Published online Jan 26, 2024. doi: 10.4330/wjc.v16.i1.16
Peer-review started: September 27, 2023
First decision: November 28, 2023
Revised: December 11, 2023
Accepted: December 28, 2023
Article in press: December 28, 2023
Published online: January 26, 2024
Processing time: 113 Days and 4.8 Hours
Although the spasm provocation test (SPT) can diagnose coronary spasms, it would be helpful if it could also predict their occurrence.
To investigate whether coronary spasms can be predicted using changes in intracoronary artery pressure measured using a pressure wire during the SPT.
Seventy patients underwent SPTs with pressure-wire measurement of intracoronary artery pressure. During each SPT, the pressure wire was advanced into the distal portion of the right coronary artery (RCA) and left anterior de
Among the 132 coronary arteries assessed using a pressure wire, there were 49 in group N, 25 in group L, and 58 in group MH. Baseline Pd/Pa was the lowest in group L (P = 0.001). The decrease in the Pd/Pa between baseline to low doses of ACh was lower in group MH than in group N (P < 0.001). A receiver-operating characteristics analysis showed that the cutoff baseline Pd/Pa value for predicting group L was 0.95, with a sensitivity of 0.600 (15/25) and a specificity of 0.713 (76/107) and that the cutoff value of Pd/Pa from baseline to low doses of ACh for predicting group MH was −0.04, with a sensitivity of 0.741 (43/58) and a specificity of 0.694 (34/49).
These findings suggest that indices of intracoronary pressure during SPT may be useful means for predicting the occurrence of coronary spasms.
Core Tip: The spasm provocation test (SPT) is a well-established tool for diagnosing coronary spasms. However, it is associated with several complications that can make the test a stressful experience. We investigated whether coronary spasms detected in the SPT can be estimated using intracoronary artery pressure measured with a pressure wire. We found that coronary spasms induced by low acetylcholine doses were associated with decreased intracoronary pressure at baseline. Coronary spasms induced by moderate-to-high acetylcholine doses showed decreased intracoronary pressure from baseline to low acetylcholine doses. These indices of intracoronary pressure may be used to predict coronary spasms.
- Citation: Teragawa H, Oshita C, Uchimura Y. Do changes in intracoronary pressure aid coronary spasm diagnosis using the spasm provocation test? World J Cardiol 2024; 16(1): 16-26
- URL: https://www.wjgnet.com/1949-8462/full/v16/i1/16.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i1.16
Vasospastic angina (VSA) is a condition in which transient vasoconstriction of the epicardial coronary arteries causes myocardial ischemia[1-4]. In recent years, VSA, angina with nonobstructive coronary artery disease (ANOCA)[5,6], and myocardial infarction with nonobstructive coronary artery (MINOCA)[7] have attracted increasing research attention. A diagnosis of VSA is made when transient electrocardiographic changes are observed in addition to typical anginal pain[8]. However, in actual clinical practice, electrocardiographic changes cannot always be detected[9,10] and the final diagnosis of VSA is made by performing a spasm provocation test (SPT).
In SPT, acetylcholine (ACh) or ergonovine maleate are used as provocative drugs. ACh is usually administered in small, gradual doses[6,11]. The physical response to this allows the diagnosis of VSA based on chest symptoms, electrocardiographic changes, and significant coronary vasoconstriction. However, VSA and/or SPT-related complications have also been reported to occur[12,13]. It is undeniable that SPT can be stressful, even for the person performing the test. Therefore, if the cardiologist performing the SPT can predict that coronary spasm will occur with the next provocation, they can respond quickly. The ability to predict coronary spasm during the test would be highly useful and allow both practical and mental preparation.
In some cases, coronary spasm tests are performed after evaluation of coronary microcirculatory dysfunction (CMD)[14,15]. In such cases, the SPT is often performed with a pressure wire in place. In our previous research, we demon
This observational retrospective study included patients who underwent coronary angiography (CAG) and the SPT using a pressure wire at our institution between January 2012 and August 2015 (n = 79). The exclusion criteria were as follows: Significant coronary stenosis (% stenosis > 50%, n = 5) or a history of percutaneous coronary intervention (n = 4). Finally, 70 patients (mean age, 68 years; 36 men, 34 women) were enrolled (Figure 1). The study protocol was approved by the ethics committee of our institution, No. 2023-11. Written informed consent was obtained from all participants for SPT. The opt-out method (http://www.jrhh.sakura.ne.jp/annnai/torikumi.html) was used to confirm final agreement to participate.
