Published online Nov 26, 2025. doi: 10.4330/wjc.v17.i11.109287
Revised: July 2, 2025
Accepted: October 21, 2025
Published online: November 26, 2025
Processing time: 198 Days and 19.8 Hours
Bioresorbable scaffolds (BRS) are a promising alternative to traditional drug-eluting stents (DES) for the treatment of acute coronary syndrome (ACS). They offer the potential for complete resorption, which may reduce long-term complications such as stent thrombosis and late restenosis. However, the safety, compatibility, and long-term outcomes of BRS in patients with intermediate to low-risk ACS have yet to be thoroughly investigated.
To investigate the safety, compatibility, and long-term outcomes of BRS in patients with intermediate to low-risk ACS.
Patients with intermediate to low-risk ACS who underwent percutaneous coronary intervention with either DES or BRS, and were continuously recruited from January 2019 to June 2022 at a single center, were analyzed. Baseline data and clinical follow-up were collected for patients who underwent DES im
A total of 128 patients were included in this study, with an average age of 63 years. Among them, 95 were male (74%). The study involved treatment of 201 blood vessels: 87 (43%) received BRS, and 114 (57%) received DES. A total of 97 patients completed the full 3-year follow-up. During this period, 5 patients (17%) in the observation group and 7 patients (16%) in the control group experienced a major cardiovascular event (DoCE). At the 1-year follow-up, 7 patients (15%) in the observation group and 6 patients (10%) in the control group experienced DoCE, and this difference was statistically significant (P < 0.05). At the 2-year follow-up, there was also a significant difference between the two groups in the number of patients who needed repeat treatment of the target blood vessel (P < 0.05). In the observation group, 18 patients (33%) underwent follow-up coronary angiography. During the follow-up period, one patient in the observation group was found to have re-narrowing in the proximal and middle segments of the left anterior descending artery, possibly due to BRS collapse. Another patient in the observation group developed chronic total occlusion in multiple vessels at the 3-year follow-up and underwent coronary artery bypass grafting.
In low- to intermediate-risk ACS patients, those who got BRS had their first major heart event sooner than those who got DES. BRS is more tissue-friendly, yet over three years both groups had about the same amount of problems - only a few BRS patients still saw the scaffold collapse or the vessel slowly block.
Core Tip: This study aimed to investigate the safety and effectiveness of bioresorbable scaffolds (BRS) compared to drug-eluting stents (DES) in patients with intermediate to low-risk acute coronary syndrome. A total of 128 patients were included in the study, with 87 receiving BRS and 114 receiving DES. The patients were followed up for a maximum of 3 years to assess the occurrence of cardiac events and complications. The results showed that the time to reach the composite endpoint of cardiac events was earlier in the BRS group compared to the DES group, although the final number did not differ significantly. BRS demonstrated better compatibility than DES. These findings suggest that BRS may be a viable alternative to DES in patients with intermediate to low-risk acute coronary syndrome, but further research is needed to address the observed complications.
- Citation: Li J, Li XR, Kong MW, Zhang J. Medium-to-long term outcomes of bioresorbable scaffold treatment in patients with acute coronary syndrome. World J Cardiol 2025; 17(11): 109287
- URL: https://www.wjgnet.com/1949-8462/full/v17/i11/109287.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i11.109287
In comparison to bare metal stents, the latest generation of drug-eluting stents (DES) significantly reduces the risk of target lesion failure[1]. However, there is still a relatively high risk of complications such as in-stent restenosis, new artery atherosclerosis, and late stent thrombosis (ScT)[2]. To overcome these limitations and avoid long-term coronary artery metalization caused by metal stents, the concept of bioresorbable scaffolds (BRS) has emerged.
The first commercially available BRS, the everolimus-eluting polymer-based BRS with polylactic acid, offers significant advantages. These advantages include promoting the restoration of normal vascular function, facilitating secondary intervention and surgical coronary artery bypass grafting, and being compatible with magnetic resonance imaging/computed tomography scans[2]. However, the medium to long-term results have shown a high incidence of complications associated with this scaffold, particularly late ScT, ultimately leading to its withdrawal from the market[3]. Since its commercial release in February 2019, the latest generation of domestically produced BRS has started to be used in domestic hospitals, and related studies have been gradually reported in recent years. A study analyzed the safety and efficacy of the novel BRS in percutaneous coronary intervention (PCI) for patients with acute myocardial infarction, concluding that BRS-PCI is safe and effective[2]. However, the latest expert consensus[3] points out that there have been significant changes in the clinical outcomes of BRS after 2-3 years post-procedure. Follow-up studies have found increased rates of adverse clinical events during the gradual degradation and decrease in scaffold support, showing statistically significant differences compared to the DES control group. In international research, debates on the safety of BRS have continued. The latest study included a population with ST-segment elevation myocardial infarction, where BRS and DES were implanted separately, and it was observed that the BRS had a higher probability of major and minor endpoints such as in-ScT compared to DES, and there are even studies that see BRS as a “disappearing technology”[4,5]. However, the results of a recent meta-analysis suggest that compared to DES, the use of BRS for treating acute coronary syndrome (ACS) does not increase patients’ mortality rate, and even demonstrates acceptable efficacy in the treatment of coronary bifurcation lesions[6,7].
Given the short time since the market launch of the new generation of BRS, the lack of consistent research conclusions, and the unclear scope of application, this study aims to include patients with low-to-intermediate-risk ACS as the study population. The study will compare the safety of BRS implantation with DES by observing the occurrence of adverse events in the BRS group and the control group. Additionally, this research will analyze the changes in serum inflammatory factor levels after stent implantation to determine the biocompatibility of BRS. Furthermore, the medium-to-long-term outcomes of patients will be assessed to provide evidence for future clinical practices.
