Published online Jan 26, 2025. doi: 10.4330/wjc.v17.i1.100886
Revised: December 18, 2024
Accepted: December 27, 2024
Published online: January 26, 2025
Processing time: 144 Days and 23.8 Hours
This article discusses the study by Grubić Rotkvić et al on the mechanisms of action of sodium-glucose cotransporter 2 inhibitors (SGLT2i) in patients with type 2 diabetes mellitus (T2DM) and heart failure (HF). T2DM and HF are highly comorbid, with a significantly increased prevalence of HF in patients with T2DM. SGLT2i exhibit potential in reducing hospitalization rates for HF and cardiova
Core Tip: Sodium-glucose cotransporter 2 inhibitor exert significant effects in patients with type 2 diabetes and heart failure (HF) through multiple mechanisms, including improving blood glucose control, promoting osmotic diuresis, reducing inflammation and oxidative stress, optimizing myocardial energy metabolism, regulating myocardial ion homeostasis, enhancing endothelial function, reducing sympathetic nervous system activity, and alleviating ventricular remodeling. These effects collectively improve cardiac function and prognosis, thereby reducing hospitalization rates and cardiovascular mortality in patients with various types of HF.
- Citation: Zhang YF, Liu YX, Yang WX. Sodium-dependent glucose transporter 2 inhibitors improve heart function in patients with type 2 diabetes and heart failure. World J Cardiol 2025; 17(1): 100886
- URL: https://www.wjgnet.com/1949-8462/full/v17/i1/100886.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i1.100886
The number of patients with heart failure (HF) remains alarmingly high, with approximately 64 million individuals affected worldwide, and this number continues to rise[1]. Among patients hospitalized for HF, whether with HF with preserved ejection fraction (HFpEF), HF with mid-range ejection fraction (HFmrEF), or HF with reduced ejection fraction (HFrEF), the 5-year mortality rate is as high as 75%[2]. Diabetes is strongly associated with the development of HF, with the prevalence of HF in diabetic patients being 2 to 5 times higher than in the general population[3,4].
The development of HF in patients with type 2 diabetes mellitus (T2DM) is the result of multiple contributing factors. In addition to the direct and indirect damage caused by hyperglycemia, other comorbid metabolic diseases, such as obesity, also contribute to myocardial injury[5]. Diabetic cardiomyopathy (DCM) refers to myocardial disease in diabetic patients that cannot be explained by hypertension, coronary artery disease, valvular heart disease, or other cardiac con
As the evidence supporting the use of sodium-glucose cotransporter 2 inhibitor (SGLT2i) in HF continues to accumulate, the recommendations for their use in authoritative guidelines have progressively strengthened. First, the EMPA-REG OUTCOME trial[12], CANVAS trial[13], DECLARE–TIMI 58 trial[14], and VERTIS-CV trial[15] all demonstrated that SGLT2i have advantages in improving cardiovascular mortality, all-cause mortality, and HF hospitalization rates in patients with T2DM. Second, the DAPA-HF trial[16] and EMPEROR-Reduced trial[17], which focused on patients with HFrEF, found that SGLT2i treatment reduced the risk of HF worsening and cardiovascular death, regardless of the presence of T2DM. Moreover, the DELIVER trial[18] and EMPEROR-Preserved trial[19] extended the applicability of SGLT2i to patients with HFmrEF and HFpEF. Meanwhile, the SOLOIST-WHF trial[20] and EMPULSE trial[21] expanded the benefits of SGLT2i to patients with T2DM who were hospitalized due to worsening HF. Additionally, while reducing cardiovascular risk in T2DM patients, SGLT2i also lower the risk of kidney failure. For example, the DECLARE–TIMI 58 trial[14] showed that, regardless of baseline estimated glomerular filtration rate or urine albumin-to-creatinine ratio, SGLT2i reduced the risk of kidney-specific endpoints in T2DM patients. The DAPA-CKD trial[22] demonstrated that SGLT2i significantly reduced the risk of heart-kidney events in patients with chronic kidney disease (CKD), with consistent benefits observed regardless of T2DM status. Based on these findings, the 2022 AHA/ACC/HFSA HF management guidelines recommend SGLT2i as a Class I, Level A recommendation for symptomatic HFrEF patients, regardless of T2DM status, to reduce HF hospitalization and cardiovascular mortality[23]. Furthermore, the 2023 ESC HF guidelines recommend the use of SGLT2i in patients with HFmrEF and HFpEF to reduce the risk of HF hospitalization or cardiovascular death, also as a Class I, Level A recommendation[24].
