Published online Jul 26, 2024. doi: 10.4330/wjc.v16.i7.397
Revised: May 20, 2024
Accepted: June 5, 2024
Published online: July 26, 2024
Processing time: 98 Days and 20.8 Hours
Peripheral artery disease (PAD) is a common condition characterized by atherosclerosis in the peripheral arteries, associated with concomitant coronary and cerebrovascular diseases. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors are a class of drugs that have shown potential in hypercholesterolemic patients. This review focuses on the efficacy, safety, and clinical outcomes of PCSK9 inhibitors in PAD based on the literature indexed by PubMed. Trials such as FOURIER and ODYSSEY demonstrate the efficacy of evolocumab and alirocu
Core Tip: Evolocumab and alirocumab, which belong to the class of proprotein convertase subtilisin/kexin type 9 inhibitors, are effective in reducing cardiovascular events and major adverse limb events in peripheral artery disease patients. Despite their proven benefits, these inhibitors are underutilized in clinical practice, often due to providers’ lack of awareness and concerns about cost. This highlights the need for further research to assess the long-term effects of these inhibitors and their cost-effectiveness for specific patient groups.
- Citation: Mohyeldin M, Abuelgasim AS, Mustafa AM. Proprotein convertase subtilisin/kexin type 9 inhibitors in peripheral artery disease: A review of efficacy, safety, and outcomes. World J Cardiol 2024; 16(7): 397-401
- URL: https://www.wjgnet.com/1949-8462/full/v16/i7/397.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i7.397
Peripheral artery disease (PAD) is a common condition characterized by atherosclerosis in the peripheral arteries, which reduces blood flow to the limbs. It is a debilitating condition affecting approximately 200 million people worldwide[1]. PAD symptoms include intermittent claudication, variable claudication, and limb pain. However, many PAD patients may present with atypical symptoms that do not align with the classic definition of claudication or remain asymptomatic; in some cases, the blood flow is severely compromised, requiring urgent surgical intervention[2]. PAD is associated with significant morbidity and mortality, as it increases the risk of cardiovascular events and limb amputation and reduces the quality of life. The prevalence of PAD is rising globally, fueled by an aging population and the growing prevalence of risk factors, including diabetes, hypertension, and smoking[3]. PAD can be managed through medical or procedural endovascular approaches such as revascularization with percutaneous angioplasty, stents, and arterectomy[4]. Medical management includes the use of statins; angiotensin-converting enzyme inhibitors; or angiotensin receptor blockers, antiplatelet therapy, and prostaglandins[5]. Our study reinforces the substantial potential benefits of the new innovative giant proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor and its role in PAD.
Drugs that inhibit the enzyme PCSK9 have shown promise in the treatment of hypercholesterolemia, particularly in familial hypercholesterolemia patients. These drugs work by inhibiting the PCSK9 enzyme, which, in turn, increases the clearance of low-density lipoprotein cholesterol (LDL-C) from the bloodstream, thereby reducing the risk of atherosclerosis and its complications[6]. The Food and Drug Administration has approved two PCSK9 inhibitors, evolocumab and alirocumab, for use in hypercholesterolemia patients[7]. Evolocumab and alirocumab are administered subcuta
In addition to antibody-based therapies, alternative approaches targeting PCSK9, including small molecules and peptides, are being explored[10,11]. Small-molecule inhibitors offer potential advantages over antibodies, such as lower manufacturing costs, oral bioavailability, and better cell permeability. These small molecules can inhibit PCSK9 through various mechanisms, such as preventing its synthesis, secretion, or interaction with LDL receptors[10]. Peptide-based strategies, such as epidermal growth factor precursor homology domain A mimetic peptides, represent another approach to blocking PCSK9-LDLR from binding with high specificity[10,11]. However, despite progress in developing these alternative PCSK9 inhibitors, challenges remain in optimizing their selectivity and potency, elucidating their effects on Lp (a) levels, and using them in homozygous familial hypercholesterolemia patients[10].
While PCSK9 inhibitory antibodies have demonstrated much clinical success thus far, small-molecule and peptide PCSK9 inhibitors are emerging as promising complementary strategies that may expand future treatment options[10,11]. This review focuses on the efficacy, safety, and clinical outcomes of PCSK9 inhibitory antibodies (evolocumab and alirocumab) in the management of PAD.
