Published online Jul 26, 2023. doi: 10.4330/wjc.v15.i7.354
Peer-review started: May 18, 2023
First decision: June 1, 2023
Revised: June 20, 2023
Accepted: July 3, 2023
Article in press: July 3, 2023
Published online: July 26, 2023
Processing time: 68 Days and 4.4 Hours
Time-restricted eating (TRE) is a dietary approach that limits eating to a set number of hours per day. Human studies on the effects of TRE intervention on cardiometabolic health have been contradictory. Heterogeneity in subjects and TRE interventions have led to inconsistency in results. Furthermore, the impact of the duration of eating/fasting in the TRE approach has yet to be fully explored.
To analyze the existing literature on the effects of TRE with different eating durations on anthropometrics and cardiometabolic health markers in adults with excessive weight and obesity-related metabolic diseases.
We reviewed a series of prominent scientific databases, including Medline, Scopus, Web of Science, Academic Search Complete, and Cochrane Library arti
Fifteen studies were included in our systematic review. TRE significantly reduces body weight, waist circumference, fat mass, lean body mass, blood glucose, insulin, and triglyceride. However, no significant changes were observed in HbA1c, HOMA-IR, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, heart rate, systolic and diastolic blood pressure. Furthermore, subgroup analyses based on the duration of the eating window revealed significant variation in the effects of TRE intervention depending on the length of the eating window.
TRE is a promising chrononutrition-based dietary approach for improving anthropometric and cardiometabolic health. However, further clinical trials are needed to determine the optimal eating duration in TRE intervention for cardiovascular disease prevention.
Core Tip: Beneficial effects of time-restricted eating (TRE) on adults with excessive weight and obesity-related metabolic diseases remain under investigation, and results are conflicting. We explored the effectiveness of TRE on anthropometric and cardiometabolic health in adults with excessive weight and obesity-related metabolic diseases. We found that TRE is an effective and sustainable dietary strategy for reducing body weight, body composition, blood glucose, insulin, and triglyceride in individuals with excessive weight or weight-related metabolic disorders. Moreover, the meta-analysis demonstrates the varying effects of fasting duration on the outcomes of interest.
- Citation: Kamarul Zaman M, Teng NIMF, Kasim SS, Juliana N, Alshawsh MA. Effects of time-restricted eating with different eating duration on anthropometrics and cardiometabolic health: A systematic review and meta-analysis. World J Cardiol 2023; 15(7): 354-374
- URL: https://www.wjgnet.com/1949-8462/full/v15/i7/354.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i7.354
The global prevalence of overweight and obesity has become a major public health problem. High body mass was responsible for over five million deaths and 160 million disability-adjusted life years worldwide[1]. According to the World Health Organization reports, the number of overweight and obese individuals has doubled globally since 1980, affecting both developed and developing countries. As a result, weight-related diseases, including obesity and related conditions such as type 2 diabetes, cardiovascular disease (CVD), and certain cancers, have become a major public health challenge worldwide and a burden on healthcare systems[2,3]. Researchers and healthcare professionals have explored multiple dietary strategies to improve weight and cardiometabolic health and prevent cardiovascular disease through caloric and macronutrient restriction, specific foods or nutrients, adherence to selected dietary patterns, and fasting. Continuous energy restriction (CER) has been frequently used to manage the body weight of individuals with excessive weight[4]. However, adherence to this diet pattern is challenging due to the daunting task of reducing daily caloric intake[5]. Additionally, CER may promote adaptive responses such as decreased physical activity, increased hunger, and deactivation of the hypothalamic-pituitary-thyroid axis, hindering weight and fat loss[6]. Moreover, CER increases the risk of adverse effects such as hypoglycemia, nutrient deficiencies, and extreme fatigue.
In recent years, intermittent fasting (IF) has emerged as an alternative weight loss and CVD prevention strategy. It refers to a cyclic eating pattern that rotates between periods of abstinence from consuming any caloric-containing food or drinks and periods of eating[7]. Current human research indicates that chrononutrition-based dietary interventions, such as time-restricted eating (TRE), have gained substantial interest from the public as one of the sustainable strategies for CVD prevention[8]. TRE is a lifestyle approach that limits eating duration to a set number of hours per day (typically within 4–12 h) during waking hours, allowing for adequate fasting[9,10]. TRE aims to align dietary intake with daily circadian rhythms. It is considered a more sustainable approach than caloric restriction, as it involves lower-intensity adaptation for long-term lifestyle modifications to reduce weight[8]. Animal studies have linked TRE to lower body weight, total cholesterol (TC), triglycerides, glucose, insulin, interleukin-6, tumor necrosis factor, and improved insulin sensitivity[11-13].
The effects of TRE with different eating durations, ranging from four to 12 h, have been studied on humans[14]. These studies have reported varying results, with some showing improvements in weight loss, insulin sensitivity, and cardiovascular markers, while others exhibiting no significant changes. Several recent systematic reviews have been conducted to review the effects of TRE interventions on anthropometric and cardiometabolic health[15-23]. The most consistent findings from these reviews were significant weight reduction with TRE intervention, with mixed results for the TRE effects on cardiometabolic health. This inconsistency is mainly due to heterogeneity in subjects and the implemented TRE interventions.
Additionally, combining results from individuals with normal body mass index (BMI) and those with metabolic dysregulation may obscure differences in the effectiveness of the intervention. To our knowledge, no systematic review has evaluated the effects of variation in TRE’s eating duration on anthropometric and cardiometabolic health. Therefore, this systematic review and meta-analysis aimed to examine the effects of varying eating durations in TRE interventions on body weight and composition, waist circumference, biomarkers of glucose metabolism, lipid metabolism, inflammatory marker, blood pressure, and heart rate in adults with excessive weight and obesity-related metabolic diseases. The findings of this review will shed light on the overall effectiveness of TRE and its optimal eating duration as a potential dietary approach for weight loss and improved cardiometabolic health in individuals with excessive weight and obesity-related metabolic diseases.
This systematic review was reported according to the updated version of the Preferred Reporting Items for Systematic Reviews and Meta-analyses Statement[24]. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (http://www.crd.york.ac.uk/PROSPERO), with a record number of CRD42022341232. Before conducting the review, PROSPERO and the Cochrane Library were searched to identify existing or ongoing similar work by other researchers.
Multiple electronic databases were queried using selected search terms until May 2022. MEDLINE Complete, Web of Science, Scopus, the Cochrane Library, Academic Search Complete, Food Science Source, OpenDissertations, Education Research Complete, and Psychology and Behavioural Sciences Collection were used for the systematic search (Supplementary Table 1). The search strategy was tailored to each database’s keywords to identify literature for the intervention of interest, TRE. The search terms included “time-restricted diet” OR “time-restricted eating” OR “time-restricted feeding” OR “time-restricted fasting” OR “time-restricted meal”, which have been identified in previous systematic reviews[19,21,22]. The search was not limited to specific years of publication or languages, and no additional terms were used to avoid filtering out relevant literature. All search outputs were exported to reference manager software (Endnote 20; endnote.com). After removing duplicates, two independent reviewers screened the articles for eligibility based on title, abstract, and full text (Zaman MK and Teng NIMF). A consensus was reached for included articles through discussions after each screening round, and a third reviewer resolved any disagreements (Juliana N).
The eligibility criteria for this systematic review were based on the predetermined inclusion and exclusion criteria. We included studies with the following characteristics: (1) Population: Adults with excess weight and obesity-related metabolic diseases; (2) Intervention: TRE, which involves daily (seven days per week) eating window restriction; (3) Comparator: The comparator accepted for this study were dietary intake with ad libitum eating window or eating window of 12 h or more; (4) Outcomes: Changes in body weight, waist circumference, fat mass, lean body mass, fasting glucose, insulin, HbA1c, homeostasis model assessment for insulin resistance (HOMA-IR), lipid profile [total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides], C-reactive protein, blood pressure (systolic and diastolic), and heart rate were identified as outcomes of interest—studies with any reported outcomes of interest were included; and (5) Study design: Only controlled/clinical trials with at least one outcome measurement performed within two weeks to 6 mo of intervention commencement were included in this review.
We excluded studies based on the following characteristics: (1) Population: Studies involving subjects younger than 18 years of age, animal models, or studies including adult subjects with normal BMI were excluded; (2) Intervention: Studies with intermittent TRE (e.g., ad libitum dietary intake during selected days) were excluded; and (3) Study design: Abstracts, and non-original articles such as expert opinions and reviews were excluded from this systematic review.
Risk of bias (RoB) assessments of the included studies were conducted and graded using version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB-2)[25]. This tool evaluates the RoB across five key domains: Randomization process, deviation from intended interventions, missing outcome data, outcome measurement, and selection of reported results. Trials were classified as having either low risk, unclear risk, or high RoB for each domain and overall. Two reviewers were involved independently in the RoB assessments of the included studies (Zaman MK and Teng NIMF). Disagreements were resolved through consensus or discussion with a third reviewer (Kasim SS).
Data collection forms were used to extract data from each study. The extraction form was piloted in at least one study included in this review. Two reviewers were involved in the data extraction from included studies (Zaman MK and Teng NIMF). Data extracted include: (1) Information about the trial: Authors, publication year, study design, sample size, and study duration; (2) Study participants: Population characteristics, location, age, gender, and BMI; (3) Intervention characteristics: Description of intervention and control arm, eating window, and timing of intervention; and (4) Outcome measures: Body weight, waist circumference, fat mass, lean body mass, fasting glucose, insulin, HbA1c, HOMA-IR, lipid profile (TC, HDL-C, LDL-C, and triglycerides), C-reactive protein, blood pressure (systolic and diastolic), and heart rate.
Standardized mean differences (SMDs), or mean differences (MDs) with 95%CI, were used to report intervention effects for each study based on pre-and post-TRE intervention. Meta-analyses were conducted, if feasible, using a minimum of two included studies with similar outcomes for each outcome of interest. Forest plots were constructed for all studies included in the meta-analysis. A two-sided P value of < 0.05 was considered statistically significant. The heterogeneity was evaluated statistically using the I² statistic, with a value greater than 50% indicating substantial heterogeneity. The random-effects model was employed when substantial heterogeneity was present. Publication bias was examined using a funnel plot visualization for outcomes with ten or more studies. Considering the heterogeneity protocol and duration of the eating window in TRE intervention, subgroup analyses were performed by applying a different range of TRE duration. All analyses were conducted using RevMan software, version 5.4.
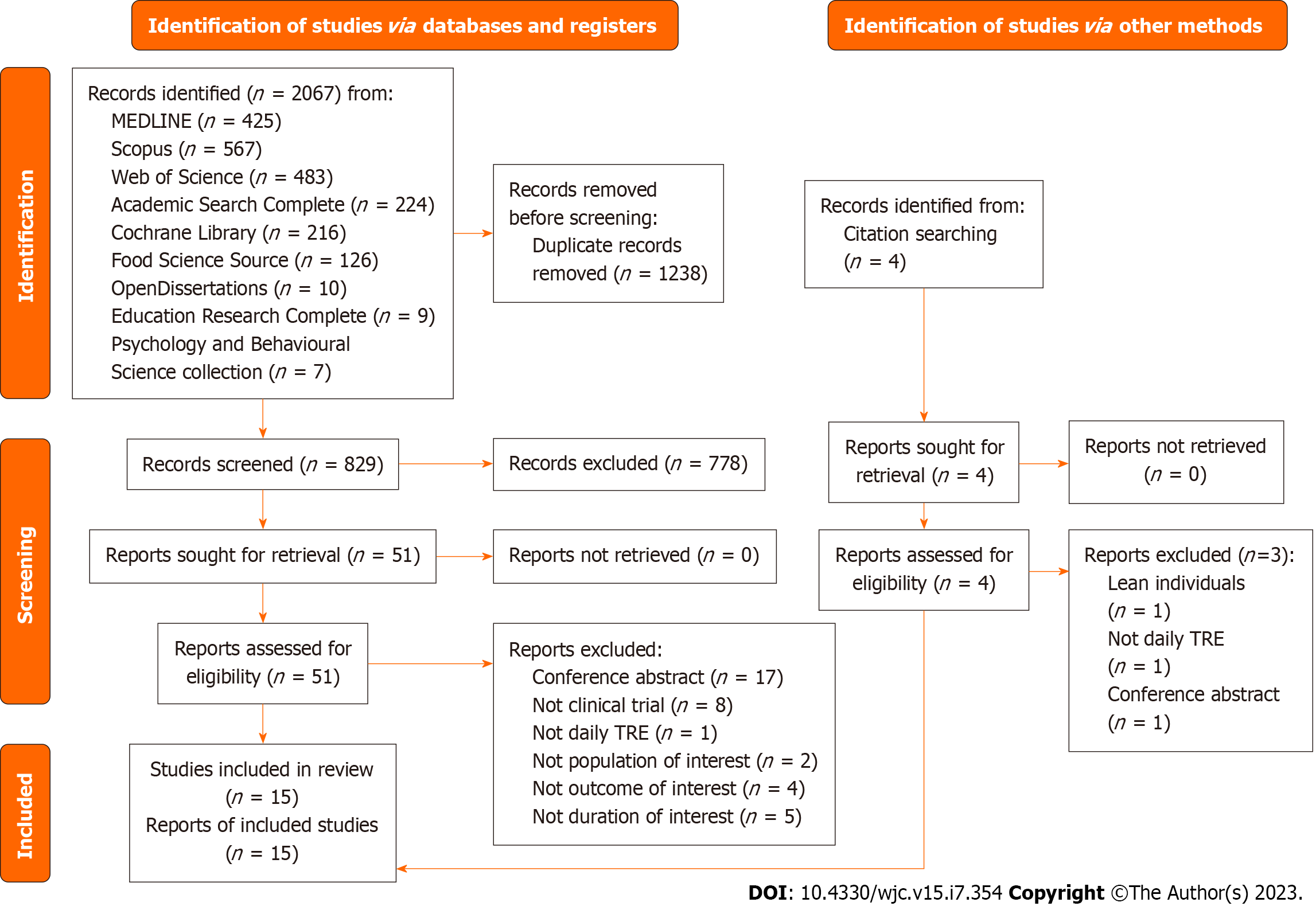
The systematic search process identified 2067 articles (Figure 1) from multiple resources, including Medline (n = 425), Scopus (n = 567), Web of Science (n = 483), Academic Search Complete (n = 224), Cochrane Library (n = 216), Food Science Source (n = 126), OpenDissertations (n = 10), Education Research Complete (n = 9), and Psychology and Behavioural Science collection (n = 7). After removing duplicates, 829 records were screened, and 51 were assessed for eligibility after excluding articles not meeting the inclusion criteria. Further screening and quality assessment resulted in 15 studies being selected for the systematic review and meta-analysis, involving 927 subjects[26-40]. The largest study recruited 139 subjects, while the smallest enrolled eight subjects[32,39]. Most studies were conducted in the United States of America (n = 7), followed by Brazil (n = 3), China (n = 3), Switzerland (n = 1), and Germany (n = 1).
