Published online Dec 26, 2024. doi: 10.4330/wjc.v16.i12.740
Revised: September 27, 2024
Accepted: October 29, 2024
Published online: December 26, 2024
Processing time: 112 Days and 20.5 Hours
Radiofrequency catheter ablation (RFCA) has become an important strategy for treating atrial fibrillation (AF), and postoperative recurrence represents a significant and actively discussed clinical concern. The recurrence after RFCA is considered closely related to inflammation. Systemic immune inflammation index (SII) is a novel inflammation predictor based on neutrophils, platelets, and lymphocytes, and is considered a biomarker that comprehensively reflects the immune inflammatory status of the body.
To explore the predictive effect of the SII on AF recurrence after RFCA and its predictive value in combination with the existing APPLE score for AF recurrence after RFCA in patients with non-valvular AF (NVAF).
We retrospectively included 457 patients with NVAF first receiving RFCA and classified them into the recurrent or non-recurrent group. We also investigated the predictive role of SII on AF recurrence following RFCA. Finally, we explored and compared the additional predictive value of the SII after combining with the APPLE score.
After 12 months of follow-up, 113 (24.7%) patients experienced recurrence. High SII has been demonstrated to be an independent predictor for postoperative AF recurrence. Receiver operating characteristic and decision curve analysis (DCA), as well as net reclassification improvement (NRI) and integrated discrimination improvement (IDI) results, showed that SII combined with the APPLE score had higher predictive efficiency than using the SII or APPLE score alone. The area under the curve of the combined model (0.662, 95% confidence interval: 0.602-0.722) significantly increased compared with that of the SII and APPLE scores alone (P < 0.001). The combined model resulted in an NRI of 29.6% and 34.1% and IDI of 4.9% and 3.5% in predicting AF recurrence compared with the SII and APPLE scores alone, respectively (all P < 0.001). The SII, APPLE score, and their combination demonstrated greater clinical utility than did the treat-all and treat-none strategies over the 20–80% risk threshold according to the DCA.
The SII was a predictor of recurrence after RFCA of AF. Moreover, the SII enhanced the predictability of the APPLE score for post-RFCA AF recurrence, providing valuable insights for physicians to optimise patient selection and develop personalised treatment plans.
Core Tip: We explored the role of the systemic immune inflammation index (SII) in predicting recurrence of atrial fibrillation (AF) after radiofrequency catheter ablation (RFCA). We show that the SII is a predictive factor for postoperative recurrence of AF after RFCA and enhances the ability of the APPLE score to predict postoperative recurrence of AF.
- Citation: Wang YJ, Liu KS, Meng XJ, Han XF, Nie LJ, Feng WJ, Chen YB. Role of a new inflammation predictor in predicting recurrence of atrial fibrillation after radiofrequency catheter ablation. World J Cardiol 2024; 16(12): 740-750
- URL: https://www.wjgnet.com/1949-8462/full/v16/i12/740.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i12.740
Atrial fibrillation (AF), the most common arrhythmia observed in clinical settings, affects 46 million people worldwide[1]. This condition is closely related to increased mortality rates and thromboembolic events, particularly stroke and heart failure, including left ventricular dysfunction, hospitalisation, reduced athletic ability, and reduced quality of life[2].
Radiofrequency catheter ablation (RFCA) is an efficacious method of controlling rhythm. Numerous studies have shown that catheter ablation has marked advantages in preventing AF recurrence, reducing AF burden, and improving long-term outcomes for patients. RFCA has proven to be the first-line therapy for paroxysmal AF, and is recommended as a Class Ia procedure according to international guidelines[3]. However, the recurrence rate of AF after initial catheter ablation has been observed to be as high as 20%-50%[4]. Therefore, the clarity of risk factors for postoperative recurrence in patients with AF after RFCA, as well as establishment of predictive models to estimate the risk of postoperative recurrence, is crucial for assisting the development of individualised treatment plans in clinical practice. Moreover, this can provide new research ideas for reducing AF recurrence after RFCA.
Although numerous studies and meta-analyses have explored the risk factors for AF recurrence after RFCA, these factors alone have limited predictive value for recurrence after RFCA. Therefore, an increasing number of studies have developed scoring systems that integrate multiple risk factors. Scoring systems commonly used in clinical practice to predict AF recurrence after RFCA include the APPLE, CAAP-AF, CHA2DS2-VASc, MB-LATER, and HATCH scores. However, the predictive power of these scoring systems is limited, and their accuracy requires improvement[5]. Therefore, further research is necessary to improve existing risk prediction models.
Recurrence after RFCA is considered closely related to inflammation. Systemic immune inflammation index (SII) is a recently proposed novel inflammation predictor based on neutrophils, platelets, and lymphocytes, and is considered a biomarker that comprehensively reflects the immune inflammatory status of the body. SII was initially used to predict the prognosis of various cancers. Recently, some studies have shown that SII can determine a patient’s risk of cardiovascular diseases[6,7]. Zhang et al[6] have demonstrated through their research that SII is an independent prognostic factor for persistent left ventricular systolic dysfunction in patients with perinatal cardiomyopathy. Therefore, SII may be a useful tool for identifying high-risk patients with perinatal cardiomyopathy. A study by Öcal et al[7] showed that SII could better predict the length of hospital stay and long-term prognosis of patients with ST-segment elevation myocardial infarction receiving primary percutaneous coronary intervention treatment. However, limited research exists on SII in predicting the recurrence of AF. Therefore, this study aimed to explore the correlation between the SII and AF recurrence after RFCA and estimate the additional predictive power of the SII when administered alongside the APPLE score. This study aimed to provide a basis for accurately assessing the recurrence risk after RFCA in patients with AF, as well as information for guiding the development of personalised treatment strategies.