The FOURIER trial investigated the efficacy of evolocumab in PAD patients. The study included 27564 atherosclerotic disease patients receiving statin therapy, of whom 3642 (13.2%) had PAD. The researchers assessed a composite primary endpoint consisting of cardiovascular mortality, stroke, myocardial infarction, hospitalization for unstable angina, or coronary revascularization. The results showed that evolocumab significantly reduced the primary endpoint in PAD patients, yielding a hazard ratio (HR) of 0.79 and a 95% confidence interval[12]. In addition, alirocumab has demonstrated efficacy in reducing cardiovascular events in these patients. In the ODYSSEY Outcomes trial, treatment with alirocumab was associated with a 15% reduction in the risk of coronary heart disease-related death, ischemic stroke, myocardial infarction, and unstable angina necessitating hospitalization[8].
Despite the significant adverse limb and cardiovascular outcomes observed in PAD patients, less attention has been paid to risk factor modification compared to other atherosclerotic diseases such as stroke or coronary artery disease. PAD patients have been consistently undertreated with lipid-lowering therapies[12].
The FOURIER trial concluded that at 48 wk, evolocumab treatment led to a 59% reduction in LDL cholesterol levels compared to placebo, with a decrease from a median baseline of 92 mg/dL (2.4 mmol/L) to 30 mg/dL (0.78 mmol/L) (P < 0.001). Compared to the placebo treatment, evolocumab significantly reduced the risk of the primary endpoint (1344 patients [9.8%] vs 1563 patients [11.3%]; HR, 0.85; 0.79 to 0.92; P < 0.001) and the secondary endpoint (816 [5.9%] vs 1013 [7.4%]; HR, 0.80; 0.73 to 0.88; P < 0.001) (Table 1)[9]. The ODYSSEY trial highlighted greater LDL-C reduction by alirocumab compared to a placebo treatment, with a decrease from 93.3 mg/dL to 37.6 mg/dL (62.7%) at 4 mo and from 101.4 mg/dL to 53.3 mg/dL (54.7%) at 48 mo (Table 1)[8].
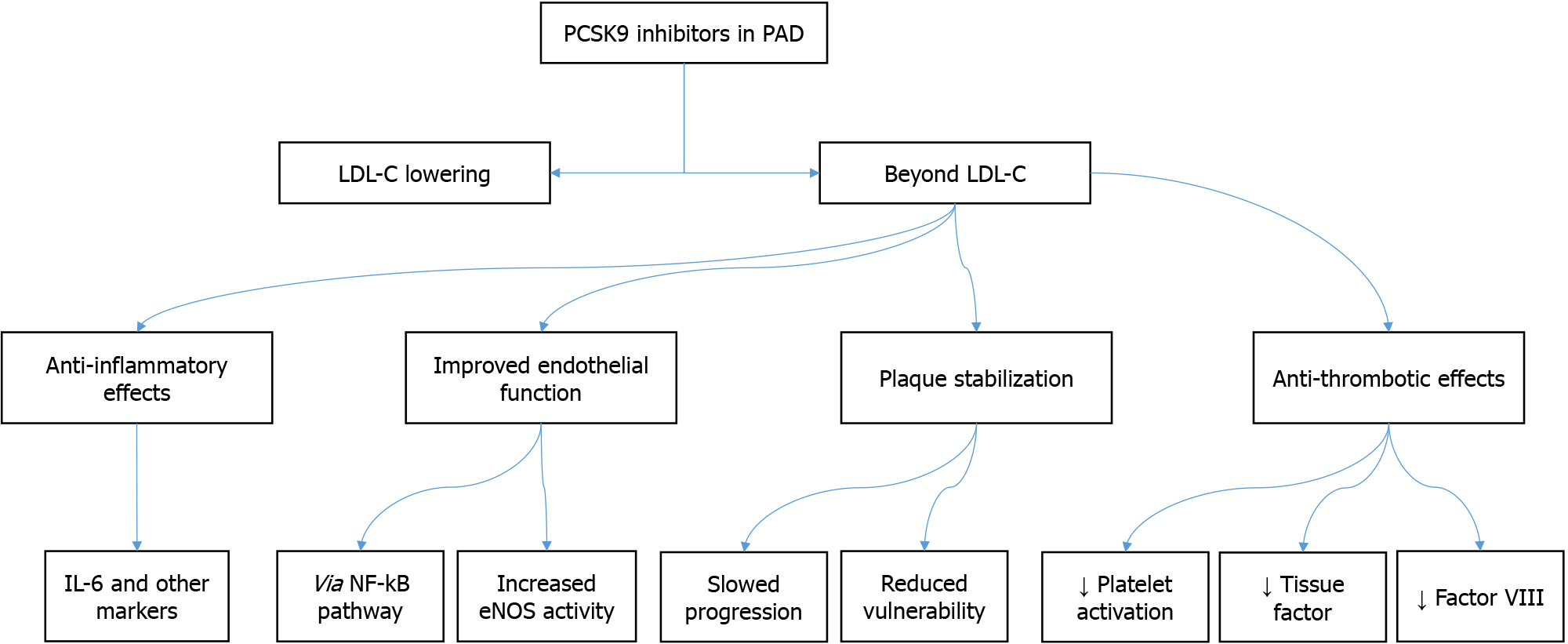
PCSK9 inhibitors may treat PAD through several mechanisms other than their potent LDL-C-lowering effects, as illustrated in Figure 1. These include anti-inflammatory effects, improved endothelial function, plaque stabilization, and anti-thrombotic effects. These pleiotropic effects could contribute to the efficacy of these inhibitors in PAD.