Table 1 displays the characteristics of the participants in the included studies. The participants were adults with overweight/obesity, prediabetes, or Type 2 Diabetes Mellitus. The participants ranged from 27 to 74 years old[26,30], with a BMI above the normal cut-off, ranging from 26.4 to 38.9 kg/m2. The majority of the included studies were parallel arms randomized controlled trials (RCT) (n = 13), randomized crossover trial (RXT) (n = 1), and non-RCT (n = 1). All studies involved at least one intervention group with a TRE regimen and a comparator group with an unrestricted time of food intake. The interventions were conducted over three weeks to twelve months, with the fasting period lasting between 12 and 20 h per day and the eating period lasting from four to 12 h daily. The timing of the start of the fasting period was either self-selected by participants or predetermined by the study (Table 1). Most TRE interventions in the included studies restricted food intake during the day, with the last meal completed by 20:00[27,29-34,37-39].
| Ref. | Study design | Sample size | Study duration | Characteristics | Location | Sex | Baseline BMI (kg/m2) | Age (yr) | Fasting: Eating period (hours) | Timing of intervention |
| Chair et al[26], 2022 | Three arm RCT | 101 | 3 wk | Prediabetes | Weight management clinic, Hunan Provincial People's Hospital, Changsha, China | M: 3, F: 64 | 35.23 ± 6.19 | 74.30 ± 8.39 | 16:08 | Free to arrange the 8-h eating window based on personal preferences |
| Che et al[27], 2021 | RCT | 120 | 14 wk | Overweight adults with type 2 diabetes | Diabetes Clinic, Zhu Xianyi Hospital of Tianjin Medical University, China | M: 65, F: 55 | TRE: 26.42 ± 1.96; Comparator: 26.08 ± 2.14 | TRE: 48.21 ± 9.32; Comparator: 48.78 ± 9.56 | 14:10 | 08:00 to 18:00 and fasted from 18:00 to 08:00 daily |
| Chow et al[28], 2020 | RCT | 20 | 12 wk | Adults with BMI ≥ 25 kg/m2 | Minnesota, United States | M: 3, F: 17 | TRE: 33.8 ± 7.6; Comparator: 34.4 ± 7.8 | TRE: 46.5 ± 9.32; Comparator: 44.2 ± 12.3 | 16:08 | Self-select |
| Cienfuegos et al[29], 2020 | RCT (3-arms) | 58 | 10 wk | Adults with BMI of 30-49.9 kg/m2 | Chicago, United States | F: 53 M:5 | 4-h TRE: 37 ± 1; 6-h TRE: 37.0 ± 1.0; Comparator: 36.0 ± 1.0 | 4-h: 47 ± 2; 6-h: 47 ± 3; Comparator: 45 ± 2 | 4-h: 20:04; 6-h: 18:06 | 4-h TRE: 15:00-19:00; 6-h TRE: 13:00-19:00 |
| Isenmann et al[30], 2021 | RCT | 35 | 16 wk | Adults with BMI ≥ 25, physically active | Gymnasium, (Windhagen, Germany) | F: 21 M: 21 | TRE: 26.3 ± 3.0; Comparator: 25.7 ± 3.3 | TRE: 27.9 ± 5.3; Comparator: 27.4 ± 5.8 | 16:08 | 12:00-20:00 |
| Kotarsky et al[31], 2021 | RCT | 21 | 8 wk | Adults with BMI 25.0 and 34.9 kg/m2 | North Dakota State university, United States | F: 18 M: 3 | 29.6 ± 2.6 kg/m2 | 44 ± 7 | 16:08 | 12:00-20:00 |
| Liu et al[32], 2022 | RCT | 139 | 6/12 mo | Adults with BMI of 28-45 kg/m2 | Guangzhou, China | F: 68 M: 71 | TRE: 31.8 ± 2.9; Comparator: 31.3 ± 2.6 | TRE: 31.6 ± 9.3; Comparator: 32.2 ± 8.8 | 16:08 | 08:00-16:00 |
| Lowe et al[33], 2020 | RCT | 116 | 12 wk | Adults with BMI 27-43 kg/m2 | United States, primarily San Francisco | F: 70 M: 46 | 32.7 ± 4.2 | 46.5 ± 10.5 | 16:08 | TRE, 8 h, 12:00-20:00 |
| Peeke et al[34], 2021 | RCT (Virtual) | 60 | 8 wk | Adults with BMI ≥ 30 kg/m2 | United States | F:69 M: 9 | 38.9 ± 7.7 | 44.0 ± 11.0 | 12:12 (14:10, with fasting snack 12 h post fasting) | Fasting began after dinner (between 17:00-20:00) |
| Phillips et al[35], 2021 | RCT | 54 | 6 mo | Adults with at least one component of metabolic syndrome | Switzerland | Not reported | TRE: 28.0 ± 4.1; Comparator: 27.0 ± 4.0 | 43.4 ± 13.3 | 12:12 | - |
| de Oliveira Maranhão Pureza et al[36], 2021 | RCT | 58 | 21/81 d | Adults with BMI 30-45 kg/m2 | Outpatient clinic of the Centro de Recuperação e Educação Nutritional, Brazil | F: 58 | TRE: 31.80 (CI: 29.25-34.36); Comparator: 31.03 (CI, 28.20-33.87) | TRE: 33.53 (CI: 32.00-35.50); Comparator: 33.12 (CI: 31.68-34.56) | 12:12 | Self-select |
| Ribeiro et al[37], 2021 | RCT | 24 | 8 wk | Physically active adults with BMI 25 kg/m2 | Brazil | F: 20 M: 4 | TRE :30.5 ± 3.5; Comparator: 31.7 ± 5,6 | TRE: 32.4 ± 5.5; Comparator: 33.0 ± 8.7 | 16:08 | 12:00-20:00 |
| Schroder et al[38], 2021 | NRCT | 40 | 3 mo | Women with BMI ≥ 30 kg/m2 | Brazil | F: 40 | TRE: 32.53 ± 1.13; Comparator: 4.55 ± 1.20 | TRE: 36.6 ± 1.6. Comparator: 42.3 ± 3.5 | 16:08 | 12:00-20:00 |
| Sutton et al[39], 2018 | RXT | 8 | 5 wk | Pre-diabetic men with BMI ≥ 25 kg/m2 | Greater Baton Rouge, United States | M: 12 | 32.2 ± 4.4 | 56.0 ± 9.0 | 18:06 | Self-select eating window between 06:30-08:30; For early TRE to end eating window by 15:00 |
| Thomas et al[40], 2022 | RCT | 81 | 12/39 wk | Adults with BMI 27-45 kg/m2 | Colorado, United States | F: 20 M: 4 | 34.1 ± 5.7 | 38.0 ± 7.8 | 14:10 | Start eating window 3 h post waking up |
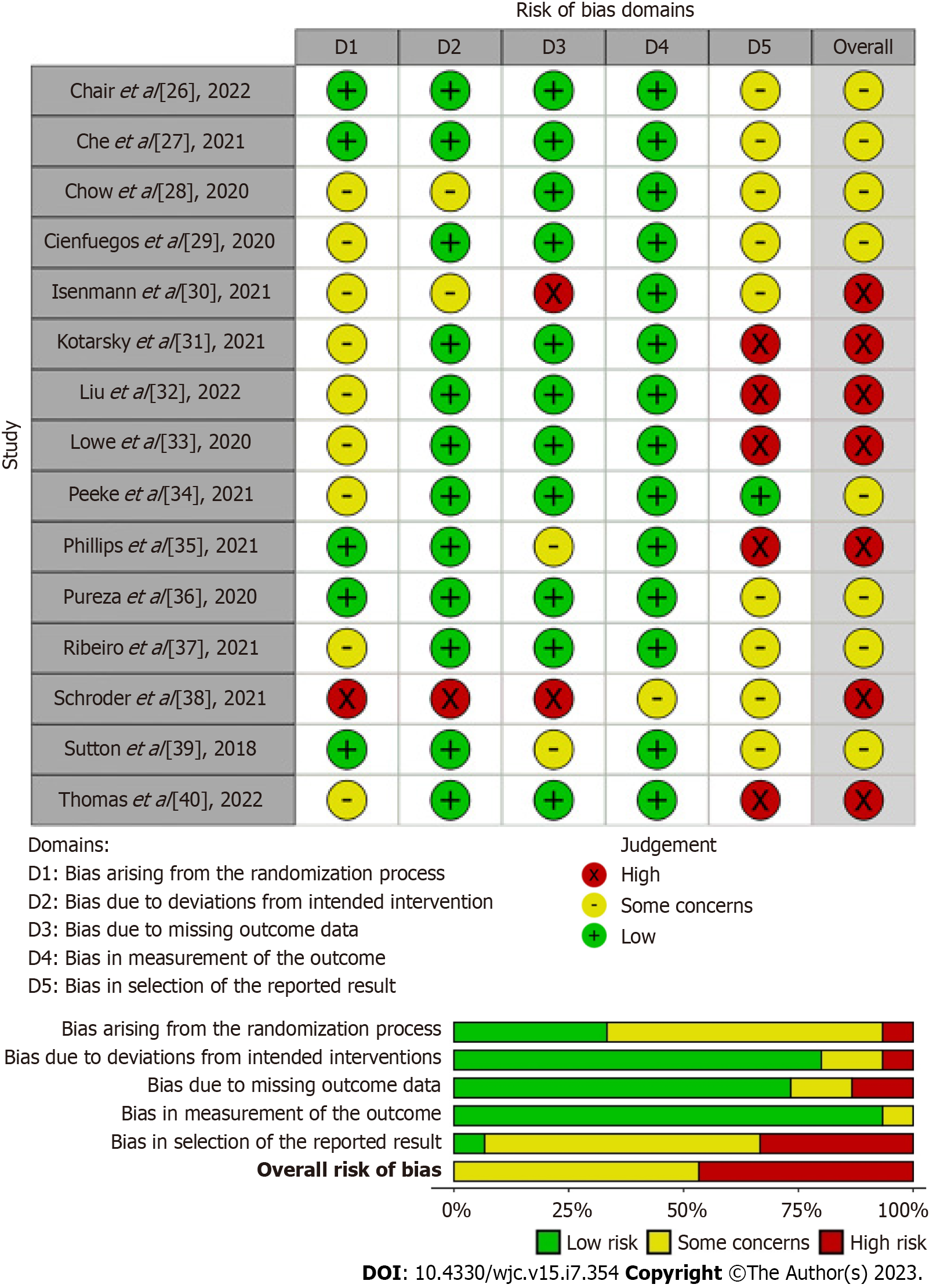
The RoB assessments for the RCTs are summarized in Figure 2. Overall, seven studies posed a high RoB[30-33,35,38,40], and eight studies posed concerns regarding the overall RoB[26-29,34,36,37,39]. In the domain of the randomization process, all included studies were identified as randomized studies except for one. Ten studies had limited or no information on randomization and concealment, leading to concerns in the domain of the randomization process[28-34,37,38,40]. Due to the nature of the intervention of interest, blinding participants may not be feasible. Information on blinding of the participants, carers, and personnel assessing in the laboratory or statistical analyses was often unknown or limited, introducing possible risk of bias due to deviations from the intended intervention. Most studies showed a low RoB due to missing outcome data[26-29,31-34,36,37,40] and outcomes measurement[26-37,39,40]. In the domain of selection of the reported result, most studies were classified as posing concern[26-30,36-39] or high risk[31-33,35,40] due to the limited availability of a prespecified analysis plan (i.e., protocol) or/and missing outcomes measurement.
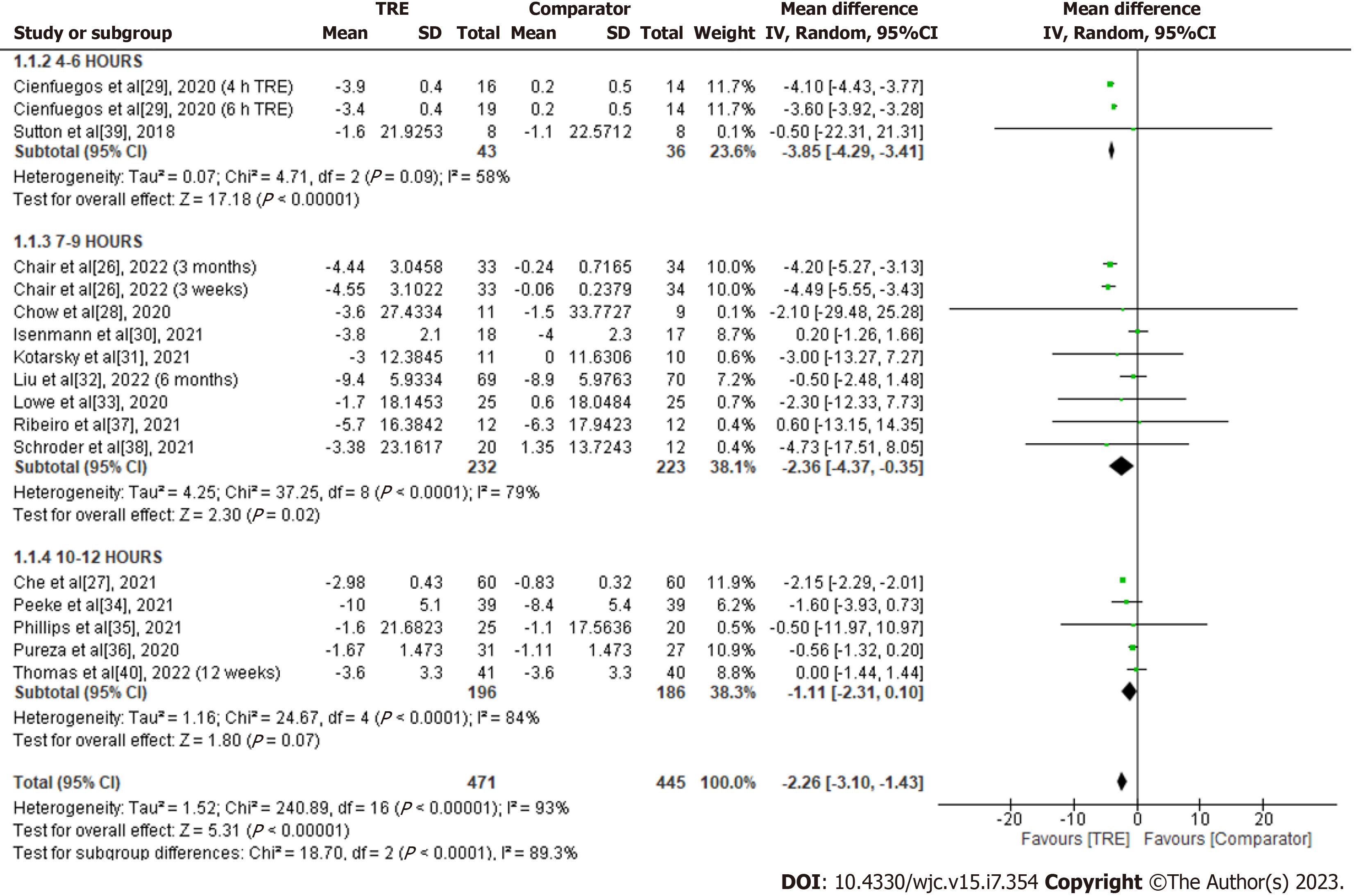
Body weight: A total of 15 studies were included in the analysis to assess the effect of TRE on body weight (kg) (Figure 3). Individuals assigned to the TRE intervention showed a significant reduction in body weight levels compared to the comparator group [MD -2.26; 95%CI: -3.10 to -1.43, P < 0.00001; I2 = 93%]. Random-effects subgroup analysis was conducted based on the duration of TRE intervention revealed no significant changes in body weight in TRE interventions ranging from ten to 12 h [MD -1.11; 95%CI: -2.31 to 0.10, P = 0.07; I2 = 84%]. Meanwhile, a significant reduction was observed in TRE interventions ranging from seven to nine hours [MD -2.36; 95%CI: -4.37 to -0.35, P = 0.02; I2 = 79%] and TRE interventions ranging from four to six hours [MD -3.85; 95%CI: -4.29 to -3.41, P < 0.00001; I2 = 58%].
