INTRODUCTION
Cardiac sarcoidosis (CS) is an inflammatory cardiomyopathy characterized by non-caseating granuloma formation in the myocardium. This granulomatous inflammation can disrupt normal cardiac structure and function, leading to conduction abnormalities, arrhythmias, heart failure, or even sudden cardiac death[1]. Cardiac involvement in systemic sarcoidosis confers a worse prognosis-one registry showed significantly higher 10-year rates of heart failure, arrhythmic complications, and mortality in sarcoidosis patients with cardiac involvement compared to those without[2]. Despite the diagnostic and therapeutic challenges posed by this heterogeneous condition, immunosuppressive therapy with corticosteroids remains the cornerstone for active CS management. Our mini review summarizes key findings on how myocardial inflammation and granulomas impact cardiac function, the efficacy and risks of steroid therapy, the role of advanced imaging in diagnosis and treatment guidance, and the implications of steroid use on arrhythmic risk and device therapy.
MYOCARDIAL INFLAMMATION, GRANULOMAS, AND CARDIAC CONDUCTION/FUNCTION
In CS, clusters of granulomas infiltrate the myocardium and can severely impair electrical conduction and contractility. Granulomatous involvement of the basal interventricular septum commonly disrupts the atrioventricular (AV) conduction system, manifesting as high-grade AV block in about 30%-40% of patients[3,4]. This occurs due to inflammation or fibrosis directly affecting the AV node and bundle branches. Beyond conduction block, active myocardial inflammation alters the electrical stability of the heart. In the acute inflammatory phase, granuloma-associated edema and cytokine release can provoke abnormal automaticity and triggered activity, increasing the risk of ventricular arrhythmias[5]. As CS progresses, the healing process of granulomatous lesions transitions from acute inflammation to a fibrotic state characterized by scar tissue formation. This fibrosis creates a substrate conducive to re-entrant arrhythmias, which contrasts sharply with the initial phases of dynamic inflammation[6,7]. Clinically, CS presentations range from asymptomatic inflammation to severe heart failure, ventricular tachycardia (VT), or sudden death. Thus, myocardial inflammation and granuloma formation underlie both the conduction disturbances and the mechanical dysfunction observed in CS.
EFFICACY OF CORTICOSTEROID THERAPY IN ACUTE INFLAMMATION
Corticosteroids are the first-line therapy to suppress active myocardial inflammation in CS, with the goal of preserving cardiac function and preventing arrhythmias[8,9]. Although no randomized controlled trials have been completed, numerous studies and clinical reports document meaningful improvements with steroid treatment. Corticosteroid therapy can reduce the burden of ventricular arrhythmias, restore AV conduction, and improve left ventricular ejection fraction (LVEF) in a substantial subset of patients[10-14]. A meta-analysis of CS cases noted that nearly half of patients with complete heart block experienced recovery of AV conduction after steroid therapy, whereas none of the untreated patients recovered conduction[15]. Early initiation of high-dose prednisone is associated with better preservation of LVEF, particularly in patients with moderate dysfunction.
Chiu et al[11] evaluated the long-term impact of corticosteroid therapy on LV remodeling over an average follow-up period of 88 months. In this retrospective study of 43 patients receiving prednisolone, participants were stratified based on baseline LVEF. Patients with preserved LVEF (> 55%) showed no significant change in LV volumes or function following treatment. In contrast, those with moderately reduced LVEF (30%-55%) experienced significant reductions in LV volume and improvements in LVEF. However, individuals with severely reduced LVEF (< 30%) did not demonstrate favorable remodeling or functional recovery; in fact, their LVEF declined from 22% to 19% (P = 0.08). Notably, lower baseline LVEF was associated with higher mortality[11].
Steroid therapy has also been linked to clinical improvements such as fewer heart failure hospitalizations[11]. In one cohort Nagai et al[14], patients receiving corticosteroids [most with active inflammation confirmed by gallium/positron emission tomography (PET) imaging] showed greater LVEF increases, and reduced heart failure events compared to those not treated with steroids. However, that study reported no significant difference in arrhythmic outcomes or cardiac mortality between treated and untreated groups[14]. Discrepancies in arrhythmic outcomes may reflect fundamental differences in the underlying disease state, such as the degree of myocardial fibrosis and disease chronicity at treatment initiation, as well as the concurrent use of other interventions, including implantable cardioverter-defibrillators and other immunosuppressants. For instance, in the presence of advanced structural remodeling or established scar tissue, the anti-inflammatory effects of corticosteroids may not translate to a reduction in the substrate for reentrant arrhythmias. This suggests that while corticosteroids effectively mitigate acute myocardial inflammation and improve pump function, they may not eliminate the risk of malignant arrhythmias, which often persist due to residual scar or other pre-existing factors. Consequently, the impact of corticosteroids on arrhythmia burden appears to be contingent upon these intrinsic disease and treatment-related variables.