This unicentric retrospective study retrospectively included patients with non-valvular AF (NVAF) who were hospitalised at Weifang People’s Hospital and underwent RFCA for AF for the first time between August 2019 and July 2022. The inclusion criteria were as follows: (1) Diagnosed with NVAF or paroxysmal or non-paroxysmal AF; (2) Age > 18 years; (3) Suitable for first-time radiofrequency ablation for AF; and (4) Provision of informed consent for this process. The exclusion criteria were as follows: (1) Acute and chronic inflammatory diseases, liver and kidney dysfunction, coagulation dysfunction, or severe heart failure indicating contraindication for surgery; (2) Incomplete clinical and imaging data; (3) Severe bradycardia, such as sick sinus syndrome or third-degree atrioventricular block; and (4) Left atrial appendage thrombosis as identified on echocardiography.
All baseline demographic, clinical, laboratory, and echocardiographic data were obtained from the electronic medical information record system of Weifang People’s Hospital. The following baseline data were collected: Sex, age, height, weight, smoking history, medical history (heart failure, hypertension, diabetes mellitus, vascular disease, coronary atherosclerotic heart disease, prior stroke/transient ischemic attack, and type of AF), and echocardiographic parameters (left atrial diameter, left ventricular ejection fraction, and left ventricular end-diastolic diameter). We collected the following laboratory parameters: High-density lipoprotein cholesterol; total cholesterol; triglyceride; low-density lipoprotein cholesterol; B-type natriuretic peptide; serum uric acid; serum creatinine; fasting blood glucose; and white blood cell, lymphocyte, monocyte, neutrophil, and platelet counts. Blood samples were collected from all included patients on the second day after admission and before RFCA.
The Chronic Kidney Disease Epidemiology Collaboration formula was applied in calculating the estimated glomerular filtration rate (eGFR)[8]. The SII level was computed using the following equation: Neutrophils × platelets ÷ lymphocytes[9]. Furthermore, CHA2DS2-VASc and APPLE scores for each patient were calculated based on relevant scoring criteria. The scoring criteria for CHA2DS2-VASc score are as follows: Hypertension, congestive heart failure (CHF), diabetes mellitus, vascular disease, age 65 to 74 years, and female sex (1 point each); and previous history of transient ischemic attack or stroke and age ≥ 75 years (2 points each)[10]. The scoring criteria for the APPLE score are as follows: Age ≥ 65 years, persistent AF, left atrial diameter ≥ 43 mm, impaired eGFR ≤ 60 mL/min/1.73 m2, and EF < 50% (1 point each)[11].
Transthoracic echocardiography was conducted before the procedure to assess heart structure and function. Before the procedure, transesophageal echocardiography or cardiac computed tomography angiography (CTA) was performed to exclude the presence of a left atrial thrombus. Before ablation, all patients underwent cardiac CTA to assess the structures of the pulmonary vein and left atrium. All operational procedures were conducted under appropriate analgesia with fentanyl. A femoral venous puncture was performed using the Seldinger technique, and a standard ten-pole catheter was interposed into the coronary sinus through the right femoral vein. Subsequently, a single trans-septal puncture was conducted under fluoroscopic direction. After a single trans-septal puncture, a pentary catheter (Biosense Webster, Irvine, CA, United States) was first inserted for modelling. After modelling was completed, the pentary catheter was replaced by an ablation catheter for ablation. Unfractionated heparin was administered intravenously before or immediately following a transseptal puncture, and an activated clotting time of 250-300 seconds was maintained. Radiofrequency ablation was conducted under the guidance of the CARTO3 navigation system (Biosense Webster, Irvine, CA, United States). The ablation catheters used during the ablation process were all catheters with pressure sensors (THERMOCOOL SMARTTOUCH, SF, Catheter).
All patients with AF underwent circumferential pulmonary vein isolation (CPVI). In cases of non-paroxysmal AF, the operator could perform additional linear ablation based on their discretion (left atrial roof, bottom, posterior wall, mitral isthmus, and tricuspid isthmus lines). After completing the ablation, if the AF did not terminate, electrical cardioversion was conducted to restore sinus rhythm. At the end of the ablation, isolation of all pulmonary veins with a bidirectional block was confirmed[12].
After RFCA, all patients were prescribed oral anticoagulants and amiodarone for 3 months. Subsequently, oral anticoagulants were continued for patients with a high risk of stroke (CHA2DS2-VASc scores ≥ 2 and ≥ 3 for male and female patients, respectively). The outpatient follow-ups at the 1st, 3th, 6th, and 12th month after ablation were conducted for 12 months. Each follow-up visit included clinical assessment, 12-lead electrocardiogram (ECG), and 24-hours Holter monitoring. If the patients had any symptoms related to AF, we performed further ECGs and Holter ECG examinations. Our endpoint included AF recurrence following catheter ablation in the 1-year follow-up period. After blanking for 3 months, any atrial arrhythmia lasting over 30 s on 12-lead ECG or 24-hours Holter monitoring was defined as AF recurrence[13]. Because all patients are regularly reviewed according to medical orders upon discharge, we can obtain this information by consulting the electronic medical information record system of Weifang People’s Hospital.
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, United States), R programming language (version 4.3.1, R Foundation for Statistical Computing, Vienna, Austria), and MedCalc software (version 20.0, MedCalc Software, Ostend, Belgium). Normally distributed continuous data were expressed as mean ± SD, while abnormally distributed data were expressed as median (interquartile range). Categorical data were indicated as numbers (percentages). Categorical data among different groups were compared using Fisher’s exact test or the χ2 test. Comparison among groups was performed using a Student’s t-test or the Mann–Whitney U test.
Factors independently predicting AF recurrence risk were identified using univariate and multivariate logistic regression. Variables satisfying P < 0.05 from univariate regression were incorporated into multivariable regression, and those associated with the SII and APPLE scores were excluded from the multivariate logistic regression analysis. Subsequently, the receiver operating characteristic (ROC) curve was plotted to calculate the best threshold of the SII to predict AF recurrence. Additionally, we utilised the area under the curve (AUC), relative integrated discrimination improvement (IDI), and net reclassification improvement (NRI) to estimate the significance of SII, APPLE score, and their combination in predicting AF recurrence following catheter ablation among patients with AF. Finally, we conducted a pairwise comparison of the ROC curves using the DeLong test, and decision curve analysis (DCA) was used to assess the clinical usefulness. Statistical significance was set at P < 0.05 (two-tailed).