Between January 2019 and December 2022, we consecutively recruited patients with low-to-intermediate-risk ACS who underwent PCI[8]. All patient data were anonymized and stored with strict security measures to ensure compliance with relevant ethical guidelines and regulatory requirements[9]. When necessary, data will be shared according to data-sharing agreements to promote research transparency and result verifiability, while ensuring the full protection of patient privacy[10]. Low-to-intermediate-risk ACS was defined as having a thrombolysis in myocardial infarction risk score of ≤ 4, as developed by Antman et al[11]. Patients were excluded if they had chronic total occlusion, left main disease, ostial lesions, bifurcation lesions with side branches > 2.0 mm in diameter requiring treatment, thrombotic lesions, severe calcified lesions, or tortuous lesions[12]. These patients agreed to undergo follow-up (including clinic visits, telephone or questionnaire surveys, etc.), and their information was included in our hospital's data management center. The data management center aimed to evaluate baseline characteristics and outcomes of patients requiring PCI for ACS. Information on the latest generation of the “Bioresorbable Rapamycin-Eluting Coronary Scaffold System (NeoVas)” developed by Leap (Beijing) Medical Devices Co., Ltd., and the next-generation “Everolimus-Eluting Coronary Stent System (SYNERGY)” developed by Boston Scientific (United States) was registered in our hospital's bioresorbable vascular scaffold registry. This registry aims to assess the safety and performance of BRS in real-world settings.
The Bioresorbable Rapamycin-Eluting Coronary Scaffold System (NeoVas) BRS utilizes a biodegradable matrix that starts to degrade in the body 6-9 months after implantation and completely disappears in approximately 3 years. Compared to metallic stents, this scaffold has a lower probability of causing vascular elastic recoil and endothelial injury-induced inflammatory reactions in the blood vessels. This, to some extent, avoids long-term risks such as plaque and thrombosis[13].
The SYNERGYTM stent incorporates the SynchronyTM polymer coating that undergoes synchronous degradation, with complete degradation of the poly lactic-co-glycolic acid coating occurring at 4-6 months after implantation, when elution of everolimus is complete. The SYNERGYTM stent exhibits more complete strut coverage (endothelialization of the vessel) and healthier tissue compared to permanent polymer-coated DES[14].
The choice of stent type is determined through discussions between the surgeon, the patient, and their family members. Regarding the surgical approach, this study followed expert consensus recommendations, including appropriate pre-dilation, the use of non-compliant balloons, optimal sizing, and post-dilation, following the “pre-dilation, sizing, post-dilation (PSP) principle” to ensure full apposition of the stent to the vessel wall[15]. In specific cases, the operator may perform a hybrid implantation of both BRS and DES to ensure adequate coverage of the target lesion. After stent implantation, it is ensured that all patients receive guideline-directed dual antiplatelet therapy with aspirin plus clopidogrel or ticagrelor for at least 12 months. For patients requiring anticoagulant therapy, the combination of oral anticoagulants with aspirin is recommended for 7-28 days, followed by clopidogrel for 12 months. Follow-up visits, telephone consultations, clinical follow-ups, and questionnaire surveys are conducted at 3 months, 6 months, and 1 year after stent implantation. Additionally, patients are encouraged to undergo coronary angiography at 1 year and 2 years after discharge for further evaluation.
The primary clinical endpoint of the study is the device-oriented composite endpoint (DoCE), which shows that patients have experienced at least one of the following outcomes during follow-up: Cardiac sudden death, ScT, target vessel myocardial infarction, and target lesion revascularization[16]. ScT is defined as the occurrence of thrombosis within the scaffold or within 4 mm of its edges, confirmed by angiography or intravascular imaging [such as optical coherence tomography (OCT)], accompanied by acute ischemic symptoms, new electrocardiographic changes indicative of ischemia, and elevated cardiac biomarkers. Secondary endpoints include target-vessel revascularization (TVR) and coronary artery bypass graft, both of which address the need for additional interventions due to restenosis or complications in the treated vessel. These clinical events are defined according to the Academic Research Consortium criteria[17]. The study outcomes were reviewed and assessed by two independent cardiology professors to ensure unbiased interpretation. These endpoints are crucial for evaluating the safety and efficacy of the BRS in patients, focusing on major adverse events and the need for re-intervention.
The biocompatibility of the stent was determined in this study by assessing the changes in serum inflammatory markers after stent implantation. Enzyme-linked immunosorbent assay (ELISA) was used to evaluate serum inflammatory factors. Two groups of patients were selected, and 4 mL of fasting venous blood was drawn on the morning of the second day after PCI. The blood samples were allowed to stand for 4 hours, centrifuged at 2000 × g for 14 minutes, and the serum was collected and stored at -70 °C until testing under standardized conditions. Clinical symptoms and serological indicators of the patients were recorded at the time of blood collection. The patients were instructed to return for a follow-up examination after one month, and the same procedure was repeated with the collection of 4 mL of fasting venous blood from both groups. ELISA was used to quantitatively detect the serum inflammatory factors interleukin (IL)-6, C-reactive protein (CRP), tumor necrosis factor (TNF)-α, and matrix metalloproteinase-9 (MMP-9)[18], following the instructions provided by the reagent kit (provided by Shenzhen Jingmei Biological Engineering Co., Ltd.). A microplate reader was used to measure the absorbance (A) value at 440 nm, which is directly proportional to the concentration. A standard curve was plotted to allow determination of the concentration of the sample.
