic Review and Meta-Analysis. J Emerg Med. 2024;67:e402-e413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 3. | Maayah M, Grubman S, Allen S, Ye Z, Park DY, Vemmou E, Gokhan I, Sun WW, Possick S, Kwan JM, Gandhi PU, Hu JR. Clinical Interpretation of Serum Troponin in the Era of High-Sensitivity Testing. Diagnostics (Basel). 2024;14:503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 4. | Ede H, Ahmed HSSS, Mahfouz ASHG, Rahhal AAA, Haider SA, Madni NA, Alkhatib MA, Elshrif HM, Al Yafei SMAA, Al Suwaidi JM, Al-Qahtani AAR, Asaad NA. The Role of Coronary Computed Tomography Angiography in Evaluation of High Troponin Patients with Narrow-Complex Supraventricular Tachycardia. Heart Views. 2021;22:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Aletras G, Koutalas E, Bachlitzanaki M, Stratinaki M, Bachlitzanaki I, Stavratis S, Garidas G, Pitarokoilis M, Foukarakis E. Paroxysmal Supraventricular Tachycardia and Troponin Elevation: Insights into Mechanisms, Risk Factors, and Outcomes. J Clin Med. 2025;14:5644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Chapman AR, Taggart C, Boeddinghaus J, Mills NL, Fox KAA. Type 2 myocardial infarction: challenges in diagnosis and treatment. Eur Heart J. 2025;46:504-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 7. | Mercik J, Radziejewska J, Pach K, Zawadzki G, Zyśko D, Gajek J. ST-segment depression in atrioventricular nodal reentrant tachycardia: Important finding or just an artifact? Medicine (Baltimore). 2022;101:e31806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Ben Yedder N, Roux JF, Paredes FA. Troponin elevation in supraventricular tachycardia: primary dependence on heart rate. Can J Cardiol. 2011;27:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 9. | Chow GV, Hirsch GA, Spragg DD, Cai JX, Cheng A, Ziegelstein RC, Marine JE. Prognostic significance of cardiac troponin I levels in hospitalized patients presenting with supraventricular tachycardia. Medicine (Baltimore). 2010;89:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Wang CH, Kuo LT, Hung MJ, Cherng WJ. Coronary vasospasm as a possible cause of elevated cardiac troponin I in patients with acute coronary syndrome and insignificant coronary artery disease. Am Heart J. 2002;144:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Bandorski D, Höltgen R, Wieczorek M, Ghofrani HA, Bogossian H, Iliodromitis K. Evaluation of troponin I serum levels in patients with arrhythmias with and without coronary artery disease. Med Klin Intensivmed Notfmed. 2024;119:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Healey JS. Troponin elevation in patients with supraventricular tachycardia: what does it mean? Can J Cardiol. 2011;27:110-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Choi EJ, Nam H, Chung CR, Yang JH, Suh GY, Park S, Lee SY, Hyun DG, Park MH, Lim CM, Ko RE; Korean Sepsis Alliance (KSA) investigators. Impact of Elevated Troponin Level at the Time of Sepsis Recognition on the Clinical Outcomes: A Propensity Score-Matched Cohort Study. J Am Heart Assoc. 2025;14:e038651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Yang L, Li B, Chen H, Belfeki N, Monchi M, Moini C. The Role of Troponin in the Diagnosis and Treatment of Acute Pulmonary Embolism: Mechanisms of Elevation, Prognostic Evaluation, and Clinical Decision-Making. Cureus. 2024;16:e67922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Elliott A, Alhuneafat L, Bartos JA. Troponin's Twist: A Sepsis Story Beyond the Heart. J Am Heart Assoc. 2025;14:e041428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Laursen SB, Pareek M, Polcwiartek C, Kristensen AMD, Tofig BJ, Hansen ML, Riahi S, Biering-Sørensen T, Torp-Pedersen C, Kragholm KH, Byrne C. High-sensitivity cardiac troponin-T concentrations and their prognostic implications in patients with paroxysmal supraventricular tachycardia. Int J Cardiol. 2025;420:132717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 17. | Sayadnik M, Shafiee A, Jenab Y, Jalali A, Sadeghian S. Predictors of High-Sensitivity Cardiac Troponin T Elevation in Patients with Acute Paroxysmal Supraventricular Tachycardia and Ischemic Heart Disease. Tex Heart Inst J. 2017;44:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Fernando H, Adams N, Mitra B. Review article: The utility of troponin and other investigations in patients presenting to the emergency department with supraventricular tachycardia. Emerg Med Australas. 2019;31:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Xue F, Jiang TB, Jiang B, Cheng XJ, He YM, Li X, Yang XJ. Cardiac troponin I elevation with supraventricular tachycardia: two case reports and review of the literature. BMC Res Notes. 2014;7:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Ghersin I, Zahran M, Azzam ZS, Suleiman M, Bahouth F. Prognostic value of cardiac troponin levels in patients presenting with supraventricular tachycardias. J Electrocardiol. 2020;62:200-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Wang CK, Yen CC, Chen SY, Lo HY, Ng CJ, Chaou CH. Prognostic value of cardiac troponin in dialysis patients with paroxysmal supraventricular tachycardia. Medicine (Baltimore). 2022;101:e30513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Murer M, Cuculi F, Toggweiler S, Weberndoerfer V, Young M, Kobza R. Elevated high-sensitivity troponin does not indicate the presence of coronary artery disease in patients presenting with supraventricular tachycardia. Cardiol J. 2017;24:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Fernando H, Adams N, Mitra B. Investigations for the assessment of adult patients presenting to the emergency department with supraventricular tachycardia. World J Emerg Med. 2020;11:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40:237-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 1809] [Article Influence: 258.4] [Reference Citation Analysis (1)] |