Baseline characteristics of the study patients are presented in Table 1. Overall, 457 patients (mean age 61.32 ± 9.97 years, 62.8% male) with NVAF who received RFCA were included in this study. The mean CHA2DS2-VASc score was 2.09 ± 1.61. The AF type was paroxysmal and persistent in 68.5% and 31.5% of the patients, respectively.
| Characteristics | All patients (n = 457) | Patients without recurrence (n = 344) | Patients with recurrence (n = 113) | P value |
| Age (years) | 61.32 ± 9.97 | 60.92 ± 10.15 | 62.56 ± 9.33 | 0.128 |
| Male sex | 287 (62.8) | 223 (64.8) | 64 (56.6) | 0.145 |
| BMI (kg/m2) | 25.68 ± 3.44 | 25.71 ± 3.51 | 25.57 ± 3.24 | 0.683 |
| Hypertension | 221 (48.4) | 161 (46.8) | 60 (53.1) | 0.245 |
| Diabetes mellitus | 87 (19) | 65 (18.9) | 22 (19.5) | 0.893 |
| Stroke/TIA | 49 (10.7) | 40 (11.6) | 9 (8) | 0.275 |
| Heart failure | 44 (9.6) | 31 (9) | 13 (11.5) | 0.436 |
| Smoking | 78 (17.1) | 60 (17.4) | 18 (15.9) | 0.711 |
| NPAF | 144 (31.5) | 92 (26.7) | 52 (46) | < 0.001 |
| CHA2DS2-VASc score | 2.09 ± 1.61 | 2.04 ± 1.62 | 2.22 ± 1.59 | 0.32 |
| APPLE | 1.004 ± 0.95 | 0.88 ± 0.86 | 1.37 ± 1.08 | < 0.001 |
| LVEF (%) | 62.48 ± 7.35 | 62.74 ± 7.18 | 61.69 ± 7.82 | 0.187 |
| LAD (mm) | 39.3 ± 5.52 | 38.71 ± 5.42 | 41.08 ± 5.45 | < 0.001 |
| LVDD (mm) | 49.91 ± 4.38 | 49.85 ± 4.18 | 50.11 ± 4.94 | 0.58 |
| TC (mmol/L) | 4.38 ± 1.01 | 4.35 ± 1.00 | 4.45 ± 1.07 | 0.409 |
| TG (mmol/L) | 1.60 ± 1.11 | 1.60 ± 1.04 | 1.62 ± 1.3 | 0.856 |
| LDL (mmol/L) | 2.6 ± 0.91 | 2.56 ± 0.88 | 2.69 ± 0.98 | 0.200 |
| HDL (mmol/L) | 1.15 ± 0.31 | 1.15 ± 0.33 | 1.15 ± 0.24 | 0.86 |
| Glucose (mmol/L) | 5.6 ± 1.74 | 5.6 ± 1.8 | 5.61 ± 1.53 | 0.978 |
| BNP (pg/mL) | 98 (51, 158) | 93 (46.25, 155.5) | 130 (61, 186.5) | 0.022 |
| eGFR (mL/min/1.73 m2) | 94.91 ± 14.18 | 95.72 ± 14.02 | 92.44 ± 14.43 | 0.033 |
| Uric acid (μmol/L) | 337.98 ± 90.37 | 338.91 ± 87.36 | 335.18 ± 99.33 | 0.704 |
| Creatinine (mmol/L) | 68.25 ± 51.62 | 68.54 ± 58.73 | 67.37 ± 16.9 | 0.834 |
| White blood cell count (× 109/L) | 6.15 ± 1.44 | 6.1 ± 1.41 | 6.3 ± 1.5 | 0.184 |
| Neutrophils (× 109/L) | 3.68 ± 1.15 | 3.6 ± 1.11 | 3.95 ± 1.26 | 0.005 |