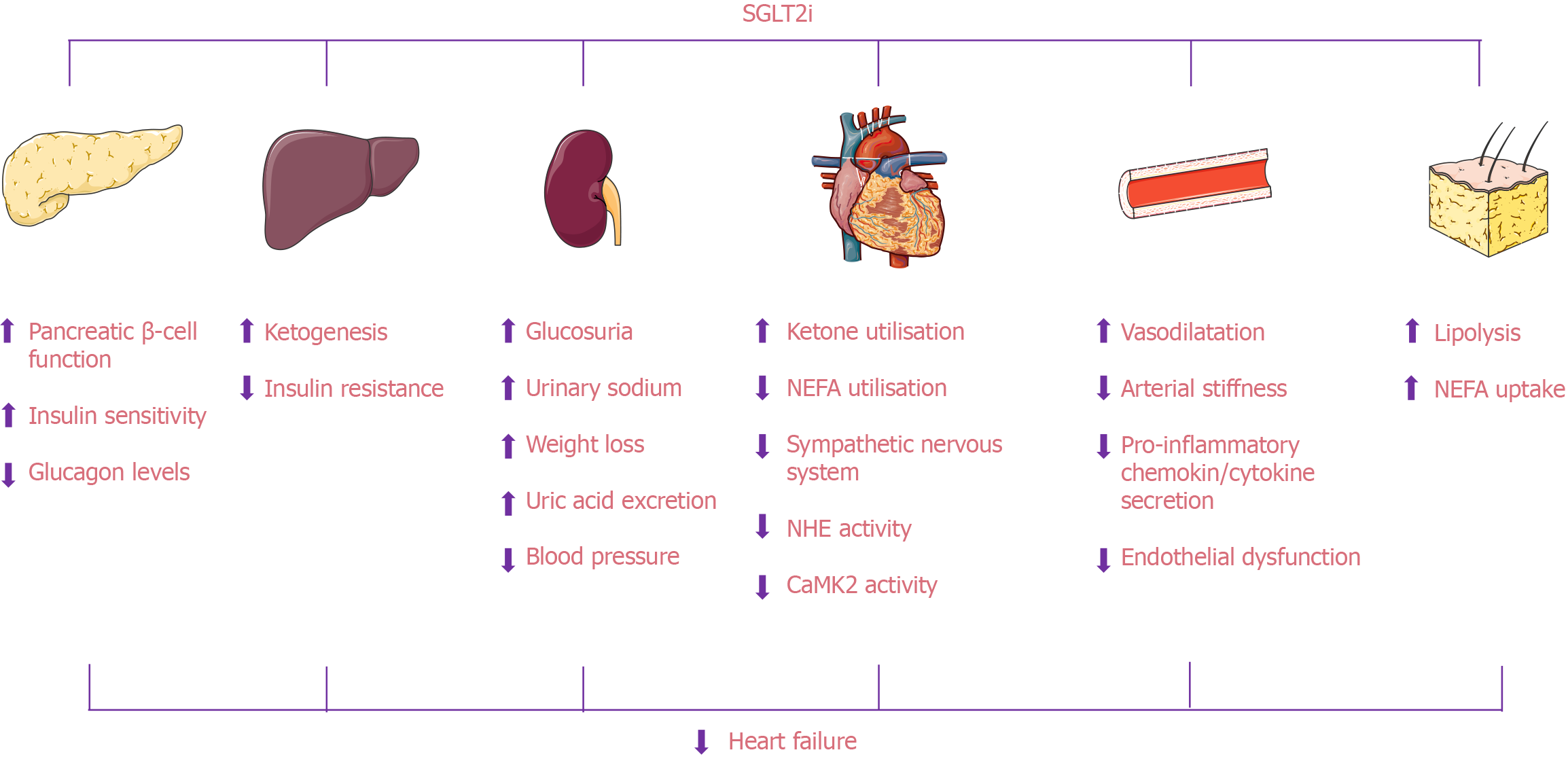
The mechanisms by which SGLT2i improve cardiac function are complex and involve multiple pathophysiological processes. These effects are primarily achieved through various pathways, including reducing water and sodium retention, alleviating inflammation and oxidative stress, and improving myocardial energy metabolism (Figure 1; Table 1).