The SPT procedure has previously been described[16]. Coronary vasodilators were withdrawn at least 48 h before each SPT. Subsequently, the SPT was performed on the right coronary artery (RCA). After the initial CAG, a 5-Fr catheter was used to insert a 0.014-inch pressure wire (PrimeWire Prestige Plus guidewire or Verrata Pressure guidewire; Phillips Volcano, Amsterdam, Holland) into the distal part of the RCA. The pressure between the catheter tips and pressure wire was previously calibrated at the ostium of the coronary arteries. The ratio of distal intracoronary pressure (Pd) detected by the pressure wire to the proximal intracoronary pressure (Pa) detected by the catheter tips was continuously monitored using the Pd/Pa indices. Then, an injection of 20 µg was made into the RCA, followed by another of 50 µg. If 50 µg ACh failed to induce coronary spasm, an 80 µg of ACh was injected. When the Pd/Pa index dropped during a coronary spasm, the lowest Pd/Pa value in response to each ACh dose was recorded, as were the Pd/Pa values immediately prior to the angiogram. CAG was performed after coronary spasm or maximum ACh infusion. When coronary spasms occurred but disappeared on their own without intracoronary 0.3 mg nitroglycerin (NTG) injection into the RCA, SPT of the left coronary artery (LCA) was performed. In such cases, NTG was injected into the RCA and, after completion of the LCA SPT, CAG was performed once more. If coronary spasms were prolonged or severe enough to result in hemodynamic instability, an intracoronary NTG injection was administered to treat them. SPT was subsequently performed on the LCA. A pressure wire was introduced into the distal segments of the left anterior descending coronary artery (LAD) during the LCA SPT, following the second pressure calibration at the LCA ostium. Similar to the RCA SPT, the LCA was infused with ACh doses. In the LCA test, the ACh doses were 50 and 100 µg, followed by 200 µg if the previous doses did not induce a coronary spasm. Following the commencement of a coronary spasm or the maximum ACh infusion, whichever came first, CAG was administered. A 0.3 mg NTG intracoronary injection and the last CAG for the LCA were then performed. According to this study, the appropriate low, moderate, and high ACh dosages for the RCA and LCA were 20, 50, and 80 µg and 50, 100, and 200 µg, respectively. Since the number of spasm-positive cases at high doses was not large, we divided the lesions into three groups: Group N consisted of lesions that did enter the coronary spasm, group L consisted of lesions in which coronary spasm was induced by the lowest dose of ACh, and group MH consisted of lesions in which coronary spasm was induced by the moderate or high-dose ACh.
When the Pd/Pa index decreased during a coronary spasm, the data immediately before the angiogram were used instead of the minimal Pd/Pa index in response to each ACh dose. The minimal Pd/Pa at low doses of ACh minus the Pd/Pa at baseline was used to define the difference in the Pd/Pa at low doses of ACh. When a PrimeWire Prestige Plus guidewire was available, the instantaneous free-wave ratio (iFR) was assessed immediately before ACh administration. The usual technique for the intravenous administration of adenosine triphosphate was used to test the fractional flow reserve (FFR) in patients with coronary atherosclerosis. We employed an autoinjector using a previously described method[16]. The diameter of the coronary artery was measured. Atherosclerotic lesions were defined as those with between a stenosis between 20% and 50%.
There are three categories of angina pectoris activity: Resting, exertion, and combined rest and exertion. When induced, VSA was defined as > 90% constriction of the coronary arteries as measured by angiogram, along with typical chest symptoms and/or an ST-segment deviation on electrocardiogram[6]. Those who had a coronary spasm in at least one major coronary artery were classified as the VSA group, and those who had no spasms in any coronary artery as the non-VSA group. Based on the classifications of the American Heart Association (AHA), focal spasms are defined as temporary arterial narrowing of more than 90% that remains within the boundaries of a single isolated coronary segment[17]. Diffuse vasoconstriction is defined by the AHA as > 90% arterial narrowing in two coronary artery segments[17]. The coronary spasm endotype observed in the RCA and LCA of each patient can differ. Coronary spasms that affect more than two major coronary arteries are referred to as multivessel spasms. We were unable to identify multivessel spasms in cases in which the unavoidable use of NTG was followed by a negative SPT. Each coronary artery at the site of the coronary spasm was separated into three sections (proximal, mid, and distal) for lesion studies, and the central area of the coronary spasm was labeled as diffuse.
Patient information was collected regarding current and former smoking habits and alcohol consumption, family history of coronary artery disease (CAD)[16], and comorbid hypertension, dyslipidemia, diabetes mellitus, or chronic kidney disease (CKD), all defined using the accepted criteria[16,18-20]. Using cardiac ultrasonography (UCG), the left ventricular ejection fraction (LVEF) was calculated using the modified Simpson’s method. Flow-mediated dilation (FMD), endothelium-dependent function, endothelium-independent function, and NTG-induced dilation (NID), were also measured and recorded in the majority of the patients (n = 62)[16,21].
For nonnormally distributed data and noncontinuous variables, data were presented as mean and SD or median and interquartile range. Student’s unpaired t-tests, Wilcoxon signed-rank tests, or 2 analyses were used for between-group comparisons of variables at baseline. The causes of coronary spasms caused by low-dose or moderate-to-high doses of ACh were identified using logistic regression analyses. Optimal cutoff values were determined using receiver-operating characteristic (ROC) analyses. JMP version 17 was used to perform statistical analyses (SAS Institute Inc., United States). Statistical significance was set as P values < 0.05.
