Published online May 26, 2023. doi: 10.4330/wjc.v15.i5.273
Peer-review started: December 19, 2022
First decision: February 8, 2023
Revised: March 24, 2023
Accepted: April 4, 2023
Article in press: April 4, 2023
Published online: May 26, 2023
Processing time: 150 Days and 13 Hours
Heart and kidney dysfunction frequently coexist in patients with acute heart failure due to the overlap between these two organ systems. Cardiorenal syn
To evaluate the use of erythropoietin (EPO) in patients with CRS and its effects on hemoglobin (Hb), major cardiovascular (CV) events, and hospitalization rates.
On February 24, 2022, searches were conducted using PubMed, MEDLINE, and EMBASE, and 148 articles were identified. A total of nine studies were considered in this systematic review. We assessed the included articles based on the National Heart, Lung, and Blood Institute quality assessment tools for controlled intervention and observational cohort or cross-sectional studies. An assessment of bias risk was conducted on the chosen studies, and data relevant to our review was extracted.
The systematic review of these studies concluded that most existing literature indicates that EPO improves baseline Hb levels and decreases myocardial remodeling and left ventricular dysfunction without reducing CV mortality. In addition, the effect of EPO on the hospitalization rate of patients with CRS needs to be further studied since this relationship is unknown. Future studies, such as randomized controlled clinical trials and prospective cohort studies, should be conducted to enhance the literature on the potential of EPO therapy in patients with CRS.
Our systematic review suggests that EPO therapy may have a significant role in managing CRS. The review highlights the potential benefits of EPO in improving baseline Hb levels, reducing the risk of major CV events, improving cardiac remodeling, myocardial function, New York Heart Association class, and B-type natriuretic peptide levels. However, the effect of EPO treatment on hospitalization remains unclear and needs further exploration.
Core Tip: Erythropoietin improves baseline hemoglobin levels and decreases the risk of major cardiovascular.
- Citation: Bhangal R, Cancarevic I, Nassar M, Umar Z. Impact of erythropoietin therapy on cardiorenal syndrome: A systematic review with meta-analysis. World J Cardiol 2023; 15(5): 273-283
- URL: https://www.wjgnet.com/1949-8462/full/v15/i5/273.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i5.273
Many patients with heart failure also experience kidney dysfunction and vice versa, as these two organs are closely related in their functions. Cardiorenal syndrome (CRS) describes a pathophysiological disorder of the heart and kidneys, whereby dysfunction of one system may contribute to dysfunction of another[1]. An estimated 50% of patients with congestive heart failure (CHF), who have both reduced and preserved left ventricular ejection fraction, have an estimated glomerular filtrate rate (eGFR) of less than 60 mL/min/1.73 m2[2]. Even moderate renal insufficiency, defined as an eGFR less than 60 mL/min/1.73 m2, is associated with an increased risk of cardiac failure, hospitalization, and all-cause mortality[2]. CRS was previously considered to indicate acute kidney injury resulting from acute cardiac disease; however, more recent research suggests that the relationship between these two organs is bi-directional, meaning either organ could be the primary culprit[1,3]. CRS is categorized into five categories: acute and chronic cardiorenal, acute and chronic nephrocardiac, and secondary cardiorenal syndrome[1,3]. Essentially, each sub-category of CRS describes the acuity and pathophysiology of cardiac and kidney dysfunction, how the two overlap, and the presence of other systemic disorders[3]. The most common subtype of CRS is type one (acute cardiorenal), with approximately 50% of patients diagnosed with this condition[4]. The risk factors associated with worsening renal function in CRS include existing chronic kidney disease, hypertension, history of heart failure, diabetes mellitus, coronary artery disease, ischemic cardiomyopathy, and history of acute kidney injury[4].
In addition, many studies have shown that kidney dysfunction in CHF is caused by poor renal plasma flow, which leads the kidneys to retain water and sodium, which in turn, allows for improved perfusion of all essential organs[1]. The presence of anemia can result in tissue hypoxia and peripheral vasodilation, resulting in lower blood pressure and renal blood flow, increased heart rate, stroke volume, renal vasoconstriction, and sodium and water reabsorption[5]. Poor renal blood flow causes increased release of renin, angiotensin, aldosterone, and antidiuretic hormone (ADH), further increasing renal vasoconstriction and sodium and water reabsorption[5]. Renin, angiotensin, aldosterone, and ADH are toxic to cardiac, renal, and endothelial cells, which results in dysfunction in these tissues[5].
Nevertheless, recent studies have revealed that kidney hemodynamic changes can occur independently of the heart’s hemodynamic changes[1]. An initial response of the kidneys to CHF is to reduce renal plasma flow and eGFR, which results in a higher filtration fraction[1]. To maintain eGFR, both efferent arteriolar resistance and glomerular capillary hydrostatic pressure must be increased, severely impairing overall cardiac function[1]. Additionally, there is evidence of increased sodium reabsorption, specifically in the Loop of Henle, as well as activation of the sympathetic nervous system and the renin-angiotensin-aldosterone system (RAAS) all play a role in the development of CRS[1,3]. Overactivation of the RAAS increases both preload and afterload, leading in the long-term to cardiac dysfunction.