| Lymphocytes (× 109/L) | 1.93 ± 0.58 | 1.97 ± 0.57 | 1.81 ± 0.58 | 0.012 |
| Monocytes (× 109/L) | 0.39 ± 0.26 | 0.4 ± 0.3 | 0.39 ± 0.11 | 0.722 |
| Platelet count (× 109/L) | 215.19 ± 53.3 | 214.87 ± 53.28 | 216.15 ± 53.59 | 0.824 |
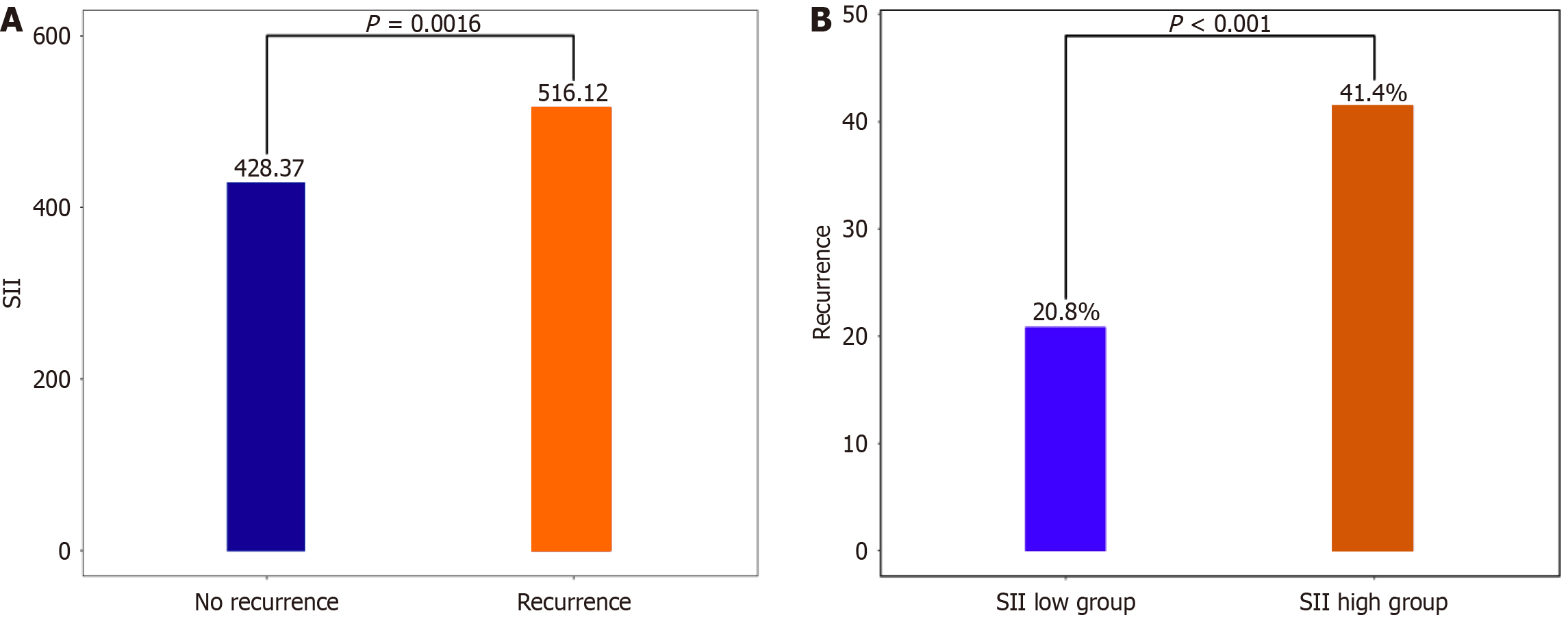
| SII | 450.06 ± 234.47 | 428.36 ± 221.24 | 516.11 ± 260.91 | 0.002 |
Patients were classified into the following two groups: Those with no AF recurrence (non-recurrence, n = 344) and those with AF recurrence (recurrence, n = 113), with an incidence of AF recurrence of 24.72%. The frequency of non-paroxysmal AF remarkably increased among patients with AF recurrence. APPLE scores remarkably increased in the recurrence group compared to that in the non-recurrence group. Additionally, the left atrial diameter in the recurrence group dramatically increased compared to that in the non-recurrence group. Furthermore, B-type natriuretic peptide, neutrophils, lymphocytes, and SII levels increased in the recurrence group, whereas eGFR level decreased compared to that in the non-recurrence group. However, no significant differences were detected in other parameters between these two groups.
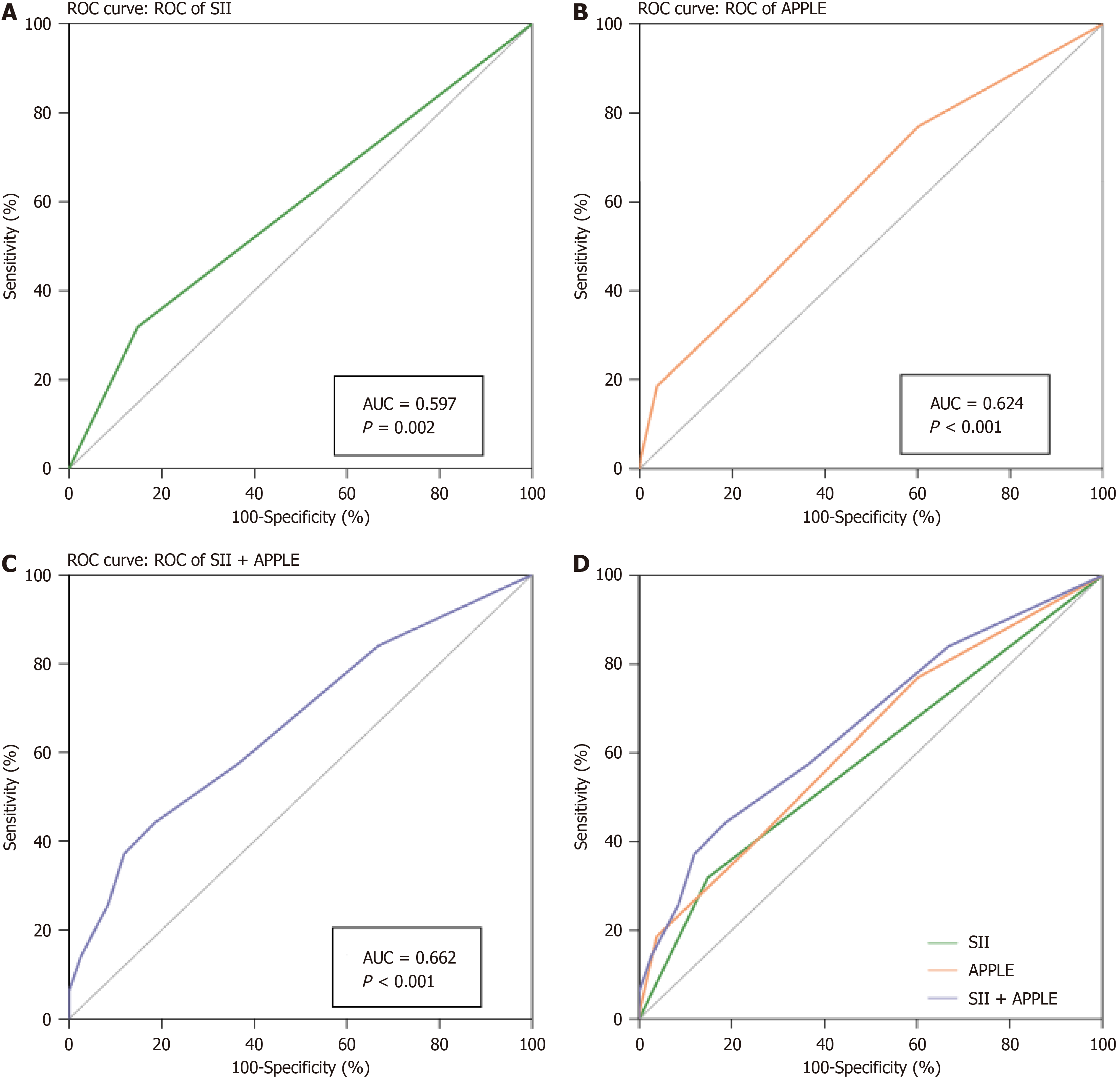
As shown in Figure 1A, the recurrence group had a significantly higher SII level than the non-recurrence group (516.11 ± 260.91 vs 428.37 ± 221.24). Additionally, based on multivariate regression, SII [odds ratio (OR): 2.257; 95% confidence interval (95%CI): 1.219-4.179, P = 0.001] and APPLE score (OR: 1.723; 95%CI: 1.338-2.219, P < 0.001) independently predicted the recurrence risk among patients following radiofrequency ablation (Table 2). ROC curve analysis indicated that the AUC of SII was 0.597 (95%CI: 0.53500.659, P < 0.001; Figure 1A), with the best truncation value of 619.27 (sensitivity: 31.86% and specificity: 85.47%). According to the best truncation value, patients were classified into the low (SII < 619.27) or high (SII ≥ 619.27) SII group. The AF recurrence rate of the high SII group significantly increased compared to that of the low SII group (20.81% vs 41.37%, P < 0.001; Figure 1B), and the AUC of the APPLE score was 0.624 (95%CI: 0.563-0.684, P < 0.001).
