Published online Dec 26, 2024. doi: 10.4330/wjc.v16.i12.776
Revised: September 21, 2024
Accepted: November 6, 2024
Published online: December 26, 2024
Processing time: 112 Days and 6.6 Hours
In this article, we evaluate the comparative efficacy and safety of mesenchymal stem cells (MSCs) derived from bone marrow (BM-MSCs) and umbilical cord (UC-MSCs) in the treatment of heart failure and myocardial infarction. MSCs have gained importance as living bio drug due to their regenerative potential, with BM-MSCs being the most extensively studied. However, UC-MSCs offer unique advantages, such as noninvasive collection and fewer ethical concerns. This systematic review and meta-analysis summarizes data from 13 randomized controlled trials, which included a total of 693 patients. Their study shows that UC-MSCs significantly improved left ventricular ejection fraction by 5.08% at 6 months and 2.78% at 12 months compared with controls, while BM-MSCs showed no significant effect. Neither cell type showed significant changes in 6-minute walk distance. In addition, UC-MSCs and BM-MSCs had comparable safety profiles, with no significant differences in major adverse cardiac events, except for a lower rehospitalization rate observed with BM-MSCs. These results position UC-MSCs as a promising alternative in MSC-based therapies for cardiac disease, offering potential improvements in cardiac function while maintaining a favorable safety profile. Future research should focus on optimizing administration protocols and further exploring the long-term benefits and mechanisms of UC-MSCs in cardiac repair.
Core Tip: This article provides a comparative analysis of umbilical cord-derived mesenchymal stem cells (UC-MSCs) and bone marrow-derived mesenchymal stem cells (BM-MSCs) for the treatment of heart failure. UC-MSCs significantly increase left ventricular ejection fraction and exhibit a favorable safety profile, positioning them as a promising alternative to BM-MSCs. Future research should focus on optimizing administration protocols and understanding the long-term benefits of UC-MSCs in cardiac therapy.
- Citation: Li P. Comparative breakthrough: Umbilical cord mesenchymal stem cells vs bone marrow mesenchymal stem cells in heart failure treatment. World J Cardiol 2024; 16(12): 776-780
- URL: https://www.wjgnet.com/1949-8462/full/v16/i12/776.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i12.776
Cardiovascular diseases (CVDs) remain the leading cause of morbidity and mortality worldwide despite advances in pharmacological treatment[1]. Heart failure (HF), a common endpoint of various CVDs, results from the progressive decline of cardiac function due to the formation of fibrous scars after myocardial infarction (MI)[2]. Conventional treatments primarily provide symptomatic relief without addressing the underlying problem of myocardial tissue loss[3]. In this context, regenerative medicine, particularly mesenchymal stem cells (MSCs) therapy, has emerged as a promising approach to repair and replace damaged myocardium[4].
MSCs are multipotent stromal cells capable of differentiating into various cell types, including cardiomyocytes[5]. They exhibit unique properties such as high proliferation rates, secretion of proangiogenic and anti-inflammatory factors, and evasion of immune surveillance, making them ideal candidates for cell-based therapies[6]. MSCs can be derived from various tissues, including bone marrow (BM-MSCs) and umbilical cord (UC-MSCs), with each tissue having different advantages and limitations[7]. BM-MSCs are the most extensively studied MSC type in clinical settings. In preclinical models, they have shown the potential to improve cardiac function and reduce scar tissue[8]. However, their therapeutic efficacy in clinical trials has been modest, possibly due to factors such as donor age, comorbidities, and quality of cell preparations[9]. In contrast, UC-MSCs derived from medical waste are increasingly recognized for their noninvasive collection procedure, lack of ethical concerns, and superior proliferative ability[10]. UC-MSCs are younger and possess embryonic cell-like properties, which may contribute to their increased therapeutic potential[11]. This article aims to evaluate the comparative safety and efficacy of BM-MSCs and UC-MSCs in the treatment of HF and MI.
