Published online Apr 26, 2023. doi: 10.4330/wjc.v15.i4.154
Peer-review started: January 11, 2023
First decision: January 31, 2023
Revised: February 6, 2023
Accepted: April 7, 2023
Article in press: April 7, 2023
Published online: April 26, 2023
Processing time: 99 Days and 4.8 Hours
We frequently encounter cases of women with vasospastic angina (VSA). Additionally, some women with VSA are younger than 60 years old. However, it is unknown whether the characteristics of VSA in women aged < 60 years are different from those in women aged ≥ 60 years.
To investigate and compare the clinical characteristics and prognosis of VSA in women aged < 60 years from those in women aged ≥ 60 years.
We enrolled 94 women with VSA who were diagnosed using the spasm provocation test. According to the age at diagnosis, the patients were divided into two groups: Group Y (age < 60 years, n = 17) and Group O (age ≥ 60 years, n = 77). Flow-mediated dilation (FMD) and nitroglycerin (NTG)-induced dilation (NID) of the brachial artery were performed and assessed using brachial ultrasonography. Moreover, conventional coronary risk factors, such as atherosclerotic lesions (stenosis > 20%) detected using coronary angiography and focal spasms (coronary spasm within one segment of one coronary artery), and major cardiovascular adverse events (MACE) were assessed in both groups.
Smoking was more prevalent in Group Y than in Group O (P = 0.04). FMD was similar in both groups (Group O: 4.3% ± 3.2%, Group Y: 4.5% ± 3.3%; P = 0.75), whereas NID was higher in Group Y (20.5% ± 8.6%) than in Group O (13.6% ± 5.3%, P < 0.01). Atherosclerosis was not detected in Group Y but was detected in Group O (61%, P < 0.01). Focal spasms were less frequent in Group Y (12%) than in Group O (38%, P = 0.04). The incidence of major adverse cardiac events did not differ between the two groups (P = 0.40).
Women aged < 60 years with VSA have less atherosclerotic lesions and focal spasms. These characteristics may be affected by smoking habits and vascular smooth muscle dysfunction.
Core Tip: We investigated whether the clinical background and prognosis of women aged < 60 years with vasospastic angina (VSA) differ from those of women aged ≥ 60 years with VSA. We showed that smoking was more frequent in women aged < 60 years with VSA. We found a significantly greater peripheral vascular response to nitroglycerin in such patients. Coronary angiography revealed fewer atherosclerotic lesions and focal spasms in such patients. Smoking status and vascular dysfunction may have influenced the above clinical characteristics in women aged < 60 years with VSA.
- Citation: Teragawa H, Oshita C, Uchimura Y. Vasospastic angina in women: Clinical backgrounds and prognoses of patients younger than and older than 60 years. World J Cardiol 2023; 15(4): 154-164
- URL: https://www.wjgnet.com/1949-8462/full/v15/i4/154.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i4.154
Vasospastic angina (VSA) is a condition characterized by transient hypercontraction of the epicardial coronary arteries, leading to myocardial ischemia[1,2]. Although the incidence of coronary artery disease is higher in men[3], the incidence of VSA is relatively higher in women[4,5]. Therefore, several reports have investigated gender differences among patients with VSA[5-9], concluding that women have a lower positivity rate than men during the spasm provocation test (SPT)[5-9], and that focal spasms occur more frequently in men than in women[8,10].
Among women with VSA, we have encountered cases of patients younger than 60 years old. In a paper by Kawana et al[6] that evaluated the characteristics of women with VSA by age, smoking was more prevalent in younger women with VSA, but the prevalence of hypertension was lower in this population. Additionally, women aged < 50 years with VSA had a worse prognosis. Aside from this study, few reports have examined the characteristics of women with VSA at an early age.
Therefore, in the present study, we retrospectively investigated the differences in clinical characteristics and prognosis between women aged < 60 years and ≥ 60 years with VSA.
