Published online Sep 26, 2023. doi: 10.4330/wjc.v15.i9.448
Peer-review started: April 27, 2023
First decision: June 1, 2023
Revised: June 16, 2023
Accepted: July 17, 2023
Article in press: July 17, 2023
Published online: September 26, 2023
Processing time: 146 Days and 18.9 Hours
Coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in a worldwide health crisis since it first appeared. Numerous studies demonstrated the virus’s predilection to cardiomyocytes; however, the effects that COVID-19 has on the cardiac conduc
To analyze the impact that COVID-19 has on the odds of major cardiovascular complications in patients with new onset heart blocks or bundle branch blocks (BBB).
The 2020 National Inpatient Sample (NIS) database was used to identify patients admitted for COVID-19 pneumonia with and without high-degree atrioven
A total of 1058815 COVID-19 hospitalizations were identified within the 2020 NIS database, of which 3210 (0.4%) and 17365 (1.6%) patients were newly diagnosed with HDAVB and BBB, respectively. We observed a significantly higher odds of in-hospital mortality, cardiac arrest, cardiogenic shock, sepsis, arrythmias, and acute kidney injury in the COVID-19 and HDAVB group. There was no statistically significant difference in the odds of cerebral infarction or pulmonary embolism. Encounters with COVID-19 pneumonia and newly diagnosed BBB had a higher odds of arrythmias, acute kidney injury, sepsis, need for mechanical ventilation, and cardiogenic shock than those without BBB. However, unlike HDAVB, COVID-19 pneumonia and BBB had no significant impact on mortality compared to patients without BBB.
In conclusion, there is a significantly higher odds of inpatient mortality, cardiac arrest, cardiogenic shock, sepsis, acute kidney injury, supraventricular tachycardia, ventricular tachycardia, THC, and LOS in patients with COVID-19 pneumonia and HDAVB as compared to patients without HDAVB. Likewise, patients with COVID-19 pneumonia in the BBB group similarly have a higher odds of supraventricular tachycardia, atrial fibrillation, atrial flutter, ventricular tachycardia, acute kidney injury, sepsis, need for mechanical ventilation, and cardiogenic shock as compared to those without BBB. Therefore, it is essential for healthcare providers to be aware of the possible worse predicted outcomes that patients with new-onset HDAVB or BBB may experience following SARS-CoV-2 infection.
Core Tip: This is the first and largest retrospective observational study based on the 2020 National Inpatient Sample database that illustrates the outcomes of patients with coronavirus disease 2019 (COVID-19) who developed new onset high degree atrioventricular blocks (HDAVB) or bundle branch blocks (BBB). We observed significantly higher rates of inpatient outcomes of interest in patients admitted for COVID-19 pneumonia and the secondary diagnosis of HDAVB or BBB compared to patients who did not. Several reports in the literature described worse outcomes experienced by this patient population. We conclude that elderly patients, whites, and males with common co-morbid conditions, hospitalized for COVID-19 pneumonia and HDAVB, seem to be at a significantly increased risk of developing cardiac complications and have a significantly increased risk of inpatient mortality, necessitating a need for preventative strategies, such as the use of temporary pacemakers or cardiac rhythm monitoring techniques.
- Citation: Shoura SJ, Teaima T, Sana MK, Abbasi A, Atluri R, Yilmaz M, Hammo H, Ali L, Kanitsoraphan C, Park DY, Alyousef T. Outcomes in patients with COVID-19 and new onset heart blocks: Insight from the National Inpatient Sample database. World J Cardiol 2023; 15(9): 448-461
- URL: https://www.wjgnet.com/1949-8462/full/v15/i9/448.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i9.448
The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has significantly impacted global health and the world’s economy[1,2]. The virus primarily targets the respiratory system, but it also elicits a robust systemic immune response, otherwise known as “cytokine storm”, leading to multi-organ dysfunction[2,3]. Although much of the focus has been on the acute respiratory distress syndrome caused by the virus, it is crucial for providers to understand the complex interplay between COVID-19 and the cardiovascular system[4,5].
Research has demonstrated that individuals with pre-existing cardiovascular disease are at a significantly higher risk of developing life-threatening SARS-CoV-2 infections and increased case fatality rates. Additionally, COVID-19 has been associated with a myriad of cardiovascular complications, including arrythmias, acute coronary syndromes, myocarditis, acute heart failure, pericarditis, cardiac tamponade, takotsubo cardiomyopathy, cor-pulmonale, cardiogenic shock, and pulmonary embolism[3,6]. These cardiovascular complications can significantly impact short and long-term mortality associated with COVID-19[7].
Of those hospitalized with COVID-19 pneumonia, 14.1% of patients experience a cardiovascular complication, with arrhythmia incidence rates ranging between 6.9-10.3%[3,8-13]. While tachyarrhythmias are the most commonly cited arrhythmias, bradyarrhythmias still represent 12% of all arrhythmias that can develop in COVID-19 affected patients[13,14]. Bradyarrhythmias can increase mortality rates, particularly in cases of new onset atrioventricular blocks. However, there is currently a lack of large-scale database analyses regarding hospital outcomes, trends, and demographics of bradyarrhythmias in patients with COVID-19.
To address this gap in knowledge, this review focuses on major cardiovascular complications in COVID-19 patients, with an emphasis on bradyarrhythmias due to high-degree atrioventricular blocks (HDAVB) (of which 1900 patients (59.2%) developed second degree atrioventricular block and 1310 (40.8%) developed complete atrioventricular block) in patients who did not have a prior permanent pacemaker or other implantable cardiac devices such as implantable cardioverter-defibrillator (ICD).
This study is a retrospective cohort analysis of admissions for COVID-19 patients in 2020 using the National Inpatient Sample (NIS) database. The NIS database is the largest publicly available all-payer inpatient healthcare database, maintained by the Agency for Healthcare Research and Quality. It was designed to assess all hospitalizations in non-federal acute care hospitals across the United States, excluding rehabilitation and long-term acute care hospitals, through a weighted probability sampling method. Data was collected from billing records submitted by hospitals to statewide data organizations, representing almost 97% of the United States (US) population. The NIS database then stratifies these hospitalizations according to bed size, teaching status, urban/rural locations, and geographic location. A sample of 20% of all hospitalizations in each stratum is collected, pooled, and weighted to ensure that it accurately represents the entire US population.
The NIS database contains patient and hospital-level information, including primary diagnosis, secondary diagnosis, primary payer type, median household income, hospital teaching status, geographic region, hospital bed size, and urban/rural location. All diagnoses are coded using the International Classification of Diseases, Tenth Revision, Clinical Modification/Procedure Coding System (ICD-10-CM/PCS).
In this database, diagnoses are divided into a single primary diagnosis and secondary diagnoses. The principal diagnosis represents the main reason for hospitalization, while secondary diagnoses include any other ICD-10 codes associated with the hospitalization. The NIS has previously been used to estimate the burden of cardiovascular diseases.