The presence of anemia with kidney dysfunction has also been shown to exacerbate CHF's progression and increase the risk of morbidity and mortality[6]. Besides contributing to tissue dysfunction by potentiating hypoxia, anemia can also increase oxidative stress, which contributes to increased hyperdynamic blood circulation and heart failure development[6]. Hemoglobin (Hb) levels are less than or equal to 12 g/dL in approximately 40% of patients with CHF[5]. Moreover, anemia is more common in the elderly, diabetics, and patients with poor baseline cardiac and/or renal function[5]. Increasing Hb levels can limit the detrimental effects of oxidative stress on the kidney and cardiac function. In a study by Pagourelias et al[6], for every 1 g/dL increase in Hb, there was a 15.8% reduction in mortality and a 14.2% reduction in mortality plus hospitalization in patients with CHF[6]. Erythropoietin (EPO) is a hematopoietic growth factor, the major function of which is to stimulate the proliferation and differentiation of erythropoiesis. EPO treatment is an efficient approach to increase Hb levels in patients with CHF whose anemia is caused by reduced EPO production. EPO can also influence other cellular processes, such as cell integrity and angiogenesis[7]. EPO has been demon
It is of great significance to investigate the role of EPO in treating patients with CRS. Recombinant human EPO (rhEPO) is a drug routinely prescribed to treat anemia in patients with end-stage renal disease (ESRD), and rhEPO treatment has been demonstrated to be beneficial for improving patients’ life quality and survival. It is reasonable to assume that rhEPO treatment is beneficial for patients with CRS. Given the high prevalence of CRS, searching for a treatment modality that can improve therapeutic responses is of high significance in clinical practice and will significantly decrease the mortality and hospitalization of CRS patients. Fewer hospitalizations would likely result in lower healthcare costs as well. Several studies are ongoing to examine the benefits of rhEPO treatment in patients with CRS. A two-part clinical trial conducted by the University of Alberta is currently examining whether treatment with EPO will increase cardiac performance and decrease renal disease progression in patients with CRS[8]. The failure to further investigate EPO for use in the treatment of CRS will result in the deprivation of a promising treatment option for many patients.
Through this systematic review, we aim to deepen our understanding of the role of EPO in managing CRS.
Our article was written according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[9]. It was not registered as a systematic review and no separate protocol was written. On February 24, 2022, a literature search was conducted on PubMed, MEDLINE, and EMBASE databases. The literature search was conducted using the following search strategy consisting of medical subject headings (MeSH) terms and regular keywords: ("Cardio-Renal Syndrome"[Mesh] OR "cardio-renal syndrome" OR "cardiorenal" OR "cardiorenal syndrome" OR "CRS" OR "reno-cardiac syndrome" OR "renocardiac syndrome") AND ("Erythropoietin"[Mesh] OR "erythropoietin" OR "EPO" OR "epoetin alfa" OR "darbepoetin alfa" OR "colony-stimulating factors").
Subsequently, the search results were imported into the Covidence platform, which automatically removed duplicate articles. Two reviewers independently examined titles and abstracts as part of an initial screening, followed by a second screening of full-length articles. Conflicts in the selected articles were discussed directly between the two reviewers, and a conclusion was drawn after each conflict article’s discussion. In the event that a conclusion could not be reached, a third reviewer was consulted to vote on the final decision. We included randomized controlled clinical trials, cross-sectional studies, prospective and retrospective case-control, and cohort studies exploring the use of erythropoietin in CRS management. The articles published up to February 24, 2022, were included. We excluded in vitro studies, animal studies, abstracts, case reports, case series, systematic reviews, and meta-analyses. Furthermore, we excluded articles in languages other than English.
Following this, a quality assessment was performed using the 2013 National Heart, Lung, and Blood Institute (NHLBI) quality assessment tools for controlled intervention studies and observational cohort or cross-sectional studies[10]. We included studies that scored 11 or higher on each quality assessment tool. Each study was then analyzed for obvious biases and confounding variables. Extrapolation of data was performed by one reviewer and displayed in a table (shown below). A qualitative analysis of the data was conducted following data extraction for the systematic review.
We performed a meta-analysis since there were a number of eligible studies. For studies included in the meta-analysis, an odds ratio (OR) was calculated. Due to the substantial heterogeneity in the concluded study, a random effects model was used for the meta-analysis. Meta-analysis was conducted using Review Manager 5.4.1 software. Meta-analysis studies with forest plots were included for the outcomes.