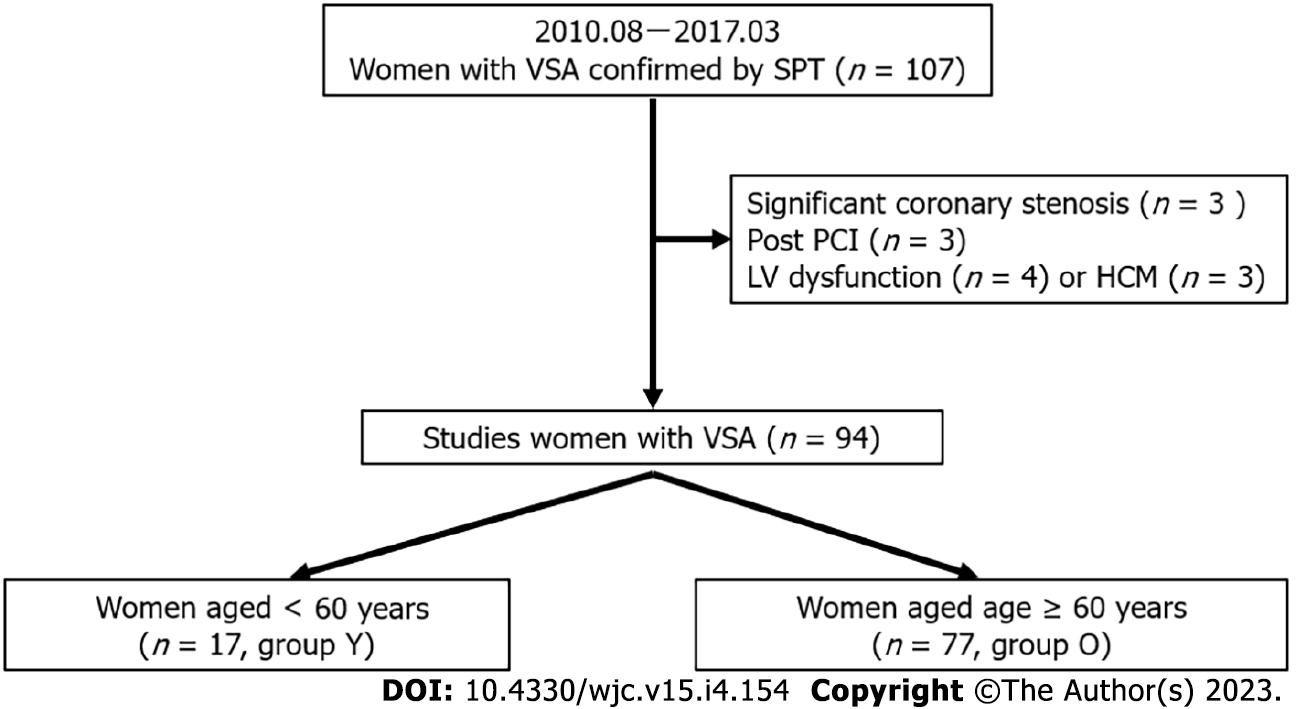
This observational retrospective study included female patients with VSA who were diagnosed using SPT at our institution between August 2010 and March 2017 (n = 107). The exclusion criteria were as follows: significant coronary stenosis (stenosis > 50%, n = 3), or a previous medical history of percutaneous coronary intervention (n = 3), heart failure (n = 4), and hypertrophic cardiomyopathy (n = 3). Ultimately, 94 women VSA were enrolled in the study. The mean and median ages of the patients at diagnosis were 69 ± 10 years and 71 (63, 76) years, respectively; the 25th percentile was 63 years. However, the cut-off age for this study was set at 60 years. Hence, the patients were classified into two groups based on the cut-off age: Group Y (age < 60 years, n = 17) and Group O (age ≥ 60 years, n = 77, Figure 1). The study protocol was approved by the ethics committee of our institution. Written informed consent was obtained from all participants.
SPT was carried out in accordance with our prior description[4,11,12]. At our institution, SPT of the right coronary artery (RCA) was carried out continuously. Acetylcholine (ACh) dosages of 20 and 50 mg were injected into the RCA following initial coronary angiography (CAG). When coronary spasm was not induced by 50 mg of ACh, ACh was continuously administered until the maximum dose of 80 mg. CAG was then performed after administration of the maximum dose of ACh or induction of coronary spasms, whichever came first. SPT of the left coronary artery (LCA) was carried out without intracoronary injection of nitroglycerin (NTG) into the RCA if the coronary spasms spontaneously resolved. In such circumstances, NTG injection into the RCA was performed after performing the SPT for the LCA. An intracoronary injection of 0.3 mg NTG was administered to ease spasms if the Ach-induced coronary spasms were severe enough to cause hemodynamic instability; this was referred to as the unavoidable use of NTG[13]. SPT of the LCA was then carried out using 50 and 100 mg of ACh. When coronary spasm was not induced by 100 mg of ACh, ACh was continuously administered until the maximum dose of 200 mg. CAG was then performed after administration of the maximum dose of ACh or the induction of coronary spasms, whichever came first. The final CAG for the LCA was performed after an intracoronary injection of 0.3 mg of NTG.
As previously shown[4], we employed an autoinjector. The coronary artery diameter was measured in accordance with the previous methods[4]. Atherosclerotic lesions were defined as those with a stenosis > 20%. We also explored the likelihood of myocardial bridging (MB), which was defined as systolic reduction > 20% in the coronary artery diameter[14].