It is worth noting that this study's manuscript is exempt from Institutional Review Board (IRB) approval since it uses de-identified data. The data used in this study is accessible online at https://www.hcup-us.ahrq.gov.
Note to reader: Although High Degree Atrioventricular Block is defined as “≥ 2 consecutive P waves at a constant physiologic rate that do not conduct to the ventricles with evidence for some atrioventricular conduction”[15] by the American Heart Association, the term High Degree Atrioventricular Block (HDAVB) throughout the entirety of the manuscript will represent patients with Mobitz I, Mobitz II and Complete heart block only. We chose to use this abbreviation to avoid word redundancy.
This retrospective cohort study focuses on adult patients aged 18 years or older who were hospitalized with COVID-19 pneumonia during the year 2020. We identified the study population by combining multiple ICD-10-CM codes, including U07.1, J12.82, U100, U49, U50, and U85, based on a literature review of similar validated studies on COVID-19, bundle branch blocks and heart blocks which included Mobitz type one, Mobitz type two and complete heart block. We further stratified the population based on the presence or absence of HDAVB without a pacemaker (I44.1, I44.2) or BBB without a pacemaker (I44.7, I45.19, I45.10) using ICD-10 codes.
Patients with prior permanent pacemakers and other intra-cardiac devices were excluded to ensure that analyzed patients had not been previously diagnosed with heart blocks and had no devices placed for this reason.
Demographic characteristics such as age, sex, race, medical insurance status, and mean household income, as well as hospital characteristics such as hospital bed size, location, and teaching status, were included as study variables. Comorbidity burden was assessed using the Charlson comorbidity index, which was adjusted for population-based research.
The study population and the stratification process are outlined in Figure 1.
The primary outcome of our analysis was to examine the mortality rate in COVID-19 pneumonia patients who had newly diagnosed HDAVB or BBB (without permanent pacemaker). In addition, we also analyzed secondary outcomes such as mean length of stay (LOS), mean total hospital charges (THC), and adjusted odds of inpatient morbidities including but not limited to cardiac arrest, respiratory failure, ventricular and supraventricular arrhythmia, acute kidney injury, and cerebral infarction.
To conduct our statistical analyses, we utilized STATA® (StataCorp, College Station, TX) version 17. The Healthcare Cost and Utilization Project (HCUP) offers year-based discharge weights, which were utilized to quantify weighted nationwide estimates. We used Fisher's exact test or Chi-square test to compare categorical variable proportions and an independent sample t-test to compare means of continuous data. We calculated unadjusted odds ratio (OR) using univariate regression analysis for every outcome. With significance of each univariate screen set to P value < 0.2, we selected variables to conduct multivariable logistic regression analysis adjusting for possible confounders. Other essential variables, based on literature review, were also included in the model. We used logistic regression analysis for binary or categorical outcomes and linear regression analysis for continuous outcomes. Two-tailed P values and a threshold of 0.05 were used to determine statistical significance.
Our manuscript is exempt from IRB approval, as NIS is a de-identified national administrative database and readily available online at https://www.hcup-us.ahrq.gov. Based on that and according to HCUP guidelines, our study did not require Institutional Review Board of Cook County Health’s approval.
According to the 2020 NIS database, out of 1058815 COVID-19 pneumonia hospitalizations, 3210 (0.4%) patients had newly diagnosed HDAVB without a pacemaker, while 17365 (1.6%) had newly diagnosed right or left BBB without a pacemaker (Figures 1 and 2). The mean age of COVID-19 patients with and without HDAVB were 72 and 64 years, while COVID-19 patients with and without BBB had a mean age of 72.5 and 64 years, respectively (P < 0.001 for both).
Compared to COVID-19 patients without HDAVB or BBB, COVID-19 patients with newly diagnosed HDAVB or BBB were more likely to be white (58% vs 52%, P = 0.0359; 62% vs 52%, P = 0.0359), be male (66.5% vs 52.7%, P < 0.001; 60.6% vs 52.7%, P < 0.001), have governmental insurance (82% vs 67%, P < 0.001; 79.5% vs 67%, P < 0.001), and be treated in large bed size hospitals (50.8% vs 45.4%, P = 0.0286; 46.8% vs 45.4%, P = 0.025) respectively.
In terms of underlying comorbidities, patients with COVID-19 pneumonia with newly diagnosed HDAVB or BBB were more likely to have complicated diabetes mellitus (55% vs 30%, P < 0.001; 43% vs 30.8%, P < 0.001), chronic kidney disease (33% vs 19%, P < 0.001; 29% vs 19%, P < 0.001), congestive heart failure (34% vs 15%, P < 0.001; 32% vs 15%, P < 0.001), prior myocardial infraction (17% vs 7%, P < 0.001; 17.5% vs 7%, P < 0.001), history of cerebrovascular accidents (5% vs 4%, P = 0.0223; 6% vs 4%, P < 0.001), peripheral vascular disease (10% vs 4.5%, P < 0.001; 10% vs 4.4%, P < 0.001), and a higher number of comorbid conditions in general (Charlson index ≥ 3) compared to COVID-19 patients without HDAVB or BBB.
COVID-19 patients with HDAVB or BBB as compared to COVID-19 patients without were less likely to be female (43.5% vs 47.2%, P < 0.001; 39.4% vs 47.3%, P < 0.001), to be African-American (18% vs 19%, P = 0.0359; 14% vs 19%, P = 0.0359), to be Hispanic (17% vs 21%, P = 0.0359; 15% vs 21%, P = 0.0359), and have private insurance (17% vs 21%, P = 0.0359; 15% vs 21%, P = 0.0359), and were less likely to have hypertension (32% vs 41%, P < 0.001; 37% vs 41%, P < 0.001) (Tables 1 and 2).