| Risk factor | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| NPAF | 2.335 (1.503-3.627) | < 0.001 | ||
| APPLE | 1.689 (1.351-2.112) | < 0.001 | 1.723 (1.338-2.219) | < 0.001 |
| LAD | 1.084 (1.041-1.129) | < 0.001 | ||
| BNP | 1.002 (1-1.003) | 0.023 | 1 (0.998-1.002) | 0.901 |
| eGFR | 0.985 (0.97-0.999) | 0.035 | ||
| Neutrophils | 1.297 (1.08-1.557) | 0.005 | ||
| Lymphocytes | 0.608 (0.412-0.899) | 0.013 | ||
| High SII | 2.686 (1.637-4.406) | < 0.001 | 2.257 (1.219-4.179) | 0.01 |
We performed an ROC curve analysis to assess the significance of SII and APPLE scores in predicting AF recurrence. The AUCs for the SII and APPLE scores were 0.597 (95%CI: 0.535–0.659, P < 0.001; Figure 2A) and 0.624 (95%CI: 0.563-0.684, P < 0.001), respectively (Figure 2B). Furthermore, we applied the best truncation value derived from the ROC curve analysis to convert the SII into a binary variable and subsequently integrated this binary SII variable into the APPLE score. The AUC of the combined model was 0.662 (95%CI: 0.602–0.722, P < 0.001; Figure 2C), remarkably increased compared with that of the SII and APPLE scores alone (P < 0.001; Figure 2D).
To further assess the additional predictive capacity of the combined model, we performed the IDI and NRI analyses. Based on the NRI and IDI analyses, the combined model significantly improved the prediction of AF recurrence after RFCA compared with the SII and APPLE scores alone. Compared with using the SII and APPLE scores alone, the use of the combined model resulted in an NRI of 29.6% and 34.1% (all P < 0.001) and an IDI of 4.9% and 3.5% (all P < 0.001), respectively, in predicting AF recurrence (Table 3).
| NRI | P value | IDI | P value | ||
| SII | APPLE | 0.127 | 0.2347 | 0.014 | 0.341 |
| SII | SII + APPLE | 0.296 | 0.00392 | 0.049 | < 0.001 |
| APPLE | SII + APPLE | 0.341 | 0.00037 | 0.035 | < 0.001 |
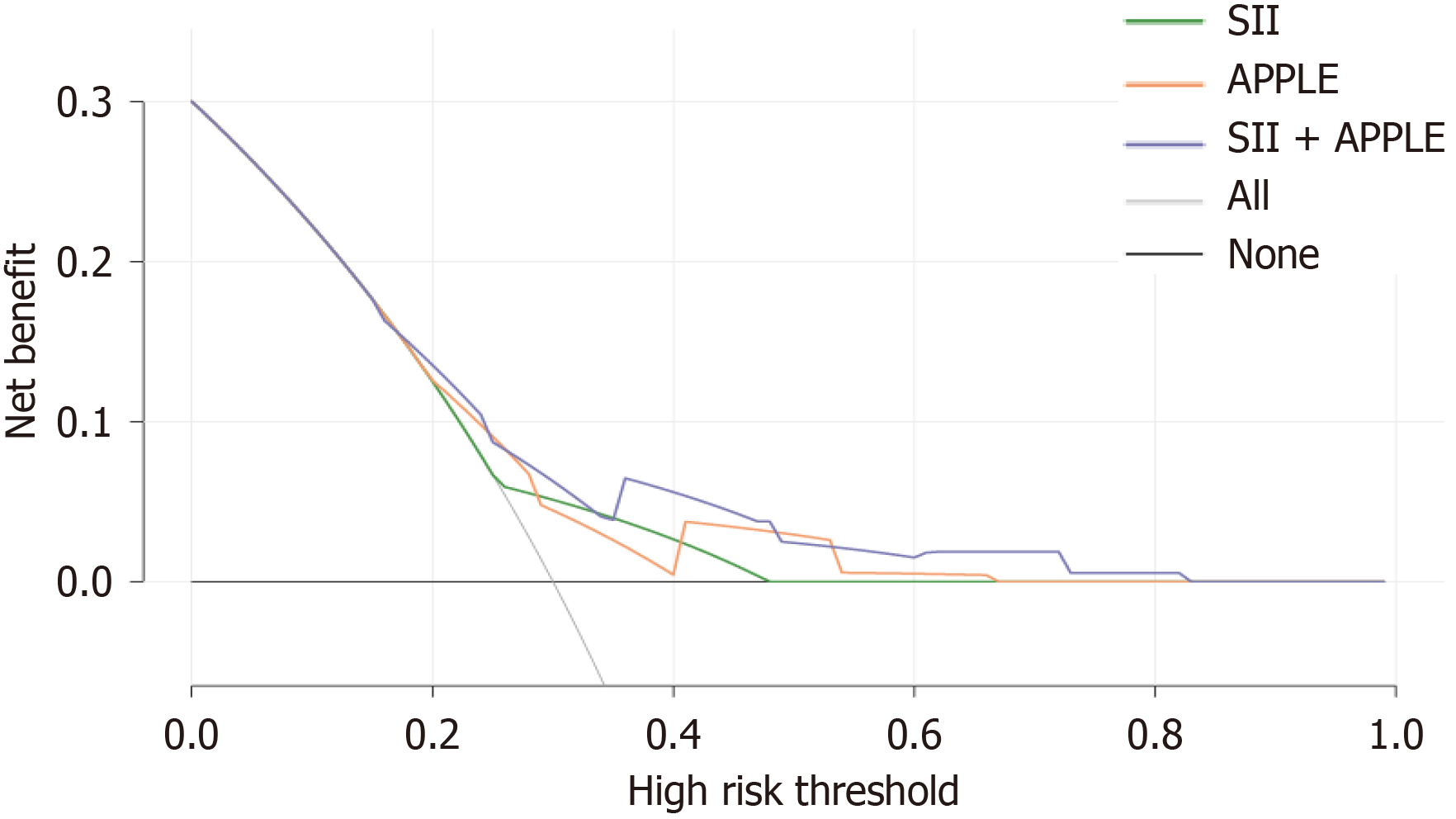
DCA was used to evaluate clinical utility (Figure 3), demonstrating that the SII, APPLE score, and their combination were effective (compared to treat-all and treat-none strategies) over the 20%-80% risk threshold. The combined model exhibited superior clinical utility compared to the SII and APPLE scores in the DCA. Therefore, this combined model is promising for clinical use.