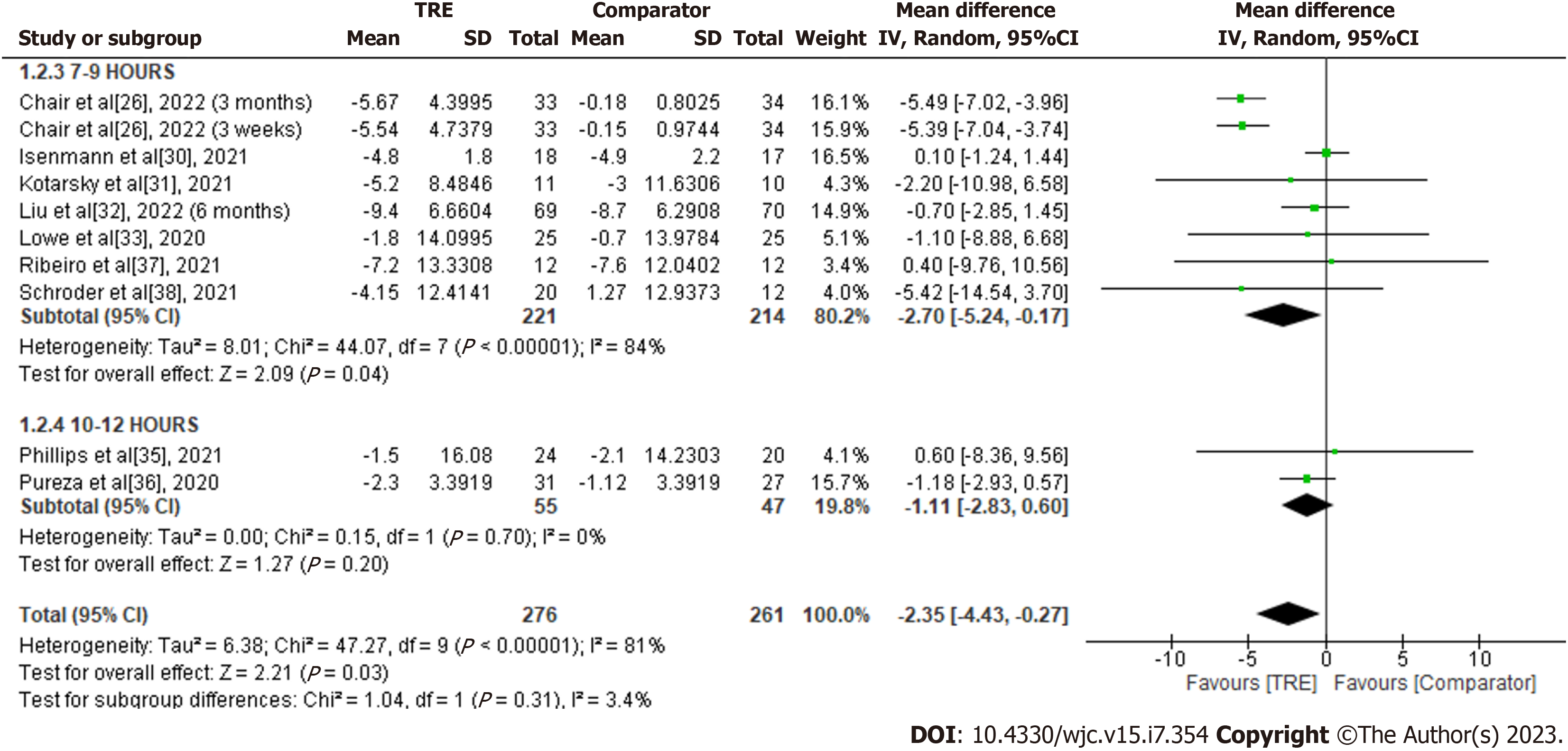
Waist circumference: A meta-analysis of nine studies demonstrated a significant overall effect of TRE on waist circumference reduction compared to 261 subjects in the comparator group [MD -2.35; 95%CI: -4.43 to -0.27, P = 0.03; I2 = 81%] (Figure 4). Random-effects subgroup analyses were conducted based on the duration of TRE intervention showed no significant changes in waist circumference following TRE interventions ranging from ten to 12 h [MD -1.11; 95%CI: -2.83 to 0.60, P = 0.20; I2 = 0%]. However, a significant reduction in waist circumference was observed in TRE interventions ranging from seven to nine hours [MD -2.70; 95%CI: -5.24 to -0.17, P = 0.04; I2 = 84%]. The effect of TRE ranging from four to six hours on the measured outcome was not reported in any of the included studies.
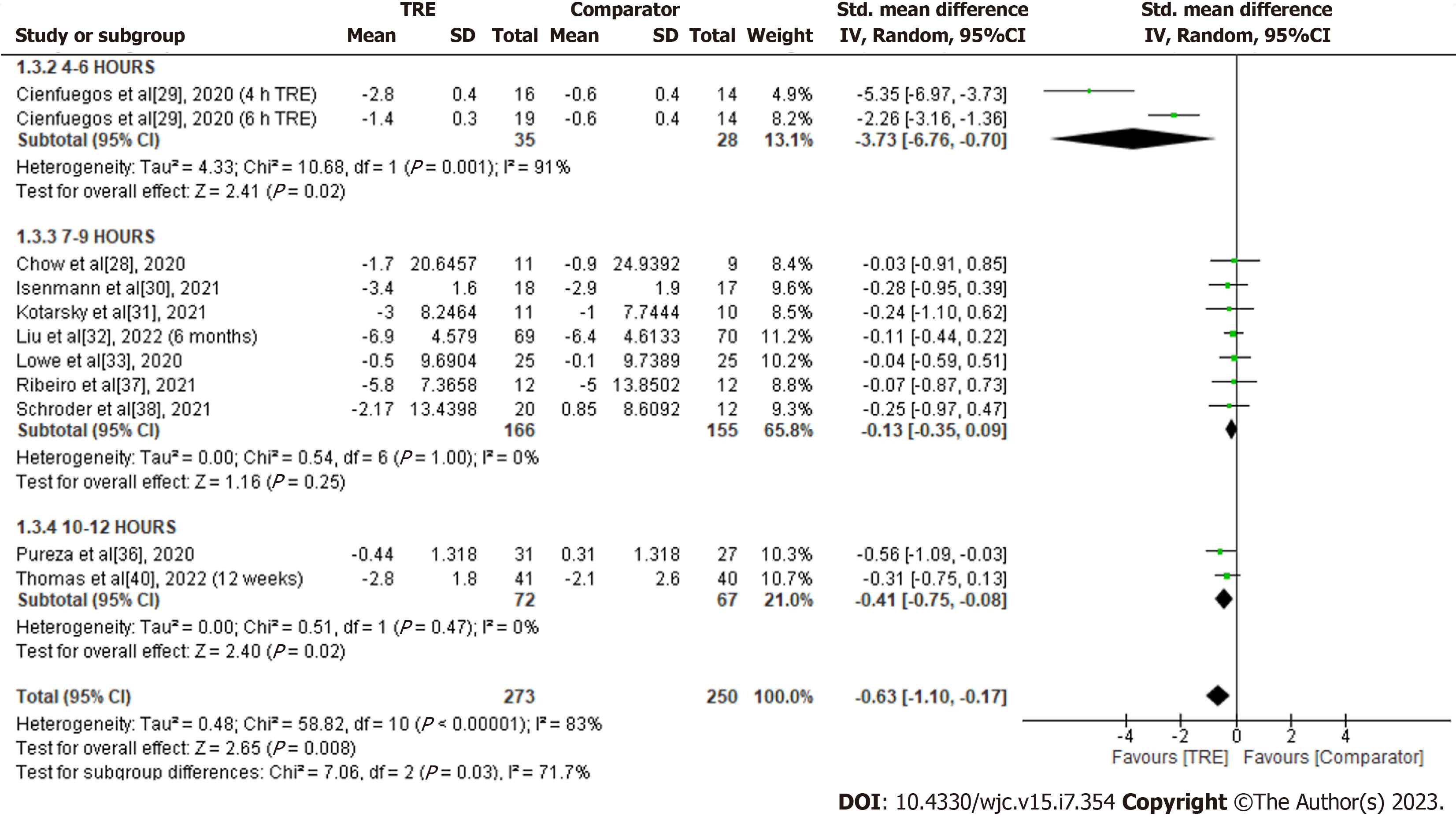
Total fat mass: A meta-analysis of ten studies evaluated the effect of TRE on total fat mass. Individuals assigned to TRE intervention showed a significant reduction in total fat mass levels compared to the comparator group [SMD -0.63; 95%CI: -1.10 to -0.17, P = 0.008; I2 = 83%] (Figure 5). Random-effects subgroup analyses were conducted based on the duration of TRE intervention, which revealed a significant reduction in total fat mass following TRE interventions ranging from ten to 12 h [SMD -0.41; 95%CI: -0.75 to -0.08, P = 0.02; I2 = 0%], and TRE interventions ranging from four to six hours [SMD -3.73; 95%CI: -6.76 to -0.70, P = 0.02; I2 = 91%]. However, no significant changes were observed in TRE interventions ranging from seven to nine hours [SMD -0.13; 95%CI: -0.35 to 0.09, P = 0.25; I2 = 0%].
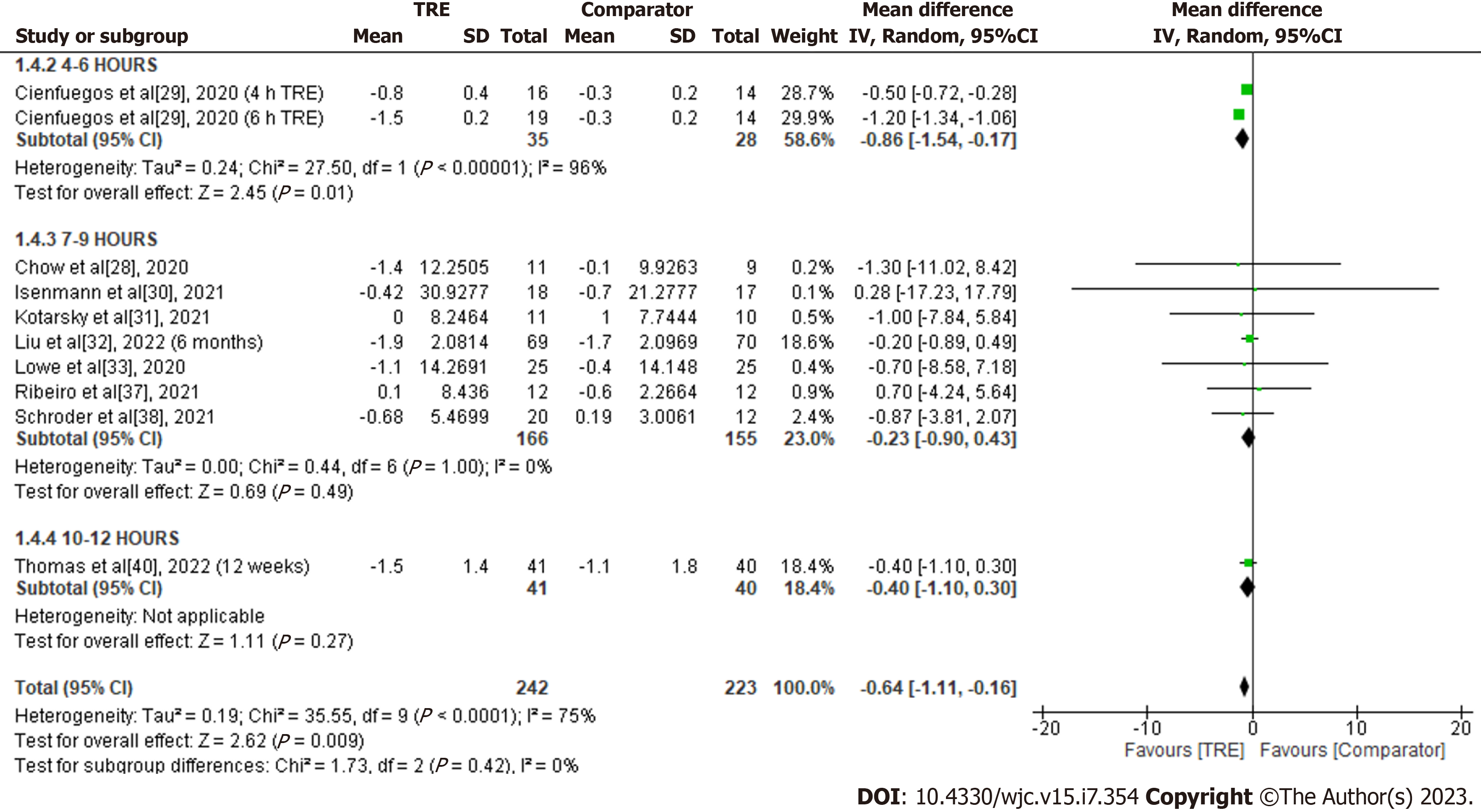
Lean body mass: A meta-analysis of nine studies was evaluated the effect of TRE on lean body mass. Individuals assigned to TRE intervention showed a significant overall reduction in lean body mass compared to the comparator group [MD -0.64; 95%CI: -1.11 to -0.16, P = 0.009; I2 = 75%] (Figure 6). Random-effects subgroup analyses were conducted based on the duration of TRE intervention demonstrated no significant changes in lean body mass following TRE interventions ranging from seven to nine hours [MD -0.23; 95%CI: -0.90 to 0.43, P = 0.49; I2 = 0%]. A significant reduction in lean body mass was observed in the TRE intervention group compared to the comparator following TRE interventions ranging from four to six hours [MD -0.86; 95%CI: -1.54 to -0.17, P = 0.01; I2 = 96%]. Subgroup analysis for TRE interventions ranging from ten to 12 h was not calculated since only one study reported this outcome.
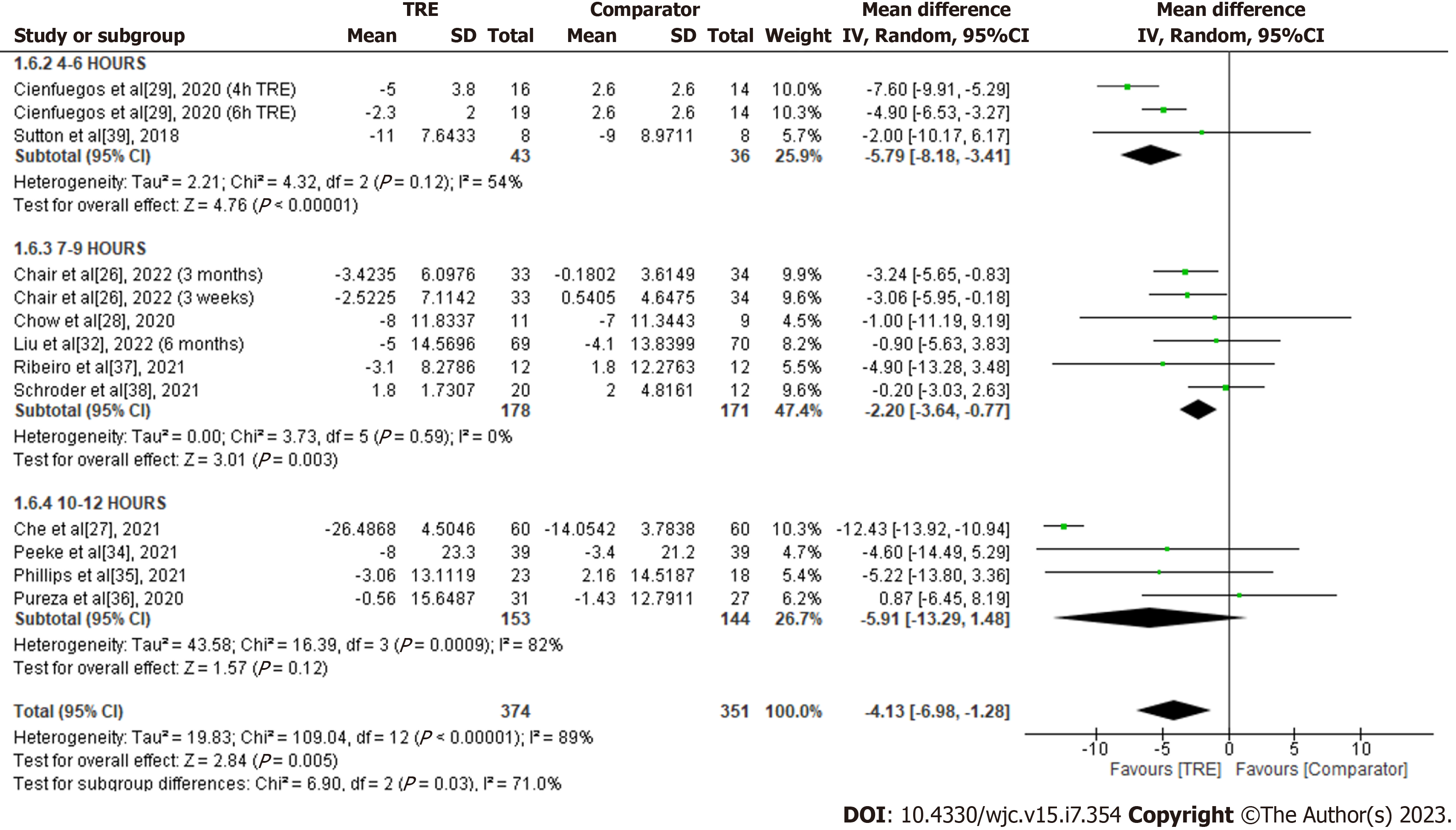
Glucose: A meta-analysis of eleven studies reported a significant overall effect of TRE on glucose levels reduction compared to the comparator group [MD -4.13; 95%CI: -6.98 to -1.28, P = 0.005; I2 = 89%] (Figure 7). Random-effects subgroup analyses were conducted based on the duration of TRE intervention showed no significant changes in glucose levels following TRE interventions ranging from ten to 12 h [MD -5.91; 95%CI: -13.29 to 1.48, P = 0.12; I2 = 82%]. However, a significant reduction was reported in TRE interventions ranging from seven to nine hours [MD -2.20; 95%CI: -3.64 to -0.77, P = 0.003; I2 = 0%] and TRE interventions ranging from four to six hours [MD -5.79; 95%CI: -8.18 to -3.41, P = 0.00001; I2 = 54%].