LONG-TERM STEROID THERAPY AND ASSOCIATED RISKS
Long-term use of high-dose corticosteroids is accompanied by substantial risks, necessitating a careful risk–benefit assessment in CS management. Prolonged steroid therapy can lead to numerous adverse effects that impact patient safety and quality of life[16]. Key among these are infectious complications by suppressing immune responses, chronic corticosteroid use predisposes patients to opportunistic infections (e.g., reactivation of latent TB, fungal infections) and poor wound healing. Metabolic side effects are also prominent; steroids induce weight gain, visceral fat deposition, hyperglycemia and insulin resistance, and dyslipidemia[17-20]. Over time, this can precipitate steroid-induced diabetes and exacerbate cardiovascular risk factors. Other long-term effects include osteoporosis and skeletal muscle weakness, hypertension, cataracts, mood and sleep disturbances, and adrenal suppression, among others[21]. Given the potential for steroid toxicity, clinicians often employ strategies to mitigate these risks. Steroid-sparing immunosuppressants (e.g. methotrexate, mycophenolate, azathioprine) are frequently introduced early in the treatment course to allow tapering to lower prednisone doses. In fact, the median duration of prednisone therapy in CS is on the order of 1.5-2 years in some series, so adjunctive therapy is used to achieve disease control while minimizing cumulative steroid exposure. Patients on long-term steroids require monitoring for complications (e.g. periodic blood glucose checks, bone density assessments, infection surveillance)[22-25]. In summary, while corticosteroids are efficacious in controlling cardiac sarcoid inflammation, their chronic use must be managed prudently to avoid serious systemic side effects.
In steroid-refractory or intolerant patients, alternative immunosuppressive agents are increasingly being employed. Methotrexate, azathioprine, and mycophenolate mofetil are commonly used as steroid-sparing therapies and are often initiated early to facilitate tapering of corticosteroids. Additionally, biologic agents are being explored in refractory CS. Tumor necrosis factor-alpha inhibitors such as infliximab have shown efficacy in extrapulmonary sarcoidosis and are being used off-label in select CS cases, particularly in those with persistent inflammation despite conventional therapy. Rituximab, a monoclonal antibody targeting CD20+ B-cells, has also been utilized with some success. While robust trial data are lacking, case reports and small series suggest these agents may provide benefit in reducing disease activity and arrhythmia burden. Further research is needed to clarify their role and long-term safety, but their inclusion in therapeutic algorithms represents a promising advancement in the management of complex or steroid-resistant CS.
ADVANCED IMAGING (CARDIAC MAGNETIC RESONANCE IMAGING AND PET) IN DIAGNOSIS AND TREATMENT DECISIONS
Cardiac magnetic resonance imaging (CMR) and PET are indispensable tools for diagnosing CS and guiding therapy. Because there is no single definitive diagnostic test for CS, a multimodality imaging approach is often employed when CS is suspected. CMR can detect myocardial scar and edema (typically via late gadolinium enhancement and T2-weighted sequences), whereas 18F-fluorodeoxyglucose (FDG) PET identifies active inflammatory metabolism in the myocardium. When used together, CMR and PET provide complementary information that significantly improves diagnostic accuracy and disease characterization[26]. Current expert criteria for clinical diagnosis of CS incorporate imaging evidence of cardiac involvement in patients with biopsy-proven extra CS, and the combination of PET and CMR helps fulfill these criteria in unclear cases[27]. Beyond diagnosis, CMR and PET provide independent prognostic information for instance, the presence of both scar (CMR) and active uptake (PET) portends a higher risk of adverse cardiac outcomes[28].