This study did not have a predetermined sample size and the statistical analysis was primarily descriptive. Categorical variables are presented as absolute numbers and percentages, while continuous variables are presented as mean (standard deviation) or median as appropriate. P values were calculated using paired t tests and χ2 tests. Kaplan-Meier curves were plotted to assess the distribution of adverse clinical outcomes over time. Univariate and multivariate Cox regression models were used to evaluate potential predictors of the primary study endpoint, DoCE. A P value < 0.05 was considered statistically significant. All analyses were performed using IBM SPSS Statistics 19.
A total of 128 patients were included in this study, with 55 patients receiving treatment with BRS (observation group) and 73 patients receiving treatment with DES (control group). The average age was 63 ± 10 years, and 95 patients (74%) were male. Overall, 84 cases (66%) were classified with intermediate-risk ACS, and 44 cases (34%) were classified with low-risk ACS. This information is shown in Table 1.
| Characteristic | Overall (n = 128) | Observation group (n = 55) | Control group (n = 73) | P value |
| Age (year), mean ± SD | 63 ± 10 | 62 ± 11 | 64 ± 6 | 0.42 |
| Male | 95 (74) | 40 (73) | 67 (92) | 0.09 |
| Type of lesion | 0.71 | |||
| Non-STEMI | 95 (74) | 40 (73) | 55 (76) | |
| STEMI | 33 (26) | 15 (27) | 18 (24) | |
| Heart rate (bpm), mean ± SD | 72 ± 16 | 71 ± 16 | 72 ± 17 | 0.01 |
| Systolic pressure (mmHg), mean ± SD | 114 ± 21 | 114 ± 20 | 119 ± 22 | 0.27 |
| Diastolic pressure (mmHg), mean ± SD | 73 ± 16 | 71 ± 9 | 76 ± 7 | 0.14 |
| Left ventricular ejection fraction (%), mean ± SD | 47 ± 10 | 47 ± 10 | 47 ± 11 | 0.47 |
| Creatinine (mmol/L), mean ± SD | 72 ± 22 | 72 ± 24 | 79 ± 10 | 0.21 |
| Pulmonary arterial hypertension | 15 (12) | 6 (11) | 9 (12) | 0.62 |
| Diabetes mellitus | 22 (17) | 9 (16) | 13 (18) | 0.76 |
| Dyslipidemia | 42 (33) | 17 (31) | 25 (34) | 0.22 |
| Smoking | 42 (33) | 24 (44) | 18 (25) | 0.62 |
| Family history of coronary artery disease | 44 (34) | 19 (35) | 25 (34) | 0.42 |
| Antithrombotics | ||||
| Aspirin | 92 (72) | 34 (62) | 58 (79) | - |
| Clopidogrel | 46 (36) | 20 (36) | 26 (36) | 1.00 |
| Direct oral anticoagulant | 6 (5) | 2 (4) | 4 (5) | 0.42 |
We successfully implanted all stents using the vascular imaging method, and treated a total of 201 Lesions, 87 of which were treated with BRS. The most common treated vessel was the left anterior descending artery (51%), and B2 type lesions accounted for approximately one quarter of all lesions. The average stent diameter was 3.25 ± 0.25 mm, and the average length was 21.1 ± 2.3 mm. Information on these lesions is shown in Table 2.
| Characteristic | Number of lesions (n = 201) | BRS treatment (n = 87) | DES treatment (n = 114) | P value |
| Treated blood vessels | 0.80 | |||
| Left anterior descending artery | 103 (51) | 43 (49) | 49 (43) | |
| Left circumflex artery | 46 (23) | 19 (22) | 33 (29) | |
| Right coronary artery | 52 (26) | 25 (19) | 32 (28) | |
| Type of lesion | 0.96 | |||
| A | 52 (26) | 20 (23) | 32 (28) | |
| B1 | 70 (35) | 32 (37) | 38 (33) | |
| B2 | 79 (39) | 35 (40) | 44 (39) | |
| Initial TIMI flow | 0.28 | |||
| 0 | 53 (26) | 27 (31) | 26 (23) | |
| 1 | 14 (7) | 6 (7) | 8 (7) | |
| 2 | 15 (8) | 7 (8) | 8 (7) | |