Angina pectoris was classified into three patterns: resting, exertion, and both resting and exertion. For anginal symptoms, the number of attacks per month, maximum attack duration (minutes), and estimated duration of disease (months) were also calculated. VSA was defined as > 90% narrowing of coronary arteries on angiograms when provoked and accompanied by the presence of usual chest pain and/or the presence of an ST-segment deviation on electrocardiogram (ECG)[15]. Focal spasm was defined as transient vessel narrowing of > 90% within the borders of one isolated coronary segment, as defined by the American Heart Association. Diffuse spasm was defined as 90% diffuse vasoconstriction observed in ≥ 2 adjacent coronary segments of the coronary arteries[10]. Multivessel spasms (MVS) were defined as coronary spasms that occurred in ≥ 2 major coronary arteries. For multivessel spasms, we could not assess when the subsequent SPT was negative after an unavoidable use of NTG[16]. Regarding the presence of coronary spasm per vessel, the frequency of coronary spasms in the left anterior descending coronary artery (LAD), left circumflex coronary artery (LCX), and RCA were reviewed.
Patients were asked about their smoking status and family history of coronary artery disease. Smoking status was classified as active smokers, former smokers (had stopped smoking for at least 1 mo), or never smokers. In the logistic analysis, smoking was defined as the combined number of active and former smokers. Hypertension, dyslipidemia, diabetes mellitus, metabolic syndrome (MtS), and chronic kidney disease (CKD) were defined based on the standard definitions described in previous papers[4]. Patients or family members were asked about their alcohol consumption, and those who drank at least once a week were defined as having alcohol consumption[17]. Blood chemical parameters, including the estimated glomerular filtration ratio (eGFR, mL/min/1.73 m2) and brain natriuretic peptide level (BNP, pg/mL), were routinely investigated on the same day of CAG. The left ventricular ejection fraction was measured using cardiac ultrasonography. On brachial echosonography, flow-mediated dilation (FMD), as an endothelium-dependent function, and NTG-induced dilation (NID), as an endothelium-independent function, were measured as previously described[18].
All study participants made at least one follow-up visit at our facility, and patients were followed-up as closely as was practical after discharge. The last date of data collection was in October 2022. Information from the medication diaries of patients who had recently made a follow-up visit was included in the follow-up assessments. We recorded the number of consumed coronary vasodilators monthly and angina events over the past 3 mo. These evaluations were performed on patients who could be assessed at least 6 mo after discharge (n = 85). The number of coronary vasodilators used was also evaluated during hospital admission, discharge, and final follow-up. For each patient, cardiac events, including readmission for angina or other cardiovascular conditions, were recorded. Readmission due to cardiovascular conditions or death from cardiac causes were considered as major adverse cardiac events (MACEs).
Data are presented as mean ± standard deviation or median with interquartile ranges for non-normally distributed data and non-continuous variables. Baseline characteristics of the groups were compared using Student’s unpaired t-tests, Wilcoxon signed-rank tests, or χ2 analysis, as appropriate. Logistic regression analysis was used to determine the presence of VAS in Group Y. MACEs were analyzed using the Kaplan–Meier survival curve and the logrank test. JMP Ver. 16 (SAS Institute Inc., Cary, NC, United States) was used to perform all statistical analyses. A P value < 0.05 was considered statistically significant.
There were 17 patients (18%) in Group Y and 77 (82%) in Group O. The patients’ characteristics are shown in Table 1. Group Y had a mean age of 54 ± 5 years and Group O had a mean age of 72 ± 7 years; the mean age was significantly lower in Group Y (P < 0.01). Although smoking was more prevalent in Group Y (P < 0.01), hypertension (P < 0.01) and CKD (P = 0.02) were less prevalent. A trend toward more frequent alcohol consumption was observed in Group Y (P = 0.09).
| Group O | Group Y | P value | ||
| No. (%) | 77 (82) | 17 (18) | ||
| Age (yr) | 72 ± 7 | 54 ± 5 | < 0.01 | |
| Body mass index | 23.7 ± 4.5 | 24.5 ± 5.3 | 0.50 | |
| Coronary risk factors (%) | ||||
| Smoking (active/former/never) | 3/5/69 | 3/4/10 | < 0.01 | |
| Hypertension | 58 (75) | 7 (41) | < 0.01 | |
| Dyslipidemia | 55 (77) | 10 (59) | 0.31 | |
| Diabetes mellitus | 12 (16) | 2 (12) | 0.68 | |
| Alcohol consumer (%) | 10 (13) | 5 (29) | 0.09 | |
| Family history of CAD (%) | 18 (23) | 5 (29) | 0.60 | |
| MtS (%) | 13 (17) | 4 (24) | 0.52 | |
| CKD (%) | 27 (35) | 1 (6) | 0.02 | |
Regarding the blood chemical parameters, eGFR was higher in Group Y than in Group O (P = 0.05), and BNP levels tended to be lower in Group Y (P = 0.09, Table 2). Regarding the brachial ultrasonographic parameters (Table 3), the brachial artery diameter at baseline (P = 0.83) and FMD (P = 0.75) were not different between the two groups, but NID was significantly higher in Group Y (20.6% ± 8.6%) than in Group O (13.6% ± 5.3%, P < 0.01). Brachial ultrasonography was performed after at least 48 h from withdrawal of coronary dilators. The results were similar in patients who had not been taking coronary dilators to rule out the effects of these drugs.