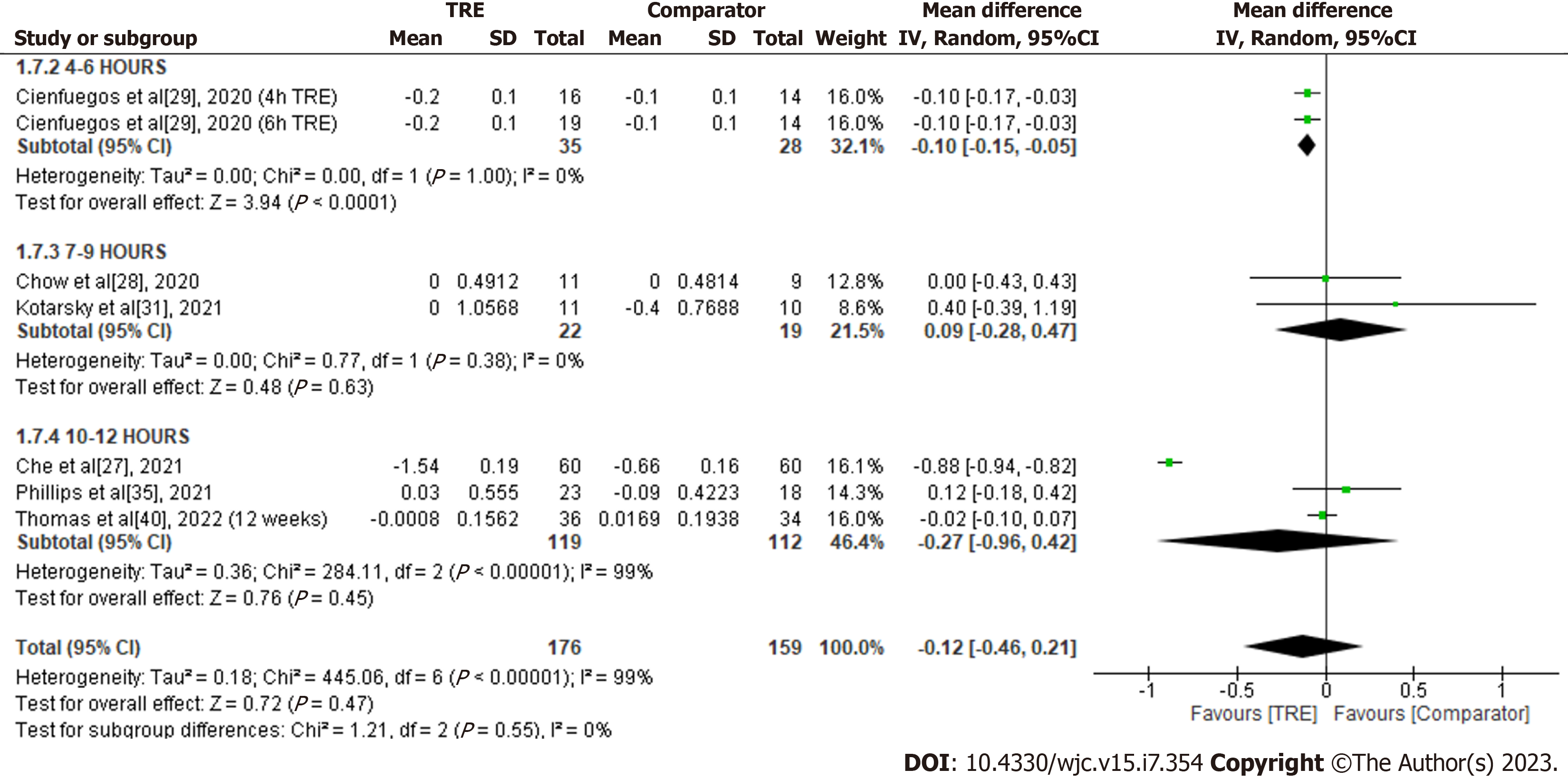
HbA1c: A meta-analysis of six studies revealed the effect of TRE on HbA1c. There was no significant difference in HbA1c levels between the test and comparator groups [MD -0.12; 95%CI: -0.46 to 0.21, P = 0.47; I2 = 99%] (Figure 8). Random-effects subgroup analyses were conducted based on the duration of TRE intervention demonstrated no significant changes in HbA1c following TRE interventions ranging from ten to 12 h [MD -0.27; 95%CI: -0.96 to 0.42, P = 0.45; I2 = 99%] and TRE interventions ranging from seven to nine hours [MD 0.09; 95%CI: -0.28 to 0.47, P = 0.63; I2 = 0%]. However, a significant reduction was reported in TRE interventions ranging from four to six hours [MD -0.10; 95%CI: -0.15 to -0.05, P < 0.0001; I2 = 0%].
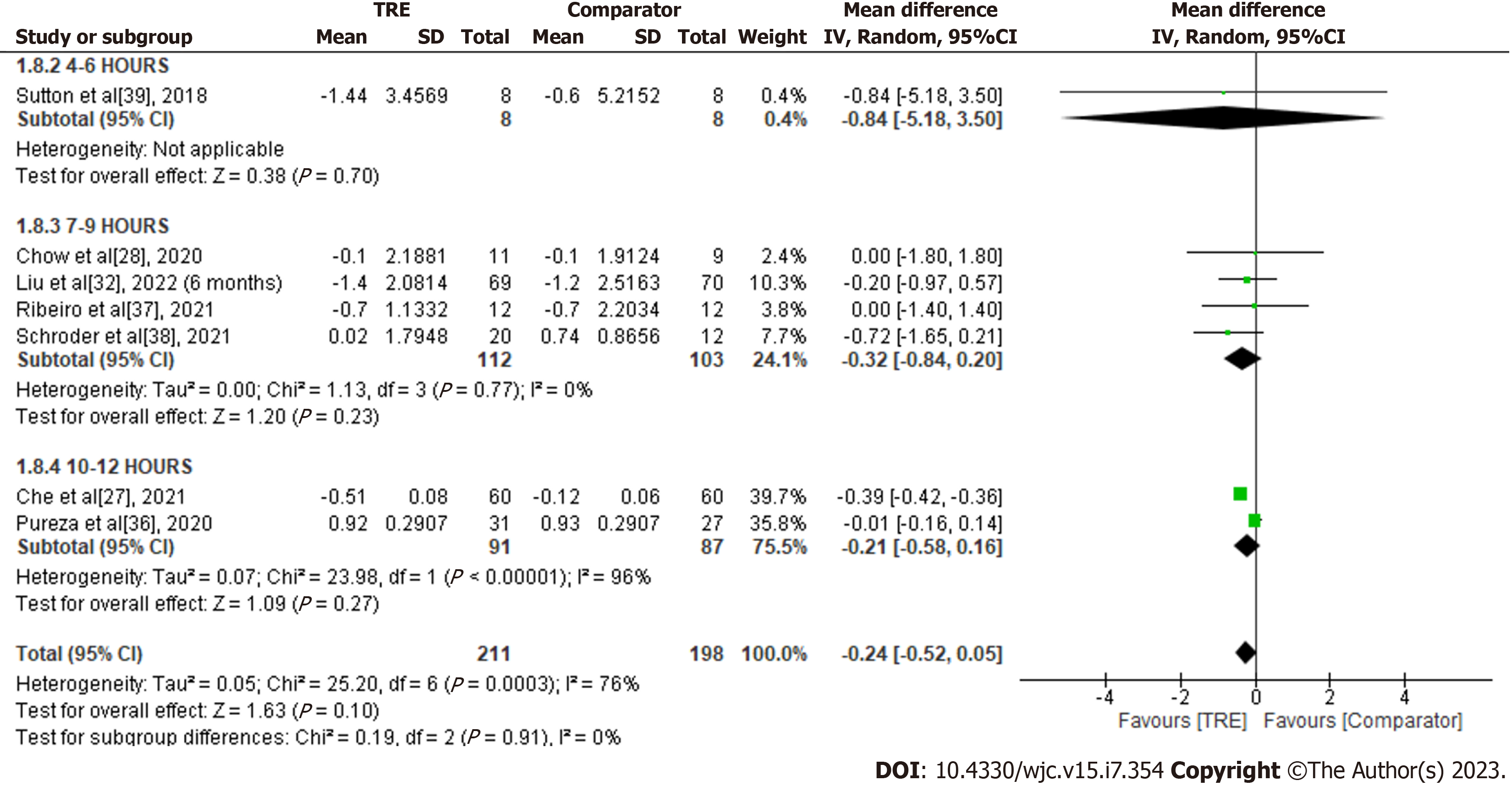
HOMA-IR: A meta-analysis of seven studies reported the effect of TRE on HOMA-IR. There was no significant difference in HOMA-IR levels compared to the comparator group [MD -0.24; 95%CI: -0.52 to 0.05, P = 0.10; I2 = 76%] (Figure 9). Random-effects subgroup analyses were conducted based on the duration of TRE intervention showed no significant changes in HOMA-IR following TRE interventions ranging from ten to 12 h [MD -0.21; 95%CI: -0.58 to 0.16, P = 0.27; I2 = 96%] and TRE interventions ranging from seven to nine hours [MD -0.32; 95%CI: -0.84 to 0.20, P = 0.23; I2 = 0%]. Subgroup analysis for TRE interventions ranging from four to six hours was not calculated since it was reported in only one study.
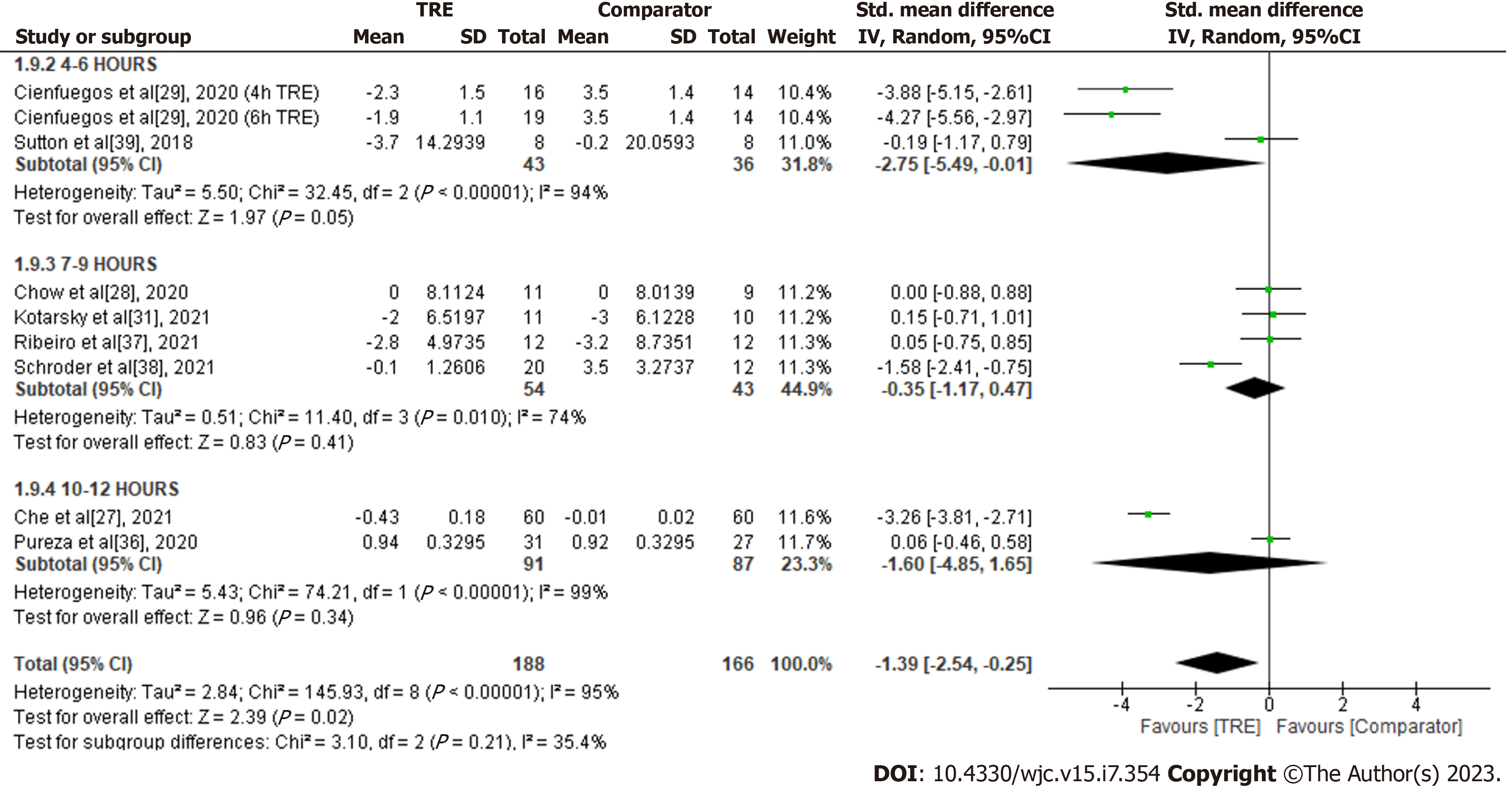
Insulin: A meta-analysis of eight studies revealed a significant overall reduction of insulin levels with TRE compared to the comparator group (SMD -1.39; 95%CI: -2.54 to -0.25, P = 0.02; I2 = 95%] (Figure 10). Random-effects subgroup analyses were conducted based on the duration of TRE intervention showed no significant changes in insulin in TRE interventions ranging from ten to 12 h (SMD -1.60; 95%CI: -4.85 to 1.65, P = 0.34; I2 = 99%] and TRE interventions ranging from seven to nine hours (SMD -0.35; 95%CI: -1.17 to 0.47, P = 0.41; I2 = 74%]. A significant reduction in insulin level was observed in TRE interventions ranging from four to six hours (SMD -2.75; 95%CI: -5.49 to -0.01, P = 0.05; I2 = 94%].