| 3 | 119 (59) | 47 (54) | 72 (63) | |
| Final TIMI flow | ||||
| 2 | 5 (2) | 3 (3) | 2 (2) | - |
| 3 | 196 (98) | 84 (97) | 112 (98) | - |
| Number of stents per lesion (bpm), mean ± SD | 1.2 ± 0.4 | 1.72 ± 0.38 | 1.21 ± 0.41 | 0.34 |
| Predilation | 201 (100) | 87 (100) | 114 (100) | - |
| Average stent diameter (mm), mean ± SD | 3.25 ± 0.25 | 3.3 ± 0.25 | 3.3 ± 0.21 | 0.32 |
| Average stent length (mm), mean ± SD | 21.1 ± 2.3 | 22.6 ± 3.3 | 21.7 ± 2.5 | 0.13 |
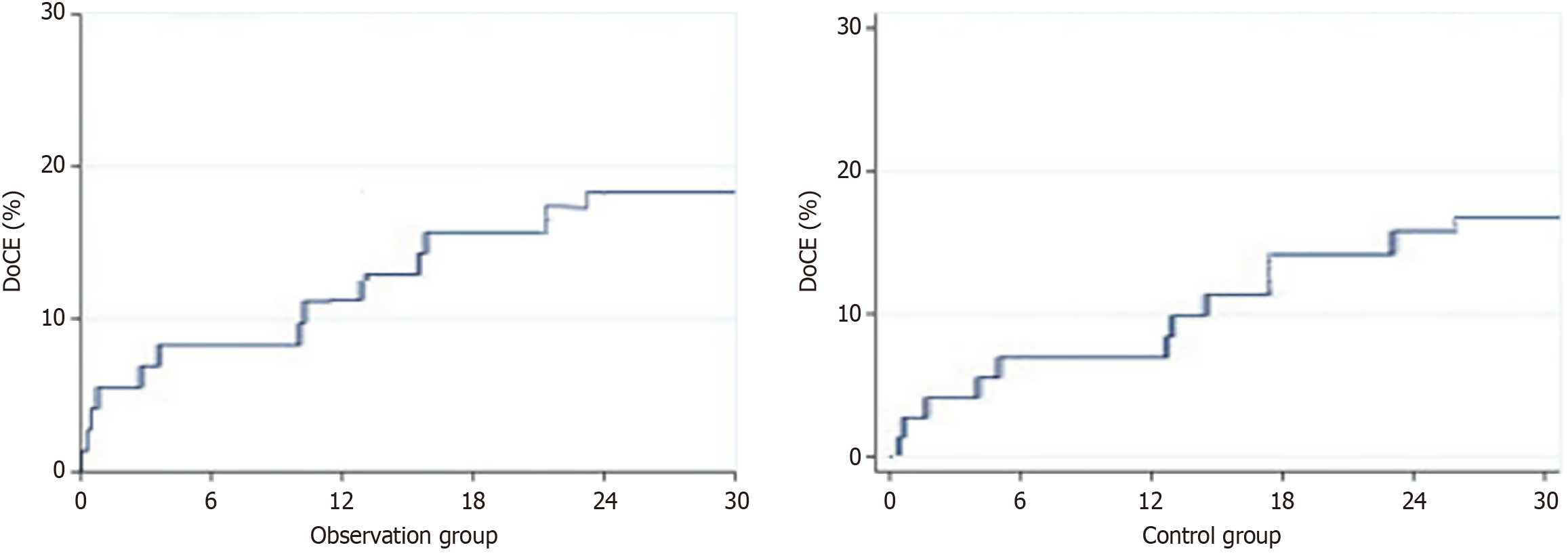
Ninety-seven (76%) patients achieved complete clinical follow-up, with a median duration of 32.5 (31-36) months. In the observation group, 5 cases (17%) reached the DoCE (disease-free survival evaluation) at 18 months, accounting for 15% of the total number of cases (P < 0.05). In the control group, 7 cases (16%) reached the DoCE, accounting for 13% at 18 months (Figure 1). The clinical follow-up results are shown in Table 3.
| Clinical outcome | 6 months | 1 year | 2 years | 3 years | ||||||||
| Observation group | Control group | P value | Observation group | Control group | P value | Observation group | Control group | P value | Observation group | Control group | P value | |
| Follow-up patients | 52 (95) | 69 (95) | 0.65 | 48 (87) | 63 (86) | 0.73 | 43 (78) | 60 (82) | 0.14 | 32 (37) | 46 (40) | 0.34 |
| Main end point | ||||||||||||
| DoCE | 2 (4) | 3 (4) | 0.19 | 7 (15) | 6 (10) | 0.03a | 9 (31) | 11 (13) | 0.08 | 5 (16) | 7 (15) | 0.22 |
| CD | 0 (0) | 0 (0) | - | 0 (0) | 0 (0) | - | 1 (2) | 1 (2) | 0.52 | 1 (3) | 2 (4) | 0.18 |
| ScT | 1 (2) | 2 (3) | 0.84 | 2 (4) | 2 (3) | 0.53 | 2 (7) | 3 (5) | 0.14 | 3 (9) | 5 (11) | 0.34 |
| TV-MI | 1 (2) | 1 (1) | 0.75 | 2 (4) | 2 (3) | 0.07 | 2 (5) | 3 (5) | 0.12 | 2 (6) | 4 (8) | 0.07 |
| TLR | 0 (0) | 0 (0) | - | 3 (6) | 3 (5) | 0.35 | 4 (9) | 4 (7) | 0.06 | 3 (9) | 5 (11) | 0.53 |
| Secondary end point | ||||||||||||
| TVR | 0 (0) | 0 (0) | - | 0 (0) | 0 (0) | - | 1 (2) | 2 (5) | 0.01a | 2 (6) | 2 (4) | 0.33 |
| CBG | 0 (0) | 0 (0) | - | 0 (0) | 0 (0) | - | 0 (0) | 0 (0) | - | 1 (3) | 0 (0) | 0.85 |
| Non-cardiogenic death | 0 (0) | 2 (3) | 0.65 | 0 (0) | 1 (2) | 0.11 | 2(5) | 1 (2) | 0.73 | 1 (3) | 2 (4) | 0.49 |
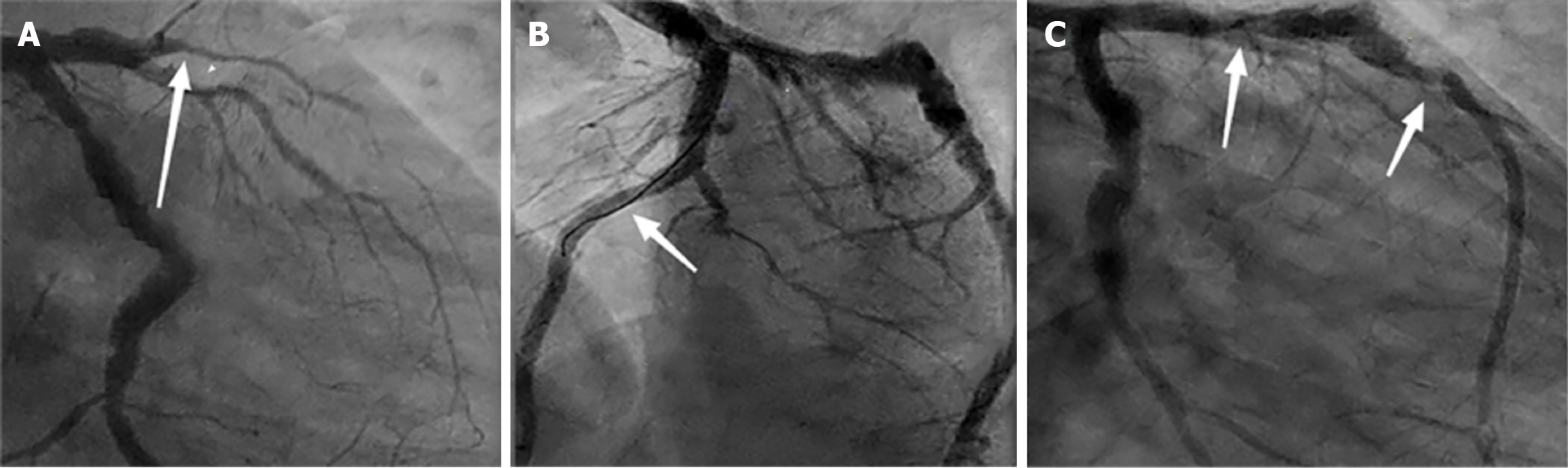
Eighteen cases (33%) in the observation group underwent re-examination by coronary angiography. Regarding adverse reactions, no significant short-term adverse reactions were observed in both groups. During the one-year follow-up period, one patient with an implanted BRS was found to have stent collapse during coronary angiography, with imaging showing discontinuous blood flow and incomplete lesion coverage in the anterior descending branch, partially showing tumor-like dilation (Figure 2). The patient had no obvious clinical symptoms and therefore did not receive treatment but was advised to undergo regular follow-up examinations. Another patient experienced chronic coronary artery occlusion in multiple vessels during the three years after BRS implantation, and underwent coronary artery bypass graft surgery.