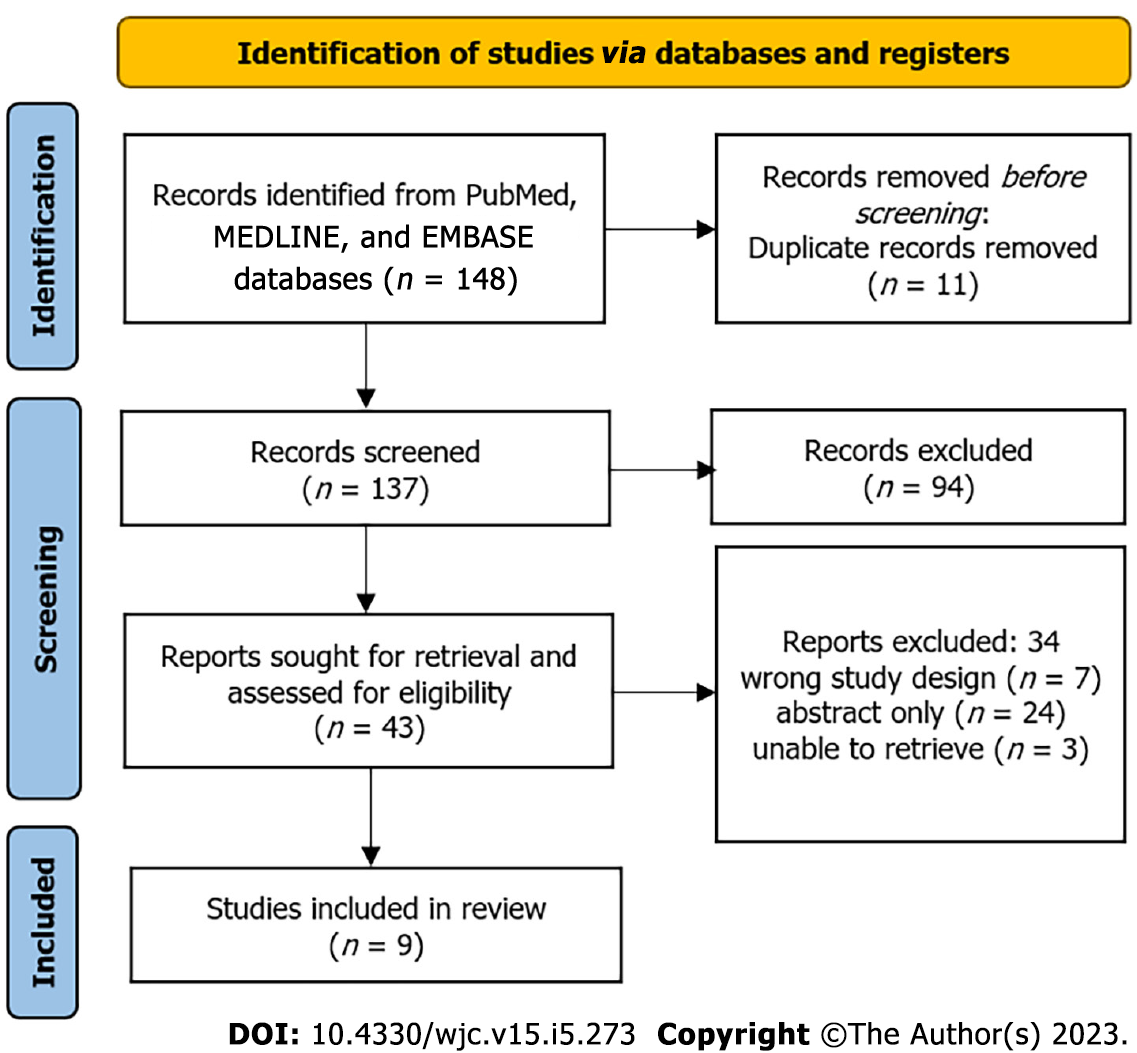
We selected 148 relevant articles from PubMed, MEDLINE, and EMBASE using an advanced search strategy combining MeSH terms and common keywords. We removed 11 duplicate articles. We screened 137 titles and abstracts before selecting 43 articles for full-text review. Of the 43 articles assessed for eligibility, 34 articles were excluded (7 were incorrect study designs, 3 were not retrievable, and 1 was an abstract). A total of nine studies were evaluated using the NHLBI quality assessment tools for controlled intervention studies and observational cohort studies or cross-sectional studies. Of the nine studies, six were randomized controlled trials, two were retrospective cohort studies, and one was a prospective cohort study. Articles that scored 11 or higher out of 14 on the assessment scale were considered high-quality articles, and therefore were included in the systematic review. Eight studies scored 11 points out of 14, whereas one study scored 12 points out of 14. A PRISMA flowchart of the reviewed literature and search strategies can be found in Figure 1. Table 1 summarizes the included studies and their statistical significance[11-19]. In addition, Table 1 illustrates how each study was scored according to the NHLBI quality assessment tools.