There were 53 patients (76%) in the VSA group and 17 patients (24%) in the non-VSA group. Patient characteristics are summarized in Table 1. There were no significant differences in age, body mass index, coronary risk factors, alcohol consumption, smoking, family history of CAD, or CKD between the two groups, but there were significantly more males in the VSA group than in the non-VSA group (P = 0.001). UCG showed no significant difference in LVEF. Brachial artery ultrasonography showed no difference in brachial artery diameter at baseline between the two groups; however, in the VSA group, FMD was significantly lower (P = 0.008) and NID tended to be lower (P = 0.061). Coronary vasodilator use, which was stopped at least 48 h before the SPT, was clearly lower in the VSA group (P = 0.018), but no differences were observed for other medications.
| Non-VSA | VSA | P value | |
| n (%) | 17 (24) | 53 (18) | |
| Age (yr) | 68 ± 10 | 68 ± 9 | 0.775 |
| Male/Female | 3/14 | 33/20 | 0.001 |
| Body mass index | 24.4 ± 4.1 | 24.5 ± 3.8 | 0.914 |
| Coronary risk factors (%) | |||
| Smoking (active/former/never) | 2/2/13 | 11/16/26 | 0.134 |
| Hypertension | 14 (82) | 39 (74) | 0.463 |
| Dyslipidemia | 11 (65) | 32 (60) | 0.750 |
| Diabetes mellitus | 4 (24) | 18 (34) | 0.420 |
| Alcohol consumer (%) | 10 (13) | 5 (29) | 0.09 |
| Family history of CAD (%) | 4 (24) | 14 (26) | 0.813 |
| CKD (%) | 7 (41) | 17 (32) | 0.492 |
| LVEF on UCG (%) | 69 ± 7 | 66 ± 9 | 0.200 |
| Brachial artery ultrasonography (n) | 14 | 48 | |
| Brachial artery diameter at baseline (mm) | 3.8 ± 0.8 | 3.9 ± 0.7 | 0.711 |
| FMD (%) | 6.2 ± 4.8 | 3.0 ± 3.6 | 0.008 |
| NID (%) | 17.7 ± 7.7 | 13.6 ± 6.9 | 0.061 |
| Medications | |||
| Any kind of coronary vasodilator (%) | 13 (76) | 23 (43) | 0.018 |
| No. of coronary vasodilators | 1 (0.5, 1) | 0 (0, 1) | 0.059 |
| Beta-receptor blockers (%) | 1 (6) | 4 (8) | 0.817 |
| RAS inhibitors (%) | 7 (41) | 12 (23) | 0.135 |
| Lipid-lowering drugs (%) | 4 (41) | 17 (32) | 0.492 |