By analyzing data from 13 randomized controlled trials (RCTs) involving 693 patients, the authors evaluate key clinical outcomes such as left ventricular ejection fraction (LVEF), 6-minute walk distance (6MWD), and major adverse cardiac events. Their study shows that UC-MSCs significantly improved LVEF by 5.08% (MD 5.08, 95%CI: 2.20%-7.95%; P = 0.0005) at 6 months and 2.78% (MD 2.78, 95%CI: 0.86-4.70; P = 0.004) at 12 months compared with controls, while BM-MSCs showed no significant effect[12]. UC-MSCs have demonstrated greater improvement in LVEF compared to BM-MSCs, likely due to several key factors. UC-MSCs exhibit enhanced proliferative capacity, allowing for increased cell viability and a greater number of cells participating in myocardial repair. They also have superior paracrine activity, which means they release higher levels of growth factors and cytokines that promote angiogenesis, reduce apoptosis, and modulate inflammation more effectively than BM-MSCs[10,13]. Additionally, UC-MSCs possess a higher immunomodulatory capacity, reducing local immune responses and creating a more favorable environment for myocardial regeneration[10,13]. These combined factors likely contribute to the observed superior improvements in LVEF with UC-MSC therapy.
The efficacy of MSC therapy for HF can vary depending on the underlying cause of the disease. Studies suggest that MSCs may be more effective in treating ischemic HF, such as that caused by MI, due to their ability to promote angiogenesis and reduce scar formation in the ischemic myocardium[13]. In contrast, for non-ischemic HF, such as those due to cardiomyopathy or valvular disease, MSCs may show more limited efficacy, as these conditions involve more complex pathological mechanisms, such as genetic mutations or mechanical abnormalities, that are less responsive to cellular therapy[7-10]. Therefore, the underlying etiology of HF should be considered when determining the potential benefits of MSCs therapy.
In addition, it would be prudent to consider treatment-related complications when comparing UC-MSCs and BM-MSCs in cardiac therapy. Although both cell types are generally regarded as safe, minor complications such as transient fever, local pain at the injection site, and mild immune responses have been reported in some studies[10,13]. Importantly, UC-MSCs are considered to have a lower risk of immune rejection due to their lower expression of major histocompatibility complex molecules, which could reduce complications compared to BM-MSCs[10]. However, there are also concerns regarding the long-term safety of MSC therapies, such as the potential for tumorigenesis or ectopic tissue formation, necessitating further investigation through longer-term studies.