| Study group | Control group | P value | |
| High degree atrioventricular block (n = 3210/0.4%) | No high degree atrioventricular block (n = 1058815/99.6%) | ||
| Age (year), mean | 72 | 64 | < 0.001 |
| Gender | < 0.001 | ||
| Female | 1075 (33.5) | 498545 (47.2) | |
| Male | (66.5) | (52.7) | |
| Ethnicity | 0.0359 | ||
| White | 1862 (58) | 550584 (52) | |
| Black | 578 (18) | 201175 (19) | |
| Hispanic | 482 (15) | 211763 (21) | |
| Other | 225 (7) | 84705 (8) | |
| Chronic kidney disease | 1084 (33) | 205000 (19) | < 0.001 |
| Hypertension | 1030 (32) | 437190 (41) | < 0.001 |
| Congestive heart failure | 1095 (34) | 162930 (15) | < 0.001 |
| Government insured | 2555 (82) | 673794 (67) | < 0.001 |
| Private insured | 480 (15) | 292190 (29) | < 0.001 |
| Self-pay | 80 (2.6) | 35875 (3.6) | 0.1828 |
| Poor socioeconomic status | 550 (17) | 168015 (16) | |
| Hospital region | 0.0144 | ||
| Northeast | 550 (17) | 186650 (17.6) | |
| Midwest | 925 (29) | 245190 (23.2) | |
| South | 1225 (38) | 441800 (42) | |
| West | 510 (16) | 181964 (17.2) | |
| Hospital bed size | 0.0286 | ||
| Small | 770 (24) | 270620 (25.6) | |
| Medium | 810 (25.2) | 304920 (29) | |
| Large | 1630 (50.8) | 480065 (45.4) | |
| Charlson index | < 0.001 | ||
| 0 | 385 (12) | 296468 (28) | |
| 1 | 642 (20) | 296468 (28) | |
| 2 | 578 (18) | 169410 (16) | |
| 3 | 1605 (50) | 296468 (28) | |
| Median household income | 0.0418 | ||
| First quartile | 931 (29) | 359997 (34) | |
| Second quartile | 995 (31) | 296468 (28) | |
| Third quartile | 738 (23) | 232939 (22) | |
| Fourth quartile | 546 (17) | 169410 (16) | |
| Myocardial infarction | 570 (17) | 77240 (7) | < 0.001 |
| Peripheral vascular disease | 320 (10) | 47355 (4.5) | < 0.001 |
| Cerebrovascular disease | 185 (5) | 42075 (4) | 0.0223 |
| Dementia | 570 (17) | 116035 (11) | < 0.001 |
| Chronic obstructive lung disease | 875 (27) | 247225 (23.4) | 0.0244 |
| Rheumatoid arthritis | 65 (2) | 30120 (3) | 0.2037 |
| Peptic ulcer disease | 20 (0.6) | 4625 (0.4) | 0.4797 |
| Mild liver disease | 95 (3) | 36275 (3.4) | 0.5039 |
| Diabetes mellitus | 680 (21) | 262850 (25) | 0.0311 |
| Diabetic complications | 1790 (55) | 327870 (30) | < 0.001 |
| Hemiplegia or paraplegia | 50 (1.5) | 16690 (1.5) | 0.9732 |
| Renal disease | 2220 (70) | 421060 (40) | < 0.001 |
| Cancer | 260 (8) | 57440 (5.4) | 0.0371 |
| Moderate/severe liver disease | 45 (1.4) | 15855 (1.5) | 0.9051 |
| Metastatic cancer | 210 (6.5) | 49170 (4.6) | 0.3633 |
| AIDS | 60 (1.9) | 13980 (1.3) | 0.6272 |
| Study group | Control group | P value | |
| All bundle branch blocks without pacemaker (n = 17365/1.6%) | No bundle branch blocks (n = 1044660/98.4%) | ||
| Age (year), mean | 72.5 | 64 | < 0.001 |
| Gender | < 0.001 | ||
| Female | 6845 (39.4) | 492775 (47.3) | |
| Male | (60.6) | (52.7) | |
| Ethnicity | 0.0359 | ||
| White | 10766 (62) | 543223 (52) | |
| Black | 2431 (14) | 198485 (19) | |
| Hispanic | 2952 (17) | 219378 (21) | |
| Other | 1216 (7) | 83573 (8) | |
| Chronic kidney disease | 5005 (29) | 201080 (19) | < 0.001 |
| Hypertension | 6390 (37) | 431830 (41) | < 0.001 |
| Congestive heart failure | 5470 (32) | 158555 (15) | < 0.001 |
| Government insured | 13360 (79.5) | 662990 (67) | < 0.001 |
| Private insured | 3065 (18.2) | 289605 (29.3) | < 0.001 |
| Self-pay | 385 (2.3) | 35570 (3.6) | < 0.001 |
| Poor socioeconomic status | 3290 | 165275 | |
| Hospital region | < 0.001 | ||
| Northeast | 3885 (22.4) | 183315 (17.6) | |
| Midwest | 4725 (27.2) | 241390 (23.2) | |
| South | 6160 (35.4) | 436866 (42) | |
| West | 2595 (15) | 179880 (17.2) | |
| Hospital bed size | 0.0025 | ||
| Small | 3900 (22.4) | 267490 (25.6) | |
| Medium | 5345 (30.8) | 300385 (29) | |
| Large | 8120 (46.8) | 473575 (45.4) | |
| Charlson index | < 0.001 | ||
| 0 | 2952 (17) | 292505 (28) | |
| 1 | 3646 (21) | 292505 (28) | |
| 2 | 3299 (19) | 147145 (16) | |
| 3 | 7466 (43) | 292505 (28) | |