| Anti-platelet drugs (%) | 4 (24) | 18 (34) | 0.420 |
In most patients, a pressure wire was inserted into the RCA and LAD. However, eight coronary vessels were excluded. This was because the RCA was too small to perform the SPT (n = 2), the catheter could not engage into the RCA (n = 2), the pressure wire could not be inserted into the distal portion of RCA due to meandering (n = 3), or the pressure wire could not be inserted into the LAD (n = 1). Thus, a pressure wire was inserted into the remaining 132 coronary vessels, including 63 RCA and 69 LAD.
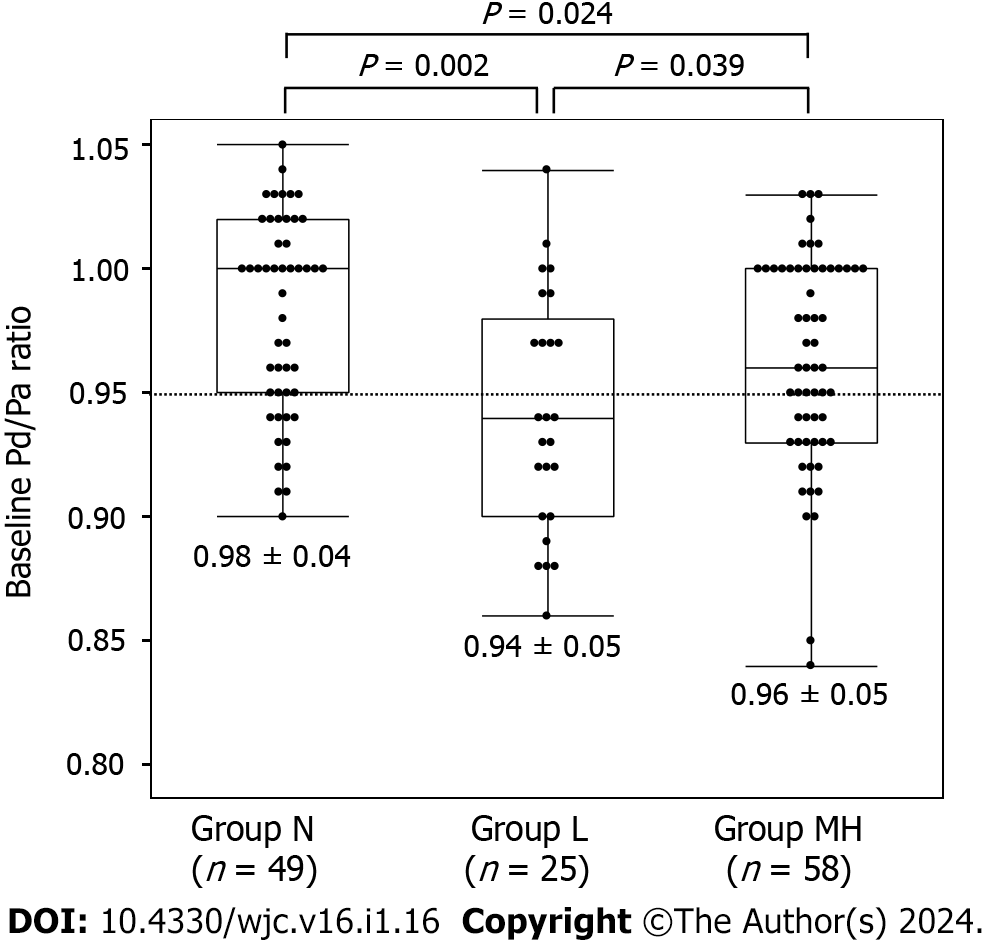
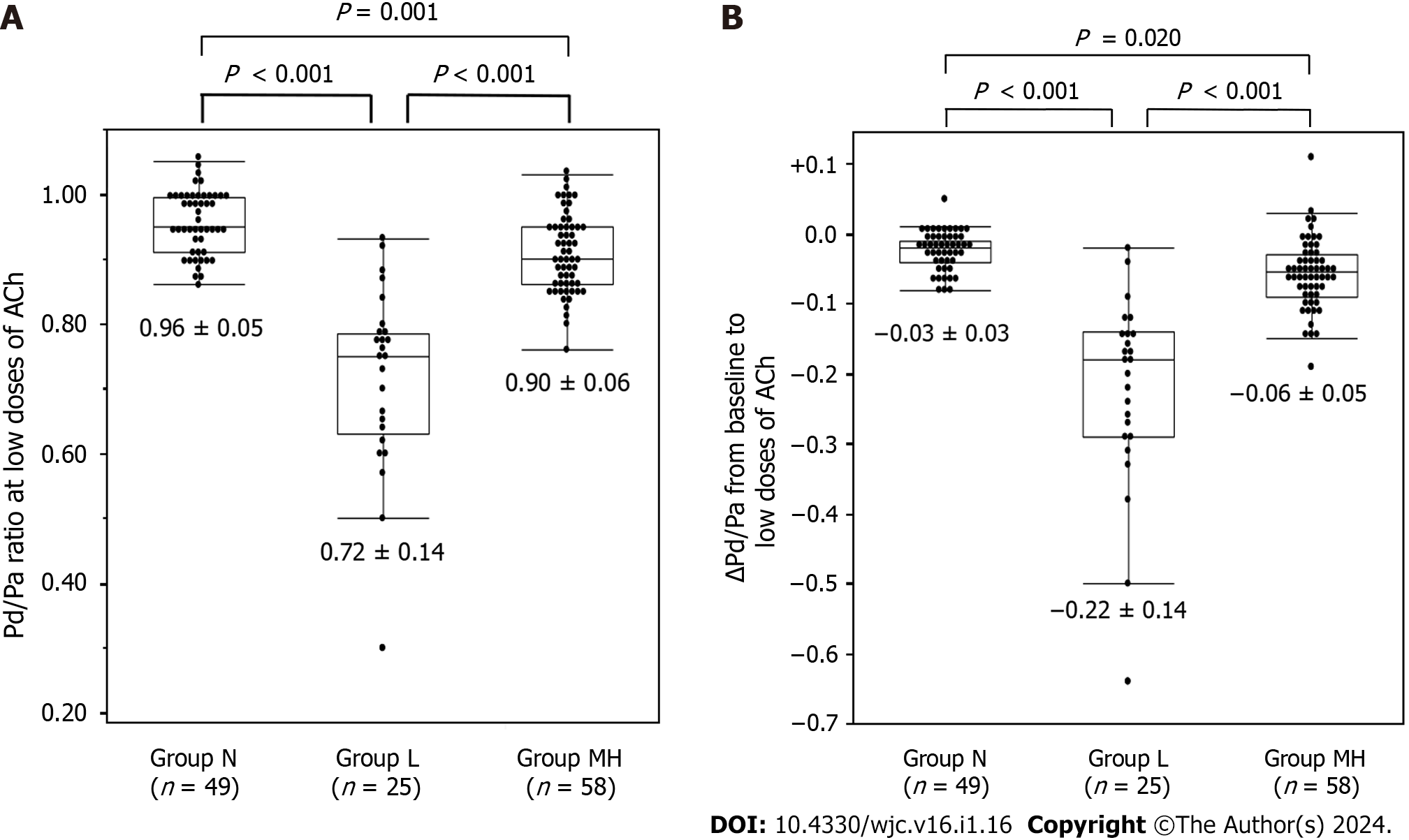
Coronary spasms were induced by low, moderate, and high doses of ACh in 25, 51, and 7 vessels, respectively. No coronary spasms occurred in the remaining 49 vessels (group N). In seven vessels, spasms were induced by the high dose but were insufficient to clarify the statistical results. Thus, as described in the Methods, vessels in which coronary spasms were induced by moderate and high doses of ACh were combined into group MH. There were 49 vessels in group N, 25 in group L, and 58 in group MH. The lesion characteristics are listed in Table 2. There were no differences between the RCA and LAD in coronary vessels, in the rate of atherosclerosis, or in the unavoidable need for NTG administration. There was no difference between group L and MH in whether induced coronary spasms were focal or diffuse (P = 0.456) and whether the coronary arteries were proximal, mid, or distal (P = 0.610). We found no significant differences in iFR (P = 0.104) or FFR (P = 0.093) among the three groups. The Pd/Pa values at baseline were the lowest in group L (P = 0.001) (Figure 2). The Pd/Pa value at low doses of ACh was lowest in group L and highest in group N (Figure 3A). The difference between baseline and low-dose ACh was lowest in group L and highest in group N (Figure 3B). Pd/Pa at moderate-to-high doses of ACh was significantly lower in group MH than in N (P < 0.001).