The expression levels of IL-6, CRP, TNF-α, and MMP-9 in the blood of the two groups of patients were compared using ELISA (Table 4). At one month, 41 cases (75%) in the observation group and 56 cases (77%) in the control group complied with medical advice and attended for re-examination. The serum expression levels of CRP in the control group were significantly reduced after PCI (P < 0.05), while the relative expression levels of IL-6, CRP, and TNF-α in the observation group were significantly lower than before treatment (P < 0.05). Compared to the control group, the changes in IL-6, CRP, and TNF-α levels in the observation group were more significant, and the differences were statistically significant (P < 0.05). However, there was no significant difference in the relative expression levels of MMP-9 between the intervention group and the control group (P > 0.05). We performed an analysis of the hazard ratio and confidence interval. The results showed that although there were no significant differences in the absolute event rates, there were differences in the relative risks of DoCE and TVR between the BRS and DES groups. Specifically, the BRS group had a higher risk of reaching DoCE at 1 year, and at 2 years, the incidence of TVR also showed a statistically significant difference (P < 0.05). This suggests that, although the differences in some clinical endpoints were small, there are still certain differences in the long-term effects between BRS and DES.
| Group | IL-6 (μg/L) | CRP (pg/mL) | TNF-α (ng/L) | MMP-9 (ng/L) | |
| Observation group | PCI postoperative | 8.4 ± 1.7 | 78.9 ± 17.0 | 129.2 ± 20.1 | 16.9 ± 1.1 |
| Review after 1 month | 6.0 ± 1.0 | 41.7 ± 6.4 | 112.9 ± 14.0 | 14.9 ± 1.1 | |
| t (P value) | 11.2 (0.21) | 19.2 (0.01a) | 9.4 (0.07) | 17.0 (0.08) | |
| Control group | PCI postoperative | 8.4 ± 1.4 | 76.1 ± 18.9 | 124.1 ± 14.7 | 16.3 ± 1.8 |
| Review after 1 month | 4.7 ± 2.4 | 23.0 ± 7.9 | 83.7 ± 10.8 | 12.3 ± 0.7 | |
| t (P value) | 22.0 (0.01a) | 24.3 (< 0.01a) | 24.0 (0.01a) | 20.2 (0.12) | |
In the latest expert consensus, it is recommended to consider moderate or lower complexity lesions as the primary indications for BRS[3]. However, there are several challenges in current research on the new generation of BRS, including the treatment of overly selected patients, short follow-up periods, and inconsistent conclusions. These factors prevent the clinical application of the new generation of BRS from having sufficient research evidence to support it. The results of this study are derived from a specific study population that included only low-to-moderate risk ACS cases and excluded complex lesions[19]. This distinct characteristic sets this study apart from most other clinical studies evaluating the performance of BRS. Furthermore, previous studies have found significant changes occurring in BRS after 2-3 years, where adverse clinical events such as gradual degradation of the stent scaffold and decreased support are more likely to occur, leading to statistically significant differences compared to DES control groups in follow-up examinations[20,21]. The divergent results in the aforementioned studies can be attributed to the selection of indications and expertise in in surgical techniques[3]. In this study, the follow-up period for patients was set within the mid to long-term range of 2-3 years, and implantation of the stent strictly followed the “PSP principle”. This approach aimed to explore whether there are still significant differences between the new generation of BRS and DES under strict control of indication selection and surgical technique.