RFCA is an effective and reliable therapy for drug-resistant AF; however, recurrence after RFCA remains a major clinical issue. In this study, the 1-year recurrence rate in patients with AF who received radiofrequency ablation for the first time was 24.7%, which was comparable to that in previous reports[4]. Thus, this study explored the correlation between simple and easily obtainable clinical variables before ablation and AF recurrence after RFCA. The SII and APPLE were found to provide important information regarding the risk of AF recurrence after RFCA. Additionally, SII was demonstrated to provide additional predictive value to the APPLE regarding recurrence after RFCA, presenting increased clinical applicability. This information is crucial for formulating treatment plans and the post-treatment follow-up of patients with AF.
Inflammatory factors, which are recognised as biological markers, can predict the incidence of AF and its recurrence after RFCA[14]. This may be due to the interaction between inflammation and oxidative stress, which further exacerbates the damage, necrosis, apoptosis, and fibrosis of atrial muscle cells, leading to electrical and structural remodelling of the atrium, thereby promoting the occurrence and maintenance of AF[15]. As inflammatory cytokines, interleukin (IL)-6, IL-1β, and tumour necrosis factor (TNF) can cause the proliferation and activation of cardiac fibroblasts, leading to myocardial fibrosis[16]. However, although cardiac magnetic resonance imaging is useful for evaluating left atrial fibrosis[17], owing to its high cost, it has not been widely used in clinical practice. Additionally, detecting inflammatory indicators, such as TNF, IL-1β, and IL-6, requires specific detection methods and unconventional testing in clinical work. Therefore, we need an easily accessible and cost-effective method to evaluate inflammation and the resulting myocardial fibrosis.
Recently, numerous studies have used inflammatory factors, such as C-reactive protein, to predict the prognosis of AF. However, the types of inflammatory cells involved in these inflammatory factors are relatively small, mostly one or two, and their response efficiency to the immune inflammatory state of the body is poor. The SII is a new and convenient inflammatory marker calculated based on neutrophils, lymphocytes, and platelets. It offers a more comprehensive reflection of the body’s inflammatory state than assessing individual white blood cells, neutrophils, and lymphocytes[18]. Elevated neutrophil and platelet counts indicate activation of the inflammatory pathway, whereas an increase in lymphocytes indicates activation of the immune pathway[19,20]. One plausible reason for heightened SII levels is the simultaneous increase in neutrophil and platelet counts, which can secrete large amounts of vascular endothelial growth factors, thereby accelerating the release of inflammatory factors[21,22]. Alternatively, a decrease in lymphocytes associated with systemic stress may contribute to an inflammatory response[22]. Building on these insights, Lin et al[23] proved that the SII is a potential biomarker of AF in patients presenting with ischemic stroke. Kaplan et al[24] expanded on this by demonstrating that, for patients with paroxysmal AF, an increase in AF recurrence following cryo-based ablation might be associated with a higher preprocedural SII. This study contributes significantly to the body of knowledge as it identifies preoperative SII after radiofrequency ablation as an independent predictor of postoperative recurrence. Moreover, the SII enhanced the significance of the APPLE score in predicting post-RFCA AF recurrence. The SII included in this study provides highly accessible data for clinical practice, enhancing the significance of the APPLE score in predicting AF recurrence without additional examination costs, thereby making it suitable for promotion in clinical practice.
This study investigated the recurrence of AF, specifically late recurrence, within 3-12 months after the first RFCA. Previous studies have shown that the APPLE score is mainly used for late AF recurrence after RFCA[11]; therefore, this score is appropriate to use for research. The results of this study also demonstrate the application value of the APPLE score in predicting the recurrence of AF after RFCA. The APPLE score contains age > 65 years (A), persistent AF (P), chronic renal insufficiency (P), left atrial diameter ≥ 43 mm (L), and left ventricular ejection fraction < 50% (E) as independent predictive factors for recurrence after radiofrequency ablation. The predictive scoring system proposed by Kornej et al[25] assigns one point to each risk factor, resulting in a possible score range of 0-5 points. Further external investigations verify the good predicting performance of the APPLE score for AF recurrence post-RFCA and provide good differentiation among populations with low, moderate, and high risk of AF recurrence following radiofrequency ablation of AF[26,27]. As demonstrated by Kornej et al[28], the APPLE score was significant for predicting left atrial low-voltage areas. Additionally, studies have shown that a surgical approach targeting low-voltage areas in the left atrium for matrix modification based on CPVI is more effective than CPVI[29]. Nevertheless, whether the SII helps predict low-voltage areas in the left atrium requires further research.