| Group N | Group L | Group MH | P value | |
| n | 49 | 25 | 58 | |
| RCA/LAD | 27/22 | 9/16 | 27/31 | 0.290 |
| Atherosclerosis (%) | 23 (47) | 9 (36) | 28 (48) | 0.568 |
| Unavoidable use of NTG | 4 (8) | 3 (12) | 5 (9) | 0.851 |
| Coronary spasm | ||||
| Focal/diffuse | 8/17 | 14/44 | ||
| Proximal/mid/distal | 4/15/6 | 5/35/18 | ||
| iFR | 0.98 ± 0.04 | 0.92 ± 0.09 | 0.98 ± 0.06 | 0.104 |
| n | 15 | 5 | 11 | |
| Pd/Pa | ||||
| Baseline | 0.98 ± 0.04 | 0.94 ± 0.05 | 0.96 ± 0.05 | 0.001 |
| Low dose of ACh | 0.96 ± 0.05 | 0.72 ± 0.14 | 0.90 ± 0.06 | < 0.001 |
| Pd/Pa (Low dose-baseline) | −0.03 ± 0.03 | −0.22 ± 0.14 | −0.06 ± 0.05 | < 0.001 |
| Moderate-high doses of ACh | 0.93 ± 0.06 | 0.78 ± 0.13 | < 0.001 | |
| FFR | 1.01 | 0.81 ± 0.09 | 0.87 ± 0.09 | 0.093 |
| n | 1 | 6 | 13 |
Predictions of coronary spasms at low and moderate-high doses of ACh were made based on Pd/Pa at baseline and changes in Pd/Pa.
In univariate analysis, the only significant indicator of coronary spasm induced by low doses of ACh was Pd/Pa at baseline (P = 0.002). Pd/Pa at baseline was negatively correlated with LAD (P < 0.001) and the presence of atherosclerosis (P = 0.030). Logistic regression analysis of positive SPT at the low dose of ACh, using Pd/Pa at baseline, the LAD, and the presence of atherosclerosis found Pd/Pa at baseline was the only significant predictor (P = 0.003). ROC analysis showed an area under the curve (AUC) of 0.688 and a cutoff Pd/Pa at baseline of 0.95 for predicting the induction of coronary spasm by low doses of ACh (group L), with sensitivity and specificity of 0.600 (15/25) and 0.713 (76/107), respectively (Figure 4A). In an analysis of the 107 Lesions in group N and MH, the factors that predicted the induction of coronary spasms by moderate or high doses of ACh were Pd/Pa at baseline (P = 0.019, AUC 0.626), Pd/Pa at low doses of ACh (P < 0.001, AUC 0.739), and the ΔPd/Pa from baseline to low ACh dose (P < 0.001, AUC 0.742). ΔPd/Pa, which had the highest AUC, did not correlate with the presence of atherosclerosis (P = 0.141) or with the LAD (P = 0.393). ROC analysis showed that the optimal ΔPd/Pa from baseline to the low dose of ACh was −0.04, with a sensitivity of 0.741 (43/58) and a specificity of 0.694 (34/49) (Figure 4B).
This study examined whether baseline Pd/Pa values and changes in this value, as measured by a pressure wire, could be used to predict the next coronary spasm when the SPT was performed using a pressure wire. We found that intracoronary pressure at baseline decreased in cases where coronary spasms were induced by a low dose of ACh, indicating that decreased intracoronary pressure at baseline may predict these coronary spasms. Furthermore, coronary spasms induced by moderate-to-high doses of ACh were not only correlated with lower intracoronary pressure at baseline, but also with lower intracoronary pressure from baseline to low-dose ACh administration, indicating that this intracoronary pressure trend is useful in the prediction of coronary spasms induced by moderate-to-high dose ACh provocation. When pressure wires are used in the SPT, these indices may be useful for predicting coronary spasms.
The SPT is a reliable and well-established test that is useful for the diagnosis of coronary spasms and the evaluation of their activity and prognosis[6,17,22-25]. However, a certain percentage of SPTs have known complications, some of which are the result of the induced spasms. In this sense, the test is by no means stress-free for either the patient or the cardiologist. The ability to predict the likelihood of coronary spasms at the next provocation in SPT has the potential to attenuate this stress to some degree. We attempted to determine whether the next coronary spasm during the SPT could be predicted based on the Pd/Pa value and its changes measured using a pressure wire.
To date, there have been no reports on the measurement of changes in intracoronary pressure during SPT. However, several studies have examined this pressure using quantitative CAG and coronary blood flow velocity using Doppler wires[26,27]. In our previous study[26], the baseline coronary artery diameter in patients with VSA (mean, 2.95 mm) was slightly lower than that of non-VSA patients (mean, 3.14 mm), although not significantly. We also showed that a 2 min infusion of ACh at a dose of 3 µg/min caused significant vasocontraction of the coronary artery but did not induce coronary spasms[26]. Similarly, Feenstra et al[27] found that an 8.6 µg/min dose of ACh for 3 min causes a nonsignificant coronary artery contraction in patients with epicardial coronary spasm. Despite the differences due to the part of the measured coronary artery and the dose of ACh infusion, the baseline coronary artery diameter and changes in this diameter in response to small doses of ACh can cause a decrease in baseline intracoronary pressure, with greater decreases in patients with VSA.