In previous studies, the compatibility of BRS has often been assessed using OCT imaging[22]. The advantage of this approach is that it allows for direct visualization of changes in the morphology of the intravascular stent and enables dynamic observation through follow-up. However, this method has clear limitations: OCT imaging is an invasive examination and is expensive, making it difficult for patients to accept. The choice of stent type was made by the clinician and patient, introducing potential bias. To address this, we used statistical adjustments, including propensity score matching, to control for observed confounders and balance the baseline characteristics between the two groups. Additionally, changes in BRS morphology typically require long-term follow-up, and apposition of the stent to the vessel wall is closely related to the operator's surgical experience. In order to eliminate these variables, this study assessed the biocompatibility of the stents by detecting factors such as IL-6, CRP, TNF-α, and MMP-9 in the patient’s serum after surgery and comparing changes in their levels. In previous studies, Cimmino et al[18] demonstrated the good tissue compatibility and safety of absorbable magnesium alloy stents by comparing the changes in inflammatory factors before and after implantation of these stents. International studies have also suggested that changes in inflammatory markers after stent implantation may be associated with complications such as ScT. In this study, the observation group showed a smaller increase in serum inflammatory markers after PCI with BRS compared to DES, indicating better biocompatibility of BRS[23,24]. The incidence of adverse reactions showed no significant difference between the observation group and the control group.
The results of this study further confirm that BRS has advantages over DES in terms of biocompatibility and affinity[22]. By evaluating the changes in postoperative inflammatory biomarkers (such as CRP, IL-6, TNF-α, and MMP-9), we found that patients in the BRS group exhibited more favorable control of inflammatory responses. This may be closely related to the degradation characteristics of BRS and its ability to promote endothelial healing. As BRS gradually degrades after implantation, it reduces long-term metal stent-induced foreign body reactions, thus promoting natural remodeling of the vascular wall. We observed that in some cases, scaffold collapse or chronic vessel occlusion occurred. However, due to the lack of systematic intravascular imaging data (such as OCT or intravascular ultrasound), we were unable to directly observe the morphological changes and endothelial healing during the degradation process of BRS. This lack of data limits our deeper understanding of the mechanisms behind scaffold failure and the vascular healing process. Future studies should incorporate intravascular imaging follow-up to clarify the characteristics of BRS in terms of long-term endothelial coverage, vascular remodeling, and degradation dynamics, thereby providing stronger evidence for optimizing patient selection and surgical strategies[23,24].
BRS patients experience more DoCE events before the 1-year follow-up, which may be related to early healing of the scaffold or thrombosis, reflecting short-term risks. However, these early events are usually manageable through appropriate interventions and do not necessarily affect the final long-term outcomes. The occurrence of late events suggests that, despite higher early risks, the final event rates in BRS patients are similar to those in DES patients as the scaffold heals and remodeling occurs, indicating long-term stability. Therefore, early complications reflect short-term risks of the scaffold, while late events indicate its long-term healing and stability, with both factors ultimately determining the final treatment outcome. From the follow-up angiography results, we observed one case of premature degradation and collapse of the stent, which has been reported in previous studies[25]. This case involved a 46-year-old patient who presented to the emergency department with acute ST-elevation myocardial infarction. Coronary angiography revealed complete occlusion of the mid left anterior descending artery. After balloon pre-dilation, two BRS were successfully implanted. The patient underwent re-examination at one-year follow-up. During the follow-up coronary angiography, we observed an aneurysm-like dilation in the mid segment of the anterior descending artery, along with narrowing at the site of the stent, indicating premature degradation and collapse of the stent. The degradation process was not uniform along the entire length of the stent and was associated with stent discontinuity and incomplete lesion coverage. The exact mechanism leading to premature degradation and stent discontinuity remains unclear. However, this case highlights the potential risks of BRS in the treatment of acute ST-elevation myocardial infarction and further research is needed to confirm these findings.
The results of this study show that there was no significant difference in the final number of patients achieving the DoCE following treatment with BRS and DES in those with moderate to low-risk ACS. However, there was a statistical difference in the number of patients reaching the DoCE in the year-long follow-up between the two groups. This may suggest that patients treated with BRS might reach the DoCE sooner than those treated with DES, but this did not affect long-term prognosis. During follow-up, there was no increase in the incidence of the “adverse clinical event rate rising in the 2-3 years after BRS surgery that’s associated with the gradual degradation and reduced support force of the stent body” as mentioned in the expert consensus[18]. This result could possibly be due to this study strictly implementing the “PSP principle” during the operation, controlling surgical indications, and ruling out complex lesions. Recent studies also indicate that patients with complex vascular lesions might have poorer stent implantation outcomes, especially in myocardial infarction patients. The presence of thrombus, impaired coronary blood flow, and coronary artery vasospasm can potentially increase the risk of target lesion failure and ScT[26]. This result is consistent with the conclusion observed by Wang et al[27] in their study of 1103 patients with coronary heart disease. However, the study by Eriksen et al[28] which followed 120 patients with ST-segment elevation myocardial infarction for 12 months, found that the DES group performed better than the BRS group in both primary and secondary endpoints. These differences may be due to variations in population and lesion characteristics: The setup in the present study was closer to that in the study by Wang et al[27], excluding high-risk ACS patients and more complex lesions, while the study by Eriksen et al[28] had a higher proportion of B/C lesions. Another possible reason is that our study included a smaller sample size, and different results could emerge in further studies with larger sample sizes. This issue requires further clarification in future studies.