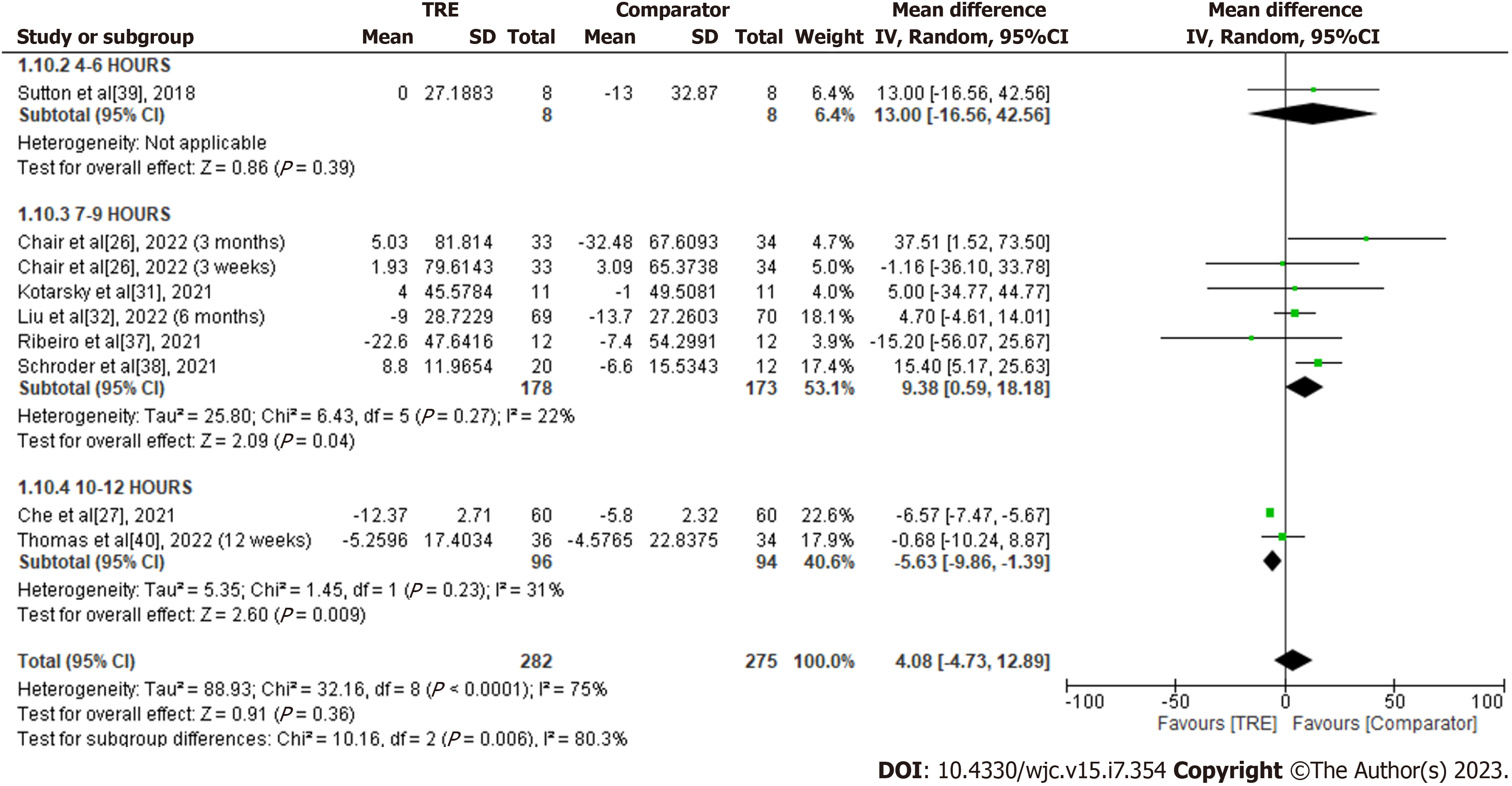
Total cholesterol: A meta-analysis of eight studies evaluated the effect of TRE on TC. There was no significant difference in TC levels between the test and comparator groups [MD 4.08; 95%CI: -4.73 to 12.89, P = 0.36; I2 = 75%] (Figure 11). Random-effects subgroup analyses were conducted based on the duration of TRE intervention showed a significant reduction in TC following TRE interventions ranging from ten to 12 h [MD -5.63; 95%CI: -9.86 to -1.39, P = 0.009; I2 = 31%]. Meanwhile, a significant increase in TC was observed in the TRE groups following TRE interventions ranging from seven to nine hours [MD 9.38; 95%CI: 0.59 to 18.18, P = 0.04; I2 = 22%]. Subgroup analysis for TRE interventions ranging from four to six hours was not calculated since it was reported only in one study.
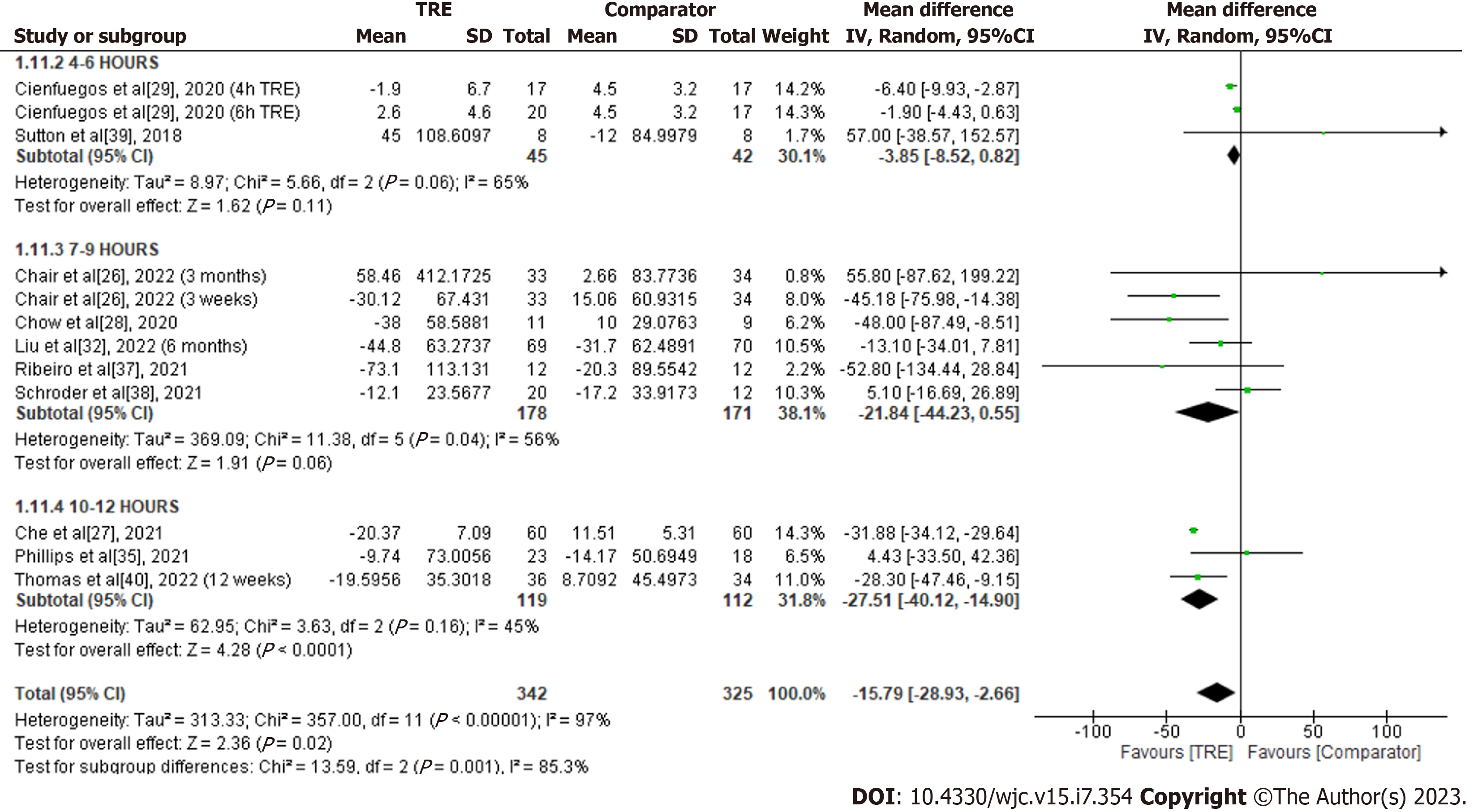
Triglycerides: A meta-analysis of ten studies evaluated the effect of TRE on triglycerides (Figure 12). Individuals assigned to TRE intervention exhibited significantly reduced triglyceride levels compared to the comparator group [MD -15.79; 95%CI: -28.93 to -2.66, P = 0.02; I2 = 97%]. Random-effects subgroup analyses were conducted based on the duration of TRE intervention revealed a significant reduction in triglycerides following TRE interventions ranging from ten to 12 h [MD -27.51; 95%CI: -40.12 to -14.90, P < 0.0001; I2 = 45%]. However, there were no significant changes in triglyceride levels observed following TRE interventions ranging from seven to nine hours [MD -21.84; 95%CI: -44.23 to 0.55, P = 0.06; I2 = 56%] and TRE interventions ranging from four to six hours [MD -3.85; 95%CI: -8.52 to 0.82), P = 0.11; I2 = 65%].
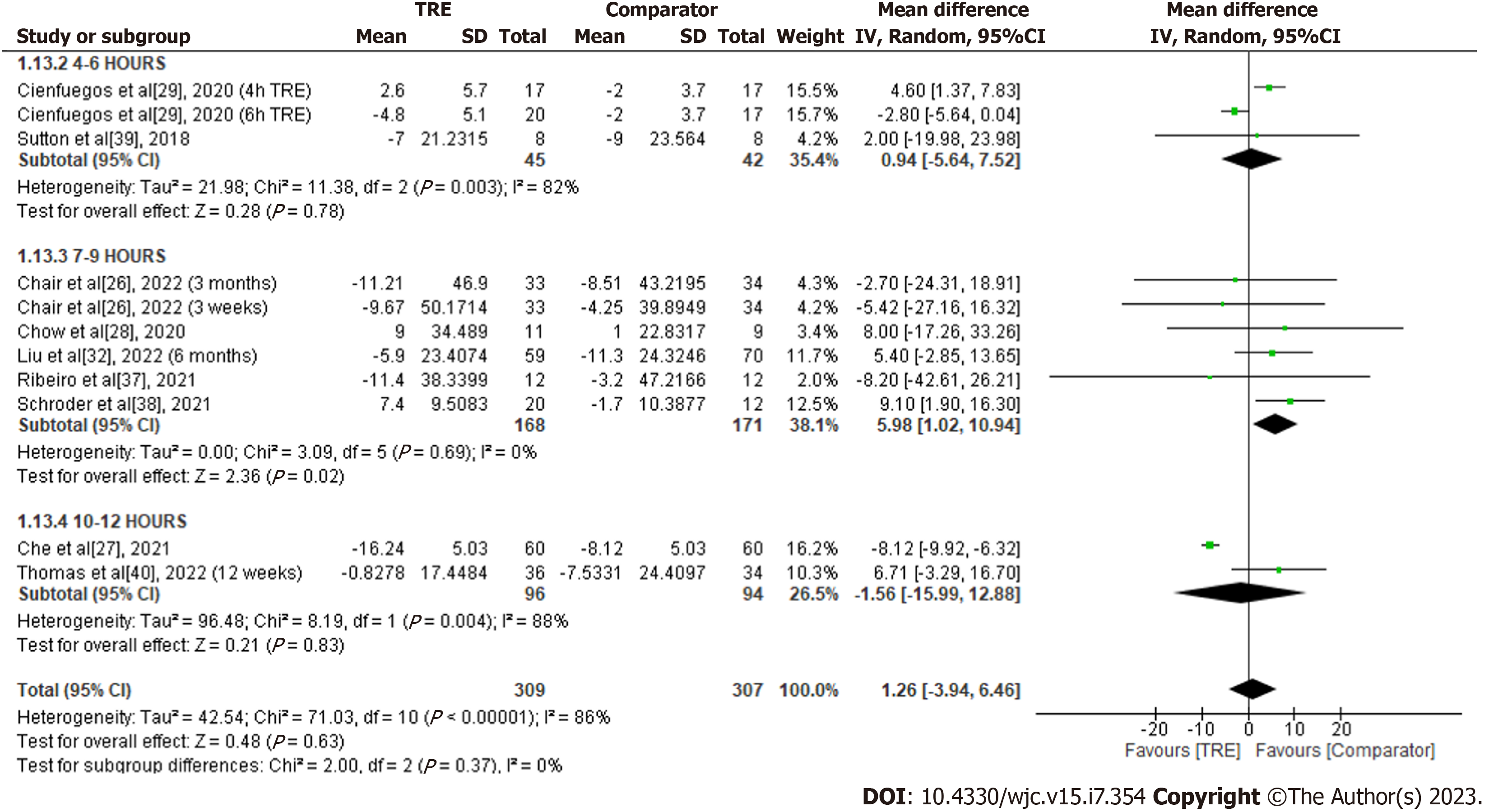
LDL-C: A meta-analysis of nine studies showed no significant overall effect of TRE on LDL-C levels compared to the comparator group [MD 1.26; 95%CI: -3.94 to 6.46, P = 0.63; I2 = 86%] (Figure 13). Random-effects subgroup analyses were conducted based on the duration of TRE intervention, which showed no significant changes in LDL-C following TRE interventions ranging from ten to 12 h [MD -1.56; 95%CI: -15.99 to 12.88, P = 0.83; I2 = 88%] and TRE interventions ranging from four to six hours [MD 0.94; 95%CI: -5.64 to 7.52, P = 0.78; I2 = 82%]. In contrast, a significant increase of LDL-C was observed in TRE interventions ranging from seven to nine hours [MD 5.98; 95%CI: 1.02 to 10.94, P = 0.02; I2 = 82%].