An analysis of data collected from the ODYSSEY and OSLER trials showed that the use of PCSK9 inhibitors in atherosclerotic cardiovascular disease patients failed to reach generally acknowledged benchmarks for incremental cost-effectiveness and was anticipated to lead to a notable rise in healthcare costs in the United States. This illustrates that the use of PCSK9 inhibitors outside of clinical trial settings could be challenging, as it may not be affordable for most healthcare systems and private insurance payers[13,14]. A systematic review of the cost-effectiveness of PCSK9 inhibitors in cardiovascular disease demonstrated that the economic evaluation of PCSK9 inhibitors has predominantly focused on direct medical costs while neglecting the potential impact of indirect costs, thereby offering an incomplete perspective. While studies have shown that PCSK9 inhibitors have unclear lifetime outcomes, particularly in younger patients, their high economic burden, owing to being priced at $7000 in developed countries and $15000 in the United States, makes them generally ineffective for the broader population. However, their use may have a better cost-effectiveness profile in specific populations, such as patients with familial hypercholesterolemia and statin intolerance. In response to concerns about high costs, Amgen Inc. reduced the price of PCSK9 inhibitors in the United States in October 2018. Future studies conducted after price reductions may yield different outcomes, and a complementary systematic review to compare the results is recommended[15].
Various studies have evaluated the safety of PCSK9 inhibitors. The FOURIER trial found evolocumab to be safe in PAD patients[12]. Injection-site reactions were the only significant adverse events reported in the FOURIER and ODYSSEY trials. In the evolocumab group, 2.1% of patients experienced injection-site reactions, compared to 1.6% in the placebo group. Approximately 90% of these reactions in both groups were mild, and only 0.1% of patients in each group discontinued the study drug due to an injection-site reaction[8,12]. Furthermore, the OSLER trial, a study reporting the efficacy and safety of evolocumab for hypercholesterolemic patients over 1 year, reported no significant adverse events between the study and control groups[13].
In the FOURIER trial, evolocumab reduced the incidence of major adverse limb events, which included acute limb ischemia, major amputation, or urgent peripheral revascularization for ischemia. Evolocumab also consistently reduced the primary endpoint in PAD patients, regardless of their prior myocardial infarction or stroke history[12]. In the ODYSSEY Outcomes trial, alirocumab also significantly reduced the risk of myocardial infarction (14%), coronary revascularization (12%), and ischemic stroke (27%)[8].
While the current research on PCSK9 inhibitors in PAD shows promising results, some limitations must be considered. Most of the trials have focused on short-term outcomes, and there is a need for longer-term studies to establish the long-term effects of PCSK9 inhibitors in PAD patients. Additionally, the cost-effectiveness of these drugs remains a concern, and more research is needed to identify the patient population that would benefit the most from this therapy while minimizing the economic burden.
Furthermore, the impact of PCSK9 inhibitors on other clinical outcomes in PAD, such as walking performance and plaque volume, remains unclear[16]. Future research should address these gaps in knowledge and provide a more comprehensive understanding of the role of PCSK9 inhibitors in the management of PAD.