| Ref. | Study design | No. of Patients | EPO dosage and treatment duration | Average Hb level (g/dL) | EPO's effect on | Other findings | ||||
| Before EPO therapy | After EPO therapy | Without EPO treatment | Hb level | Major CV event | Hospitalization rate | |||||
| Jackevicius et al[12], 2015 | Retrospective cohort | 2058 | N/A | N/A | 10.5 | 11.1 | Inverse relationship between EPO therapy and Hb levels | N/A | N/A | The strongest predictors of EPO use include iron supplementation, markers of declining renal function, and patients concurrently taking hydralazine, nitrates, ARBs, and/or aspirin |
| Eisenga et al[13], 2019 | Open-label, prospective, randomized trial | 56 | N/A | 11.7 | 13.2 | 11.8 | Increased Hb levels | Increased cFGFR23 and iFGFR23, increased risk of CV events and mortality | N/A | N/A |
| McMurray et al[14], 2011 | Randomized trial | 3847 | N/A | N/A | N/A | N/A | N/A | Patients with CKD and anemia have an 11.2% higher risk for a major CV event. History of CHF was the highest-ranked predictor of future CV events, followed by age (hazard increase by 74% and 27%, respectively) | N/A | N/A |
| Fazlibegović et al[15], 2006 | Prospective cohort | 90 | 2000 to 6000 international units (I.U.) for 1-3 times per week, depending on the Hb level | 8.7 | 10.1 | N/A | Increased Hb levels | Improved functional ability of the myocardium, LV function, and quality of life | N/A | The average NYHA class before treatment with EPO was class II, with a range of I-IV; after treatment, the average class was I, with a range of I-II |
| Palazzuoli et al[11], 2007 | A randomized, double-blind controlled study | 51 | 6000 I.U. twice weekly | 10.4 | 11.2 | 10.6 | Increased Hb levels | Improved LV systolic function, LV remodeling, NYHA class, BNP levels, and quality of life compared to control group | N/A | N/A |
| Jie et al[16], 2011 | Open-label randomized trial | 65 | N/A | 7.5 | 8.4 | 6.9 | Increased Hb levels with long term use | N/A | N/A | Reduced CD34+ KRD-EPC levels, which was associated with decreased vascular regenerative potential |
| Jackevicius et al[17], 2014 | Retrospective cohort study | 2058 | 10.5: | 11.1 | Increased Hb levels | Increased risk of major CV and acute coronary syndrome events | Increased risk of an all-cause hospitalization rate | EPO use increases the risk of mortality | ||
| Jie et al[18], 2012 | Randomized trial | 30 | 50 I.U./kg once weekly | 11.8 | 12.3 | N/A | Increased Hb levels | Modest alteration in monocyte transcriptomes, indicating imprints of inflammation and oxidative stress. The increase in oxidative stress, in turn, increased the risk of CV events | N/A | Variable response in gene expression. Unable to conclude whether EPO improved hematopoietic effects in CRS patients with such gene mutations |
| Emans et al[19], 2013 | Open-label prospective randomized trial | 62 | 50 I.U./kg once weekly | 13.7 | 11.8 | Increased Hb levels | N/A | N/A | Elevated serum NGAL is inversely correlated with baseline EPO levels, independent of renal function. Low-dose EPO treatment caused a moderate decrease in serum NGAL levels, therefore reflecting potential improvement in renal tubular damage | |