Imaging is also critical in guiding treatment decisions. Identifying active inflammation on PET/CMR helps select patients who are likely to respond to immunosuppressive therapy. Baseline FDG-PET scan before starting therapy to map the extent of active cardiac sarcoid inflammation[29-31]. Serial imaging is then used to monitor response: A repeat PET scan about 3-6 months into steroid treatment can demonstrate whether myocardial inflammation has abated[32]. A significant reduction in FDG uptake on follow-up PET correlates with effective suppression of inflammation, at which point tapering of steroids to a maintenance dose (to complete about 12 months of therapy) is often undertaken[27]. CMR may also be repeated intermittently to assess changes in ventricular function or scar burden, though implanted devices [implantable cardioverter-defibrillators (ICDs)/pacemakers] can limit CMR utility[33,34]. Advanced imaging also aids in prognostication. For instance, patients with active inflammation in the basal septum on PET were more likely to recover AV conduction after steroid therapy, highlighting the importance of metabolic activity over fibrosis in predicting reversibility[35]. Such findings illustrate how CMR/PET can inform prognosis and tailor management (e.g. identifying patients in whom aggressive immunosuppression is warranted vs. those where permanent pacing and other measures should be prioritized). In summary, CMR and PET are essential in confirming the diagnosis of CS, assessing disease activity, stratifying risk, and dynamically guiding immunosuppressive therapy.
STEROID THERAPY, ARRHYTHMIC RISK, AND THE USE OF ICDS
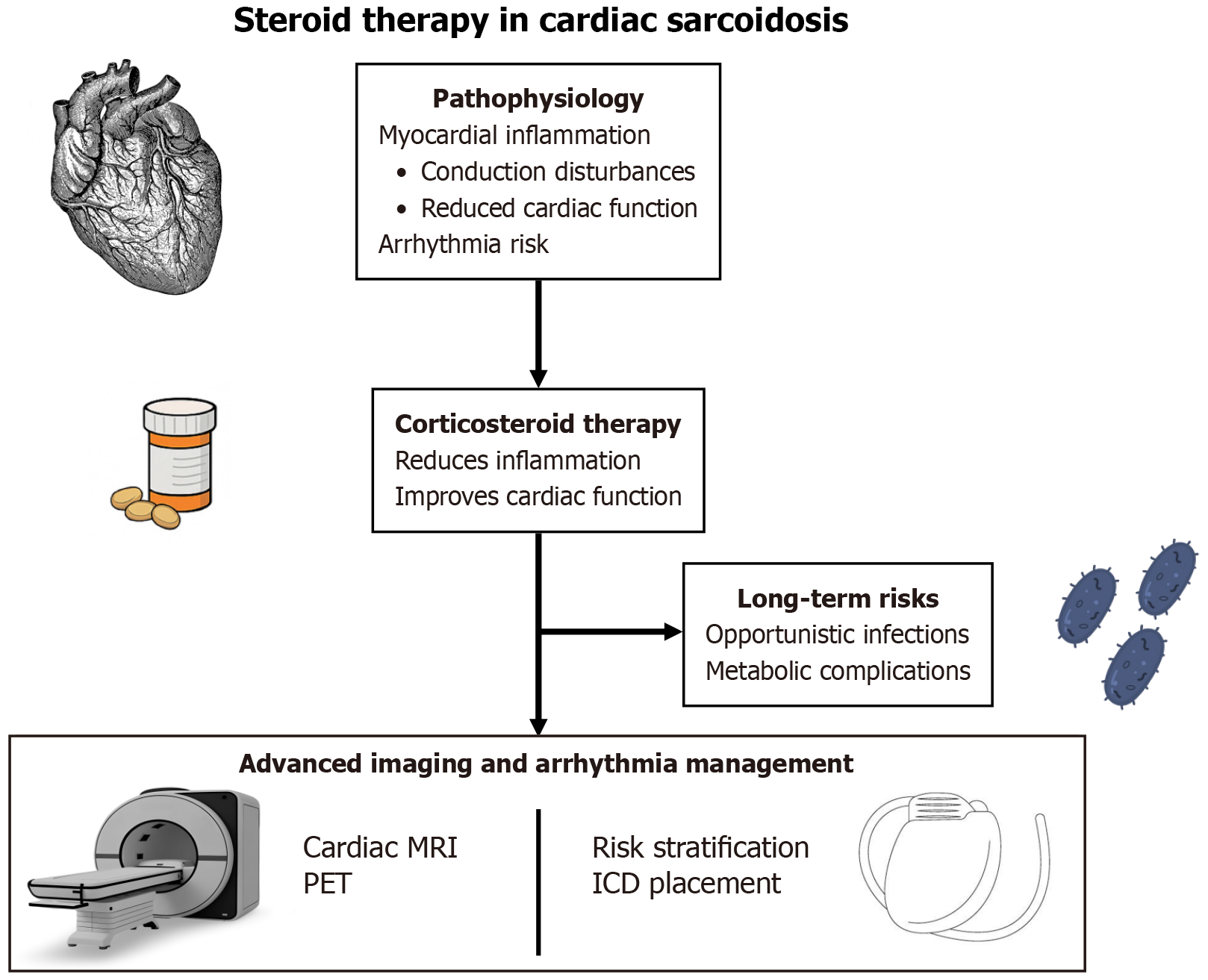
A central challenge in CS management is mitigating the high arrhythmic risk-a challenge that persists even with successful suppression of inflammation. Corticosteroid therapy can quiet active myocarditis and in some cases reduce arrhythmia frequency (for instance, resolving VT storm or eliminating frequent ventricular ectopy)[36,37]. However, immunosuppression alone cannot reliably prevent life-threatening arrhythmias in CS. Studies have shown that patients remain at significant risk for malignant ventricular arrhythmias and sudden cardiac death despite steroid treatment. In a Japanese series of CS patients with complete heart block, 41% experienced a fatal cardiac event [mostly VT/ventricular fibrillation (VF)] over about 3 years, and this high event rate was equally observed even among those whose AV block had resolved with steroids[38]. In other words, restoring conduction with therapy did not eliminate the underlying arrhythmic propensity. This finding reinforces guideline recommendations that device therapy should be pursued in parallel with medical therapy for CS. Experts advocate early implantation of pacemakers/ICDs when indicated, rather than waiting to see the full effect of steroids, given the unpredictable course of arrhythmias. For example, in a patient who presents with Mobitz type II or third-degree AV block due to sarcoid, a permanent pacemaker is indicated promptly, and adding an ICD for primary prevention is considered prudent even if AV conduction later improves with steroids[39,40]. Likewise, anyone surviving cardiac arrest or presenting with sustained VT fulfills standard criteria for an ICD (secondary prevention) regardless of inflammatory status. Even in the absence of documented