This study has several limitations. Firstly, due to its observational nature, we cannot establish causality, and interpretation of the results is limited. Secondly, the lack of randomization may lead to bias in treatment allocation, affecting the accurate assessment of treatment effects. Therefore, we cannot draw definitive conclusions on one treatment being superior to another. Additionally, the non-random selection of patients introduces potential selection bias, which may influence the validity of the results and limit the generalizability of the study. This study was conducted at a single center, which may limit the applicability of the findings, and future multi-center studies may provide more reliable evidence. The small sample size may also affect statistical power, and a larger sample size might provide more precise results. The conclusions in this study are as follows: Patients with intermediate to low-risk ACS treated with BRS reached the discontinuation of dual antiplatelet therapy (DoCE) earlier than those treated with DES, but the final number did not show a significant difference. In terms of compatibility, BRS demonstrated stronger compatibility compared to DES. During the 3-year follow-up, both groups of patients showed similar types and numbers of complications. However, in the BRS group, there were still isolated cases of scaffold collapse and chronic coronary vessel occlusion. Isolated scaffold collapse is a key safety signal in BRS technology, and its occurrence may be associated with several factors. Firstly, uneven degradation is a potential issue during the degradation process of the BRS polymer substrate, which may lead to the loss of support in certain areas, increasing the risk of collapse. Secondly, polymer fatigue is another potential mechanism, where the scaffold material may experience fatigue under repeated mechanical stress, affecting its stability and leading to scaffold collapse. Additionally, vascular recoil is also a contributing factor in scaffold collapse, as vascular recoil after scaffold implantation may be influenced by the mechanical properties of the scaffold, resulting in morphological changes. With regard to risk factors, small vessel diameter (< 2.75 mm), overlapping scaffolds, and suboptimal expansion (failure to achieve adequate expansion diameter) all increase the likelihood of isolated scaffold collapse.
These findings support the recommendations of the latest expert guidelines, suggesting that BRS should be used only in highly selected patients and require strict pre-implantation preparation using the “PSP” technique. From the perspective of historical research, newer PCI devices, especially stents, should be cautiously introduced into clinical practice. Our study confirms that in simple lesions, through strict pre-operative preparation, BRS has the same level of safety as DES, with better biocompatibility with blood vessels. However, further research is needed to determine the appropriate indications for BRS treatment, to guide patient and lesion selection, and to further investigate the exact mechanisms leading to premature degradation and collapse of the scaffold in a few cases.
| 1. | Haude M, Wlodarczak A, van der Schaaf RJ, Torzewski J, Ferdinande B, Escaned J, Iglesias JF, Bennett J, Toth G, Joner M, Toelg R, Wiemer M, Olivecrona G, Vermeersch P, Garcia-Garcia HM, Waksman R. Safety and performance of the third-generation drug-eluting resorbable coronary magnesium scaffold system in the treatment of subjects with de novo coronary artery lesions: 6-month results of the prospective, multicenter BIOMAG-I first-in-human study. EClinicalMedicine. 2023;59:101940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 2. | Hanna J, Smolderen KG, Castro-Dominguez Y, Romain G, Lee M, Turner J, Mena-Hurtado C. Drug-Coated Balloon and Drug-Eluting Stent Safety in Patients With Femoropopliteal and Severe Chronic Kidney Disease. J Am Heart Assoc. 2023;12:e028622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Chinese Society of Cardiology of Chinese Medical Association. [Chinese expert consensus on the clinical application of coronary bioresorbable scaffold]. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:350-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Seguchi M, Aytekin A, Lenz T, Nicol P, Alvarez-Covarrubias HA, Xhepa E, Klosterman GR, Beele A, Sabic E, Utsch L, Alyaqoob A, Joner M. Challenges of the newer generation of resorbable magnesium scaffolds: Lessons from failure mechanisms of the past generation. J Cardiol. 2023;81:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Zuo J, Wang P, Xue K, Tan Y, Zhang T, Li Y, He F, Wu W, Yan Z, Cong L, Li G. Lipid alterations in acute myocardial infarction are associated with gut microbiota. Microbiol Spectr. 2025;13:e0237024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Zhang T, Li B, Wuethrich A, Liu H, Zhou Q, Hu Z, Wang H, Trau M, Bao F, Li J, Sun Y, Li J. Multiplex Cardiac Biomarker Profiling for Precision Coronary Artery Disease Risk Assessment Using SERS with Cubic Core-Satellite Nanostructures. Anal Chem. 2025;97:14750-14760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Ezad SM, O'Kane P. Bioresorbable Vascular Scaffolds: A Disappearing Technology, But Should We Let It Vanish? J Invasive Cardiol. 2023;35:E151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Verdoia M, Kedhi E, Suryapranata H, Galasso G, Dudek D, De Luca G. Poly (l-lactic acid) bioresorbable scaffolds versus metallic drug-eluting stents for the treatment of coronary artery disease: A meta-analysis of 11 randomized trials. Catheter Cardiovasc Interv. 2020;96:813-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Liang XY, Li Y, Zhang WJ, Qiao X, Yang RR, Wang ZL. Efficacy and safety of bioresorbable scaffolds in patients with coronary bifurcation lesions: A systematic review and meta-analysis. Cardiol J. 2022;29:563-573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Li CJ, Xu B, Song L, Yu MY, Yan HB, Qiu H, Mu CW, Cui JG, Guan CD, Sun ZW, Qiao SB, Gao RL. [The safety and efficacy of Firesorb bioresorbable scaffold in first-in-man study for coronary artery disease: the four-year outcomes]. Zhonghua Xin Xue Guan Bing Za Zhi. 2021;49:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284:835-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2176] [Cited by in RCA: 2183] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 12. | Kamel M, Zaheer M, Ahmed M, Ali H. Letter to the editor: Prognostic utility of hybrid coronary computed tomography angiography and myocardial perfusion imaging in elderly patients with suspected coronary artery disease. Int J Cardiol. 