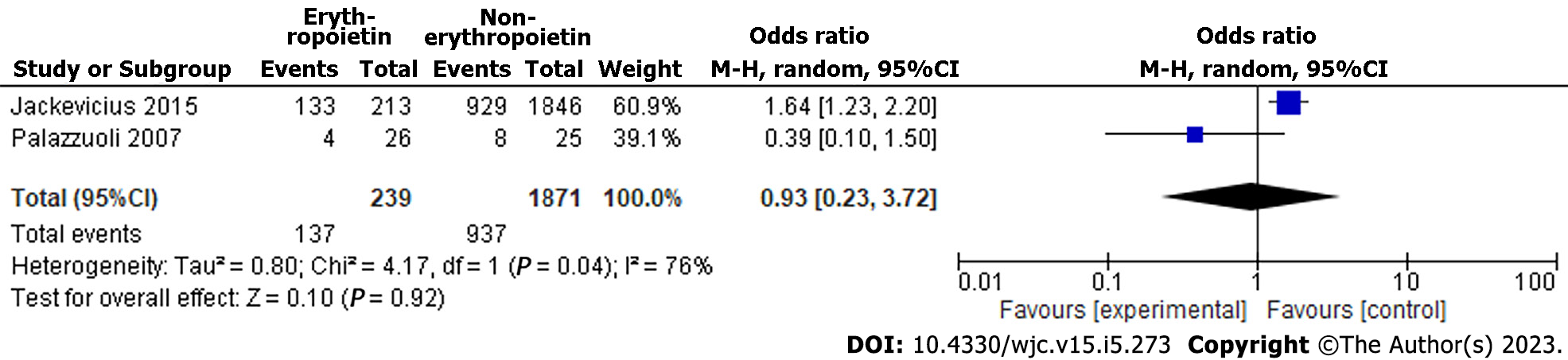
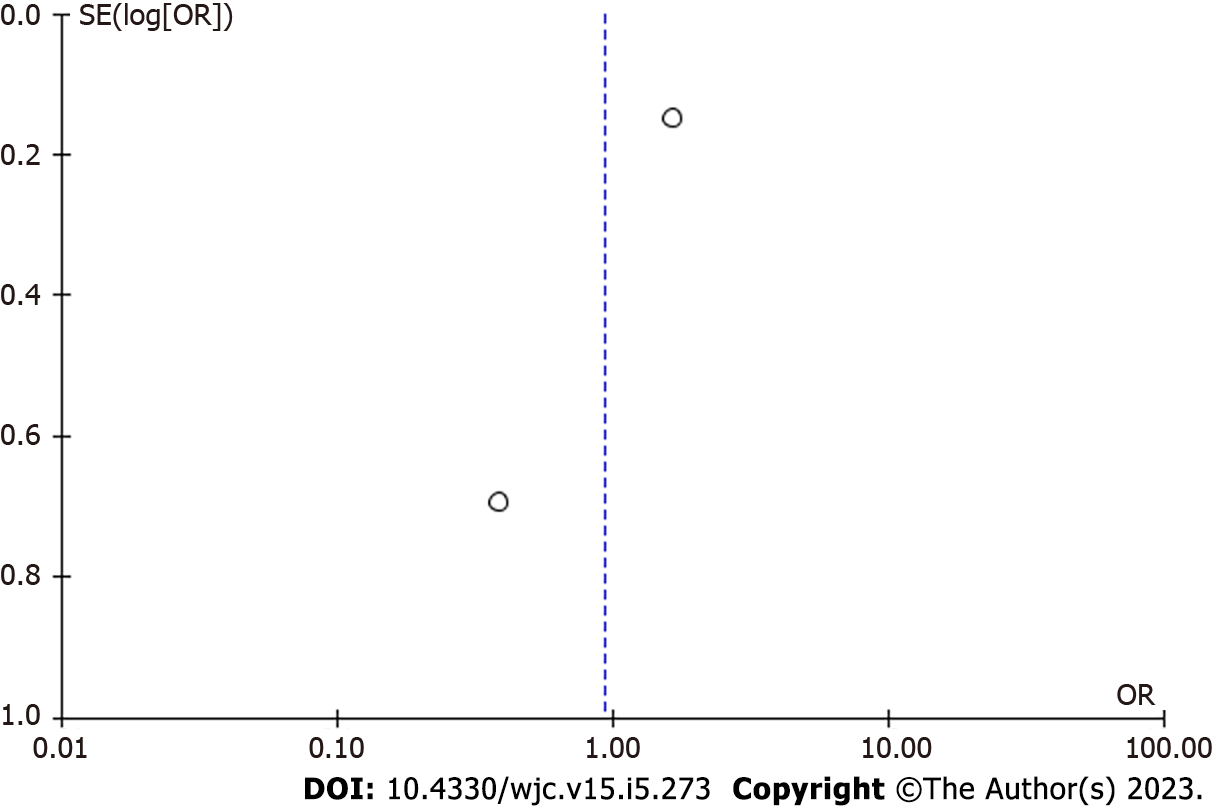
There were two studies included in the meta-analysis[11,12]. Study results showed a nonsignificant decrease in all causes of hospitalization (OR = 0.93 [95% confidence interval (CI), 0.23, 3.72]; P = 0.92). There was a high level of heterogeneity among the included studies (I² = 76%) (Figure 2). The asymmetry in the funnel plot indicates the existence of publication or selective reporting bias (Figure 3).
Elevated serum neutrophil-gelatinase-associated lipocalin (NGAL) is inversely correlated with baseline EPO levels, independent of renal function. Low-dose EPO treatment caused a moderate decrease in serum NGAL levels, therefore reflecting potential improvement in renal tubular damage.
As expected, the majority of studies concluded that EPO therapy increased Hb levels in patients with CRS. Furthermore, it would be expected that lower Hb levels would be associated with a higher rate of EPO prescription. This was confirmed in a study by Jackevicius et al[12] that found EPO therapy to be inversely related to Hb levels, such that patients with lower Hb levels are more likely to be prescribed EPO. On average, Hb levels are found to increase approximately 6 wk after EPO treatment, at which point most patients experience symptomatic improvement[20]. The need for prolonged treatment was confirmed by Jie et al[16], who found that short-term EPO use (3 wk) did not result in any change in Hb levels in patients with CRS, whereas long-term EPO therapy (52 wk) resulted in a significant increase in both Hb and reticulocyte count. Further, the strongest predictors of EPO use include iron supplementation, markers of declining renal function, and patients taking hydralazine, nitrates, angiotensin II receptor blockers (ARBs), and/or aspirin concurrently[12]. Hydralazine and nitrates are typically prescribed to patients with heart failure once patients’ renal function no longer permits RAAS inhibitor usage, at which point they are also far more likely to be prescribed EPO for advanced chronic kidney disease[12]. The temporal relationship is also important because hydralazine is a drug used to treat hypertension, and hypertension is a known side effect of EPO. No clear conclusions regarding aspirin or ARBs were drawn.
Likewise, in another study by Jie et al[18], EPO not only provided a significant rise in Hb levels compared to the non-EPO therapy but more interestingly, altered the monocyte transcriptomes that played a role in inflammation and oxidative stress. The increase in oxidative stress, in turn, increased the risk of cardiovascular (CV) events[18]. In this study, EPO’s role in oxidative stress was found to be associated with improving Hb level and alterations in gene expression directly involved in the inflammatory response. The effects were, however, very variable and inconsistent between individuals. The authors were unable to demonstrate a beneficial non-hematopoietic effect of EPO, although no harmful effects were found[18].