VT/VF, patients with severe LV dysfunction (LVEF < 35%) after initial therapy meet primary prevention criteria for ICD placement[41]. Current consensus guidelines therefore call for a tandem approach: Treat active inflammation with immunosuppressants and aggressively manage arrhythmic risk with antiarrhythmic drugs and/or device implantation as needed. Notably, immunosuppressive therapy should accompany but not replace ICD therapy when warranted – steroid use does not obviate the need for an ICD, since scar-mediated reentrant circuits can cause ventricular tachyarrhythmias even after inflammation subsides[42]. In practice, this means that a CS patient’s care often involves a multidisciplinary strategy: Corticosteroids (sometimes escalated to high-dose IV pulses for acute VT storm) to control inflammation, plus electrophysiological interventions (ICDs, pacemakers, or ablations) to protect against arrhythmias. This comprehensive approach addresses both the substrate (granulomatous inflammation) and the consequence (arrhythmia/sudden cardiac death risk) of CS (Figure 1).
Figure 1 Steroid therapy in cardiac sarcoidosis.
MRI: Magnetic resonance imaging; PET: Positron emission tomography; ICD: Implantable cardioverter-defibrillators.
CONCLUSION
CS is a complex disease in which granulomatous myocardial inflammation can lead to conduction block, arrhythmias, and heart failure. Corticosteroid therapy plays a pivotal role in managing acute myocardial inflammation in CS, it can improve AV nodal conduction and LV function, particularly when instituted early in the disease course. Steroids remain the first-line treatment for active CS and have proven efficacy in ameliorating cardiac inflammation and dysfunction. However, long-term steroid use must be balanced against its significant risks, including infections and metabolic complications, prompting the use of steroid-sparing agents and diligent monitoring to minimize toxicity. Advanced cardiac imaging (CMR and FDG-PET) has become indispensable for diagnosing CS, identifying active inflammation, guiding biopsy and therapy decisions, and monitoring treatment response. These tools ensure that immunosuppressive therapy is appropriately targeted and adjusted based on disease activity. Finally, given the persistent arrhythmic risks in CS, steroid therapy is integrated with arrhythmia management rather than used in isolation. Patients are risk-stratified for sudden death, and indications for implantable cardioverter-defibrillators are carefully considered alongside medical therapy. In summary, effective management of CS requires a multifaceted, evidence-guided approach: Quelling myocardial inflammation with corticosteroids (and other immunosuppressants when needed), employing advanced imaging for diagnosis and follow-up, and proactively addressing conduction and arrhythmic issues with device therapy. This combined strategy offers the best chance to improve cardiac outcomes and reduce life-threatening events in patients with this challenging inflammatory heart disease.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report’s classification
Scientific Quality: Grade A, Grade A
Novelty: Grade A, Grade A
Creativity or Innovation: Grade A, Grade A
Scientific Significance: Grade A, Grade A
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Xu JW, MD, Associate Chief Physician, China S-Editor: Liu H L-Editor: A P-Editor: Wang CH