| Median household income | < 0.001 | ||
| First quartile | 5088 (29.3) | 355184 (34) | |
| Second quartile | 4705 (27.1) | 292505 (28) | |
| Third quartile | 4202 (24.4) | 229825 (22) | |
| Fourth quartile | 3334 (19.2) | 147145 (16) | |
| Myocardial infarction | 3035 (17.5) | 74775 (7) | < 0.001 |
| Peripheral vascular disease | 1530 (9) | 46145 (4.4) | < 0.001 |
| Cerebrovascular disease | 1045 (6) | 41215 (4) | < 0.001 |
| Dementia | 2835 (16) | 113770 (11) | < 0.001 |
| Chronic obstructive lung disease | 4300 (25) | 243800 (23.4) | 0.0592 |
| Rheumatoid arthritis | 495 (3) | 29690 (3) | 0.9993 |
| Peptic ulcer disease | 60 (0.4) | 4585 (0.4) | 0.4011 |
| Mild liver disease | 670 (3.8) | 35700 (3.4) | 0.1675 |
| Diabetes mellitus | 4005 (23) | 259525 (25) | 0.0137 |
| Diabetic complications | 7420 (43) | 322240 (30.8) | < 0.001 |
| Hemiplegia or paraplegia | 320 (1.8) | 16420 (1.6) | 0.3728 |
| Renal disease | 10370 (60) | 412910 (39.5) | < 0.001 |
| Cancer | 970 (5.6) | 56730 (5.4) | 0.7950 |
| Moderate/Severe liver disease | 240 (1.4) | 15660 (1.5) | 0.7335 |
| Metastatic cancer | 840 (4.8) | 48540 (4.6) | 0.8478 |
| AIDS | 210 (1.2) | 13830 (1.3) | 0.8074 |
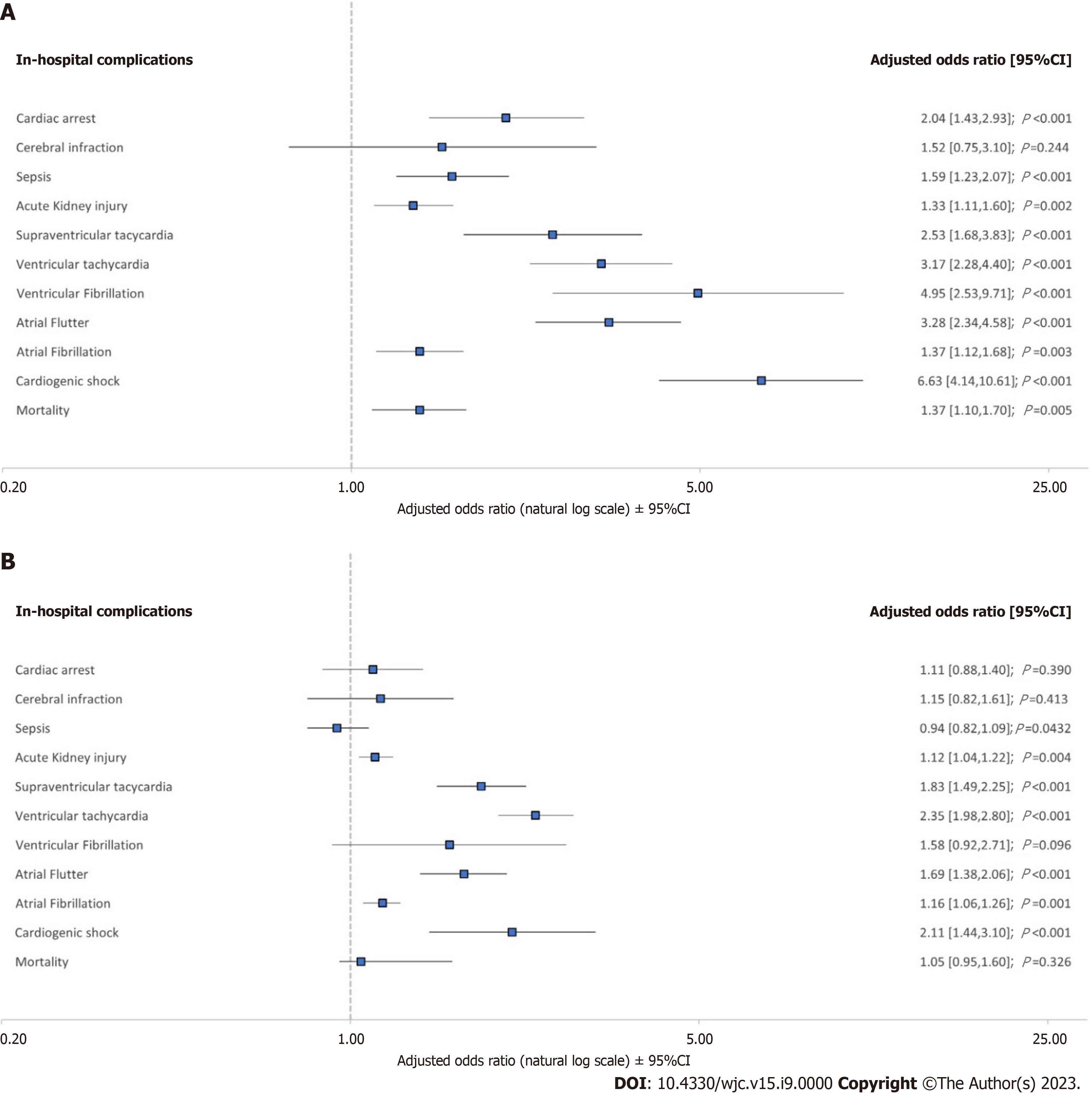
On multivariant regression analysis of encounters with COVID-19 and newly diagnosed HDAVB without pacemakers as demonstrated in Figure 2A, they were found to have a longer mean LOS of 2.28 d [95% confidence interval (CI): 1.49-3.07, P < 0.001], higher mean THC of $33801 (95%CI: 17314 -50261, P < 0.001), higher adjusted odds (aOR) of mortality (aOR: 1.36, 95%CI: 1.1-1.69, P = 0.005), cardiac arrest (aOR: 2.04, 95%CI: 1.42-2.92, P < 0.001), atrial fibrillation (aOR: 1.36, 95%CI: 1.12-1.68, P = 0.003), atrial flutter (aOR: 3.27, 95%CI: 2.33-4.58, P < 0.001), ventricular fibrillation (aOR: 4.95, 95%CI: 2.52-9.71, P < 0.001), supraventricular tachycardia (aOR: 2.53, 95%CI: 1.68-3.82, P < 0.001), ventricular tachycardia (aOR: 3.16, 95%CI: 2.27-4.4, P < 0.001), acute kidney injury (aOR: 1.33, 95%CI: 1.11-1.6, P = 0.002), sepsis (aOR: 1.59, 95%CI: 1.22-2.06, P < 0.001), mechanical ventilation (aOR: 1.41, 95%CI: 1.08-1.84, P = 0.011), and cardiogenic shock (aOR: 6.62, 95%CI: 4.14-10.6, P < 0.001), compared to those without HDAVB.
Meanwhile, patients with COVID-19 and BBB without pacemakers as demonstrated in Figure 2B had an increased odds of atrial fibrillation (aOR: 1.15, 95%CI: 1.05-1.26, P = 0.001), atrial flutter (aOR: 1.68, 95%CI: 1.37-2.06, P < 0.001), supraventricular tachycardia (aOR: 1.83, 95%CI: 1.48-2.5, P < 0.001), ventricular tachycardia (aOR: 2.35, 95%CI: 1.97-2.8, P < 0.001), acute kidney injury (aOR: 1.12, 95%CI: 1.03-1.21, P = 0.004), sepsis (aOR: 1.59, 95%CI: 1.22-2.06, P < 0.001), mechanical ventilation (aOR: 1.22, 95%CI: 1.08-1.39, P = 0.001), and cardiogenic shock (aOR: 2.11, 95%CI: 1.44-3.08, P < 0.001), than those without BBB without pacemaker but with a similar odds of mortality (aOR: 1.05, 95%CI: 0.95-1.16, P = 0.326).
On univariate regression analysis of encounters with COVID-19 and HDAVB as demonstrated in Table 3, they were found to have a longer mean LOS of 3.07 d (95%CI: 2.32-3.84, P < 0.001), higher mean THC of $40692 (95%CI: 24708-56674, P < 0.001), increased odds of mortality (aOR: 2.18, 95%CI: 1.8-2.64, P < 0.001), cardiac arrest (aOR: 2.78, 95%CI: 2-3.86, P < 0.001), atrial fibrillation (aOR: 2.46, 95%CI: 2.1-2.9, P < 0.001), atrial flutter (aOR: 5.45, 95%CI: 4-7.42, P < 0.001), ventricular fibrillation (aOR: 6.33, 95%CI: 3.27-12.27, P < 0.001), supraventricular tachycardia (aOR: 3.39, 95%CI: 2.31-4.96, P < 0.001), ventricular tachycardia (aOR: 4.75, 95%CI: 3.05-6.46, P < 0.001), acute kidney injury (aOR: 1.73, 95%CI: 1.47-2.03, P < 0.001), sepsis (aOR: 1.85, 95%CI: 1.44-2.39, P < 0.001), mechanical ventilation (aOR: 1.72, 95%CI: 1.33-2.22, P < 0.001), and cardiogenic shock (aOR: 8.75, 95%CI: 5.61-13.63, P < 0.001), as compared to those without HDAVB.