| Beneficial factors of SGLT2i with ability to improve T2DM with HF |
| 1 Reducing inflammation |
| 2 Inhibiting oxidative stress |
| 3 Attenuating myocardial fibrosis and apoptosis |
| 4 Improving myocardial energetics |
| 5 Improving myocardial fatty acid and glucose metabolism |
| 6 Improving mitochondrial function |
| 7 Normalizing intracellular Ca2+ handling in cardiomyocytes |
| 8 Inhibiting the sympathetic nervous system |
| 9 Improving adverse cardiac remodeling |
| 10 Reducing hyperglycemic toxicity |
| 11 Osmotic diuretics |
| 12 Lowering blood pressure |
SGLT2is produce an osmotic diuretic effect by inhibiting sodium reabsorption in the renal proximal tubules, promoting urinary sodium excretion. This helps improve water and sodium retention, reduce blood pressure, and decrease both the preload and afterload on the heart, thereby benefiting HF patients[25]. In a study by Grubić Rotkvić et al[26], a significant and meaningful antihypertensive effect was observed in patients with initially high systolic blood pressure (> 131 mmHg) and diastolic blood pressure (> 80 mmHg). In the subgroup with an initial systolic blood pressure ≤ 131 mmHg, an increase in systolic blood pressure was noted. Similar to this study, the EMPA-REG OUTCOME trial found that empag
In a study by Grubić Rotkvić et al[26], it was observed that SGLT2is can reduce the expression of circulating inflammatory markers, such as myeloperoxidase and high-sensitivity C-reactive protein, thereby lowering levels of inflammation and oxidative stress, which benefits cardiovascular health. The potential mechanisms underlying the anti-inflammatory effects of SGLT2is are multifaceted. First, SGLT2is improve glycemic control by promoting urinary glucose excretion[30], reducing hyperglycemic toxicity[31], and preventing systemic metabolic abnormalities. Second, SGLT2is can reduce body weight[32,33] and improve insulin sensitivity[31]. Additionally, SGLT2is promote uric acid excretion by SGLT2 in the kidneys, which increases urinary glucose excretion while simultaneously promoting uric acid excretion. SGLT2is also reduce uric acid synthesis by acting on different pathways of uric acid metabolism, lowering intracellular hypoxanthine levels and inhibiting purine synthesis, which helps prevent and improve hyperuricemia[34-36]. However, it remains unclear how long the serum uric acid-lowering effect of SGLT2is lasts, and whether there are ethnic or gender differences in this effect.
Basic research has found that SGLT2is exert beneficial effects on cardiac cells through various mechanisms, such as reducing oxidative stress and inflammation. The specific pathophysiological mechanisms include inhibiting fibrosis, improving mitochondrial function, regulating ion transport, suppressing endoplasmic reticulum stress, modulating apoptosis and autophagy, alleviating cellular hypertrophy, and possessing anti-inflammatory and antioxidant properties, all of which contribute to improved cardiac function[37]. Overall, SGLT2is alleviate oxidative stress and inflammation in HF through multiple molecular pathways. However, these effects are unlikely to be mediated by cardiac SGLT2, as SGLT2 is not expressed in the heart[38-40].