There are several reasons why performing the SPT with a pressure wire can be useful: (1) A decrease in intracoronary pressure when a coronary spasm occurs, indicating that a spasm has occurred before CAG; (2) Improvements in intracoronary pressure following NTG administration shows whether a coronary spasm is immediately relieved, reducing the need for additional NTG and/or contrast medium; and (3) The insertion of a pressure wire stabilizes the catheter's engagement, especially in the RCA. The present study showed the feasibility of predicting coronary spasm provocation using intracoronary pressure at baseline and its changes during low-dose ACh infusion. However, we do not recommend routine use of a pressure wire with SPT. Insertion of a pressure wire prolongs fluoroscopy time and increases medical costs. In addition, there is a risk of coronary artery trauma due to pressure-wire insertion, which cannot always be inserted all the way to the distal end of the coronary artery. The insertion of the wire can potentially obscure the findings of the SPT or cause the accordion phenomenon. Hence, there are many possible disadvantages of pressure-wire insertion. In recent years, there has been increased research attention on the SPT and the functional testing of CMD, as many patients with ANOCA or MINOCA conditions do not have significant stenosis of the coronary arteries[5,6] and clarification of causes can improve prognosis[28]. A pressure wire is sometimes used to evaluate CMD before the SPT[14,15]. The number of cases in which a pressure wire is used to induce coronary spasm is likely to increase in the future. In such cases, the results of the present study can be used as a reference for predicting coronary spasms. We hope that the validity of the results of this study will be strengthened in future research.
This study had several limitations. First, because this was a single-center study with a small sample size, our results may not be generalizable to all patients with coronary spasms. Second, although coronary artery diameters should be measured during drug provocation from quantitative CAG, in asymptomatic cases, no electrocardiographic changes, or no changes in intracoronary pressure, CAG was omitted or only a test shot was taken, and changes in coronary artery diameters were not evaluated. This was a significant impediment to our findings. Third, some negative coronary spasm cases may have completed provocation following a moderate dose of ACh. Recently, the usefulness of high-dose ACh provocation[29,30] and sequential provocation proposed by Sueda et al[31] was reported, and it is possible that such provocation would have changed the results to positive. The rates of coronary vasodilators in the VSA and non-VSA groups differed, which may have had some effect on the SPT results, although their use was stopped for at least 48 h before the SPT. Fourth, the present study included patients with chest symptoms but no significant stenosis in the coronary arteries. Some patients had positive Holter ECG or exercise stress ECG results, but not all patients had confirmed ischemic findings. From this point of view, the patients included in the study were ANOCA patients. Finally, the use of pressure wires during the SPT may have been higher in our sample because the SPT was performed after CMD assessment, in which case, ACh provocation would occur after NTG administration. Because of the small number of patients in this study who required NTG, the changes in intracoronary pressure during ACh provocation recorded after NTG administration may not have been consistent with our main results.
We performed SPT using a pressure wire to investigate whether coronary spasm could be predicted from changes in the intracoronary artery pressure during the test. Decreased intracoronary pressure at baseline may be a useful means of inferring coronary spasms induced by a low-dose ACh provocation. A decrease in intracoronary pressure from baseline following low-dose ACh provocation may be useful for predicting subsequent moderate-to-high dose ACh-induced coronary spasms. Although pressure wires are not recommended for routine use in the SPT, when they are used, the intracoronary pressure findings appear to be useful in the prediction of coronary spasms. We hope that the validity of our results will be evaluated in future studies.
Although the spasm provocation test (SPT) can diagnose coronary spasms, there are some complications related to SPT.
To reduce complications related to SPT, it would be helpful if it could also predict the occurrence of coronary spasm during the SPT.
We investigated whether coronary spasms can be predicted using changes in intracoronary artery pressure measured using a pressure wire during the SPT.
Seventy patients underwent SPTs with pressure-wire measurement of intracoronary artery pressure. During each SPT, the pressure wire was advanced into the distal portion of the right coronary artery (RCA) and left anterior descending coronary artery, and the ratio of intracoronary pressure to aortic pressure (Pd/Pa) was monitored. Coronary spasm was defined as an arterial narrowing of > 90% in response to the administration of acetylcholine (ACh), with chest symptoms and/or ischemic electrocardiographic changes. ACh was administered to the RCA at low, moderate, or high doses of 20, 50, or 80 µg, respectively, and to the left coronary artery (LCA) at low, moderate, or high doses of 50, 100, or 200 µg, respectively. Coronary arteries with coronary spasms at low doses of ACh were defined as group L, and those with coronary spasms at moderate or high doses were defined as group MH. Those who did not occur coronary spasms at any ACh dose were designated as group N.
Among the 132 coronary arteries assessed using a pressure wire, there were 49 in group N, 25 in group L, and 58 in group MH. Baseline Pd/Pa was the lowest in group L (P = 0.001). The decrease in the Pd/Pa between baseline to low doses of ACh was lower in group MH than in group N (P < 0.001). A receiver-operating characteristics analysis showed that the cutoff baseline Pd/Pa value for predicting group L was 0.95, with a sensitivity of 0.600 (15/25) and a specificity of 0.713 (76/107) and that the cutoff value of Pd/Pa from baseline to low doses of ACh for predicting group MH was −0.04, with a sensitivity of 0.741 (43/58) and a specificity of 0.694 (34/49).