| Group O | Group Y | P value | |
| Total cholesterol (mg/dL) | 202 ± 33 | 196 ± 37 | 0.53 |
| Triglyceride (mg/dL) | 131 ± 73 | 119 ± 46 | 0.54 |
| HDL-cholesterol (mg/dL) | 63 ± 17 | 62 ± 18 | 0.82 |
| LDL-cholesterol (md/dL) | 113 ± 29 | 111 ± 32 | 0.82 |
| Fasting blood sugar (md/dL) | 100 ± 16 | 101 ± 17 | 0.93 |
| Hemoglobin A1C (%) | 6.0 ± 0.7 | 5.7 ± 0.6 | 0.10 |
| C-reactive protein (mg/dL) | 0.05 (0.02, 0.13) | 0.07 (0.02, 0.15) | 0.81 |
| eGFR (mL/min/1.73 m2) | 68.8 ± 16.8 | 77.5 ± 13.2 | 0.05 |
| BNP (pg/mL) | 22 (14, 54) | 15 (10, 29) | 0.09 |
| Group O | Group Y | P value | |||
| UCG | |||||
| LVEF (%) | 68 ± 9 | 66 ± 6 | 0.46 | ||
| Brachial ultrasonography | |||||
| All studied patients | |||||
| No. | 77 | 17 | |||
| Heart rate (/min) | 66 ± 10 | 67 ± 13 | 0.85 | ||
| Mean blood pressure | 100 ± 14 | 94 ± 14 | 0.16 | ||
| Brachial blood flow | |||||
| Baseline (mL/min) | 61 ± 44 | 59 ± 52 | 0.87 | ||
| % increase | 384 ± 490 | 327 ± 265 | 0.59 | ||
| Brachial artery diameter (mm) | |||||
| Baseline | 3.5 ± 0.5 | 3.5 ± 0.5 | 0.83 | ||
| Hyperemia | 3.7 ± 0.5 | 3.6 ± 0.5 | 0.88 | ||
| After NTG | 4.0 ± 0.5 | 4.2 ± 0.4 | 0.25 | ||
| FMD (%) | 4.3 ± 3.2 | 4.5 ± 3.3 | 0.75 | ||
| NID (%) | 13.6 ± 5.4 | 20.5 ± 8.6 | < 0.01 | ||
| Patients who did not take any coronary vasodilators | |||||
| No. | 39 | 14 | |||
| Brachial artery diameter (mm) | |||||
| Baseline | 3.5 ± 0.5 | 3.6 ± 0.4 | 0.68 | ||
| Hyperemia | 3.6 ± 0.6 | 3.7 ± 0.4 | 0.60 | ||
| After NTG | 4.0 ± 0.5 | 4.2 ± 0.4 | 0.21 | ||
| FMD (%) | 4.1 ± 3.1 | 4.3 ± 3.5 | 0.82 | ||
| NID (%) | 14.6 ± 5.7 | 18.5 ± 6.5 | 0.04 | ||
Logistic regression analysis showed that NID [odds ratio (OR): 5.1, P = 0.02] and absence of CKD (OR: 7.5, P < 0.01) and hypertension (OR: 4.5, P = 0.03) were factors responsible for the presence of women aged < 60 years (R2 = 0.32), while smoking tended to be associated with it (OR: 3.7, P = 0.06).