In HF, myocardial energy metabolism is impaired, with a decline in glucose utilization and an increase in non-esterified fatty acid (NEFA) oxidation. This leads to elevated reactive oxygen species (ROS) generation in cardiomyocytes, exacerbating oxidative stress and lipid peroxidation damage. These changes contribute to mitochondrial dysfunction, cell necrosis or apoptosis, ventricular remodeling, and ultimately, cardiac dysfunction.
Beta-hydroxybutyrate is considered a "super fuel" that can substitute glucose and NEFAs as an energy source for the myocardium. SGLT2 inhibitors can increase fatty acid oxidation and ketone body production, reducing the toxic effects of hyperglycemia on cardiomyocytes, enhancing myocardial utilization of ketone bodies, and thereby improving myocar
SGLT2is can improve myocardial contraction and active relaxation by regulating myocardial ion homeostasis. In HF patients, the upregulation of Na+-H+ exchanger (NHE) activity leads to increased cytoplasmic Na+ concentration, calcium (Ca2+) overload, and reduced mitochondrial Ca2+ concentration. This not only accelerates the progression of HF but also increases the risk of sudden cardiac death in patients with arrhythmias. Studies have shown that SGLT2is reduce cytoplasmic Na+ and Ca2+ concentrations by inhibiting NHE, while increasing mitochondrial Ca2+ levels[43]. The increase in mitochondrial Ca2+ concentration is crucial for myocardial cell excitability, contraction, and mitochondrial antioxidant capacity. Additionally, SGLT2is can inhibit calcium/calmodulin-dependent protein kinase 2 (CaMK2), a key regulator. Overexpression and activation of CaMK2 are markers of HF, leading to reduced calcium concentration in the sarcoplas
In this study, Grubić Rotkvić et al[26] observed that after SGLT2i treatment, left ventricular longitudinal strain (GLS) showed beneficial improvements compared to pre-treatment levels, as previously reported[46,47], indicating that SGLT2is improved left ventricular function. GLS, which assesses left ventricular myocardial function, can detect early subtle abnormalities and helps predict outcomes in various cardiac conditions. For HF patients, GLS can be used to predict subclinical left ventricular dysfunction or to better identify the severity or prognosis of the disease, outperforming traditional echocardiographic parameters[47].
A key potential mechanism of SGLT2is in HF management lies in their ability to improve adverse cardiac remodeling. SGLT2is enhance cardiac autonomic function in patients with T2DM[48], reduce levels of tyrosine hydroxylase and norepinephrine in the heart and kidneys[49], thereby inhibiting the sympathetic nervous system and improving adverse cardiac remodeling. Preclinical studies have also shown that empagliflozin can reduce cardiomyocyte apoptosis, decrease fibroblast activation, and attenuate extracellular matrix remodeling, thus exerting antifibrotic effects[50].
As SGLT2is become increasingly popular, clinicians need to be aware of certain rare side effects and further investigate their underlying causes and mechanisms. For instance, in patients who have recently started SGLT2i treatment, it is important to promptly identify the cause and suspend the use of the drug during an acute pancreatitis episode[51]. Additionally, some experts recommend temporarily withholding SGLT2i in patients with advanced CKD within 24 hours before or after receiving high-dose contrast agents or undergoing selective coronary interventions[52]. Furthermore, theoretically, SGLT2i may increase the risk of dehydration, orthostatic hypotension, or falls due to osmotic diuresis and reduced blood volume, which could lead to a higher risk of fractures[53]. It is also important to note that SGLT2i can potentially lead to diabetic ketoacidosis, which requires healthcare providers to consider whether to pause treatment in patients undergoing any acute events, including surgery[54].