Several studies have shown that Hb levels improve after sufficient treatment with EPO. Exogenous EPO stimulates erythropoiesis, which increases red blood cell production and Hb levels. By reducing the degree of anemia among patients with CRS, oxidative stress and inflammatory response can be reduced. As a result, anemia symptoms such as fatigue, malaise, weakness, and dizziness are also improved, thus enhancing patients' overall quality of life. Similarly, since the data presented clearly points towards increasing Hb levels, it is reasonable to expect that those suffering from symptomatic anemia would experience clinical improvement, likely prompting them to be compliant with EPO therapy. In our review, the degree of Hb improvement varied significantly between the studies that were analyzed, and it was highly dependent on the dose of EPO given and the frequency of EPO administration. There is still insufficient data to predict an individual's response to therapy.
The effect of EPO on CV mortality is at this time difficult to establish, considering the differences in outcomes reported by currently available studies[11,13-15]. The results of a randomized study by McMurray et al[14] revealed that patients with both chronic kidney disease and anemia are at an increased risk of major CV events by 11.2%. In addition, a history of CHF was the strongest predictor of CV events in the future (hazard increased by 74%), followed by age (hazard increased by 27%)[14]. Moreover, a prospective cohort study involving EPO therapy ranging from 2000 to 6000 international units (I.U.) for 1-3 times per week found that EPO treatment concurrently with iron supplementation improved the functional ability of the myocardium, left ventricular (LV) function, and overall quality of life among patients with chronic forms of the cardiorenal syndrome[15]. Palazzuoli et al[11] also compared the efficacy of combination therapy with EPO and iron to iron alone in patients with cardiorenal syndrome with anemia. They found that concurrent EPO and iron supplementation improved LV systolic function, LV remodeling, New York Heart Association (NYHA) class, B-type natriuretic peptide (BNP) levels, and quality of life compared to a control group that took only oral iron[11]. Eisenga et al[13] found that EPO caused a significant increase in C-terminal FGF23 (cFGF23) and a smaller increase in intact FGF23 (iFGF23). A higher level of cFGF23 has been associated with increased risk of CV events and mortality; however, the underlying mechanism remains unknown[13].
As demonstrated in this review, the exact effects of EPO supplementation, with or without iron supplementation, are difficult to determine. Some studies have even suggested worse CV outcomes, whereas others did not show statistically significant differences in CV mortality. The effects on myocardial function and the reported quality of life appear more promising.
Our review included studies on the effects of EPO therapy on hospitalization rates in patients with CRS. For example, a study by Jackevicius et al[17] found that EPO use was associated with an increased risk of all-cause hospitalization, major CV events, acute coronary syndrome, and mortality. The risk of hospitalization may be due in part to patients receiving EPO having a poor renal function and/or myocardial dysfunction, which places them at an increased risk of hospitalization even before receiving EPO. However, a study by Emans et al[19] showed that baseline EPO levels inversely correlated with serum neutrophil gelatinase-associated lipocalin (NGAL) levels, regardless of renal function, and that low-dose EPO treatment caused a moderate decrease in serum NGAL levels, reflecting a potential improvement in renal tubular damage. NGAL is a protein from the lipocalin family that inhibits bacterial uptake of iron and is a biomarker reflective of tubular damage as it can detect early-stage acute kidney injury[19].
The effects of EPO treatment on the hospitalization rates of patients with CRS remain variable and should therefore be further investigated. Interestingly, EPO reduces NGAL levels, thereby minimizing renal tubular destruction and increasing iron availability. Therefore, this is likely to minimize hospitalization rates as well. The effect of EPO on renal tubular function should be further explored, as this is a potentially significant benefit of EPO therapy in patients with CRS and other renal conditions. For the time being, physicians should determine whether the potential benefits of EPO use in patients with CRS outweigh the risks.
In summary, this systematic review studied the overall significance of EPO when managing patients with CRS. The literature demonstrates that not only does EPO increase patients baseline Hb levels but it also lowers the risk of major CV events from occurring in patients with CRS. EPO improves the heart failure aspect of CRS by both improving cardiac remodeling and overall myocardial function, and by doing so, EPO is able to minimize the CV mortality as well. Moreover, the reviewed literature does not demonstrate a clear effect of EPO on hospitalization rates and hence this effect should be further analyzed in future studies. Lowering hospitalization rates would potentially decrease healthcare expenses and, more importantly, improve the quality of life of patients with this condition. In conclusion, the literature included in this review clearly demonstrates how EPO has several significant benefits when used to treat patients with CRS.
The strongest aspect of this systematic review is that it primarily focused on randomized controlled clinical trials and cohort studies, which provide a more accurate description of the temporal sequence among exposure, EPO treatment, and CRS effects.
This systematic review had some limitations that should be highlighted. A limited amount of total data available regarding this topic increased the risk of publication bias. In addition, some articles discussing this topic had small sample sizes, raising the possibility of cognitive bias. Moreover, the articles were selected from three separate databases with a constriction of only selecting articles written in English.
The literature regarding patients with CRS and the use of EPO therapy should be expanded with more randomized controlled clinical trials and prospective cohort studies. Moreover, since the protocols used for EPO administration varied significantly between studies, further research should be conducted regarding EPO's most optimal dosage and treatment duration. The effect on mortality does not appear to reach statistical significance at this time, which may change with a larger sample size. It is also important for further research to be performed examining the effects of EPO treatment on hospitalization rates, as this association remains unknown at this time due to insufficient data.