| 25. | Peacock WF, Baumann BM, Bruton D, Davis TE, Handy B, Jones CW, Hollander JE, Limkakeng AT, Mehrotra A, Than M, Ziegler A, Dinkel C. Efficacy of High-Sensitivity Troponin T in Identifying Very-Low-Risk Patients With Possible Acute Coronary Syndrome. JAMA Cardiol. 2018;3:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 26. | Liu L, Cheng YT, Xu A, Cheung BMY. Association between high sensitivity cardiac troponin and mortality risk in the non-diabetic population: findings from the National Health and Nutrition Examination Survey. Cardiovasc Diabetol. 2023;22:296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Apple FS, Schulz K, Schmidt CW, van Domburg TSY, Fonville JM, de Theije FK. Determination of sex-specific 99th percentile upper reference limits for a point of care high sensitivity cardiac troponin I assay. Clin Chem Lab Med. 2021;59:1574-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 28. | Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, Claeys MJ, Dan GA, Dweck MR, Galbraith M, Gilard M, Hinterbuchner L, Jankowska EA, Jüni P, Kimura T, Kunadian V, Leosdottir M, Lorusso R, Pedretti RFE, Rigopoulos AG, Rubini Gimenez M, Thiele H, Vranckx P, Wassmann S, Wenger NK, Ibanez B; ESC Scientific Document Group. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44:3720-3826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 2807] [Article Influence: 935.7] [Reference Citation Analysis (0)] |
| 29. | Camp S, Elhamdani A, Zinabu S, McGinnis P, Mearns-Escobar N, Michael M, Pourmand A, Brent BA, Myers BA, Tran QK. Serum troponin testing and adverse cardiovascular outcomes in supraventricular tachycardia: A retrospective study from TriNetX. Am J Emerg Med. 2025;95:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Chen JL, Hsiao CH, Yen CC. Prognostic value of cardiac troponin in elderly patients with paroxysmal supraventricular tachycardia: A multicenter study. Am J Emerg Med. 2023;69:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 31. | Noorvash D, Ramos R, Hatch L, Muck A, Olson AS. Assessment of the Utility of Ordering a Troponin in Low- and Intermediate-Risk Patients Presenting to the Emergency Department with Supraventricular Tachycardia: A Retrospective Chart Review. J Emerg Med. 2018;55:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Carlberg DJ, Tsuchitani S, Barlotta KS, Brady WJ. Serum troponin testing in patients with paroxysmal supraventricular tachycardia: outcome after ED care. Am J Emerg Med. 2011;29:545-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Bukkapatnam RN, Robinson M, Turnipseed S, Tancredi D, Amsterdam E, Srivatsa UN. Relationship of myocardial ischemia and injury to coronary artery disease in patients with supraventricular tachycardia. Am J Cardiol. 2010;106:374-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Ashok A, Cabalag M, Taylor DM. Usefulness of laboratory and radiological investigations in the management of supraventricular tachycardia. Emerg Med Australas. 2017;29:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Gabrielli M, Cucurachi R, Lamendola P, Candelli M, Pignataro G, Del Bono G, Franceschi F. Troponin Testing in Adult Patients Presenting to the Emergency Department for Paroxysmal Supraventricular Tachycardia: A Review. Cardiol Rev. 2023;31:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Özlek B, Tanık VO, Barutçu S. Reassessing the role of troponin in supraventricular tachycardia: Clinical utility or contextual signal? Int J Cardiol. 2025;436:133439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Mcgarry T. B-PO04-158 Troponin Elevation During Episodes of Supraventricular Tachycardia. Heart Rhythm. 2021;18:S342-S343. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Miccichè S, Pelaggi G, Lo Nigro MC, Celeste S, Moncada A, La Cognata O, Lo Savio A, Bonanno S, Bellocchi P, Agnelli G, Currò A. High-Sensitivity Cardiac Troponin T Elevation and Paroxysmal Supraventricular Tachycardia. Is it Always Acute Coronary Syndrome? Cor Vasa. 2025;67:77-81. [DOI] [Full Text] |
| 39. | Brugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomström-Lundqvist C, Calkins H, Corrado D, Deftereos SG, Diller GP, Gomez-Doblas JJ, Gorenek B, Grace A, Ho SY, Kaski JC, Kuck KH, Lambiase PD, Sacher F, Sarquella-Brugada G, Suwalski P, Zaza A; ESC Scientific Document Group. 2019 ESC Guidelines for the management of patients with supraventricular tachycardiaThe Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur Heart J. 2020;41:655-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 711] [Article Influence: 118.5] [Reference Citation Analysis (0)] |
| 40. | Kamel H, Elkind MS, Bhave PD, Navi BB, Okin PM, Iadecola C, Devereux RB, Fink ME. Paroxysmal supraventricular tachycardia and the risk of ischemic stroke. Stroke. 2013;44:1550-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/