Finally, we are pleased to see a series of recent studies investigating the use of SGLT2is in patients with acute myocardial infarction. These studies have shown a reduction in NT-proBNP levels and improvements in some echocardiographic parameters in the short term. However, whether this will significantly improve patient prognosis and quality of life still requires validation through future clinical trials.
In summary, SGLT2is have emerged as a standout treatment for patients with T2DM and HF, significantly reducing cardiovascular mortality and hospitalization rates in HF patients. They benefit various types of HF patients, whether acute or chronic, and regardless of ejection fraction status, whether preserved or reduced. Furthermore, SGLT2is exert cardioprotective effects through multiple mechanisms, such as controlling blood glucose, inducing osmotic diuresis, lowering blood pressure, improving inflammation and oxidative stress, optimizing myocardial energy metabolism, regulating myocardial ion homeostasis, and improving ventricular remodeling, all of which contribute to enhanced heart function and prognosis. Although there are still some challenges in their clinical application, such as rare adverse effects and usage restrictions, these issues are expected to be better addressed with further research and the accumulation of clinical experience.
| 1. | Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2023;118:3272-3287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 1907] [Article Influence: 635.7] [Reference Citation Analysis (0)] |
| 2. | Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J Am Coll Cardiol. 2017;70:2476-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 817] [Article Influence: 90.8] [Reference Citation Analysis (1)] |
| 3. | Huynh K, Bernardo BC, McMullen JR, Ritchie RH. Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol Ther. 2014;142:375-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 446] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 4. | Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1406] [Cited by in RCA: 1483] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 5. | Battault S, Renguet E, Van Steenbergen A, Horman S, Beauloye C, Bertrand L. Myocardial glucotoxicity: Mechanisms and potential therapeutic targets. Arch Cardiovasc Dis. 2020;113:736-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord. 2010;11:31-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 566] [Cited by in RCA: 572] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 7. | Wang M, Li Y, Li S, Lv J. Endothelial Dysfunction and Diabetic Cardiomyopathy. Front Endocrinol (Lausanne). 2022;13:851941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 113] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 8. | Salvatore T, Pafundi PC, Galiero R, Albanese G, Di Martino A, Caturano A, Vetrano E, Rinaldi L, Sasso FC. The Diabetic Cardiomyopathy: The Contributing Pathophysiological Mechanisms. Front Med (Lausanne). 2021;8:695792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 9. | Radzioch E, Dąbek B, Balcerczyk-Lis M, Frąk W, Fularski P, Młynarska E, Rysz J, Franczyk B. Diabetic Cardiomyopathy-From Basics through Diagnosis to Treatment. Biomedicines. 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 10. | Kaur N, Guan Y, Raja R, Ruiz-Velasco A, Liu W. Mechanisms and Therapeutic Prospects of Diabetic Cardiomyopathy Through the Inflammatory Response. Front Physiol. 2021;12:694864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Miki T, Yuda S, Kouzu H, Miura T. Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev. 2013;18:149-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 357] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 12. | Wanner Ch, Inzucchi SE, Zinman B. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375:1801-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 13. | Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380:2295-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2826] [Cited by in RCA: 4332] [Article Influence: 618.9] [Reference Citation Analysis (0)] |
| 14. | Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4652] [Cited by in RCA: 4538] [Article Influence: 648.3] [Reference Citation Analysis (0)] |