| High degree atrioventricular block without pacemakers | Adjusted OR | 95%CI | P value |
| Mortality | 2.18 | 1.80-2.64 | < 0.001 |
| Cardiac arrest | 2.78 | 2.00-3.86 | < 0.001 |
| Atrial fibrillation | 2.46 | 2.10-2.90 | < 0.001 |
| Atrial flutter | 5.45 | 4.00-7.42 | < 0.001 |
| Ventricular fibrillation | 6.33 | 3.27-12.27 | < 0.001 |
| Supraventricular tachycardia | 3.39 | 2.31-4.96 | < 0.001 |
| Ventricular tachycardia | 4.75 | 3.05-6.46 | < 0.001 |
| Acute kidney injury | 1.73 | 1.47-2.03 | < 0.001 |
| Sepsis | 1.85 | 1.44-2.39 | < 0.001 |
| Mechanical ventilation | 1.72 | 1.33-2.22 | < 0.001 |
| Cardiogenic shock | 8.75 | 5.61-13.63 | < 0.001 |
Encounters with COVID-19 and with BBB without pacemaker as demonstrated in Table 4 had an increased odds of mortality (aOR: 1.63, 95%CI: 1.42-1.89, p < 0.001), atrial fibrillation (aOR: 2.12, 95%CI: 1.88-2.40, P < 0.001), atrial flutter (aOR: 2.32, 95%CI: 1.7-3.17, P < 0.001), supraventricular tachycardia (aOR: 2.22, 95%CI: 1.64-2.99, P < 0.001), ventricular tachycardia (aOR: 4.09, 95%CI: 3.24-5.14, P < 0.001), acute kidney injury (aOR: 1.71, 95%CI: 1.53-1.9, P < 0.001), sepsis (aOR: 1.81, 95%CI: 1.52-2.26, P < 0.001), mechanical ventilation (aOR: 1.67, 95%CI: 1.38-2.23, P < 0.001), and cardiogenic shock (aOR: 3.29, 95%CI: 2.04-5.31, P < 0.001), than those without BBB without pacemaker.
| Right or left bundle branch blocks without pacemaker | Adjusted OR | 95%CI | P value |
| Mortality | 1.63 | 1.42-1.89 | < 0.001 |
| Cardiac arrest | 1.43 | 1.04-1.97 | 0.026 |
| Atrial fibrillation | 2.12 | 1.88-2.40 | < 0.001 |
| Atrial flutter | 2.32 | 1.70-3.17 | < 0.001 |
| Ventricular fibrillation | 2.31 | 1.11-4.82 | 0.024 |
| Supraventricular tachycardia | 2.22 | 1.64-2.99 | < 0.001 |
| Ventricular tachycardia | 4.09 | 3.24-5.14 | < 0.001 |
| Acute kidney injury | 1.71 | 1.53-1.90 | < 0.001 |
| Sepsis | 1.81 | 1.52-2.26 | < 0.001 |
| Mechanical ventilation | 1.67 | 1.38-2.23 | < 0.001 |
| Cardiogenic shock | 3.29 | 2.04-5.31 | < 0.001 |
Absolute values of outcomes such as mortality amongst other outcomes of interest comparing both groups (HDAVB and BBB) can be found in Table 5.
| In-hospital outcomes Rates | High degree atrioventricular block (n = 3210)
| All bundle branch blocks without pacemaker (n = 17365) |
| Mortality | 665 (21.3) | 2952 (17.0) |
| Cardiac arrest | 175 (5.6) | 504 (2.9) |
| Atrial fibrillation | 172 (5.5) | 799 (4.6) |
| Atrial flutter | 209 (6.7) | 538 (3.1) |
| Ventricular fibrillation | 44 (1.4) | 87 (0.5) |
| Supraventricular tachycardia | 150 (4.8) | 555 (3.2) |
| Ventricular Tachycardia | 222 (7.1) | 799 (4.6) |
| Sepsis | 365 (11.6) | 1233 (7.1) |
| Mechanical ventilation | 324 (10.4) | 1528 (8.8) |
| Cardiogenic shock | 103 (3.3) | 174 (1.0) |
| Pulmonary embolism | 106 (3.4) | 521 (3.0) |
| Acute kidney injury | 1298 (41.6) | 5956 (34.3) |
| Cerebrovascular accidents | 50 (1.6) | 191 (1.1) |
This analysis is the most comprehensive and updated study to date that examined newly diagnosed HDAVB and BBB in SARS-CoV-2 positive patients who have not previously received a permanent pacemaker. Several cases in the literature suggesting poor predicted outcomes in this patient population have been described.
Our analysis uncovered several significant findings in patient demographics and major clinical outcomes, summarized in Tables 1 and 2, respectively.
We found that patients with COVID-19 and newly diagnosed HDAVB had a significantly higher odds of inpatient mortality and cardiac arrest. Furthermore, COVID-19 patients with either HDAVB or BBB had a significantly higher odds of cardiac arrest, cardiogenic shock, sepsis, acute kidney injury, and requirement for ventilatory support. Additionally, they had higher total hospital charges and a longer length of stay compared to patients without HDAVB or BBB. Furthermore, we observed a higher burden of atrial flutter, atrial fibrillation, supraventricular tachycardias, and ventricular tachycardias in patients with HDAVB or BBB without pre-existing pacemakers. Patients with COVID-19 and newly diagnosed HDAVB or BBB may have higher mortality rates and adverse clinical outcomes due to a higher burden of co-morbidities, such as chronic kidney disease, diabetes mellitus, and heart failure[7,16]. These conditions may increase the risk of complications and make it difficult for patients to recover from the virus. The increased burden of arrhythmias in patients with HDAVB or BBB could be due to the effect of the virus on the heart's electrophysiology. Previous studies suggested that SARS-CoV-2 can cause myocarditis, which may lead to arrhythmias and other cardiac complications[17,18].