We conducted a systematic review to investigate the role of EPO in CRS management. The benefits of EPO therapy in patients with ESRD and CHF are well-established. Thus, it is highly likely that EPO would also be beneficial for patients with CRS. The literature review indicates that EPO improves baseline Hb levels and decreases the risk of major CV events in patients with CRS. The ability of EPO to improve cardiac remodeling, myocardial function, NYHA class, and BNP levels is significant since this will improve the heart failure aspect of CRS and, more importantly, lower the likelihood of death for patients who have this condition. Additionally, the effect of EPO treatment on hospitalization remains unclear and should be further explored as it would benefit patients and potentially reduce healthcare costs. Our review had a few limitations, including the possibility of publication bias, cognitive bias, and the possibility of missing some studies due to the criteria used for selecting articles. Additionally, future randomized controlled clinical trials would benefit the medical community in assessing the use of EPO in treating patients with CRS and specifically examine different EPO dosages and their effects on CV risk, mortality risk, and hospitalization rates. Since CRS is a prevalent condition, the discovery of an effective adjunctive treatment option will significantly affect the clinical outcome of patients with this condition.
Cardiorenal syndrome (CRS) describes a pathophysiological disorder of the heart and kidneys, whereby dysfunction of one system may contribute to dysfunction of another. Many patients with heart failure also experience kidney dysfunction and vice versa, as these two organs are closely related in their functions. Poor renal plasma flow in congestive heart failure (CHF) causes the kidneys to retain water and sodium, which allows for improved perfusion of all essential organs, leading to kidney dysfunction.
Anemia exacerbates CHF's progression and increases the risk of morbidity and mortality, and increasing hemoglobin (Hb) levels can limit the detrimental effects of oxidative stress on the kidney and cardiac function. Erythropoietin (EPO) treatment is an efficient approach to increase Hb levels in patients with CHF whose anemia is caused by reduced EPO production. Investigating the role of EPO in treating patients with CRS is of great significance as it could significantly decrease the mortality and hospitalization of CRS patients and lead to lower healthcare costs.
This systematic review evaluated the use of erythropoietin (EPO) in patients with CRS and its effects on Hb, major cardiovascular (CV) events, and hospitalization rates. The study aimed to deepen our understanding of the role of EPO in managing CRS and whether it can be a promising treatment option for many patients. The primary objective was to assess the efficacy of EPO therapy in improving cardiac and renal function in CRS patients.
The article was written according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The literature search was conducted on PubMed, MEDLINE, and EMBASE databases using a search strategy consisting of medical subject headings terms and regular keywords. Two reviewers independently examined titles and abstracts, followed by a second screening of full-length articles. Quality assessment was performed using the 2013 National Heart, Lung, and Blood Institute (NHLBI) quality assessment tools for controlled intervention studies and observational cohort or cross-sectional studies. A meta-analysis was performed using Review Manager 5.4.1 software, and meta-analysis studies with forest plots were included for the outcomes. In vitro studies, animal studies, abstracts, case reports, case series, systematic reviews, and meta-analyses were excluded. Furthermore, articles in languages other than English were excluded.
This study selected 148 relevant articles and narrowed them down to nine studies that were evaluated using the NHLBI quality assessment tools. The majority of the studies concluded that EPO therapy increased Hb levels in patients with CRS and reduced anemia symptoms such as fatigue, malaise, weakness, and dizziness. However, the degree of Hb improvement varied significantly between the studies and was highly dependent on the dose and frequency of EPO administration. The effect of EPO on CV mortality is difficult to establish as different studies have reported varying outcomes. EPO use was associated with an increased risk of hospitalization, major CV events, acute coronary syndrome, and mortality in some studies. However, other studies found potential benefits of EPO on myocardial function and quality of life. The study's strength lies in its focus on randomized controlled clinical trials and cohort studies, while its limitations include publication bias and small sample sizes.
The literature review provides evidence supporting the use of EPO therapy as a potential treatment option for patients with CRS. The benefits of EPO treatment in improving Hb levels, reducing major CV events, and improving cardiac remodeling, myocardial function, NYHA class, and BNP levels are significant, indicating a positive impact on the heart failure aspect of CRS and lowering the likelihood of death for patients. However, further studies are needed to investigate the effect of EPO treatment on hospitalization rates and potential side effects. Overall, the findings of this review suggest that EPO therapy may be a promising adjunctive treatment option for CRS, and more research is needed to confirm its effectiveness and optimize its use in clinical practice.