| 15. | Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R, Gallo S, Cosentino F, Shih WJ, Gantz I, Terra SG, Cherney DZI, McGuire DK; VERTIS CV Investigators. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N Engl J Med. 2020;383:1425-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 1088] [Article Influence: 181.3] [Reference Citation Analysis (0)] |
| 16. | McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 4895] [Article Influence: 699.3] [Reference Citation Analysis (0)] |
| 17. | Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383:1413-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1875] [Cited by in RCA: 3526] [Article Influence: 587.7] [Reference Citation Analysis (0)] |
| 18. | Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Desai AS, Jhund PS, Belohlavek J, Chiang CE, Borleffs CJW, Comin-Colet J, Dobreanu D, Drozdz J, Fang JC, Alcocer-Gamba MA, Al Habeeb W, Han Y, Cabrera Honorio JW, Janssens SP, Katova T, Kitakaze M, Merkely B, O'Meara E, Saraiva JFK, Tereshchenko SN, Thierer J, Vaduganathan M, Vardeny O, Verma S, Pham VN, Wilderäng U, Zaozerska N, Bachus E, Lindholm D, Petersson M, Langkilde AM; DELIVER Trial Committees and Investigators. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med. 2022;387:1089-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 1804] [Article Influence: 451.0] [Reference Citation Analysis (0)] |
| 19. | Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M; EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021;385:1451-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3009] [Cited by in RCA: 3231] [Article Influence: 646.2] [Reference Citation Analysis (0)] |
| 20. | Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B; SOLOIST-WHF Trial Investigators. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N Engl J Med. 2021;384:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 753] [Cited by in RCA: 1354] [Article Influence: 270.8] [Reference Citation Analysis (0)] |
| 21. | Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J, Ferreira JP, Nassif ME, Psotka MA, Tromp J, Borleffs CJW, Ma C, Comin-Colet J, Fu M, Janssens SP, Kiss RG, Mentz RJ, Sakata Y, Schirmer H, Schou M, Schulze PC, Spinarova L, Volterrani M, Wranicz JK, Zeymer U, Zieroth S, Brueckmann M, Blatchford JP, Salsali A, Ponikowski P. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28:568-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 594] [Article Influence: 148.5] [Reference Citation Analysis (0)] |
| 22. | Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383:1436-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1586] [Cited by in RCA: 3629] [Article Influence: 604.8] [Reference Citation Analysis (1)] |
| 23. | Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW; ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895-e1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1618] [Cited by in RCA: 1660] [Article Influence: 415.0] [Reference Citation Analysis (0)] |
| 24. | McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Skibelund AK; ESC Scientific Document Group. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023;44:3627-3639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 1380] [Article Influence: 460.0] [Reference Citation Analysis (3)] |
| 25. | Chin KL, Ofori-Asenso R, Hopper I, von Lueder TG, Reid CM, Zoungas S, Wang BH, Liew D. Potential mechanisms underlying the cardiovascular benefits of sodium glucose cotransporter 2 inhibitors: a systematic review of data from preclinical studies. Cardiovasc Res. 2019;115:266-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Grubić Rotkvić P, Rotkvić L, Đuzel Čokljat A, Cigrovski Berković M. Sodium-dependent glucose transporter 2 inhibitors effects on myocardial function in patients with type 2 diabetes and asymptomatic heart failure. World J Cardiol. 2024;16:448-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 27. | Zinman B, Lachin JM, Inzucchi SE. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2016;374:1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 28. | Serenelli M, Böhm M, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, DeMets DL, Bengtsson O, Sjöstrand M, Langkilde AM, Anand IS, Chiang CE, Chopra VK, de Boer RA, Diez M, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Verma S, Docherty KF, Jhund PS, McMurray JJV. Effect of dapagliflozin according to baseline systolic blood pressure in the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial (DAPA-HF). Eur Heart J. 2020;41:3402-3418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 29. | Häring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Woerle HJ, Broedl UC; EMPA-REG METSU Trial Investigators. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36:3396-3404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 308] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 30. | Verbrugge FH. Role of SGLT2 Inhibitors in Patients with Diabetes Mellitus and Heart Failure. Curr Heart Fail Rep. 2017;14:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, Xiong J, Perez Z, Norton L, Abdul-Ghani MA, DeFronzo RA. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 649] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 32. | Bolinder J, Ljunggren Ö, Kullberg J, Johansson L, Wilding J, Langkilde AM, Sugg J, Parikh S. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 664] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 33. | Sargeant JA, Henson J, King JA, Yates T, Khunti K, Davies MJ. A Review of the Effects of Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors on Lean Body Mass in Humans. Endocrinol Metab (Seoul). 2019;34:247-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 150] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 34. | La Grotta R, de Candia P, Olivieri F, Matacchione G, Giuliani A, Rippo MR, Tagliabue E, Mancino M, Rispoli F, Ferroni S, Berra CC, Ceriello A, Prattichizzo F. Anti-inflammatory effect of SGLT-2 inhibitors via uric acid and insulin. Cell Mol Life Sci. 2022;79:273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 35. | Xing YJ, Liu BH, Wan SJ, Cheng Y, Zhou SM, Sun Y, Yao XM, Hua Q, Meng XJ, Cheng JH, Zhong M, Zhang Y, Lv K, Kong X. A SGLT2 Inhibitor Dapagliflozin Alleviates Diabetic Cardiomyopathy by Suppressing High Glucose-Induced Oxidative Stress in vivo and in vitro. Front Pharmacol. 2021;12:708177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 36. | Lu YP, Zhang ZY, Wu HW, Fang LJ, Hu B, Tang C, Zhang YQ, Yin L, Tang DE, Zheng ZH, Zhu T, Dai Y. SGLT2 inhibitors improve kidney function and morphology by regulating renal metabolic reprogramming in mice with diabetic kidney disease. J Transl Med. 2022;20:420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 37. | Erdogan BR, Arioglu-Inan E. SGLT2 inhibitors: how do they affect the cardiac cells. Mol Cell Biochem. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Abdulrahman N, Ibrahim M, Joseph JM, Elkoubatry HM, Al-Shamasi AA, Rayan M, Gadeau AP, Ahmed R, Eldassouki H, Hasan A, Mraiche F. Empagliflozin inhibits angiotensin II-induced hypertrophy in H9c2 cardiomyoblasts through inhibition of NHE1 expression. Mol Cell Biochem. 2022;477:1865-1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Kondo H, Akoumianakis I, Badi I, Akawi N, Kotanidis CP, Polkinghorne M, Stadiotti I, Sommariva E, Antonopoulos AS, Carena MC, Oikonomou EK, Reus EM, Sayeed R, Krasopoulos G, Srivastava V, Farid S, Chuaiphichai S, Shirodaria C, Channon KM, Casadei B, Antoniades C. Effects of canagliflozin on human myocardial redox signalling: clinical implications. Eur Heart J. 2021;42:4947-4960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 40. | Sayour AA, Oláh A, Ruppert M, Barta BA, Horváth EM, Benke K, Pólos M, Hartyánszky I, Merkely B, Radovits T. Characterization of left ventricular myocardial sodium-glucose cotransporter 1 expression in patients with end-stage heart failure. Cardiovasc Diabetol. 2020;19:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 41. | Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, Ishikawa K, Watanabe S, Picatoste B, Flores E, Garcia-Ropero A, Sanz J, Hajjar RJ, Fuster V, Badimon JJ. Empagliflozin Ameliorates Adverse Left Ventricular Remodeling in Nondiabetic Heart Failure by Enhancing Myocardial Energetics. J Am Coll Cardiol. 