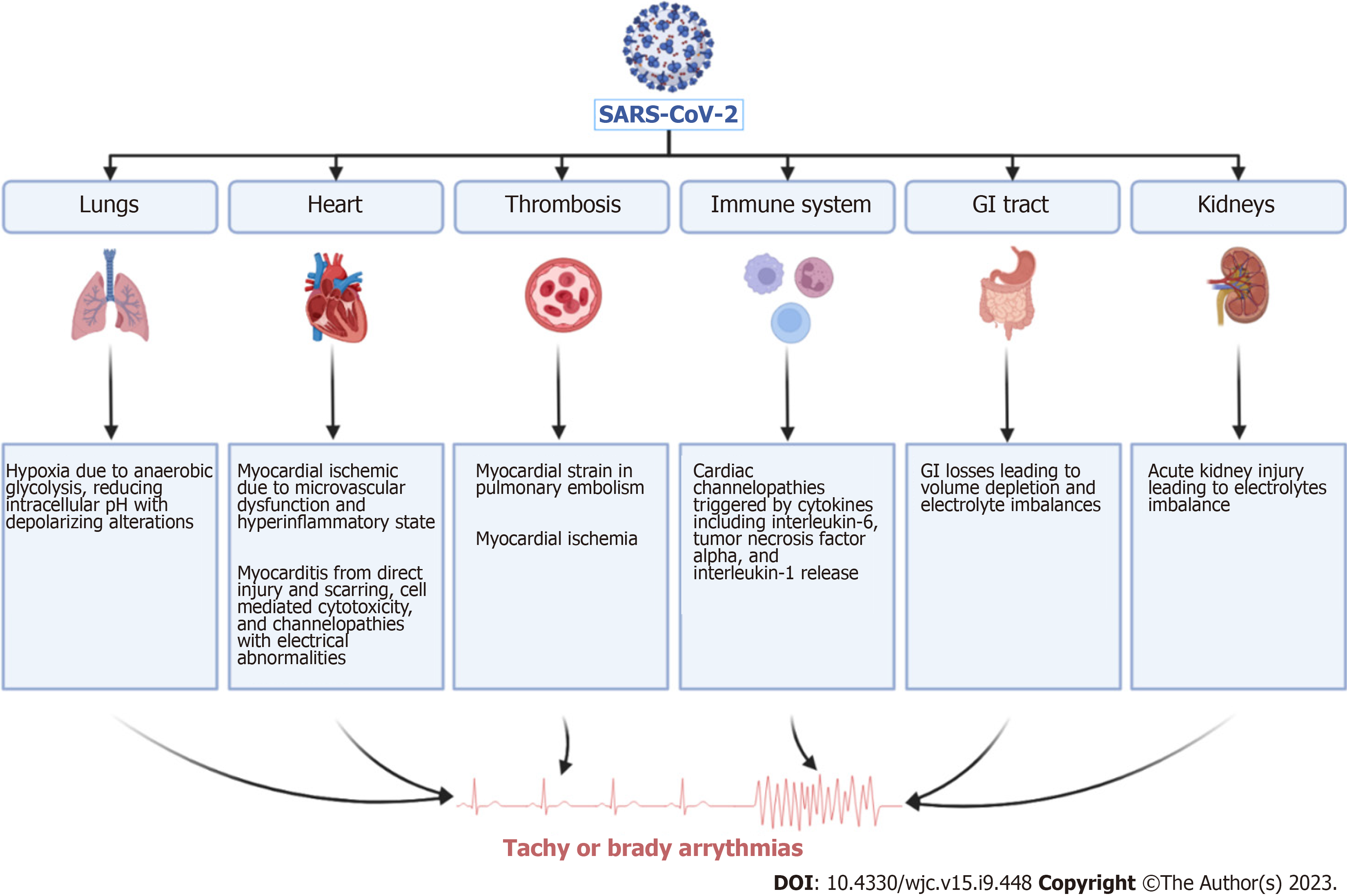
A retrospective study that included 756 COVID-19 patients demonstrated a 2-fold increase in the risk of death in the presence of atrioventricular block[7]. Multiple underlying mechanisms behind the development of atrioventricular block in COVID-19 patients have been postulated but incompletely understood. Inflammatory mediated injury to the myocardial cells and the intrinsic cardiac conduction system whether at the supra-Hisian or infra-Hisian level and severe hypoxia seem to be the main driving triggers to the development of heart blocks in COVID-19 patients[19]. Other possible underlying mechanisms mostly observed in patients with myocarditis due to COVID-19 are caused by the interruption of the electrical impulse generation or propagation throughout the cardiac conduction system[18]. Other suggested mechanisms in the literature are illustrated in Figure 3. It is also pertinent to mention that the use of hydroxychloroquine and azithromycin in year 2020 and the use of atrioventricular blocking agents such as beta blockers, which can prolong QTc interval and delay conduction at the level of the atrioventricular node, respectively, may have exaggerated the incidence of arrhythmias particularly in COVID-19 patients[20]. Therefore, to prevent the unnecessary over-utility of permanent pacemakers in these patients, it is imperative to re-evaluate this patient population for indications of permanent pacemaker placement after their recovery[21].
Our results suggest that patients with COVID-19 pneumonia who developed new-onset HDAVB or BBB without the presence of prior pacemakers and with concomitant positive SARS-CoV-2 results on admission, regardless of symptom severity, consisted of an older population with a higher comorbidity burden, as evidenced by a higher prevalence of chronic kidney disease, diabetes mellitus, and heart failure. Hypertension, however, was less commonly observed in patients who developed HDAVB. The absence of hypertension in patients who developed HDAVB could be due to the fact that hypertension is a risk factor for other types of heart disease, such as coronary artery disease and heart failure, which may have masked the association with HDAVB in this study. White race and male gender were associated with a higher risk for developing HDAVB compared to other races and female gender. This higher incidence of HDAVB in white and female patients could be related to differences in genetic susceptibility or hormonal factors. However, further research is required to confirm this hypothesis.
Previous studies have shown that older age, male gender, and diabetes mellitus were associated with a higher risk of atrioventricular block. Additionally, a population-based cohort study established a longitudinal increase in the risk of atrioventricular blocks with each 20 mg/dL increase in fasting blood glucose level[22].
We also report a higher odds of tachyarrhythmias in patients with HDAVB as compared to patients without HDAVB. Given the higher burden of co-morbidities and older age in the former, the likelihood of fibrosis in the cardiac conduction system is high, which can lead to the development of HDAVB. Given these findings, it is understandable that higher mortality and major adverse cardiovascular events were more commonly observed in patients with a higher comorbidity burden.
Moreover, patients with heart failure are more likely to be on atrioventricular nodal blocking agents precipitating heart blocks as well. HDAVB has been reported with COVID-19 though the incidence is rare and limited to case reports and series. The nature of HDAVB associated with COVID-19 is mostly regarded as transient with spontaneous recovery (incidence and percentages might add reliability) with mere observation[19,23] but has been treated with permanent pacemakers[24-26] and rarely with ablation as well[27].
Several studies have highlighted the association between COVID-19 infection and worse clinical outcomes in patients with new-onset bundle branch blocks. A meta-analysis encompassing 2539 hospitalized COVID-19 patients revealed a higher prevalence of LBBB among those experiencing unfavorable clinical outcomes[28]. Another meta-analysis comprising 1580 patients with COVID-19 infection demonstrated a mortality risk associated with LBBB, indicating its potential as a predictive marker[29]. Similarly, a meta-analysis involving 1904 COVID-19 patients revealed a significantly increased risk of short-term mortality in those with right bundle branch block (RBBB)[30]. Our own study further confirms a high risk of mortality in COVID-19 pneumonia patients with secondary diagnoses of new-onset bundle branch blocks and no prior intracardiac devices. The observed association between COVID-19 infection and new-onset BBB in our study opens up new avenues for understanding potential myocardial injury mechanisms in this context. Acute BBB may signify acute myocardial injury, possibly arising from various etiologies, including COVID-induced ischemic heart disease, inflammation or myocarditis, medication-related side effects, and electrolyte imbalances. Recognizing these potential mechanisms is crucial in guiding appropriate management strategies for these patients, especially those with significant cardiovascular comorbidities.