There were no differences between the two groups on whether angina occurred at rest or with exertion nor in the number of attacks, maximum attack duration, or estimated duration of illness (Table 4). The frequency of coronary dilator intake (P = 0.02) and number of coronary dilators taken before admission (P = 0.01) were significantly lower in Group Y.
| Group O | Group Y | P value | |||
| Chest symptoms | |||||
| Rest/Exercise/Both | 60/9/9 | 14/1/2 | 0.78 | ||
| Maximum duration of attack (min) | 20 ± 27 | 16 ± 28 | 0.10 | ||
| Diseased duration (M) | 5 (1, 48) | 12 (3, 42) | 0.78 | ||
| No. of anginal attacks (/M) | |||||
| At admission | 4 (1, 10) | 4 (1, 10) | 0.43 | ||
| At follow-up | 0 (0, 1) | 2 (0.1, 2.8) | < 0.01 | ||
| No. | 69 | 16 | |||
| Medications | |||||
| Taking statins at admission (%) | 36 (47) | 7 (46) | 0.68 | ||
| Taking antiplatelet drugs at admission (%) | 20 (26) | 1 (6) | 0.07 | ||
| Taking vasodilators at admission (%) | 38 (49) | 3 (18) | 0.02 | ||
| No. coronary vasodilators | |||||
| At admission | 0 (0,1) | 0 (0, 0) | 0.01 | ||
| 0.6 ± 0.7 | 0.2 ± 0.4 | 0.01 | |||
| At discharge | 1 (1, 1) | 1 (1, 1) | 0.01 | ||
| 1.2 ± 0.5 | 0.9 ± 0.3 | 0.02 | |||
| At follow-up | 1 (1, 2) | 1.5 (1, 2) | 0.52 | ||
| 1.5 ± 0.9 | 1.6 ± 0.9 | 0.53 | |||
Regarding CAG (Table 5), the prevalence of atherosclerosis was significantly lower in Group Y (P < 0.01), but that of MB did not differ between the two groups (P = 0.94). Regarding SPT (Table 5), the frequency of focal spasms was significantly lower in Group Y (P = 0.04), while the frequency of MVS was not significantly different among those that underwent evaluation (P = 0.56). The frequency of coronary spasms in the LAD and RCA was not different between the two groups; however, the frequency of coronary spasms in the LCX was significantly higher in Group Y (P < 0.01). The frequency of unavoidable use of NTG was also significantly higher in Group Y (P = 0.01). The incidence of ST-segment elevation on ECG during coronary spasms tended to be higher in Group Y (P = 0.09).
| Group O | Group Y | P value | ||
| CAG | ||||
| Atherosclerotic change (%) | 47 (61) | 0 (0) | < 0.01 | |
| Myocardial bridging (%) | 13 (17) | 3 (18) | 0.94 | |
| SPT | ||||
| Focal/diffuse/focal and diffuse | 16/48/33 | 0/15/2 | 0.08 | |
| Presence of focal spasm (%) | 29 (38) | 2 (12) | 0.04 | |
| Multi-vessels spasm (%, No.) | 39 (57, 68) | 8 (67, 12) | 0.56 | |
| Vessels of spasm | ||||
| RCA (%, No.) | 44 (62, 71) | 10 (67, 15) | 0.72 | |
| LAD (%, No.) | 70 (96, 73) | 13 (93, 14) | 0.62 | |
| LCX (%, No.) | 5 (7, 72) | 5 (38, 13) | < 0.01 | |
| An unavoidable use of NTG (%) | 14 (18) | 8 (47) | 0.01 | |
| ST deviation during SPT (%) | 10 (13) | 5 (29) | 0.09 | |
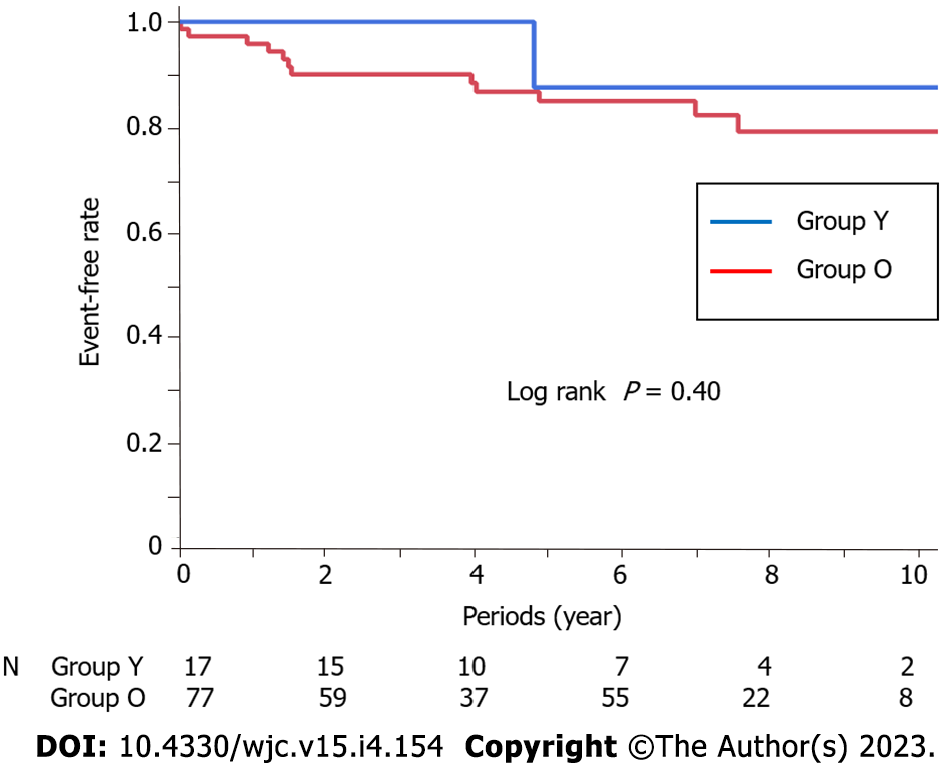
The number of prescribed coronary dilators at discharge was significantly lower in Group Y (P = 0.01). The median follow-up period was 6.4 (3.9, 8.4) years, with no difference between the two groups (Group Y: 4.3 years, Group O: 6.8 years, P = 0.12). There was no difference in the number of coronary dilators taken at the time of the last follow-up in patients who had been followed for more than 6 mo (P = 0.52), but the number of chest symptoms per month was significantly higher in Group Y (P < 0.01). There was no significant difference in the number of MACEs between the two groups (Figure 2, Logrank P = 0.40).