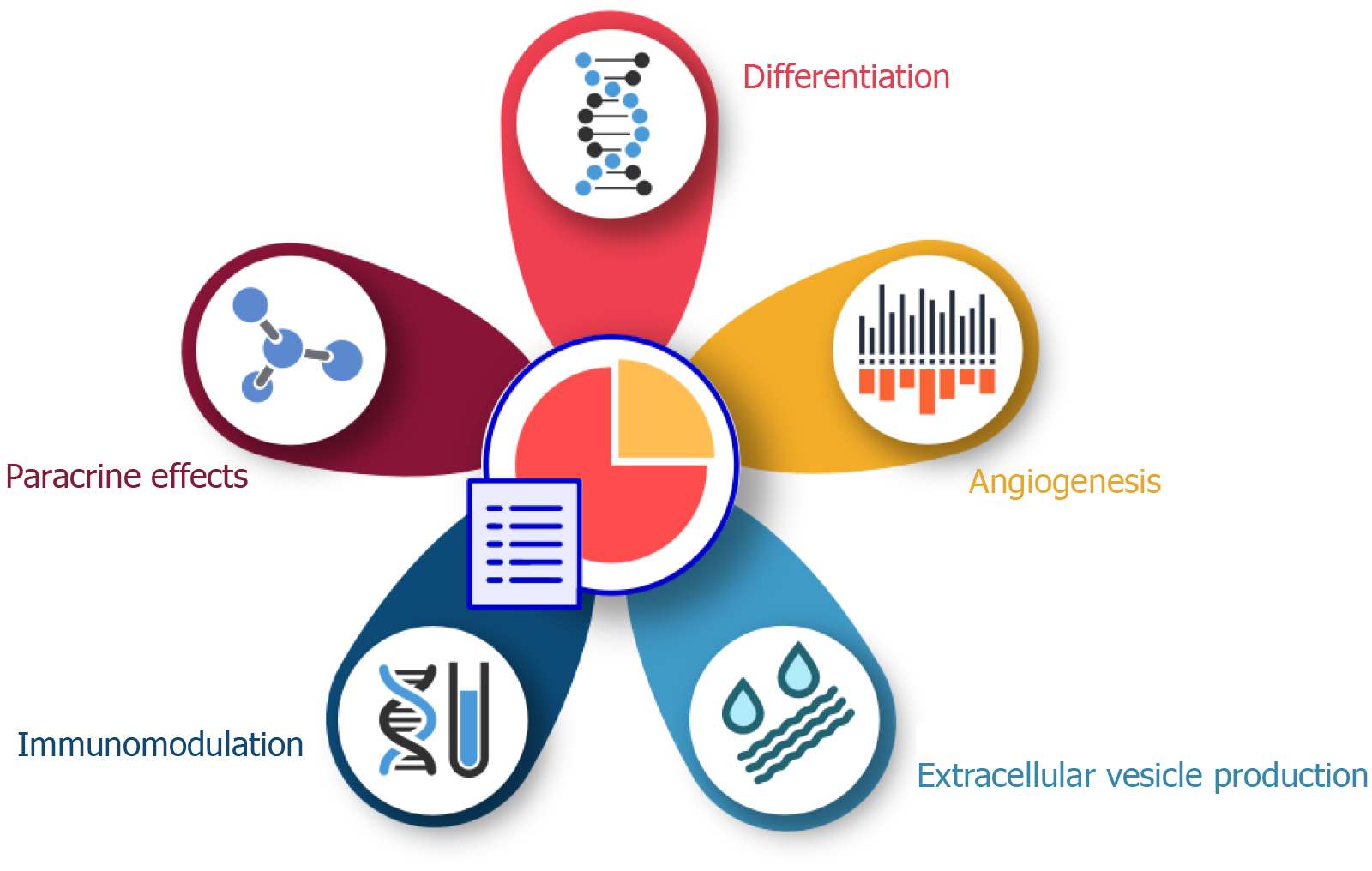
MSCs have attracted considerable attention due to their cardioprotective properties, which are mainly attributed to their multiple mechanisms of action. These mechanisms include direct differentiation, paracrine signaling, immunomodulation, and extracellular vesicle (EV) production, resillience and adaptablity, and together contribute to myocardial repair and functional recovery after cardiac injury[14] (Figure 1).
One of the main mechanisms by which MSCs exert cardioprotective effects is their ability to differentiate into cardiomyocytes and vascular endothelial cells. This differentiation ability enables MSCs to replace damaged myocardial tissue and contribute to the formation of new blood vessels, thereby improving cardiac repair and perfusion[15]. However, the extent of direct differentiation is limited, and the predominant mechanism of action is thought to be paracrine signaling[16]. MSCs secrete a variety of bioactive molecules, including growth factors, cytokines and chemokines, which mediate their paracrine effects. These secreted factors promote angiogenesis, reduce apoptosis, inhibit fibrosis and stimulate endogenous cardiac progenitor cells, thereby facilitating myocardial repair[17,18].
Immunomodulation is another crucial mechanism by which MSCs confer cardioprotection. MSCs interact with various immune cells, including T cells, B cells, natural killer cells and macrophages, and modulate their activity to create an anti-inflammatory environment that favors tissue repair[19]. For example, MSCs can induce the polarization of macrophages toward the M2 phenotype, which is associated with tissue repair and regeneration[20]. In addition, MSCs secrete anti-inflammatory cytokines such as interleukin-10 and transforming growth factor β, which further attenuates the inflammatory response[21].
EVs released by MSCs also play a crucial role in their cardioprotective effects. EVs, including exosomes and microvesicles, are rich in proteins, lipids, and nucleic acids that can affect target cells and tissues. MSC-derived EVs have been shown to deliver microRNAs and other regulatory molecules to cardiac cells, thereby modulating gene expression and promoting cardiac repair[22]. For example, miR-126 and miR-210, which are present in MSC-derived exosomes, have been associated with enhancing angiogenesis and reducing apoptosis in ischemic cardiac tissue[23,24]. In addition, MSCs secrete angiogenic factors such as vascular endothelial growth factor, fibroblast growth factor, and hepatocyte growth factor, which promote the formation of new blood vessels in the damaged myocardium. This improves blood supply and oxygen delivery to the heart tissue[25].