According to the available literature, PCSK9 inhibitors, such as evolocumab and alirocumab, have demonstrated efficacy in reducing cardiovascular events and major adverse limb events in PAD patients. These drugs have also shown a favorable safety profile, with injection-site reactions being the most frequently reported adverse event. Despite these positive findings, PCSK9 inhibitors continue to be underutilized in clinical practice, possibly due to providers’ lack of awareness and cost concerns. Further research is needed to establish knowledge and provide a more comprehensive understanding of the role of PCSK9 inhibitors in the management of PAD, to investigate the long-term effects and cost-effectiveness of PCSK9 inhibitors in PAD patients, and to identify the patient population that would benefit the most from this therapy. Overall, PCSK9 inhibitors represent a promising therapeutic option for internal medicine physicians, cardiologists, and vascular surgeons managing PAD patients.
| 1. | El-ashmawy HM, Omar REH, Almazouq FMJ, Ahmed AM. Possible Role of Fatty Acid Binding Protein in Type 2 Diabetes and Peripheral Artery Disease: Review Article. Egypt J Hosp Med. 2022;89:4270-4272. [DOI] [Full Text] |
| 2. | Zemaitis MR, Boll JM, Dreyer MA. Peripheral Arterial Disease. 2023 May 23. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 3. | Fowkes FG, Aboyans V, Fowkes FJ, McDermott MM, Sampson UK, Criqui MH. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol. 2017;14:156-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 511] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 4. | Patel RAG, Sakhuja R, White CJ. The Medical and Endovascular Treatment of PAD: A Review of the Guidelines and Pivotal Clinical Trials. Curr Probl Cardiol. 2020;45:100402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Conte SM, Vale PR. Peripheral Arterial Disease. Heart Lung Circ. 2018;27:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 6. | Schwartz GG, Steg PG, Szarek M, Bittner VA, Diaz R, Goodman SG, Kim YU, Jukema JW, Pordy R, Roe MT, White HD, Bhatt DL; ODYSSEY OUTCOMES Committees and Investigators*. Peripheral Artery Disease and Venous Thromboembolic Events After Acute Coronary Syndrome: Role of Lipoprotein(a) and Modification by Alirocumab: Prespecified Analysis of the ODYSSEY OUTCOMES Randomized Clinical Trial. Circulation. 2020;141:1608-1617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 7. | Jia X, Al Rifai M, Saeed A, Ballantyne CM, Virani SS. PCSK9 Inhibitors in the Management of Cardiovascular Risk: A Practical Guidance. Vasc Health Risk Manag. 2022;18:555-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 8. | Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med. 2018;379:2097-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2585] [Cited by in RCA: 2503] [Article Influence: 312.9] [Reference Citation Analysis (0)] |
| 9. | Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR; FOURIER Steering Committee and Investigators. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376:1713-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3898] [Cited by in RCA: 4335] [Article Influence: 481.7] [Reference Citation Analysis (0)] |
| 10. | Ahamad S, Mathew S, Khan WA, Mohanan K. Development of small-molecule PCSK9 inhibitors for the treatment of hypercholesterolemia. Drug Discov Today. 2022;27:1332-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Evison BJ, Palmer JT, Lambert G, Treutlein H, Zeng J, Nativel B, Chemello K, Zhu Q, Wang J, Teng Y, Tang W, Xu Y, Rathi AK, Kumar S, Suchowerska AK, Parmar J, Dixon I, Kelly GE, Bonnar J. A small molecule inhibitor of PCSK9 that antagonizes LDL receptor binding via interaction with a cryptic PCSK9 binding groove. Bioorg Med Chem. 2020;28:115344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Skeik N, Nowariak ME, Smith JE, Alexander JQ, Manunga JM, Mirza AK, Sullivan TM. Lipid-lowering therapies in peripheral artery disease: A review. Vasc Med. 2021;26:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Kosmas CE, Skavdis A, Sourlas A, Papakonstantinou EJ, Peña Genao E, Echavarria Uceta R, Guzman E. Safety and Tolerability of PCSK9 Inhibitors: Current Insights. Clin Pharmacol. 2020;12:191-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Arbel R, Hammerman A, Triki N, Greenberg D. PCSK9 inhibitors may improve cardiovascular outcomes-Can we afford them? Int J Cardiol. 2016;220:242-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Azari S, Rezapour A, Omidi N, Alipour V, Behzadifar M, Safari H, Tajdini M, Bragazzi NL. Cost-effectiveness analysis of PCSK9 inhibitors in cardiovascular diseases: a systematic review. Heart Fail Rev. 2020;25:1077-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Rrapo-Kaso E, Loffler AI, Petroni GR, Meyer CH, Walker M, Kay JR, DiMaria JM, Domanchuk K, Carr JC, McDermott MM, Kramer CM. Alirocumab and plaque volume, calf muscle blood flow, and walking performance in peripheral artery disease: A randomized clinical trial. Vasc Med. 2023;28:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/