The present study investigated the clinical characteristics and prognosis of women aged < 60 years with VSA compared to those in women aged ≥ 60 years with VSA. Our results showed that women aged < 60 years with VSA were more likely to be smokers and less likely to have hypertension and CKD. Additionally, they had very good peripheral vascular function as indicated by their response to NTG. The results of CAG and SPT showed that there was less atherosclerosis and less focal spasm in women aged < 60 years with VSA. However, the frequency of coronary spasms in the LCX was high, and NTG was unavoidably used in this population. Additionally, the prognosis of women aged < 60 years with VSA was favorable as long as coronary dilators were strictly administered, although their chest symptoms persisted. These clinical characteristics should be considered in the treatment and follow-up of such patients.
Although there have been several reports on the characteristics of women with VSA[5-10], few reports have explored the characteristics of VSA by age[6]. Kawana et al[6] classified patients with VSA based on age: Under 50 years, 50–64 years, and over 65 years, and they found that although the prevalence of hypertension and dyslipidemia was lower in younger patients, the prevalence of smoking was higher. In the present study, the prevalence of hypertension and CKD, which was possibly induced by hypertension itself, was also significantly lower in women aged < 60 years, but these findings appear to be age-related and not limited to the presence of VSA or gender[19,20]. Meanwhile, the same was true for the prevalence of smoking in the present study, confirming that smoking is more frequent in younger age groups. Smoking is a risk factor for coronary spasms even in young women[21], and it was reported that smoking causes hypercontraction of vascular smooth muscles through the activation of Rho kinase[22] and/or vascular endothelial dysfunction through increased production of reactive oxygen species[23]. Thus, smoking may be an etiologic factor of VSA in women aged < 60 years.
On the other hand, NID of the brachial artery was higher in women aged < 60 years with VSA and was still a significant and influential factor even when smoking was included in the logistic regression analysis. Meanwhile, FMD did not differ between the two groups. These findings cannot be fully explained but may indicate relative vascular endothelial dysfunction and/or vascular smooth muscle hypercontraction. Smoking may have caused these vascular dysfunctions, but it is also possible that the relative decline in sex hormones during menopause may cause these changes[24]. Furthermore, it is also possible that there is a genetic problem with eNOS that may have caused vascular dysfunction[25], although we have not found significant differences in the family history of CAD between the two groups. Future large studies or registries should carefully evaluate age-specific vascular dysfunction in women with VSA.
Regarding CAD and SPT, women aged < 60 years with VSA had less atherosclerosis, which could be explained by age-related changes regardless of the presence of VSA or gender. Focal spasms were also less frequent in women aged < 60 years, which may also be related to fewer atherosclerotic lesions. Several studies have shown that focal spasm is more likely to occur at sites with atherosclerotic lesions[26,27]. However, the frequency of coronary spasm in the LCX was significantly higher in women aged < 60 years. Sueda et al[28] showed that coronary spasms in the LCX was significantly less than those in the RCA or LAD (28%), suggesting that the distribution of muscarinic receptors may differ according to the coronary artery vessel. Furthermore, Sueda et al[7] did not report any differences in terms of sex regarding the frequency of coronary spasms in the LCX. In the present study, SPT was initiated in the RCA and shifted to the LCA; it is possible that the frequency of coronary spasms in the LCX may differ depending on where SPT is initiated. In any case, the fact that coronary spasms in the LCX were more frequent in women aged < 60 years suggests that muscarinic receptor distribution may change with age in women with VSA. The unavoidable use of NTG was reported to be associated with more active coronary spasms[13], which may suggest that women aged < 60 years with VSA have more active coronary spasms.