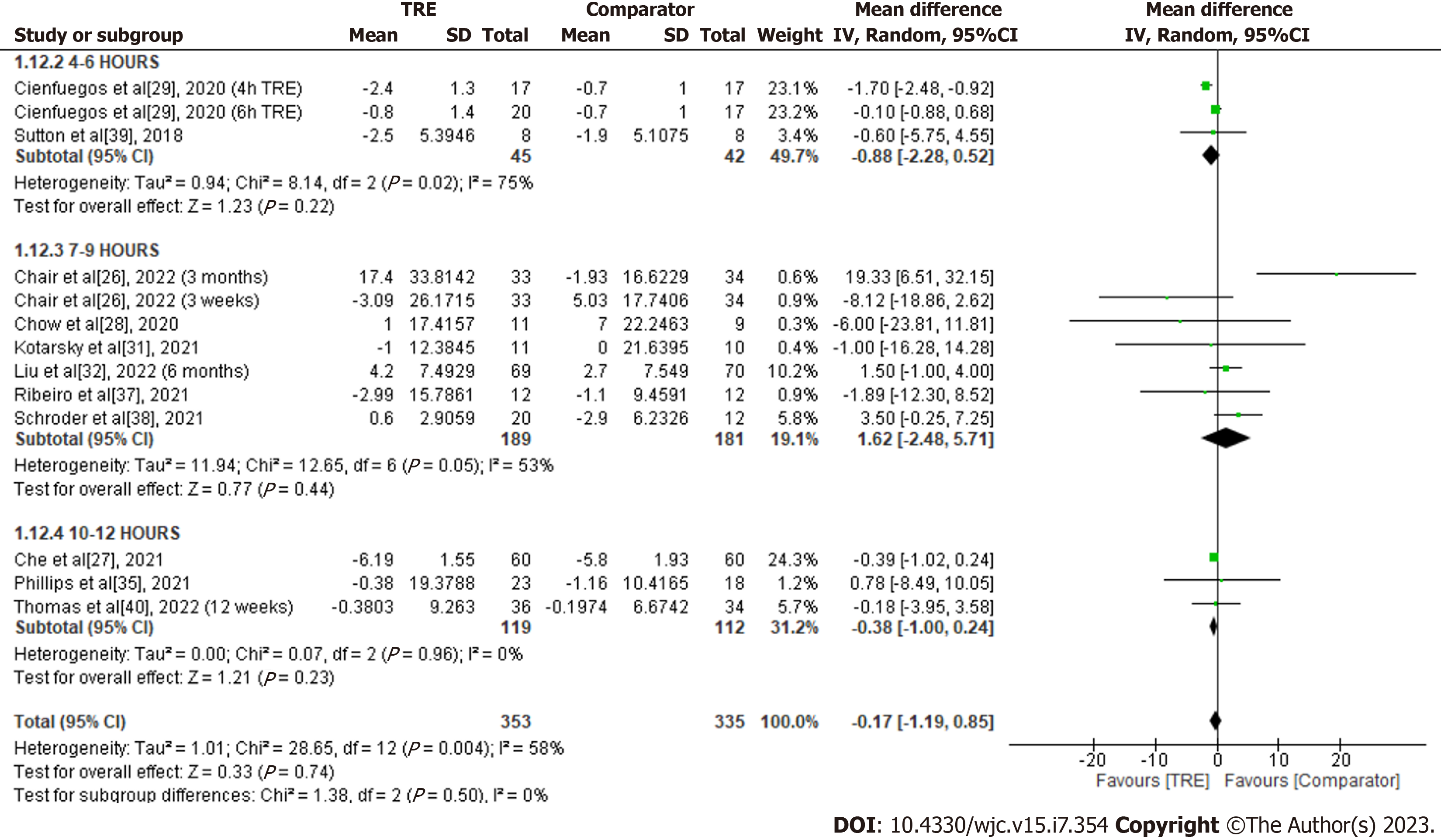
HDL-C: A meta-analysis of ten studies showed no significant overall effect of TRE on HDL-C levels compared to the comparator group [MD -0.17; 95%CI: -1.19 to 0.85, P = 0.74; I2 = 58%] (Figure 14). Random-effects subgroup analyses were conducted based on the duration of TRE intervention demonstrated no significant changes in HDL-C following TRE interventions ranging from ten to 12 h [MD -0.38; 95%CI: -1.00 to 0.24, P = 0.23; I2 = 0%], TRE interventions ranging from seven to nine hours [MD 1.62; 95%CI: -2.48 to 5.71, P = 0.44; I2 = 53%], and TRE interventions ranging from four to six hours [MD -0.88; 95%CI: -2.28 to 0.52, P = 0.22; I2 = 75%].
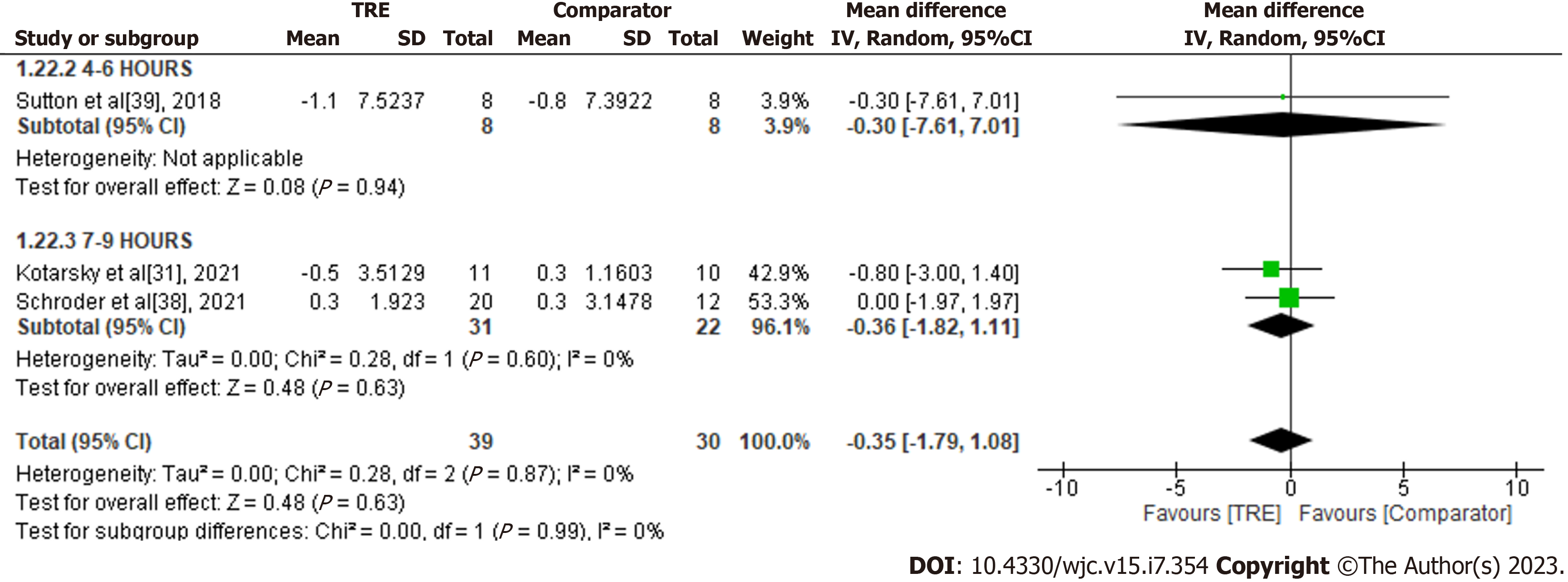
C-reactive protein: A meta-analysis of three studies evaluated the effect of TRE on C-reactive protein (Figure 15). There was no significant difference in C-reactive protein levels between the test and comparator groups [MD -0.35; 95%CI: -1.79 to 1.08, P = 0.63; I2 = 0%]. No subgroup analysis was conducted due to the limited included studies reporting this outcome.
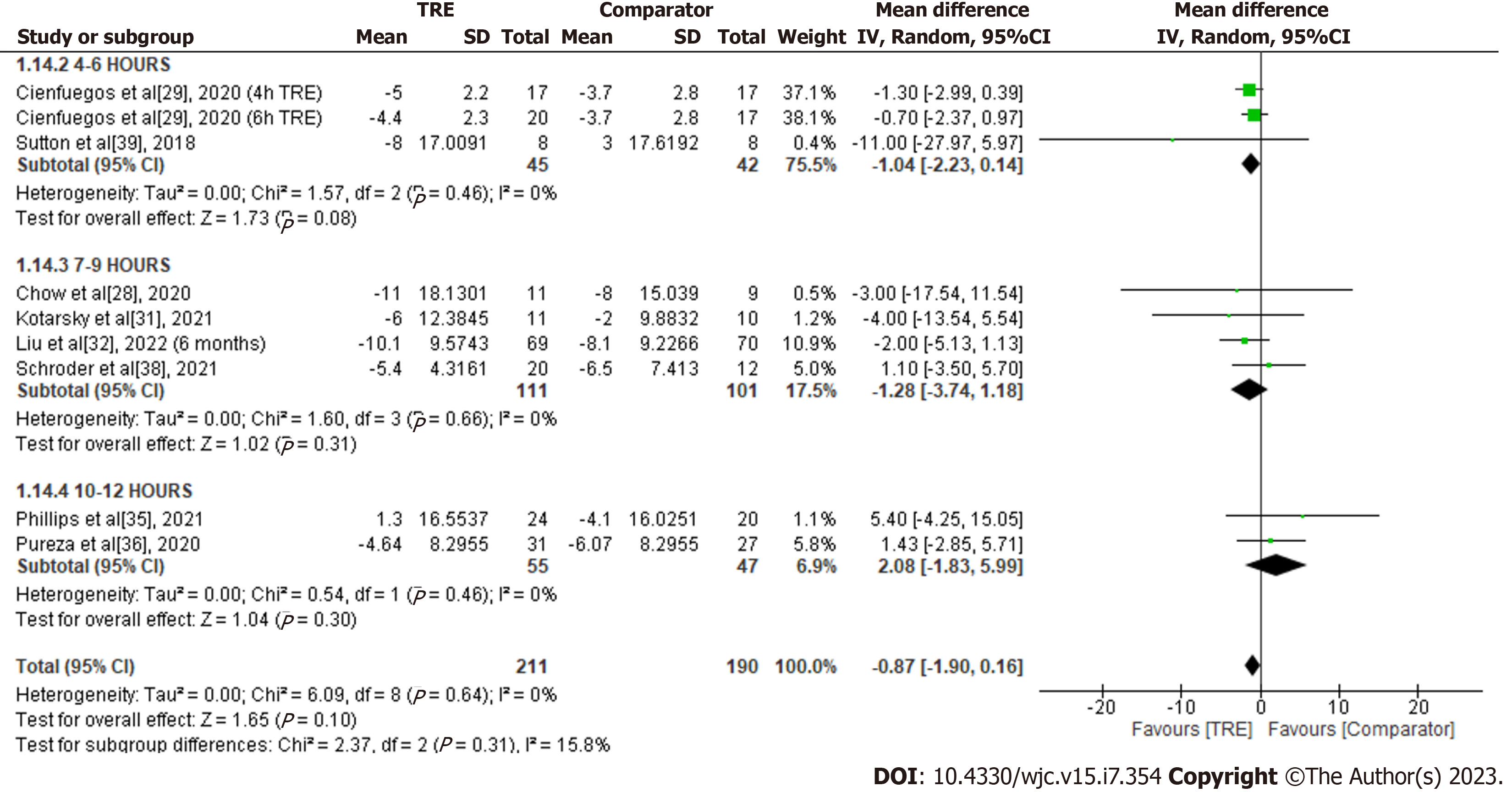
Systolic blood pressure: A meta-analysis of eight studies evaluated the effect of TRE on systolic blood pressure (Figure 16). There was no significant difference in systolic blood pressure levels between groups [MD -0.87; 95%CI: -1.90 to 0.16, P = 0.10; I2 = 0%]. Random-effects subgroup analyses were conducted based on the duration of TRE intervention showed no changes in systolic blood pressure following TRE interventions ranging from ten to 12 h [MD 2.08; 95%CI:
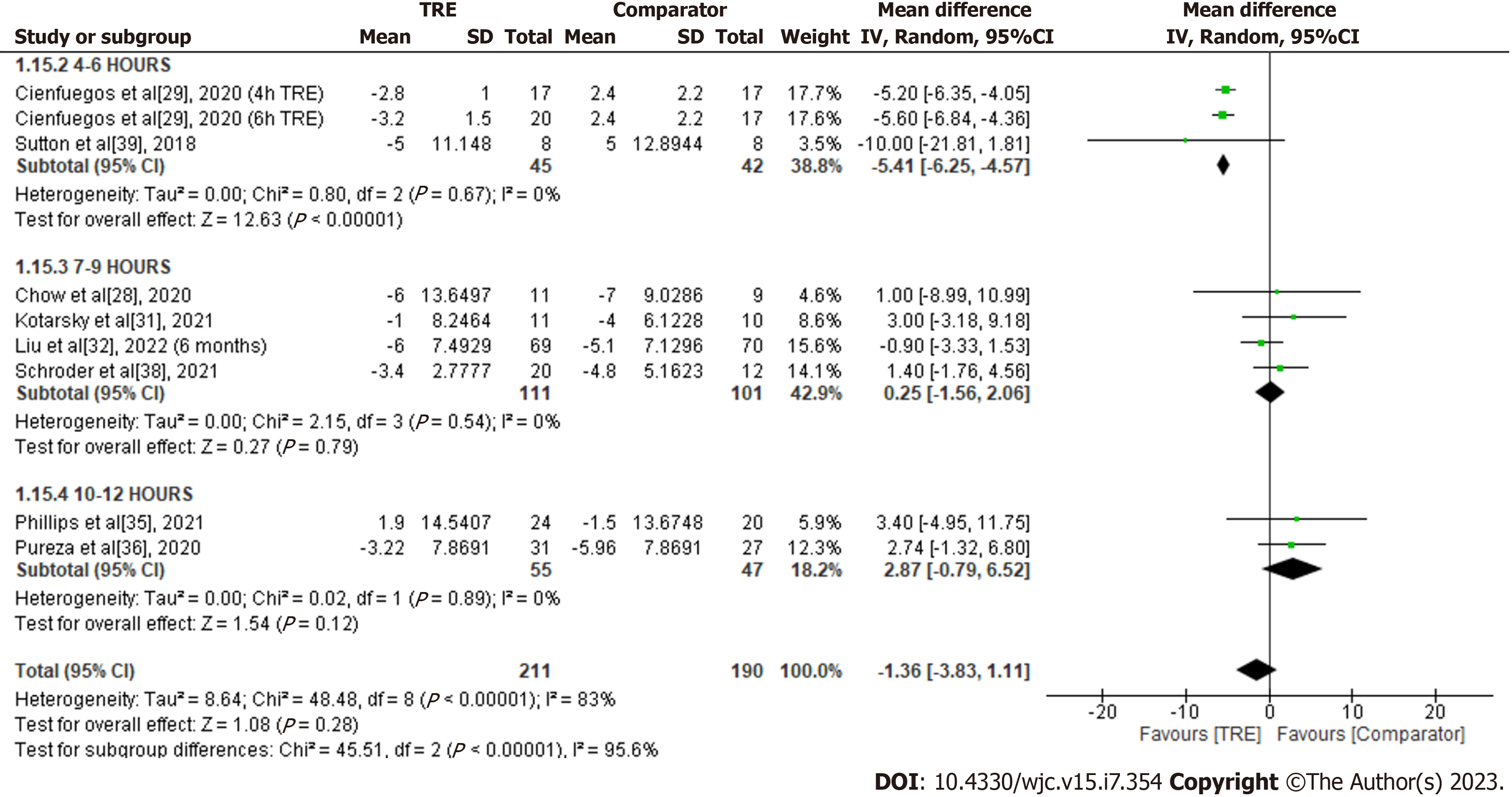
Diastolic blood pressure: A meta-analysis of eight studies evaluated the effect of TRE on diastolic blood pressure (Figure 17). There was no significant difference in diastolic blood pressure levels between the test and comparator groups [MD -1.36; 95%CI: -3.83 to 1.11, P = 0.28; I2 = 83%]. Random-effects subgroup analyses based on the duration of TRE intervention showed no significant changes in diastolic blood pressure following TRE interventions ranging from ten to 12 h [MD 2.87; 95%CI: -0.79 to 6.52, P = 0.12; I2 = 0%] and TRE interventions ranging from seven to nine hours [MD 0.25; 95%CI: -1.56 to 2.06, P = 0.79; I2 = 0%]. In contrast, there was a significant reduction observed in TRE interventions ranging from four to six hours [MD -5.41; 95%CI: -6.25 to -4.57, P = < 0.00001; I2 = 0%].