Despite several significant findings, our study has several limitations as well. Since its a NIS based study, the limitations of database analysis apply here as well. The diagnosis depends on the ICD-10 coding and may not be representative of the actual real-world figures given the potential for human errors. The NIS database is limited in the use of therapeutic medications that the patients are on and therefore confounders with use of atrioventricular nodal blockers cannot be mitigated. Therefore, due to the unavailability of such data, we were unable to estimate medication effect on the deve
To ensure that all the patients admitted with COVID-19 pneumonia had a secondary diagnosis of new onset HDAVB, we decided to exclude patients with pacemakers or other intracardiac devices which may suggest a prior diagnosis of HDAVB. It is worth noting that due to the possibility that some patients without prior pacemakers may have had undiagnosed asymptomatic atrioventricular heart blocks prior to the diagnosis of COVID-19, those patients cannot be identified nor can be excluded.
As SARS-CoV-2 continues to loom with potential for future resurgence, there is a dire need for long term observational studies to better recognize patients at risk of developing fatal arrythmias such as HDAVB or BBB and to validate management strategies. We conclude that elderly patients, whites, and males with underlying diabetes, chronic kidney disease, congestive heart failure, prior myocardial infarction, history of cerebrovascular accidents, or peripheral artery disease admitted for COVID-19 pneumonia who develop new onset HDAVB are at least six times more likely to develop cardiogenic shock and three times more likely to develop ventricular tachycardia or ventricular fibrillation and have a significantly increased risk of inpatient mortality. Meanwhile, patients who developed new onset BBB had an at least two times increased risk of developing cardiogenic shock and ventricular tachycardia compared to patients who did not develop BBB, albeit without significant increase in inpatient mortality.
Therefore, it is crucial for healthcare providers to recognize the potential for worse outcomes in patients with COVID-19 who develop new HDAVB or right or Left BBB, given their significant co-morbidities and predicted worse outcomes. By identifying the disease process early in its course, through monitoring techniques and temporary pacemakers, it may be possible to initiate treatment early and prevent the development of adverse outcomes in high-risk patients.
Finally, the management of COVID-19 has evolved over time, including the use of different medications with varying cardiac effects. This evolution may impact the incidence and severity of bradyarrhythmias. Therefore, our dataset from 2020 may not be fully representative of the current situation.
Coronavirus disease 2019 (COVID-19) tremendously impacted patients worldwide. While most research has focused on the virus's effects on the respiratory system, we set to understand the impact that the virus has on the cardiac conductive system. Research has strongly suggested that COVID-19 has a predilection to cardiac tissue. Fewer studies, however, have looked into the effect of the virus on the cardiovascular conductive system. With the availability of pacemakers, cardiac monitoring techniques, and the increasing burden of cardiac arrhythmias triggered by COVID-19, there is a dire need for new studies to establish this association on a larger patient population.
By identifying the gaps in the literature, and with the emergence of case reports and series reported about this topic, we acknowledged the need for large scale studies to draw statistically significant conclusions which could give rise to new interventions that could possibly mitigate life threatening cardiovascular outcomes in high risk patients.
The aim of our study was to analyze the impact that COVID-19 had on the odds of major cardiovascular complications in patients with newly diagnosed heart blocks and bundle branch blocks on a large patient sample. Our analysis was successful in measuring the cardiovascular impact caused by this virus with significance of our results supported by our large sample of patients and patient selection process. We included only patients with new onset high degree atrioventricular blocks or bundle branch blocks, presumed to be triggered by COVID-19 or its treatment. Regardless of causality, which was not the aim of our study, we demonstrated the large burden of serious and life-threatening complications inflicting our patient population. Therefore, our results may suggest that high-risk patients may benefit from early use of temporary pacemakers to mitigate the negative impact coronavirus has on patients with newly diagnosed heart blocks and bundle branch blocks.
Using the 2020 National Inpatient Sample database, we selected our patient population of interest, which included all patients hospitalized for COVID-19 pneumonia as the primary diagnosis, utilizing ICD-10 codes. To conduct our statistical analysis, we utilized STATA® (StataCorp, College Station, TX, United States) version 17. We further stratified our sample into patients who had a secondary diagnosis of either high degree atrioventricular blocks or bundle branch blocks. We excluded patients with prior pacemakers using ICD-10 procedure codes. Inpatient mortality was our primary outcome of interest while secondary outcomes included significant cardiac and noncardiac outcomes. Finally, multivariant and univariate regression analyses were conducted on both patient groups.
Our analysis demonstrated that patients with coronavirus 2019 pneumonia and newly diagnosed high degree atrioventricular blocks had a significantly increased odds of inpatient mortality, cardiac arrest, life-threatening tachyarrhythmias, need for mechanical ventilation, and cardiogenic shock. Patients with COVID-19 pneumonia and newly diagnosed bundle branch blocks experienced no significant increase in mortality on multivariate regression analysis however had similar other outcomes.
Our study identified high risk groups or patients prone to poor clinical outcomes. Elderly white males with common medical co-morbidities such as diabetes, chronic kidney disease, and peripheral artery disease who were hospitalized for COVID-19 pneumonia and developed high degree atrioventricular block or bundle branch blocks experienced worse clinical outcomes, and thus may benefit from temporary pacemaker placement or long term cardiac monitoring techniques. However, additional research is required to establish clear benefit from the utility of temporary pacemakers or continuous cardiac monitoring techniques on the outcomes experienced in this patient population. Although we identified the increased odds of possibly fatal complications experienced by this patient population, we were unable to measure the contribution of medications used during our patients' hospitalizations due to the limitations of the National Inpatient Sample database. Furthermore, we have no available data to discern the outcomes of these patients following their discharge. This information would be crucial in determining the predicted course of disease in these patients.