These findings suggest that indices of intracoronary pressure during SPT may be useful for predicting coronary spasms induced by both low doses and moderate-high doses of ACh.
We do not recommend that all patients undergo SPT testing with a pressure wire in all cases. However, if SPT is performed after evaluation of coronary microvascular function using a pressure wire, it may be possible to leave the pressure wire in place, which may help predict coronary spasm. It is necessary to confirm the results of this study by accumulating more data in the future.
We thank Ms. Akemi Seno for her secretarial assistance. We also thank the staff of the catheterization laboratory, cardiovascular ward, and cardiovascular outpatient clinic at our institution.
| 1. | Yasue H, Nakagawa H, Itoh T, Harada E, Mizuno Y. Coronary artery spasm--clinical features, diagnosis, pathogenesis, and treatment. J Cardiol. 2008;51:2-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 383] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 2. | Jewulski J, Khanal S, Dahal K. Coronary vasospasm: A narrative review. World J Cardiol. 2021;13:456-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (2)] |
| 3. | Rehan R, Weaver J, Yong A. Coronary Vasospastic Angina: A Review of the Pathogenesis, Diagnosis, and Management. Life (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 4. | Beltrame JF. Management of vasospastic angina. Heart. 2022;109:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J, Maas AHEM, Prescott E, Karam N, Appelman Y, Fraccaro C, Louise Buchanan G, Manzo-Silberman S, Al-Lamee R, Regar E, Lansky A, Abbott JD, Badimon L, Duncker DJ, Mehran R, Capodanno D, Baumbach A. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41:3504-3520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 637] [Cited by in RCA: 572] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 6. | Hokimoto S, Kaikita K, Yasuda S, Tsujita K, Ishihara M, Matoba T, Matsuzawa Y, Mitsutake Y, Mitani Y, Murohara T, Noda T, Node K, Noguchi T, Suzuki H, Takahashi J, Tanabe Y, Tanaka A, Tanaka N, Teragawa H, Yasu T, Yoshimura M, Asaumi Y, Godo S, Ikenaga H, Imanaka T, Ishibashi K, Ishii M, Ishihara T, Matsuura Y, Miura H, Nakano Y, Ogawa T, Shiroto T, Soejima H, Takagi R, Taruya A, Tsuda E, Wakabayashi K, Yokoi K, Minamino T, Nakagawa Y, Sueda S, Shimokawa H, Ogawa H; Japanese Circulation Society and Japanese Association of Cardiovascular Intervention and Therapeutics and Japanese College of Cardiology Joint Working Group. JCS/CVIT/JCC 2023 Guideline Focused Update on Diagnosis and Treatment of Vasospastic Angina (Coronary Spastic Angina) and Coronary Microvascular Dysfunction. Circ J. 2023;87:879-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 125] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 7. | Montone RA, Niccoli G, Fracassi F, Russo M, Gurgoglione F, Cammà G, Lanza GA, Crea F. Patients with acute myocardial infarction and non-obstructive coronary arteries: safety and prognostic relevance of invasive coronary provocative tests. Eur Heart J. 2018;39:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 8. | JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J. 2014;78:2779-2801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 361] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 9. | Sueda S, Miyoshi T, Sasaki Y, Ohshima K, Sakaue T, Habara H, Kohno H. Complete definite positive spasm on acetylcholine spasm provocation tests: comparison of clinical positive spasm. Heart Vessels. 2016;31:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Sueda S, Miyoshi T, Sasaki Y, Sakaue T, Habara H, Kohno H. Approximately half of patients with coronary spastic angina had pathologic exercise tests. J Cardiol. 2016;68:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Suzuki S, Kaikita K, Yamamoto E, Jinnouchi H, Tsujita K. Role of acetylcholine spasm provocation test as a pathophysiological assessment in nonobstructive coronary artery disease. Cardiovasc Interv Ther. 2021;36:39-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Sueda S, Saeki H, Otani T, Mineoi K, Kondou T, Yano K, Ochi T, Ochi N, Hayashi Y, Tsuruoka T, Kawada H, Matsuda S, Uraoka T. Major complications during spasm provocation tests with an intracoronary injection of acetylcholine. Am J Cardiol. 2000;85:391-394, A10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Takahashi T, Samuels BA, Li W, Parikh MA, Wei J, Moses JW, Fearon WF, Henry TD, Tremmel JA, Kobayashi Y; Microvascular Network. Safety of Provocative Testing With Intracoronary Acetylcholine and Implications for Standard Protocols. J Am Coll Cardiol. 2022;79:2367-2378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 14. | Ford TJ, Ong P, Sechtem U, Beltrame J, Camici PG, Crea F, Kaski JC, Bairey Merz CN, Pepine CJ, Shimokawa H, Berry C; COVADIS Study Group. Assessment of Vascular Dysfunction in Patients Without Obstructive Coronary Artery Disease: Why, How, and When. JACC Cardiovasc Interv. 2020;13:1847-1864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 15. | Feenstra RGT, Seitz A, Boerhout CKM, Bukkems LH, Stegehuis VE, Teeuwisse PJI, de Winter RJ, Sechtem U, Piek JJ, van de Hoef TP, Ong P, Beijk MAM. Principles and pitfalls in coronary vasomotor function testing. EuroIntervention. 