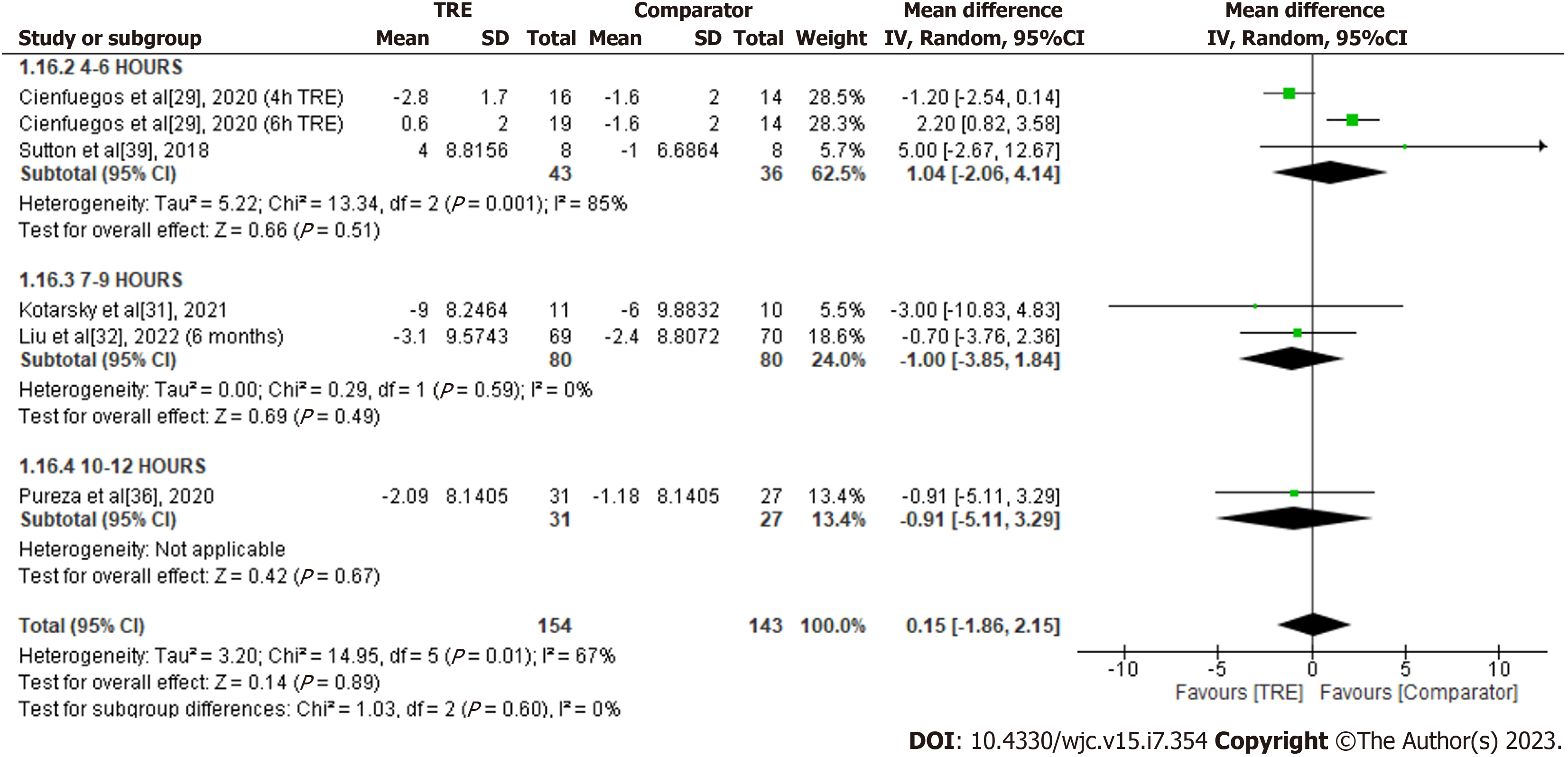
Heart rate: A meta-analysis of five studies reported no significant overall effect of TRE on heart rate levels in comparison to the comparator group [MD 0.15; 95%CI: -1.86 to 2.15, P = 0.89; I2 = 67%] (Figure 18). Random-effects subgroup analyses based on the duration of TRE intervention showed no significant changes in heart rate following TRE interventions ranging from seven to nine hours [MD -1.00; 95%CI: -3.85 to 1.84, P = 0.49; I2 = 0%] and TRE interventions ranging from four to six hours [MD 1.04; 95%CI: -2.06 to 4.14, P = 0.51; I2 = 85%]. Subgroup analysis for TRE interventions ranging from ten to 12 h was not calculated since only one study reported the outcome for this particular duration.
The potential publication biases were assessed using funnel plots based on the outcomes of interest (Supple
Adopting IF, including time-restricted eating interventions, to potentially optimize metabolic health by altering the duration of food consumption is a topic of increasing interest in research and others[41]. The present review analyzed the effects of TRE intervention on anthropometrics and cardiometabolic health markers in adults with excessive weight and obesity-related metabolic diseases. The meta-analysis showed that TRE significantly reduced body weight, waist circumference, fat mass, lean body mass, blood glucose, insulin, and triglyceride. However, no changes were observed in HbA1c, HOMA-IR, TC, LDL-C, HDL-C, heart rate, systolic and diastolic blood pressure. Interestingly, subgroup analyses based on the duration of the eating window revealed that TRE interventions with shorter eating windows (4-6 h) resulted in a more pronounced effect size than longer eating windows as measured for all outcomes. The meta-analysis results suggest that TRE is an effective treatment strategy for adults with excessive weight and obesity-related metabolic diseases as it improves specific metabolic parameters and potentially decreases the risk of atherosclerotic cardiovascular disease.
Limiting food intake to a shorter duration, without explicitly attempting to reduce energy intake, induces fasting physiology. This adaptive mechanism in the human body has evolved to cope with periods of food scarcity and prolonged fasting and is critical for survival[42]. A fasting regime, including TRE, activates metabolic switching from energy production through liver-derived glucose to adipose cell–derived ketones[43,44]. At the molecular level, TRE triggers circadian coordination with nutrient-sensing pathways to regulate metabolic health and protects against metabolic disorders induced by poor dietary intake[45]. Findings from this review are consistent with previous meta-analyses where TRE was shown effective in weight reduction despite mixed findings on body composition[16,18,21,22]. Compared to CER, weight loss achieved through IF is comparable to[46], if not superior to CER[47]. TRE may spontaneously decrease energy intake by 20%-30% under ad libitum conditions, resulting in weight loss of 1%-4%[48]. During periods of fasting and CER, macro- and micronutrients are less accessible to cells and tissues. Hence, several pathways play comparable roles in mediating CER and IF effects. Decreased glucose levels or decreased protein and amino acid availability, as generated by caloric restriction or fasting, activate AMP-activated protein kinase (AMPK) and inhibit mTOR, resulting in reduced protein synthesis and ribosome biogenesis, as well as the activation of autophagy[49].
Nutrient timing has been proposed as a potential approach to restoring metabolic health by synchronizing dietary intake with the circadian clock[50]. TRE interventions consistently improved glucose metabolism by reducing glucose levels in human studies, as confirmed in the current meta-analysis[16,18,19,21,22]. The glucoregulatory mechanisms of TRE demonstrate that eating within a limited eating window during the day restores cAMP Response Element-Binding Protein phosphorylation, decreases gluconeogenesis, and increases glucogenesis during the fed state via enhanced autophagic flux, mild production in ketone bodies, reduced oxidative stress, and promotion of β-cell responsiveness[51]. However, the effects of TRE on lipid profiles, blood pressure, and heart rate have been inconsistent[16,18,19,21,22]. Nonetheless, the meta-analyses revealed that TRE did not worsen any outcomes studied. While it is widely accepted that TRE improves circadian rhythms, it remains unknown whether the metabolic improvements are the result of calorie limitation or time restriction[52].
The duration of the eating window in TRE interventions on humans varies, which has led to heterogeneous results between studies. This systematic review suggests that TRE’s beneficial effects may be time-dependent, with a shorter eating window resulting in better weight management and cardiometabolic health than a longer eating duration. The mechanisms by which this occurs have yet to be fully understood. An animal study revealed that a 4-h time-restricted feeding could reprogram the circadian clock by restoring the expression phase of clock genes, despite the high-fat diet[11]. At the cellular level, prolonged fasting leads to increased AMP levels, gene expression, and activation of AMPK, a critical intracellular energy sensor that regulates processes associated with energy metabolism[11,53]. This results in reduced fatty acid synthesis and enhanced fatty acid oxidation in the liver[54]. Similar to AMPK, Sirtuin 1 (SIRT1) activity increases in response to prolonged fasting[55]. SIRT1 regulates numerous biological processes, such as insulin response, glycolysis, apoptosis, antioxidative defense, DNA repair, inflammatory response, metabolism, cancer, and stress, improving cardiometabolic health and CVD prevention[56-58].
Jamshed et al[59] conducted a 4-d randomized crossover study to elucidate the possible mechanisms of actions of TRE with short eating duration in humans. This study revealed that TRE with a short eating window improved multiple health aspects via circadian and fasting-related mechanisms[59]. The authors postulated that eating earlier in the day and having shorter inter-meal intervals could help minimize glycemic excursions, suggesting that TRE interventions with longer inter-meal intervals may be less effective in lowering glucose levels. Additionally, the study found that TRE may alter diurnal patterns in fasting cholesterol, ketones, cortisol, and circadian clock genes, particularly by increasing ketone levels in the morning and improving the amplitude of the cortisol rhythm. The study also demonstrated that six hours of TRE may produce a favorable effect on hormones and genes related to lifespan and autophagy, such as brain-derived neurotrophic factor, SIRT1, and LC3A, the autophagosome protein.
The findings of this meta-analysis provide evidence to support the hypothesis that longer fasting duration is associated with better weight control[60,61]. Contrary to animal studies, restricting eating duration does not affect 24-h energy expenditure in humans[62,63]. Animal studies have suggested that time-restricted feeding may increase energy expenditure by enhancing oxidative metabolism and expression of the mitochondrial uncoupling protein, which is responsible for non-shivering thermogenesis in brown and white adipose tissue[13,64,65]. Nevertheless, a human study detected an increased thermic effect of food during the early postprandial period[63]. The study demonstrated that short TRE interventions primarily promote weight loss by decreasing appetite, as evidenced by reduced ghrelin levels and normalized hunger, which tend to promote fullness and reduced appetite. Furthermore, TRE with a short eating window leads to alterations in substrate oxidation, with an increase in 24-h protein oxidation and a decrease in 24-h non-protein Respiratory Quotient (npRQ), indicative of increased fat oxidation. This metabolic alteration is likely attributed to the prolonged daily fasting phase rather than circadian effects[43]. Furthermore, the short TRE group showed higher metabolic flexibility, defined as the difference between the maximum and minimum values of the npRQ, indicating a better ability to switch between different oxidizing substrates than the ad libitum group.
It is important to note that the associations between fasting duration and weight and cardiometabolic health may vary depending on the time of fasting and eating[15]. The early fasting window, characterized by breakfast consumption and early evening meals, may have different metabolic consequences than the late fasting window, characterized by breakfast omission and night-time snacking[60]. Since humans are diurnal organisms, eating closer to daylight is consistent with the 24-h circadian rhythms of metabolism, leading to better metabolic health. The alignment of meals with typical circadian oscillations of hormonal profiles is necessary for TRE to be considered a nutritional strategy utilizing chrononutrition concepts. For example, plasma glucose concentration exhibits diurnal fluctuation, with peak values occurring at the start of the activity phase[66]. Since food intake promotes insulin production, plasma insulin levels reflect the daily rhythm of food intake. Thus, night eating results in a misalignment of central and peripheral endogenous glucose circadian rhythms and impaired glucose tolerance, while restricting meals to the daytime prevents such dysregulation[67]. Accordingly, the current pool of evidence suggests that later or self-selected TRE periods are less effective in improving metabolic health markers[68].
Although the findings of this meta-analysis suggest that short TRE may improve cardiometabolic outcomes, it is crucial to consider the sustainability of a restrictive eating pattern. The primary concern with a short eating window is that it may be too limiting for many individuals, making it challenging to adhere to over the long term. Adherence is a crucial factor in the success of any dietary intervention, as a lack of adherence can lead to the failure of the intervention. Additionally, limiting eating periods may lead to disordered eating patterns or restrictive dieting behaviors. A recent study found that individuals who engaged in TRE were at a higher risk for disordered eating behaviors, such as overeating, losing control, binge eating, vomiting, laxative use, and compulsive exercise[69]. The application of short TRE may not be suitable for all individuals, particularly those with certain medical conditions or who are pregnant or breastfeeding. Alteration in the metabolism and nutrient needs of these individuals may necessitate a more frequent or longer eating duration than the general population.
We have identified several strengths in our meta-analysis. We utilized multiple databases to search existing literature and identify eligible studies related to TRE conducted on individuals with excessive weight or weight-related metabolic diseases while excluding individuals with a normal BMI. This approach ensured the homogeneity of the population of interest, as this group of individuals may have a higher tendency to experience metabolic disturbances than individuals with a normal BMI. Additionally, we performed subgroup analyses based on arbitrary clustering of the eating window duration of TRE intervention to explore methodological heterogeneity. Furthermore, we only included studies involving clinical trials that lasted two weeks up to six months to reduce heterogeneity from short-term interventions (i.e., less than seven days) and studies reporting long-term effects of TRE. However, this meta-analysis has some limitations. Most included studies had small sample sizes, with several posing a high RoB in some domains. Blinding participants was impossible due to the nature of behavioral interventions. Nonetheless, this factor is unlikely to affect the results as outcomes were objectively measured, with some studies executed blinding of assessors. Additionally, there was high heterogeneity in some of the outcomes, which could be due to differences in population, fasting/eating duration, duration of the intervention, meal timing, meal frequency, co-interventions, and level of adherence. Data on such factors, including dietary intake, physical activity, and adherence level, were unavailable in some reports, which might have resulted in biased conclusions. Future large-randomized-controlled trials with rigorous methodology are necessary to elucidate the role of different TRE duration on cardiometabolic health and determine the optimal TRE duration to translate into clinical practice.
In conclusion, findings from this meta-analysis demonstrate that TRE is an effective and sustainable dietary strategy for reducing body weight, body composition, blood glucose, insulin, and triglyceride in individuals with excessive weight or weight-related metabolic disorders. Moreover, this study demonstrated that the favorable benefits of TRE on health are dependent on eating duration, with shorter durations resulting in more significant changes in anthropometric and cardiometabolic health markers. However, due to the challenges of adhering to a strict regimen, the TRE interventions with short eating windows may only suit specific individuals and must be monitored vigilantly. Therefore, extensive studies with larger sample sizes and higher quality are required to confirm the findings of this meta-analysis and determine the optimal duration of the eating window for primary and secondary CVD prevention.
There is growing interest in time-based dietary intervention as an alternative to caloric restriction or nutrient-based dietary intervention for cardiovascular disease prevention.
Time-restricted eating (TRE) is considered a mild form of intermittent fasting and has shown conflicting cardiometabolic health outcomes in humans.
Our study aimed to explore the overall effectiveness of TRE and its optimal duration as a potential dietary approach for weight loss and improved cardiometabolic health in individuals with excessive weight and obesity-related metabolic diseases.
Systematic searches were conducted via multiple databases (MEDLINE Complete, Web of Science, Scopus, the Cochrane Library, Academic Search Complete, Food Science Source, OpenDissertations, Education Research Complete, and Psychology and Behavioural Sciences Collection) to identify the relevant articles. The methodological quality of the included studies was assessed using the Cochrane risk-of-bias tool for randomized trials (RoB-2). Meta-analyses were conducted depending on feasibility. Analysis was performed using RevMan software.
TRE significantly decreased body weight, waist circumference, adipose mass, lean body mass, blood glucose, insulin, and triglyceride. HbA1c, homeostasis model assessment for insulin resistance, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, heart rate, systolic and diastolic blood pressure showed no significant changes with the treatment. In addition, subgroup analyses based on the eating duration revealed significant variation in the effects of the TRE intervention on the measured outcomes.
TRE is an effective and sustainable dietary strategy to improve the anthropometric and cardiometabolic health of individuals with excessive weight or weight-related metabolic disorders.
A larger sample size and higher quality studies are necessary to corroborate the findings of this meta-analysis and define the optimal duration of the eating window for cardiovascular disease prevention.