The efficacy of SII in enhancing the prediction of AF recurrence after RFCA using the APPLE score may be related to the following aspects: First, studies have shown that SII is positively correlated with age, with higher levels of SII corresponding with an increase in age[30]. Age is also an independent risk factor for APPLE score. Second, a study on the adult population in the United States found a positive correlation between SII and chronic kidney disease (CKD), and SII can be considered a positive indicator for timely identification and treatment guidance of CKD[31]. Additionally, chronic renal insufficiency is an independent risk factor for the APPLE score. Furthermore, Tang et al[32]’s research indicates that SII is associated with CHF. The high level of SII is closely related to the poor short-term prognosis in critically ill patients with CHF, including 30-and 90-day and hospital all-cause mortalities, as well as the occurrence of major cardiovascular adverse events, and is expected to be a simple and effective prognostic evaluation indicator. Similarly, left ventricular ejection fraction < 50% is an independent risk factor for APPLE score. Finally, studies have shown that SII is significantly elevated in patients with persistent AF[33]. Moreover, persistent AF is an independent risk factor for the APPLE score. Therefore, the SII enhanced the significance of the APPLE score in predicting AF recurrence after RFCA.
A study compared the predictive abilities of known AF scoring systems (MB-LATER, CHADS2, CHA2DS2-VASc, BASE-AF2, CAAP-AF, APPLE, and HATCH) in a large Chinese cohort of patients with AF who underwent RFCA[34]. According to NRI and IDI, the MB-LATER score demonstrated superior performance in predicting post-ablation AF recurrence in this population. However, in this study, owing to the lack of statistical analysis of patients with early recurrence after RFCA, MB-LATER scores could not be obtained. In the future, we plan to conduct further research to estimate the additional predictive value of the SII for the MB-LATER score.
This study had some limitations. First, it relied exclusively on research data derived from a solitary retrospective study conducted at a single tertiary hospital. The small sample size and a lack of long-term continuous recording of heart rhythm post-ablation introduce a potential source of unavoidable bias. Therefore, further validation through multicentre prospective studies is required to address these limitations and enhance the generalisability of the findings. Second, in this study, an intracardiac monitor was not used to perform long-term continuous heart rhythm monitoring on patients after RFCA, which may lead to missed diagnosis in some asymptomatic patients with paroxysmal AF and result in a low recurrence rate. Finally, this study was conducted during the COVID-19 pandemic, which may have led to missing follow-ups of some patients and resulting in bias.
The SII, as a new inflammatory marker, contributes to predicting AF recurrence post-RFCA. Moreover, the SII enhanced the significance of the APPLE score in predicting AF recurrence after RFCA. This marker can help physicians optimise patient selection and develop personalised treatment plans.
The authors are grateful for the clinical data provided by the Department of Cardiology of Weifang People’s Hospital.
| 1. | Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. Epidemiology of Atrial Fibrillation in the 21st Century: Novel Methods and New Insights. Circ Res. 2020;127:4-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 1054] [Article Influence: 175.7] [Reference Citation Analysis (0)] |
| 2. | European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery, Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369-2429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3227] [Cited by in RCA: 3360] [Article Influence: 210.0] [Reference Citation Analysis (0)] |
| 3. | Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3176] [Cited by in RCA: 6903] [Article Influence: 1380.6] [Reference Citation Analysis (1)] |
| 4. | Ayzenberg O, Swissa M, Shlezinger T, Bloch S, Katzir I, Chodick G, Caspi A, Vered Z. Atrial Fibrillation Ablation Success Rate - A Retrospective Multicenter Study. Curr Probl Cardiol. 2023;48:101161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 5. | Mulder MJ, Kemme MJB, Hopman LHGA, Kuşgözoğlu E, Gülçiçek H, van de Ven PM, Hauer HA, Tahapary GJM, Götte MJW, van Rossum AC, Allaart CP. Comparison of the predictive value of ten risk scores for outcomes of atrial fibrillation patients undergoing radiofrequency pulmonary vein isolation. Int J Cardiol. 2021;344:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Zhang Y, Liu W, Yu H, Chen Z, Zhang C, Ti Y, Bu P. Value of the Systemic Immune-Inflammatory Index (SII) in Predicting the Prognosis of Patients With Peripartum Cardiomyopathy. Front Cardiovasc Med. 2022;9:811079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Öcal L, Keskin M, Cerşit S, Eren H, Özgün Çakmak E, Karagöz A, Çakir H, Gürsoy MO, Doğan S, Zhalilov M, Türkmen MM. Systemic immune-inflammation index predicts in-hospital and long-term outcomes in patients with ST-segment elevation myocardial infarction. Coron Artery Dis. 2022;33:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20319] [Cited by in RCA: 21048] [Article Influence: 1238.1] [Reference Citation Analysis (0)] |
| 9. | Zhong JH, Huang DH, Chen ZY. Prognostic role of systemic immune-inflammation index in solid tumors: a systematic review and meta-analysis. Oncotarget. 2017;8:75381-75388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 10. | Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4381] [Cited by in RCA: 5366] [Article Influence: 315.6] [Reference Citation Analysis (0)] |
| 11. | Kornej J, Hindricks G, Shoemaker MB, Husser D, Arya A, Sommer P, Rolf S, Saavedra P, Kanagasundram A, Patrick Whalen S, Montgomery J, Ellis CR, Darbar D, Bollmann A. The APPLE score: a novel and simple score for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation. Clin Res Cardiol. 2015;104:871-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (1)] |