2022;17:1271-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Teragawa H, Oshita C, Uchimura Y. Does the intracoronary pressure differ according to two types (diffuse or focal) of coronary spasm? World J Cardiol. 2023;15:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 17. | Sato K, Kaikita K, Nakayama N, Horio E, Yoshimura H, Ono T, Ohba K, Tsujita K, Kojima S, Tayama S, Hokimoto S, Matsui K, Sugiyama S, Yamabe H, Ogawa H. Coronary vasomotor response to intracoronary acetylcholine injection, clinical features, and long-term prognosis in 873 consecutive patients with coronary spasm: analysis of a single-center study over 20 years. J Am Heart Assoc. 2013;2:e000227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 18. | Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A; Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5325] [Cited by in RCA: 5477] [Article Influence: 322.2] [Reference Citation Analysis (0)] |
| 19. | National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 534] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 20. | Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20005] [Cited by in RCA: 21074] [Article Influence: 390.3] [Reference Citation Analysis (0)] |
| 21. | Maruhashi T, Kajikawa M, Kishimoto S, Takaeko Y, Yamaji T, Harada T, Hashimoto Y, Han Y, Aibara Y, Yusoff FM, Nakano Y, Chayama K, Nakashima A, Goto C, Yoshimura K, Higashi Y. Serum Potassium Levels of 4.5 to Less Than 5.0 mmol/L Are Associated with Better Vascular Function. J Atheroscler Thromb. 2022;29:1588-1602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Okumura K, Yasue H, Matsuyama K, Goto K, Miyagi H, Ogawa H. Sensitivity and specificity of intracoronary injection of acetylcholine for the induction of coronary artery spasm. J Am Coll Cardiol. 1988;12:883-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 241] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Takagi Y, Takahashi J, Yasuda S, Miyata S, Tsunoda R, Ogata Y, Seki A, Sumiyoshi T, Matsui M, Goto T, Tanabe Y, Sueda S, Sato T, Ogawa S, Kubo N, Momomura S, Ogawa H, Shimokawa H; Japanese Coronary Spasm Association. Prognostic stratification of patients with vasospastic angina: a comprehensive clinical risk score developed by the Japanese Coronary Spasm Association. J Am Coll Cardiol. 2013;62:1144-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Nishimiya K, Suda A, Fukui K, Hao K, Takahashi J, Matsumoto Y, Mitsuishi K, Watanabe T, Ohyama K, Sugisawa J, Tsuchiya S, Satoh K, Shindo T, Godo S, Kikuchi Y, Shiroto T, Yasuda S, Shimokawa H. Prognostic Links Between OCT-Delineated Coronary Morphologies and Coronary Functional Abnormalities in Patients With INOCA. JACC Cardiovasc Interv. 2021;14:606-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Ishii M, Kaikita K, Sato K, Tanaka T, Sugamura K, Sakamoto K, Izumiya Y, Yamamoto E, Tsujita K, Yamamuro M, Kojima S, Soejima H, Hokimoto S, Matsui K, Ogawa H. Acetylcholine-Provoked Coronary Spasm at Site of Significant Organic Stenosis Predicts Poor Prognosis in Patients With Coronary Vasospastic Angina. J Am Coll Cardiol. 2015;66:1105-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Teragawa H, Mitsuba N, Ishibashi K, Nishioka K, Kurisu S, Kihara Y. Evaluation of coronary microvascular function in patients with vasospastic angina. World J Cardiol. 2013;5:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Feenstra RGT, Boerhout CKM, Vink CEM, Woudstra J, Wittekoek ME, de Waard GA, Appelman Y, Eringa EC, Marques KMJ, de Winter RJ, van de Hoef TP, Beijk MAM, Piek JJ. Haemodynamic characterisation of different endotypes in coronary artery vasospasm in reaction to acetylcholine. Int J Cardiol Heart Vasc. 2022;42:101105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 28. | Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, Hood S, McGeoch R, McDade R, Yii E, Sidik N, McCartney P, Corcoran D, Collison D, Rush C, McConnachie A, Touyz RM, Oldroyd KG, Berry C. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J Am Coll Cardiol. 2018;72:2841-2855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 597] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 29. | Sueda S, Kohno H, Miyoshi T, Sakaue T, Sasaki Y, Habara H. Maximal acetylcholine dose of 200 μg into the left coronary artery as a spasm provocation test: comparison with 100 μg of acetylcholine. Heart Vessels. 2015;30:771-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Sueda S, Fujimoto K, Sasaki Y, Sakaue T, Kohno H. Dose maximal acetylcholine dose into the left coronary artery affect the positive provoked spasm in the left circumflex artery? Coron Artery Dis. 2019;30:547-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Sueda S, Miyoshi T, Sasaki Y, Sakaue T, Habara H, Kohno H. Sequential spasm provocation tests might overcome a limitation of the standard spasm provocation tests. Coron Artery Dis. 2015;26:490-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Serafy AS, Egypt S-Editor: Li L L-Editor: A P-Editor: Zhao S