Regarding the prognosis, Kawana et al[6] reported that women aged < 50 years with VSA had poorer prognoses than those aged ≥ 50 years. In the present study, the prognoses of patients aged < 60 years and ≥ 60 years were similar. This may be due to the small number of cases and the cut-off age of 60 years in this study rather than 50 years. Nevertheless, the fact that focal spasm, a marker of poor prognosis[10,27], was less frequent in patients younger than 60 years and that younger women with VSA have a poorer prognosis[6] and required more coronary dilators may explain the similar prognosis in the two groups. Chest symptoms were significantly more frequent at follow-up in younger patients with VSA, possibly indicating that these patients had more active coronary spasms.
The implications of the present study are as follows: vascular dysfunction is present in relatively young patients with VSA, and since smoking may be a risk factor, it may be important to encourage women to quit smoking immediately. Additionally, because these patients may have more active coronary spasms, it is important to monitor and maintain them with increasing doses of coronary dilators to improve chest symptoms.
This study had several limitations. First, it was a single-center study with a small number of patients, and the distribution of patients was unequal in the studied groups. Thus, the results may not be applicable to all patients experiencing coronary spasms. Furthermore, due to the small number of cases, it was not possible to classify the patients into three groups as in the study of Kawana et al[6]. More studies with considerable sample size are needed to support the findings in this study. Second, this study was conducted on women with VSA, and we do not have data from our institution regarding vascular function in men with VSA or in healthy women. Therefore, it is difficult to conclude whether the findings in this study are truly characteristic of women aged < 60 years with VSA. Future large studies and multicenter registries should clarify this issue. Finally, brachial artery echocardiography was performed on the day before SPT and after discontinuation of coronary dilators. We concluded that these findings were true because the results were similar in patients who were not taking coronary dilators. Nevertheless, we cannot rule out the possibility that the results of brachial artery echocardiography may have been influenced by the residual effects of withdrawal of coronary vasodilators.
In conclusion, we examined the clinical characteristics and prognosis of women aged < 60 years with VSA and compared them to women aged ≥ 60 years with VSA, revealing that these patients were more likely to be smokers and have vascular dysfunction. The frequency of atherosclerosis and focal spasms was low, but the frequency of coronary spasms in the LCX was high. They were also more likely to unavoidably use NTG, suggesting that they may have more active coronary spasms. Such patients should be carefully monitored by increasing the use of coronary dilators and encouraged to quit smoking, if they smoke. Cardiologists need to be reminded that young women with VSA have high coronary spasm activity.
We frequently encounter cases of women with vasospastic angina (VSA). Additionally, some women with VSA are younger than 60 years old.
However, it is unknown whether the characteristics of VSA in women aged <60 years are different from those in women aged ≥ 60 years.
The objective of the present study was to investigate and compare the clinical characteristics and prognosis of VSA in women aged < 60 years from those in women aged ≥ 60 years.
We enrolled 94 women with VSA who were diagnosed using the spasm provocation test (SPT). According to the age at diagnosis, the patients were divided into two groups: Group Y (age < 60 years, n = 17) and Group O (age ≥ 60 years, n = 77). Flow-mediated dilation (FMD) and nitroglycerin (NTG)-induced dilation (NID) of the brachial artery were performed and assessed using brachial ultrasonography. Moreover, conventional coronary risk factors, such as atherosclerotic lesions (stenosis > 20%) detected using coronary angiography and focal spasms (coronary spasm within one segment of one coronary artery), and major cardiovascular adverse events (MACE) were assessed in both groups.
Smoking was more prevalent in Group Y than in Group O (P = 0.04). FMD was similar in both groups (Group O: 4.3% ± 3.2%, Group Y: 4.5% ± 3.3%; P = 0.75), whereas NID was higher in Group Y (20.5% ± 8.6%) than in Group O (13.6% ± 5.3%, P < 0.01). Atherosclerosis was not detected in Group Y but was detected in Group O (61%, P < 0.01). Focal spasms were less frequent in Group Y (12%) than in Group O (38%, P = 0.04). The incidence of MACEs did not differ between the two groups (P = 0.40).
Women aged < 60 years with VSA have less atherosclerotic lesions and focal spasms. These characteristics may be affected by smoking habits and vascular smooth muscle dysfunction.
Vascular dysfunction is present in relatively young patients with VSA, and since smoking may be a risk factor, it may be important to encourage women to quit smoking immediately. Additionally, because these patients may have more active coronary spasms, it is important to monitor and maintain them with increasing doses of coronary dilators to improve chest symptoms.