2025;438:133558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Kereiakes DJ, Meredith IT, Masotti M, Carrié D, Moreno R, Erglis A, Mehta SR, Elhadad S, Berland J, Stein B, Airaksinen J, Jobe RL, Reitman A, Janssens L, Christen T, Dawkins KD, Windecker S. Safety and efficacy of a bioabsorbable polymer-coated, everolimus-eluting coronary stent in patients with diabetes: the EVOLVE II diabetes substudy. EuroIntervention. 2017;12:1987-1994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Kang J, Han JK, Yang HM, Park KW, Kang HJ, Koo BK, Kim HS. Bioresorbable Vascular Scaffolds- Are We Facing a Time of Crisis or One of Breakthrough? Circ J. 2017;81:1065-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Haude M, Ince H, Abizaid A, Toelg R, Lemos PA, von Birgelen C, Christiansen EH, Wijns W, Neumann FJ, Kaiser C, Eeckhout E, Lim ST, Escaned J, Garcia-Garcia HM, Waksman R. Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 month results of a prospective, multicentre, non-randomised, first-in-man trial. Lancet. 2016;387:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 231] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 16. | Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW; Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4265] [Cited by in RCA: 4762] [Article Influence: 250.6] [Reference Citation Analysis (0)] |
| 17. | Hu Z, Wang H, Fan G, Zhang H, Wang X, Mao J, Zhao Y, An Y, Huang Y, Li C, Chang L, Chu X, LiLi, Li Y, Zhang Y, Qin G, Gao X, Zhang B. Danhong injection mobilizes endothelial progenitor cells to repair vascular endothelium injury via upregulating the expression of Akt, eNOS and MMP-9. Phytomedicine. 2019;61:152850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Cimmino G, Ragni M, Cirillo P, Petrillo G, Loffredo F, Chiariello M, Gresele P, Falcinelli E, Golino P. C-reactive protein induces expression of matrix metalloproteinase-9: a possible link between inflammation and plaque rupture. Int J Cardiol. 2013;168:981-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Lee JM, Joh HS, Choi KH, Hong D, Park TK, Yang JH, Song YB, Choi JH, Choi SH, Jeong JO, Lee JY, Choi YJ, Chae JK, Hur SH, Bae JW, Oh JH, Chun KJ, Kim HJ, Cho BR, Shin D, Lee SH, Hwang D, Lee HJ, Jang HJ, Kim HK, Ha SJ, Shin ES, Doh JH, Hahn JY, Gwon HC; SMART-REWARD Investigators. Safety and Efficacy of Everolimus-Eluting Bioresorbable Vascular Scaffold Versus Second-Generation Drug-Eluting Stents in Real-World Practice. J Korean Med Sci. 2023;38:e34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Kereiakes DJ, Ellis SG, Metzger C, Caputo RP, Rizik DG, Teirstein PS, Litt MR, Kini A, Kabour A, Marx SO, Popma JJ, McGreevy R, Zhang Z, Simonton C, Stone GW; ABSORB III Investigators. 3-Year Clinical Outcomes With Everolimus-Eluting Bioresorbable Coronary Scaffolds: The ABSORB III Trial. J Am Coll Cardiol. 2017;70:2852-2862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 208] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 21. | Stone GW, Kimura T, Gao R, Kereiakes DJ, Ellis SG, Onuma Y, Chevalier B, Simonton C, Dressler O, Crowley A, Ali ZA, Serruys PW. Time-Varying Outcomes With the Absorb Bioresorbable Vascular Scaffold During 5-Year Follow-up: A Systematic Meta-analysis and Individual Patient Data Pooled Study. JAMA Cardiol. 2019;4:1261-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 22. | Wilson GJ, Marks A, Berg KJ, Eppihimer M, Sushkova N, Hawley SP, Robertson KA, Knapp D, Pennington DE, Chen YL, Foss A, Huibregtse B, Dawkins KD. The SYNERGY biodegradable polymer everolimus eluting coronary stent: Porcine vascular compatibility and polymer safety study. Catheter Cardiovasc Interv. 2015;86:E247-E257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Rampat R, Williams T, Mayo T, Mengozzi M, Ghezzi P, Hildick-Smith D, Cockburn J. Association between inflammatory biomarkers and neointimal response following elective implantation of the ABSORB bioresorbable vascular scaffold. Coron Artery Dis. 2019;30:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Fedele G, Castiglioni S, Maier JAM, Locatelli L. The Effects of Sirolimus and Magnesium on Primary Human Coronary Endothelial Cells: An In Vitro Study. Int J Mol Sci. 2023;24:2930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Yildiz M, Guddeti RR, Shivapour D, Smith L, Sharkey SW, Schmidt CW, Okeson BK, Dworak M, Garberich RF, Rohm HS, Pacheco-Coronado R, Smith TD, Kereiakes DJ, Garcia S, Henry TD. Frequency, Etiology, and Impact of Unplanned Repeat Coronary Angiography After ST-Elevation Myocardial Infarction. Am J Cardiol. 2022;163:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Bossard M, Madanchi M, Avdijaj D, Attinger-Toller A, Cioffi GM, Seiler T, Tersalvi G, Kobza R, Schüpfer G, Cuculi F. Long-Term Outcomes After Implantation of Magnesium-Based Bioresorbable Scaffolds-Insights From an All-Comer Registry. Front Cardiovasc Med. 2022;9:856930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Wang X, Li Y, Fu G, Xu B, Zhou Y, Su X, Liu H, Zhang Z, Yu B, Tao L, Zheng Q, Li L, Xu K, Han Y; NeoVas OPC Investigators. Three-year clinical outcomes of the novel sirolimus-eluting bioresorbable scaffold for the treatment of de novo coronary artery disease: A prospective patient-level pooled analysis of NeoVas trials. Catheter Cardiovasc Interv. 2023;101:967-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Eriksen E, Neghabat O, Saeed S, Herstad J, Nordrehaug JE, Tuseth V, Holm NR, Holck EN, Sejr-Hansen M, Maule CF, Barkholt TØ, Andreasen LN, Christiansen EH, Bleie Ø. Everolimus-eluting bioresorbable scaffold versus everolimus-eluting metallic stent in primary percutaneous coronary intervention of ST-segment elevation myocardial infarction: a randomized controlled trial. Coron Artery Dis. 2023;34:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/