| 12. | Dong JZ, Sang CH, Yu RH, Long DY, Tang RB, Jiang CX, Ning M, Liu N, Liu XP, Du X, Tse HF, Ma CS. Prospective randomized comparison between a fixed '2C3L' approach vs. stepwise approach for catheter ablation of persistent atrial fibrillation. Europace. 2015;17:1798-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL. Corrigendum to: 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:4194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 296] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 14. | Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, Chen PS, Chen SA, Chung MK, Nielsen JC, Curtis AB, Davies DW, Day JD, d'Avila A, de Groot NMSN, Di Biase L, Duytschaever M, Edgerton JR, Ellenbogen KA, Ellinor PT, Ernst S, Fenelon G, Gerstenfeld EP, Haines DE, Haissaguerre M, Helm RH, Hylek E, Jackman WM, Jalife J, Kalman JM, Kautzner J, Kottkamp H, Kuck KH, Kumagai K, Lee R, Lewalter T, Lindsay BD, Macle L, Mansour M, Marchlinski FE, Michaud GF, Nakagawa H, Natale A, Nattel S, Okumura K, Packer D, Pokushalov E, Reynolds MR, Sanders P, Scanavacca M, Schilling R, Tondo C, Tsao HM, Verma A, Wilber DJ, Yamane T. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. Heart Rhythm. 2017;14:e445-e494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 15. | Jalife J, Kaur K. Atrial remodeling, fibrosis, and atrial fibrillation. Trends Cardiovasc Med. 2015;25:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 16. | McGarry TJ, Narayan SM. The anatomical basis of pulmonary vein reconnection after ablation for atrial fibrillation: wounds that never felt a scar? J Am Coll Cardiol. 2012;59:939-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Baek YS, Choi JI, Kim YG, Lee KN, Roh SY, Ahn J, Kim DH, Lee DI, Hwang SH, Shim J, Kim JS, Kim DH, Park SW, Kim YH. Atrial Substrate Underlies the Recurrence after Catheter Ablation in Patients with Atrial Fibrillation. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, Zhang X, Wang WM, Qiu SJ, Zhou J, Fan J. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212-6222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1414] [Cited by in RCA: 1558] [Article Influence: 129.8] [Reference Citation Analysis (2)] |
| 19. | Adamo M, Gardner RS, McDonagh TA, Metra M. The 'Ten Commandments' of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2022;43:440-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Wada H, Dohi T, Miyauchi K, Nishio R, Takeuchi M, Takahashi N, Endo H, Ogita M, Iwata H, Kasai T, Okazaki S, Isoda K, Suwa S, Daida H. Neutrophil to Lymphocyte Ratio and Long-Term Cardiovascular Outcomes in Coronary Artery Disease Patients with Low High-Sensitivity C-Reactive Protein Level. Int Heart J. 2020;61:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Lau WY, Lai ECH. Loco-regional intervention for hepatocellular carcinoma. J Interv Med. 2019;2:43-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | Ang JJ, Chia DKA, Chan DKH. Lymphocyte-White Cell Ratio Is a Novel Marker of Morbidity Following Colorectal Cancer Surgery. J Surg Res. 2021;259:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Lin KB, Fan FH, Cai MQ, Yu Y, Fu CL, Ding LY, Sun YD, Sun JW, Shi YW, Dong ZF, Yuan MJ, Li S, Wang YP, Chen KK, Zhu JN, Guo XW, Zhang X, Zhao YW, Li JB, Huang D. Systemic immune inflammation index and system inflammation response index are potential biomarkers of atrial fibrillation among the patients presenting with ischemic stroke. Eur J Med Res. 2022;27:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 24. | Kaplan E, Ekızler FA, Saribaş H, Tak BT, Cay S, Korkmaz A, Ozeke O, Ozcan F, Topaloglu S, Aras D. Effectiveness of the systemic immune inflammation index to predict atrial fibrillation recurrence after cryoablation. Biomark Med. 2023;17:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 25. | Kornej J, Hindricks G, Kosiuk J, Arya A, Sommer P, Husser D, Rolf S, Richter S, Huo Y, Piorkowski C, Bollmann A. Comparison of CHADS2, R2CHADS2, and CHA2DS2-VASc scores for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation: the Leipzig Heart Center AF Ablation Registry. Circ Arrhythm Electrophysiol. 2014;7:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 26. | Kornej J, Schumacher K, Sommer P, Potpara T, Arya A, Dagres N, Bollmann A, Husser-Bollmann D, Lip GYH, Hindricks G. Very late arrhythmia recurrences in patients with sinus rhythm within the first year after catheter ablation: The Leipzig Heart Center AF Ablation Registry. Europace. 2019;21:1646-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Deng H, Shantsila A, Guo P, Potpara TS, Zhan X, Fang X, Liao H, Liu Y, Wei W, Fu L, Xue Y, Wu S, Lip GYH. Sex-related risks of recurrence of atrial fibrillation after ablation: Insights from the Guangzhou Atrial Fibrillation Ablation Registry. Arch Cardiovasc Dis. 2019;112:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Kornej J, Büttner P, Sommer P, Dagres N, Dinov B, Schumacher K, Bollmann A, Hindricks G. Prediction of electro-anatomical substrate using APPLE score and biomarkers. Europace. 2019;21:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Blandino A, Bianchi F, Grossi S, Biondi-Zoccai G, Conte MR, Gaido L, Gaita F, Scaglione M, Rametta F. Left Atrial Substrate Modification Targeting Low-Voltage Areas for Catheter Ablation of Atrial Fibrillation: A Systematic Review and Meta-Analysis. Pacing Clin Electrophysiol. 2017;40:199-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 30. | Fest J, Ruiter R, Ikram MA, Voortman T, van Eijck CHJ, Stricker BH. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: a population-based prospective cohort study. Sci Rep. 2018;8:10566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 185] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 31. | Li L, Chen K, Wen C, Ma X, Huang L. Association between systemic immune-inflammation index and chronic kidney disease: A population-based study. PLoS One. 2024;19:e0292646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 32. | Tang Y, Zeng X, Feng Y, Chen Q, Liu Z, Luo H, Zha L, Yu Z. Association of Systemic Immune-Inflammation Index With Short-Term Mortality of Congestive Heart Failure: A Retrospective Cohort Study. Front Cardiovasc Med. 2021;8:753133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 33. | Naser A, Sayilan S, Güven O, Şengör BG, Biçici A, Uzun Y, Ekmekçi A, Kılıçgedik A. Inflammation Burden and Atrial Fibrillation Burden: A Bidirectional Relationship. Arq Bras Cardiol. 2024;121:e20230680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Deng H, Shantsila A, Xue Y, Potpara TS, Bai Y, Zhan X, Fang X, Liao H, Wei W, Wu S, Lip GYH. Using the MB-LATER score for predicting arrhythmia outcome after catheter ablation for atrial fibrillation: The Guangzhou atrial fibrillation project. Int J Clin Pract. 2018;72:e13247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/