2019;73:1931-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 501] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 42. | Ekanayake P, Hupfeld C, Mudaliar S. Sodium-Glucose Cotransporter Type 2 (SGLT-2) Inhibitors and Ketogenesis: the Good and the Bad. Curr Diab Rep. 2020;20:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Chung CC, Lin YK, Chen YC, Kao YH, Yeh YH, Trang NN, Chen YJ. Empagliflozin suppressed cardiac fibrogenesis through sodium-hydrogen exchanger inhibition and modulation of the calcium homeostasis. Cardiovasc Diabetol. 2023;22:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 44. | Mustroph J, Wagemann O, Lücht CM, Trum M, Hammer KP, Sag CM, Lebek S, Tarnowski D, Reinders J, Perbellini F, Terracciano C, Schmid C, Schopka S, Hilker M, Zausig Y, Pabel S, Sossalla ST, Schweda F, Maier LS, Wagner S. Empagliflozin reduces Ca/calmodulin-dependent kinase II activity in isolated ventricular cardiomyocytes. ESC Heart Fail. 2018;5:642-648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 45. | Trum M, Wagner S, Maier LS, Mustroph J. CaMKII and GLUT1 in heart failure and the role of gliflozins. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Hu M, Cai X, Yang W, Zhang S, Nie L, Ji L. Effect of Hemoglobin A1c Reduction or Weight Reduction on Blood Pressure in Glucagon-Like Peptide-1 Receptor Agonist and Sodium-Glucose Cotransporter-2 Inhibitor Treatment in Type 2 Diabetes Mellitus: A Meta-Analysis. J Am Heart Assoc. 2020;9:e015323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 47. | Tanaka H, Soga F, Tatsumi K, Mochizuki Y, Sano H, Toki H, Matsumoto K, Shite J, Takaoka H, Doi T, Hirata KI. Positive effect of dapagliflozin on left ventricular longitudinal function for type 2 diabetic mellitus patients with chronic heart failure. Cardiovasc Diabetol. 2020;19:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 48. | Shimizu W, Kubota Y, Hoshika Y, Mozawa K, Tara S, Tokita Y, Yodogawa K, Iwasaki YK, Yamamoto T, Takano H, Tsukada Y, Asai K, Miyamoto M, Miyauchi Y, Kodani E, Ishikawa M, Maruyama M, Ogano M, Tanabe J; EMBODY trial investigators. Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol. 2020;19:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (5)] |
| 49. | Matthews VB, Elliot RH, Rudnicka C, Hricova J, Herat L, Schlaich MP. Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J Hypertens. 2017;35:2059-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 167] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 50. | Kang S, Verma S, Hassanabad AF, Teng G, Belke DD, Dundas JA, Guzzardi DG, Svystonyuk DA, Pattar SS, Park DSJ, Turnbull JD, Duff HJ, Tibbles LA, Cunnington RH, Dyck JRB, Fedak PWM. Direct Effects of Empagliflozin on Extracellular Matrix Remodelling in Human Cardiac Myofibroblasts: Novel Translational Clues to Explain EMPA-REG OUTCOME Results. Can J Cardiol. 2020;36:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 51. | Li R, Luo P, Guo Y, He Y, Wang C. Clinical features, treatment, and prognosis of SGLT2 inhibitors induced acute pancreatitis. Expert Opin Drug Saf. 2024;1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 52. | Heyman SN, Aronson D, Abassi Z. SGLT2 Inhibitors and the Risk of Contrast-Associated Nephropathy Following Angiographic Intervention: Contradictory Concepts and Clinical Outcomes. Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 53. | Bourdel-Marchasson I, Maggi S, Abdelhafiz A, Bellary S, Demurtas J, Forbes A, Ivory P, Rodríguez-Mañas L, Sieber C, Strandberg T, Tessier D, Vergara I, Veronese N, Zeyfang A, Christiaens A, Sinclair A. Essential steps in primary care management of older people with Type 2 diabetes: an executive summary on behalf of the European geriatric medicine society (EuGMS) and the European diabetes working party for older people (EDWPOP) collaboration. Aging Clin Exp Res. 2023;35:2279-2291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 54. | Handelsman Y, Henry RR, Bloomgarden ZT, Dagogo-Jack S, DeFronzo RA, Einhorn D, Ferrannini E, Fonseca VA, Garber AJ, Grunberger G, LeRoith D, Umpierrez GE, Weir MR. American Association of Clinical Endocrinologists and American College of Endocrinology Position Statement on The Association of Sglt-2 Inhibitors and Diabetic Ketoacidosis. Endocr Pract. 2016;22:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 259] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/