In terms of future research perspectives, it is crucial to investigate the potential long-term effects of EPO therapy in patients with CRS. Since CRS is a chronic condition, examining the long-term effects of EPO treatment will provide valuable insights into its efficacy and safety. Additionally, future studies should explore the potential of combining EPO therapy with other treatments for CRS, such as angiotensin-converting enzyme inhibitors and beta blockers, to determine if combination therapy could improve clinical outcomes. Finally, future research should also aim to identify biomarkers that can predict a patient's response to EPO therapy, which would allow for more personalized treatment approaches.
| 1. | Ronco C, Bellasi A, Di Lullo L. Cardiorenal Syndrome: An Overview. Adv Chronic Kidney Dis. 2018;25:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 2. | Costanzo MR. The Cardiorenal Syndrome in Heart Failure. Heart Fail Clin. 2020;16:81-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 3. | Kumar U, Wettersten N, Garimella PS. Cardiorenal Syndrome: Pathophysiology. Cardiol Clin. 2019;37:251-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 4. | Uduman J. Epidemiology of Cardiorenal Syndrome. Adv Chronic Kidney Dis. 2018;25:391-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Silverberg DS, Wexler D, Iaina A, Steinbruch S, Wollman Y, Schwartz D. Anemia, chronic renal disease and congestive heart failure--the cardio renal anemia syndrome: the need for cooperation between cardiologists and nephrologists. Int Urol Nephrol. 2006;38:295-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Pagourelias ED, Koumaras C, Kakafika AI, Tziomalos K, Zorou PG, Athyros VG, Karagiannis A. Cardiorenal anemia syndrome: do erythropoietin and iron therapy have a place in the treatment of heart failure? Angiology. 2009;60:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Nangaku M, Fliser D. Erythropoiesis-stimulating agents: past and future. Kidney Int Suppl. 2007;S1-S3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | van der Putten K, Jie KE, Emans ME, Verhaar MC, Joles JA, Cramer MJ, Velthuis BK, Meiss L, Kraaijenhagen RJ, Doevendans PA, Braam B, Gaillard CA. Erythropoietin treatment in patients with combined heart and renal failure: objectives and design of the EPOCARES study. J Nephrol. 2010;23:363-368. [PubMed] |
| 9. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 52342] [Article Influence: 10468.4] [Reference Citation Analysis (2)] |
| 10. | Study Quality Assessment Tools. (2021). Accessed: March 20, 2022, 2022. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. |
| 11. | Palazzuoli A, Silverberg DS, Iovine F, Calabrò A, Campagna MS, Gallotta M, Nuti R. Effects of beta-erythropoietin treatment on left ventricular remodeling, systolic function, and B-type natriuretic peptide levels in patients with the cardiorenal anemia syndrome. Am Heart J. 2007;154:645.e9-645.15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Jackevicius CA, Co MJ, Warner AL. Predictors of erythropoietin use in patients with cardiorenal anaemia syndrome. Int J Pharm Pract. 2015;23:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Eisenga MF, Emans ME, van der Putten K, Cramer MJ, Diepenbroek A, Velthuis BK, Doevendans PA, Verhaar MC, Joles JA, Bakker SJL, Nolte IM, Braam B, Gaillard CAJM. Epoetin Beta and C-Terminal Fibroblast Growth Factor 23 in Patients With Chronic Heart Failure and Chronic Kidney Disease. J Am Heart Assoc. 2019;8:e011130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | McMurray JJ, Uno H, Jarolim P, Desai AS, de Zeeuw D, Eckardt KU, Ivanovich P, Levey AS, Lewis EF, McGill JB, Parfrey P, Parving HH, Toto RM, Solomon SD, Pfeffer MA. Predictors of fatal and nonfatal cardiovascular events in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia: an analysis of the Trial to Reduce cardiovascular Events with Aranesp (darbepoetin-alfa) Therapy (TREAT). Am Heart J. 2011;162:748-755.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Fazlibegović E, Hadziomerović M, Corić S, Babić E, Fazlibegović F. Erythropoietin in cardiorenal anemia syndrome. Bosn J Basic Med Sci. 2006;6:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Jie KE, van der Putten K, Bergevoet MW, Doevendans PA, Gaillard CA, Braam B, Verhaar MC. Short- and long-term effects of erythropoietin treatment on endothelial progenitor cell levels in patients with cardiorenal syndrome. Heart. 2011;97:60-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Jackevicius CA, Fan CS, Warner A. Clinical outcomes of erythropoietin use in heart failure patients with anemia of chronic kidney disease. J Card Fail. 2014;20:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Jie KE, van der Putten K, Wesseling S, Joles JA, Bergevoet MW, Pepers-de Kort F, Doevendans PA, Yasui Y, Liu Q, Verhaar MC, Gaillard CA, Braam B. Short-term erythropoietin treatment does not substantially modulate monocyte transcriptomes of patients with combined heart and renal failure. PLoS One. 2012;7:e41339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Emans ME, Braam B, Diepenbroek A, van der Putten K, Cramer MJ, Wielders JP, Swinkels DW, Doevendans PA, Gaillard CA. Neutrophil gelatinase-associated lipocalin (NGAL) in chronic cardiorenal failure is correlated with endogenous erythropoietin levels and decreases in response to low-dose erythropoietin treatment. Kidney Blood Press Res. 2012;36:344-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Auerbach M, Ballard H, Trout JR, McIlwain M, Ackerman A, Bahrain H, Balan S, Barker L, Rana J. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: a multicenter, open-label, randomized trial. J Clin Oncol. 2004;22:1301-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 285] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wu YJ, China; Zhao XC, China S-Editor: Liu JH L-Editor: Filipodia P-Editor: Yu HG