Future research with different methodology should focus on comparing outcomes of patients admitted with COVID-19 pneumonia with newly diagnosed heart blocks secondary to the virus and undergo temporary pacemaker placement to controls who do not. This will help draw conclusions and establish guidelines that can standardize the approach and utility of temporary pacemakers in high risk patients.
| 1. | Lazzerini PE, Boutjdir M, Capecchi PL. COVID-19, Arrhythmic Risk, and Inflammation: Mind the Gap! Circulation. 2020;142:7-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 189] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 2. | Shah PB, Welt FGP, Mahmud E, Phillips A, Kleiman NS, Young MN, Sherwood M, Batchelor W, Wang DD, Davidson L, Wyman J, Kadavath S, Szerlip M, Hermiller J, Fullerton D, Anwaruddin S; American College of Cardiology and the Society for Cardiovascular Angiography and Interventions. Triage Considerations for Patients Referred for Structural Heart Disease Intervention During the COVID-19 Pandemic: An ACC/SCAI Position Statement. JACC Cardiovasc Interv. 2020;13:1484-1488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, Brown TS, Der Nigoghossian C, Zidar DA, Haythe J, Brodie D, Beckman JA, Kirtane AJ, Stone GW, Krumholz HM, Parikh SA. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J Am Coll Cardiol. 2020;75:2352-2371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1381] [Cited by in RCA: 1374] [Article Influence: 229.0] [Reference Citation Analysis (0)] |
| 4. | Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7635] [Cited by in RCA: 6191] [Article Influence: 1031.8] [Reference Citation Analysis (0)] |
| 5. | Dou Q, Wei X, Zhou K, Yang S, Jia P. Cardiovascular Manifestations and Mechanisms in Patients with COVID-19. Trends Endocrinol Metab. 2020;31:893-904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Hua A, O'Gallagher K, Sado D, Byrne J. Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur Heart J. 2020;41:2130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 7. | McCullough SA, Goyal P, Krishnan U, Choi JJ, Safford MM, Okin PM. Electrocardiographic Findings in Coronavirus Disease-19: Insights on Mortality and Underlying Myocardial Processes. J Card Fail. 2020;26:626-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 8. | Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38:1504-1507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 668] [Cited by in RCA: 654] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 9. | Creel-Bulos C, Hockstein M, Amin N, Melhem S, Truong A, Sharifpour M. Acute Cor Pulmonale in Critically Ill Patients with Covid-19. N Engl J Med. 2020;382:e70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 10. | Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:819-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1111] [Cited by in RCA: 1273] [Article Influence: 212.2] [Reference Citation Analysis (0)] |
| 11. | Singh S, Desai R, Gandhi Z, Fong HK, Doreswamy S, Desai V, Chockalingam A, Mehta PK, Sachdeva R, Kumar G. Takotsubo Syndrome in Patients with COVID-19: a Systematic Review of Published Cases. SN Compr Clin Med. 2020;2:2102-2108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Malaty M, Kayes T, Amarasekera AT, Kodsi M, MacIntyre CR, Tan TC. Incidence and treatment of arrhythmias secondary to coronavirus infection in humans: A systematic review. Eur J Clin Invest. 2021;51:e13428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Garcia-Zamora S, Lee S, Haseeb S, Bazoukis G, Tse G, Alvarez-Garcia J, Gul EE, Çinier G, Alexander B, Martins Pinto-Filho M, Liu T, Baranchuk A. Arrhythmias and electrocardiographic findings in Coronavirus disease 2019: A systematic review and meta-analysis. Pacing Clin Electrophysiol. 2021;44:1062-1074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Wang Y, Wang Z, Tse G, Zhang L, Wan EY, Guo Y, Lip GYH, Li G, Lu Z, Liu T. Cardiac arrhythmias in patients with COVID-19. J Arrhythm. 2020;36:827-836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, Goldschlager NF, Hamilton RM, Joglar JA, Kim RJ, Lee R, Marine JE, McLeod CJ, Oken KR, Patton KK, Pellegrini CN, Selzman KA, Thompson A, Varosy PD. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;140:e382-e482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 193] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 16. | CDC. [Accessed 2023]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. |
| 17. | Kimball E, Buchwalder K, Upchurch C, Kea B. Intermittent complete heart block with ventricular standstill after Pfizer COVID-19 booster vaccination: A case report. J Am Coll Emerg Physicians Open. 2022;3:e12723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Manolis AS, Manolis AA, Manolis TA, Apostolopoulos EJ, Papatheou D, Melita H. COVID-19 infection and cardiac arrhythmias. Trends Cardiovasc Med. 2020;30:451-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 19. | Dagher L, Wanna B, Mikdadi G, Young M, Sohns C, Marrouche NF. High-degree atrioventricular block in COVID-19 hospitalized patients. Europace. 2021;23:451-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | O'Connell TF, Bradley CJ, Abbas AE, Williamson BD, Rusia A, Tawney AM, Gaines R, Schott J, Dmitrienko A, Haines DE. Hydroxychloroquine/Azithromycin Therapy and QT Prolongation in Hospitalized Patients With COVID-19. JACC Clin Electrophysiol. 2021;7:16-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Dherange P, Lang J, Qian P, Oberfeld B, Sauer WH, Koplan B, Tedrow U. Arrhythmias and COVID-19: A Review. JACC Clin Electrophysiol. 2020;6:1193-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 22. | Viskin D, Halkin A, Sherez J, Megidish R, Fourey D, Keren G, Topilsky Y. Heart Failure Due to High-Degree Atrioventricular Block: How Frequent Is It and What Is the Cause? Can J Cardiol. 2021;37:1562-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 23. | Dehghani Firouzabadi M, Goudarzi S, Dehghani Firouzabadi F, Moosaie F. Complete heart block and itchy rash in a patient with COVID-19. Caspian J Intern Med. 2020;11:569-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 24. | Ashok V, Loke WI. Case report: high-grade atrioventricular block in suspected COVID-19 myocarditis. Eur Heart J Case Rep. 2020;4:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Aryanti RR, Hermanto DY, Yuniadi Y. Dynamic changes of atrioventricular conduction during Covid-19 infection: Does inflammation matter? Int J Arrhythmia. 2022;23:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 26. | Maisuradze N, Rehaw O, Maglakelidze D, Budzikowski AS, Jallad A. High-Degree Atrioventricular Block in a Patient With Asymptomatic COVID-19 Infection: A Case Report. Cureus. 2022;14:e24397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 27. | LaPage MJ, Bradley DJ, Dechert BE. Successful treatment of acquired heart block with ablation. HeartRhythm Case Rep. 2022;8:745-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 28. | Alsagaff MY, Oktaviono YH, Dharmadjati BB, Lefi A, Al-Farabi MJ, Gandi P, Marsudi BA, Azmi Y. Electrocardiography on admission is associated with poor outcomes in coronavirus disease 2019 (COVID-19) patients: A systematic review and meta-analysis. J Arrhythm. 2021;37:877-885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Zuin M, Rigatelli G, Roncon L, Zuliani G. Left Bundle Branch Block and Mortality in COVID-19 Patients. Am J Cardiol. 2021;153:149-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Zuin M, Rigatelli G, Roncon L, Zuliani G, Mortality risk in COVID-19 patients with right bundle branch block. Mortality risk in COVID-19 patients with right bundle branch block. Rev Esp Cardiol (Engl Ed). 2021;74:1122-1124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Quesada A, Spain; Sutton R, United Kingdom; Yarmahmoodi F, Iran S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Xu ZH