UC-MSCs and BM-MSCs have shown potential in myocardial regeneration and cardiac remodeling, with both therapies demonstrating improvements in cardiac function and reductions in scar size. UC-MSCs have been noted for their greater proliferative capacity and lower immunogenicity, which may enhance myocardial repair by promoting angiogenesis and reducing fibrosis. BM-MSCs, while effective, tend to have reduced differentiation potential with age, possibly limiting their regenerative abilities compared to UC-MSCs[26]. Further clinical trials are needed to validate these findings and establish standardized protocols for their application in HF and MI[27].
The advent of regenerative medicine, particularly the use of MSCs, offers a promising therapeutic approach to address the underlying myocardial damage and improve cardiac function. Despite no observed differences in the 6MWD between the two MSC types, UC-MSCs present a more favorable safety profile and are associated with a lower rate of rehospitalization. The promising results of UC-MSCs can be attributed to their unique characteristics, including noninvasive collection, fewer ethical concerns, higher proliferative capacity, and embryonic-cell-like properties. These advantages position UC-MSCs as a viable and potentially superior alternative to BM-MSCs for cardiac regeneration therapy. However, several challenges remain, including the need for standardized cell isolation, administration protocols, and long-term studies to validate these findings and optimize clinical applications[28,29]. Future research should focus on enhancing the therapeutic efficacy of MSCs through preconditioning strategies, identifying biomarkers for predicting treatment outcomes, and understanding the long-term effects and underlying mechanisms of MSCs therapy in cardiac repair. Additionally, large-scale RCTs are necessary to confirm the benefits of UC-MSCs and establish them as a standard treatment for HF and MI. In conclusion, UC-MSCs hold significant promise for advancing cardiac regeneration therapy, offering a new avenue for improving heart function and patient outcomes. By addressing the current challenges and continuing to explore innovative approaches, MSC-based therapies have the potential to revolutionize the treatment landscape for HF and MI.
| 1. | Li P, Luo Y, Chen YM. B-type natriuretic peptide-guided chronic heart failure therapy: a meta-analysis of 11 randomised controlled trials. Heart Lung Circ. 2013;22:852-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 2. | Pharoun J, Berro J, Sobh J, Abou-Younes MM, Nasr L, Majed A, Khalil A, Joseph, Stephan, Faour WH. Mesenchymal stem cells biological and biotechnological advances: Implications for clinical applications. Eur J Pharmacol. 2024;977:176719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 3. | Berebichez-Fridman R, Montero-Olvera PR. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ Med J. 2018;18:e264-e277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 308] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 4. | Haider KH. Priming mesenchymal stem cells to develop "super stem cells". World J Stem Cells. 2024;16:623-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 5. | Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 773] [Cited by in RCA: 1359] [Article Influence: 194.1] [Reference Citation Analysis (0)] |
| 6. | Chen XM, Wang X, Hou Z. Editorial: MSC-derived exosomes in tissue regeneration. Front Cell Dev Biol. 2023;11:1293109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Klimczak A. Mesenchymal Stem/Progenitor Cells and Their Derivates in Tissue Regeneration-Part II. Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 8. | Jihwaprani MC, Sula I, Charbat MA, Haider KH. Establishing delivery route-dependent safety and efficacy of living biodrug mesenchymal stem cells in heart failure patients. World J Cardiol. 2024;16:339-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Li Z, Zhang Z, Ren Y, Wang Y, Fang J, Yue H, Ma S, Guan F. Aging and age-related diseases: from mechanisms to therapeutic strategies. Biogerontology. 2021;22:165-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 370] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 10. | Mebarki M, Abadie C, Larghero J, Cras A. Human umbilical cord-derived mesenchymal stem/stromal cells: a promising candidate for the development of advanced therapy medicinal products. Stem Cell Res Ther. 2021;12:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 11. | Sharma P, Maurya DK. Wharton's jelly mesenchymal stem cells: Future regenerative medicine for clinical applications in mitigation of radiation injury. World J Stem Cells. 2024;16:742-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (17)] |