We would like to thank Prof. Dr. Susan Armijo-Olivo for providing intensive systematic review training, which enabled us to complete the work.
| 1. | GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204-1222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13226] [Cited by in RCA: 12082] [Article Influence: 2013.7] [Reference Citation Analysis (42)] |
| 2. | Gregg EW, Shaw JE. Global Health Effects of Overweight and Obesity. N Engl J Med. 2017;377:80-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 217] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 3. | Agha M, Agha R. The rising prevalence of obesity: part A: impact on public health. Int J Surg Oncol (N Y). 2017;2:e17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 203] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 4. | Wharton S, Lau DCW, Vallis M, Sharma AM, Biertho L, Campbell-Scherer D, Adamo K, Alberga A, Bell R, Boulé N, Boyling E, Brown J, Calam B, Clarke C, Crowshoe L, Divalentino D, Forhan M, Freedhoff Y, Gagner M, Glazer S, Grand C, Green M, Hahn M, Hawa R, Henderson R, Hong D, Hung P, Janssen I, Jacklin K, Johnson-Stoklossa C, Kemp A, Kirk S, Kuk J, Langlois MF, Lear S, McInnes A, Macklin D, Naji L, Manjoo P, Morin MP, Nerenberg K, Patton I, Pedersen S, Pereira L, Piccinini-Vallis H, Poddar M, Poirier P, Prud'homme D, Salas XR, Rueda-Clausen C, Russell-Mayhew S, Shiau J, Sherifali D, Sievenpiper J, Sockalingam S, Taylor V, Toth E, Twells L, Tytus R, Walji S, Walker L, Wicklum S. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192:E875-E891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 894] [Article Influence: 178.8] [Reference Citation Analysis (3)] |
| 5. | Golbidi S, Daiber A, Korac B, Li H, Essop MF, Laher I. Health Benefits of Fasting and Caloric Restriction. Curr Diab Rep. 2017;17:123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 6. | Seimon RV, Roekenes JA, Zibellini J, Zhu B, Gibson AA, Hills AP, Wood RE, King NA, Byrne NM, Sainsbury A. Do intermittent diets provide physiological benefits over continuous diets for weight loss? A systematic review of clinical trials. Mol Cell Endocrinol. 2015;418 Pt 2:153-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 7. | Mandal S, Simmons N, Awan S, Chamari K, Ahmed I. Intermittent fasting: eating by the clock for health and exercise performance. BMJ Open Sport Exerc Med. 2022;8:e001206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Katsi V, Papakonstantinou IP, Soulaidopoulos S, Katsiki N, Tsioufis K. Chrononutrition in Cardiometabolic Health. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Charlot A, Hutt F, Sabatier E, Zoll J. Beneficial Effects of Early Time-Restricted Feeding on Metabolic Diseases: Importance of Aligning Food Habits with the Circadian Clock. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 10. | Christensen RAG, Kirkham AA. Time-Restricted Eating: A Novel and Simple Dietary Intervention for Primary and Secondary Prevention of Breast Cancer and Cardiovascular Disease. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012;26:3493-3502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 286] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 12. | Rothschild J, Hoddy KK, Jambazian P, Varady KA. Time-restricted feeding and risk of metabolic disease: a review of human and animal studies. Nutr Rev. 2014;72:308-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 13. | Chaix A, Lin T, Le HD, Chang MW, Panda S. Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab. 2019;29:303-319.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 487] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 14. | Soliman GA. Intermittent fasting and time-restricted eating role in dietary interventions and precision nutrition. Front Public Health. 2022;10:1017254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 15. | Xie Z, He Z, Ye Y, Mao Y. Effects of time-restricted feeding with different feeding windows on metabolic health: A systematic review of human studies. Nutrition. 2022;102:111764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 16. | Wang W, Wei R, Pan Q, Guo L. Beneficial effect of time-restricted eating on blood pressure: a systematic meta-analysis and meta-regression analysis. Nutr Metab (Lond). 2022;19:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 17. | Tsitsou S, Zacharodimos N, Poulia KA, Karatzi K, Dimitriadis G, Papakonstantinou E. Effects of Time-Restricted Feeding and Ramadan Fasting on Body Weight, Body Composition, Glucose Responses, and Insulin Resistance: A Systematic Review of Randomized Controlled Trials. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 18. | Liu L, Chen W, Wu D, Hu F. Metabolic Efficacy of Time-Restricted Eating in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Clin Endocrinol Metab. 2022;107:3428-3441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 19. | Pureza IROM, Macena ML, da Silva Junior AE, Praxedes DRS, Vasconcelos LGL, Bueno NB. Effect of early time-restricted feeding on the metabolic profile of adults with excess weight: A systematic review with meta-analysis. Clin Nutr. 2021;40:1788-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 20. | Kang J, Ratamess NA, Faigenbaum AD, Bush JA, Beller N, Vargas A, Fardman B, Andriopoulos T. Effect of Time-Restricted Feeding on Anthropometric, Metabolic, and Fitness Parameters: A Systematic Review. J Am Nutr Assoc. 2022;41:810-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Pellegrini M, Cioffi I, Evangelista A, Ponzo V, Goitre I, Ciccone G, Ghigo E, Bo S. Effects of time-restricted feeding on body weight and metabolism. A systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21:17-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 22. | Moon S, Kang J, Kim SH, Chung HS, Kim YJ, Yu JM, Cho ST, Oh CM, Kim T. Beneficial Effects of Time-Restricted Eating on Metabolic Diseases: A Systemic Review and Meta-Analysis. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 168] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 23. | Adafer R, Messaadi W, Meddahi M, Patey A, Haderbache A, Bayen S, Messaadi N. Food Timing, Circadian Rhythm and Chrononutrition: A Systematic Review of Time-Restricted Eating's Effects on Human Health. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 24. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51476] [Article Influence: 10295.2] [Reference Citation Analysis (2)] |
| 25. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 18757] [Article Influence: 2679.6] [Reference Citation Analysis (0)] |
| 26. | Chair SY, Cai H, Cao X, Qin Y, Cheng HY, Ng MT. Intermittent Fasting in Weight Loss and Cardiometabolic Risk Reduction: A Randomized Controlled Trial. J Nurs Res. 2022;30:e185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Che T, Yan C, Tian D, Zhang X, Liu X, Wu Z. Time-restricted feeding improves blood glucose and insulin sensitivity in overweight patients with type 2 diabetes: a randomised controlled trial. Nutr Metab (Lond). 2021;18:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 28. | Chow LS, Manoogian ENC, Alvear A, Fleischer JG, Thor H, Dietsche K, Wang Q, Hodges JS, Esch N, Malaeb S, Harindhanavudhi T, Nair KS, Panda S, Mashek DG. Time-Restricted Eating Effects on Body Composition and Metabolic Measures in Humans who are Overweight: A Feasibility Study. Obesity (Silver Spring). 2020;28:860-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 274] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 29. | Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Wiseman E, Pavlou V, Lin S, Oliveira ML, Varady KA. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020;32:366-378.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 405] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 30. | Isenmann E, Dissemond J, Geisler S. The Effects of a Macronutrient-Based Diet and Time-Restricted Feeding (16:8) on Body Composition in Physically Active Individuals-A 14-Week Randomised Controlled Trial. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 31. | Kotarsky CJ, Johnson NR, Mahoney SJ, Mitchell SL, Schimek RL, Stastny SN, Hackney KJ. Time-restricted eating and concurrent exercise training reduces fat mass and increases lean mass in overweight and obese adults. Physiol Rep. 2021;9:e14868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 32. | Liu D, Huang Y, Huang C, Yang S, Wei X, Zhang P, Guo D, Lin J, Xu B, Li C, He H, He J, Liu S, Shi L, Xue Y, Zhang H. Calorie Restriction with or without Time-Restricted Eating in Weight Loss. N Engl J Med. 2022;386:1495-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 344] [Article Influence: 86.0] [Reference Citation Analysis (2)] |
| 33. | Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, Philip E, Vittinghoff E, Heymsfield SB, Olgin JE, Shepherd JA, Weiss EJ. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men With Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern Med. 2020;180:1491-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 404] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 34. | Peeke PM, Greenway FL, Billes SK, Zhang D, Fujioka K. Effect of time restricted eating on body weight and fasting glucose in participants with obesity: results of a randomized, controlled, virtual clinical trial. Nutr Diabetes. 2021;11:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 35. | Phillips NE, Mareschal J, Schwab N, Manoogian ENC, Borloz S, Ostinelli G, Gauthier-Jaques A, Umwali S, Gonzalez Rodriguez E, Aeberli D, Hans D, Panda S, Rodondi N, Naef F, Collet TH. The Effects of Time-Restricted Eating versus Standard Dietary Advice on Weight, Metabolic Health and the Consumption of Processed Food: A Pragmatic Randomised Controlled Trial in Community-Based Adults. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 36. | de Oliveira Maranhão Pureza IR, da Silva Junior AE, Silva Praxedes DR, Lessa Vasconcelos LG, de Lima Macena M, Vieira de Melo IS, de Menezes Toledo Florêncio TM, Bueno NB. Effects of time-restricted feeding on body weight, body composition and vital signs in low-income women with obesity: A 12-month randomized clinical trial. Clin Nutr. 2021;40:759-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 37. | Ribeiro DE, Santiago AF, Abreu WCd. Continuous energy restriction (CER) plus 16/8 time-restricted feeding improve body composition and metabolic parameters in overweight and obese, but no more than CER alone. Nutr Healthy Aging. 2021;6:147-156. [DOI] [Full Text] |
| 38. | Schroder JD, Falqueto H, Mânica A, Zanini D, de Oliveira T, de Sá CA, Cardoso AM, Manfredi LH. Effects of time-restricted feeding in weight loss, metabolic syndrome and cardiovascular risk in obese women. J Transl Med. 2021;19:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 39. | Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018;27:1212-1221.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 834] [Cited by in RCA: 1051] [Article Influence: 131.4] [Reference Citation Analysis (0)] |
| 40. | Thomas EA, Zaman A, Sloggett KJ, Steinke S, Grau L, Catenacci VA, Cornier MA, Rynders CA. Early time-restricted eating compared with daily caloric restriction: A randomized trial in adults with obesity. Obesity (Silver Spring). 2022;30:1027-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 41. | Patikorn C, Roubal K, Veettil SK, Chandran V, Pham T, Lee YY, Giovannucci EL, Varady KA, Chaiyakunapruk N. Intermittent Fasting and Obesity-Related Health Outcomes: An Umbrella Review of Meta-analyses of Randomized Clinical Trials. JAMA Netw Open. 2021;4:e2139558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 42. | Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19:181-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 965] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 43. | Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, Mainous AG 3rd, Leeuwenburgh C, Mattson MP. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity (Silver Spring). 2018;26:254-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 464] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 44. | Schuppelius B, Peters B, Ottawa A, Pivovarova-Ramich O. Time Restricted Eating: A Dietary Strategy to Prevent and Treat Metabolic Disturbances. Front Endocrinol (Lausanne). 2021;12:683140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 45. | Zeb F, Wu X, Fatima S, Zaman MH, Khan SA, Safdar M, Alam I, Feng Q. Time-restricted feeding regulates molecular mechanisms with involvement of circadian rhythm to prevent metabolic diseases. Nutrition. 2021;89:111244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 46. | Cioffi I, Evangelista A, Ponzo V, Ciccone G, Soldati L, Santarpia L, Contaldo F, Pasanisi F, Ghigo E, Bo S. Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: a systematic review and meta-analysis of randomized controlled trials. J Transl Med. 2018;16:371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 47. | Zhang Q, Zhang C, Wang H, Ma Z, Liu D, Guan X, Liu Y, Fu Y, Cui M, Dong J. Intermittent Fasting versus Continuous Calorie Restriction: Which Is Better for Weight Loss? Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 48. | Gabel K, Varady KA. Current research: effect of time restricted eating on weight and cardiometabolic health. J Physiol. 2022;600:1313-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Hofer SJ, Carmona-Gutierrez D, Mueller MI, Madeo F. The ups and downs of caloric restriction and fasting: from molecular effects to clinical application. EMBO Mol Med. 2022;14:e14418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 143] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 50. | Smith HA, Betts JA. Nutrient timing and metabolic regulation. J Physiol. 2022;600:1299-1312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 51. | Cienfuegos S, McStay M, Gabel K, Varady KA. Time restricted eating for the prevention of type 2 diabetes. J Physiol. 2022;600:1253-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 52. | Huang Y, Liu D, Wei X, Huang C, Li C, Zhang H. Time-restricted eating on weight loss: implications from the TREATY study. Life Med. 2022;1:58-60. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Lei L, Lixian Z. Effect of 24 h Fasting on Gene Expression of AMPK, Appetite Regulation Peptides and Lipometabolism Related Factors in the Hypothalamus of Broiler Chicks. Asian-Australas J Anim Sci. 2012;25:1300-1308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Foretz M, Even PC, Viollet B. AMPK Activation Reduces Hepatic Lipid Content by Increasing Fat Oxidation In Vivo. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 55. | Regmi P, Heilbronn LK. Time-Restricted Eating: Benefits, Mechanisms, and Challenges in Translation. iScience. 2020;23:101161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 56. | Han D, Wang J, Ma S, Chen Y, Cao F. SIRT1 as a Promising Novel Therapeutic Target for Myocardial Ischemia Reperfusion Injury and Cardiometabolic Disease. Curr Drug Targets. 2017;18:1746-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 57. | Jiao F, Gong Z. The Beneficial Roles of SIRT1 in Neuroinflammation-Related Diseases. Oxid Med Cell Longev. 2020;2020:6782872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 212] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 58. | Zhu Y, Yan Y, Gius DR, Vassilopoulos A. Metabolic regulation of Sirtuins upon fasting and the implication for cancer. Curr Opin Oncol. 2013;25:630-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 477] [Article Influence: 68.1] [Reference Citation Analysis (1)] |
| 60. | Xiao Q, Bauer C, Layne T, Playdon M. The association between overnight fasting and body mass index in older adults: the interaction between duration and timing. Int J Obes (Lond). 2021;45:555-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 61. | Kahleova H, Lloren JI, Mashchak A, Hill M, Fraser GE. Meal Frequency and Timing Are Associated with Changes in Body Mass Index in Adventist Health Study 2. J Nutr. 2017;147:1722-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 62. | Bao R, Sun Y, Jiang Y, Ye L, Hong J, Wang W. Effects of Time-Restricted Feeding on Energy Balance: A Cross-Over Trial in Healthy Subjects. Front Endocrinol (Lausanne). 2022;13:870054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 63. | Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM. Early Time-Restricted Feeding Reduces Appetite and Increases Fat Oxidation But Does Not Affect Energy Expenditure in Humans. Obesity (Silver Spring). 2019;27:1244-1254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 218] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 64. | Aouichat S, Chayah M, Bouguerra-Aouichat S, Agil A. Time-Restricted Feeding Improves Body Weight Gain, Lipid Profiles, and Atherogenic Indices in Cafeteria-Diet-Fed Rats: Role of Browning of Inguinal White Adipose Tissue. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 65. | Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1519] [Article Influence: 108.5] [Reference Citation Analysis (0)] |
| 66. | Kalsbeek A, la Fleur S, Fliers E. Circadian control of glucose metabolism. Mol Metab. 2014;3:372-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 240] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 67. | Chellappa SL, Qian J, Vujovic N, Morris CJ, Nedeltcheva A, Nguyen H, Rahman N, Heng SW, Kelly L, Kerlin-Monteiro K, Srivastav S, Wang W, Aeschbach D, Czeisler CA, Shea SA, Adler GK, Garaulet M, Scheer FAJL. Daytime eating prevents internal circadian misalignment and glucose intolerance in night work. Sci Adv. 2021;7:eabg9910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 68. | Parr EB, Devlin BL, Hawley JA. Perspective: Time-Restricted Eating-Integrating the What with the When. Adv Nutr. 2022;13:699-711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 69. | Ganson KT, Cuccolo K, Hallward L, Nagata JM. Intermittent fasting: Describing engagement and associations with eating disorder behaviors and psychopathology among Canadian adolescents and young adults. Eat Behav. 2022;47:101681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Malaysia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pahlavani HA, Iran; Skrlec I, Croatia S-Editor: Li L L-Editor: A P-Editor: Xu ZH