| 12. | Bakinowska E, Kiełbowski K, Boboryko D, Bratborska AW, Olejnik-Wojciechowska J, Rusiński M, Pawlik A. The Role of Stem Cells in the Treatment of Cardiovascular Diseases. Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 13. | Pearson MJ, Smart NA. Reported methods for handling missing change standard deviations in meta-analyses of exercise therapy interventions in patients with heart failure: A systematic review. PLoS One. 2018;13:e0205952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Li X, Zhang D, Yu Y, Wang L, Zhao M. Umbilical cord-derived mesenchymal stem cell secretome promotes skin regeneration and rejuvenation: From mechanism to therapeutics. Cell Prolif. 2024;57:e13586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 15. | Cheng YC, Hsieh ML, Lin CJ, Chang CMC, Huang CY, Puntney R, Wu Moy A, Ting CY, Herr Chan DZ, Nicholson MW, Lin PJ, Chen HC, Kim GC, Zhang J, Coonen J, Basu P, Simmons HA, Liu YW, Hacker TA, Kamp TJ, Hsieh PCH. Combined Treatment of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes and Endothelial Cells Regenerate the Infarcted Heart in Mice and Non-Human Primates. Circulation. 2023;148:1395-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 16. | Zheng H, Liang X, Liu B, Huang X, Shen Y, Lin F, Chen J, Gao X, He H, Li W, Hu B, Li X, Zhang Y. Exosomal miR-9-5p derived from iPSC-MSCs ameliorates doxorubicin-induced cardiomyopathy by inhibiting cardiomyocyte senescence. J Nanobiotechnology. 2024;22:195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 17. | Ala M. The beneficial effects of mesenchymal stem cells and their exosomes on myocardial infarction and critical considerations for enhancing their efficacy. Ageing Res Rev. 2023;89:101980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 18. | Huerta CT, Voza FA, Ortiz YY, Liu ZJ, Velazquez OC. Targeted cell delivery of mesenchymal stem cell therapy for cardiovascular disease applications: a review of preclinical advancements. Front Cardiovasc Med. 2023;10:1236345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 19. | Li P, Ou Q, Shi S, Shao C. Immunomodulatory properties of mesenchymal stem cells/dental stem cells and their therapeutic applications. Cell Mol Immunol. 2023;20:558-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 155] [Reference Citation Analysis (0)] |
| 20. | Guo Y, Peng Y, Zeng H, Chen G. Progress in Mesenchymal Stem Cell Therapy for Ischemic Stroke. Stem Cells Int. 2021;2021:9923566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Tan X, Gong YZ, Wu P, Liao DF, Zheng XL. Mesenchymal stem cell-derived microparticles: a promising therapeutic strategy. Int J Mol Sci. 2014;15:14348-14363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Zou J, Yang W, Cui W, Li C, Ma C, Ji X, Hong J, Qu Z, Chen J, Liu A, Wu H. Therapeutic potential and mechanisms of mesenchymal stem cell-derived exosomes as bioactive materials in tendon-bone healing. J Nanobiotechnology. 2023;21:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 202] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 23. | Zhang M, Johnson-Stephenson TK, Wang W, Wang Y, Li J, Li L, Zen K, Chen X, Zhu D. Mesenchymal stem cell-derived exosome-educated macrophages alleviate systemic lupus erythematosus by promoting efferocytosis and recruitment of IL-17(+) regulatory T cell. Stem Cell Res Ther. 2022;13:484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 109] [Reference Citation Analysis (0)] |
| 24. | Yang Y, Lee EH, Yang Z. Hypoxia-Conditioned Mesenchymal Stem Cells in Tissue Regeneration Application. Tissue Eng Part B Rev. 2022;28:966-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 25. | Menasché P. Mesenchymal Stromal Cell Therapy for Heart Failure: Never Stop DREAMing. J Am Coll Cardiol. 2023;81:864-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 26. | Yang GD, Ma DS, Ma CY, Bai Y. Research Progress on Cardiac Tissue Construction of Mesenchymal Stem Cells for Myocardial Infarction. Curr Stem Cell Res Ther. 2024;19:942-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Zhu D, Cheng K. Cardiac Cell Therapy for Heart Repair: Should the Cells Be Left Out? Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Li P. Aficamten for Obstructive Hypertrophic Cardiomyopathy. N Engl J Med. 2024;391:664-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 29. | Li P. FFR-Guided Complete or Culprit-Only PCI in Patients with Myocardial Infarction. N Engl J Med. 2024;391:287-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/