We thank Ms. Akemi Seno for her secretarial assistance. We also thank the staff of the catheterization laboratory, cardiovascular ward, and cardiovascular outpatient clinic.
| 1. | Yasue H, Nakagawa H, Itoh T, Harada E, Mizuno Y. Coronary artery spasm--clinical features, diagnosis, pathogenesis, and treatment. J Cardiol. 2008;51:2-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 383] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 2. | Jewulski J, Khanal S, Dahal K. Coronary vasospasm: A narrative review. World J Cardiol. 2021;13:456-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (2)] |
| 3. | Yasuda S, Kaikita K, Akao M, Ako J, Matoba T, Nakamura M, Miyauchi K, Hagiwara N, Kimura K, Hirayama A, Matsui K, Ogawa H; AFIRE Investigators. Antithrombotic Therapy for Atrial Fibrillation with Stable Coronary Disease. N Engl J Med. 2019;381:1103-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 414] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 4. | Teragawa H, Oshita C, Ueda T. History of gastroesophageal reflux disease in patients with suspected coronary artery disease. Heart Vessels. 2019;34:1631-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Saito Y, Saito Y, Kato K, Kobayashi Y. Gender differences in factors associated with vasospastic angina. Int J Cardiol. 2022;349:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 6. | Kawana A, Takahashi J, Takagi Y, Yasuda S, Sakata Y, Tsunoda R, Ogata Y, Seki A, Sumiyoshi T, Matsui M, Goto T, Tanabe Y, Sueda S, Kubo N, Momomura S, Ogawa H, Shimokawa H; Japanese Coronary Spasm Association. Gender differences in the clinical characteristics and outcomes of patients with vasospastic angina--a report from the Japanese Coronary Spasm Association. Circ J. 2013;77:1267-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Sueda S, Miyoshi T, Sasaki Y, Sakaue T, Habara H, Kohno H. Gender differences in sensitivity of acetylcholine and ergonovine to coronary spasm provocation test. Heart Vessels. 2016;31:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Sueda S, Sakaue T. Sex-related Differences in Patients with Positive Coronary Spasm as Identified by Acetylcholine Testing. Intern Med. 2021;60:2357-2365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Park JY, Choi SY, Rha SW, Choi BG, Noh YK, Kim YH. Sex Difference in Coronary Artery Spasm Tested by Intracoronary Acetylcholine Provocation Test in Patients with Nonobstructive Coronary Artery Disease. J Interv Cardiol. 2022;2022:5289776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Sato K, Kaikita K, Nakayama N, Horio E, Yoshimura H, Ono T, Ohba K, Tsujita K, Kojima S, Tayama S, Hokimoto S, Matsui K, Sugiyama S, Yamabe H, Ogawa H. Coronary vasomotor response to intracoronary acetylcholine injection, clinical features, and long-term prognosis in 873 consecutive patients with coronary spasm: analysis of a single-center study over 20 years. J Am Heart Assoc. 2013;2:e000227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 11. | Teragawa H, Oshita C, Orita Y. Clinical significance of prolonged chest pain in vasospastic angina. World J Cardiol. 2020;12:450-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Suzuki S, Kaikita K, Yamamoto E, Jinnouchi H, Tsujita K. Role of acetylcholine spasm provocation test as a pathophysiological assessment in nonobstructive coronary artery disease. Cardiovasc Interv Ther. 2021;36:39-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Teragawa H, Oshita C, Uchimura Y. Clinical Characteristics and Prognosis of Patients with Vasospastic Angina Subjected to the Spasm Provocation Test and the Unavoidable Use of Nitroglycerin. J Cardiovasc Dev Dis. 2023;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 14. | Teragawa H, Oshita C, Uchimura Y. The Impact of Myocardial Bridging on the Coronary Functional Test in Patients with Ischaemia with Non-Obstructive Coronary Artery Disease. Life (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J. 2014;78:2779-2801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 361] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 16. | Teragawa H, Oshita C, Uchimura Y. Clinical Characteristics and Prognosis of Patients with Multi-Vessel Coronary Spasm in Comparison with Those in Patients with Single-Vessel Coronary Spasm. J Cardiovasc Dev Dis. 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 17. | Teragawa H, Fukuda Y, Matsuda K, Higashi Y, Yamagata T, Matsuura H, Chayama K. Effect of alcohol consumption on endothelial function in men with coronary artery disease. Atherosclerosis. 2002;165:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Teragawa H, Oshita C, Uchimura Y, Akazawa R, Orita Y. Coronary Microvascular Vasodilatory Function: Related Clinical Features and Differences According to the Different Coronary Arteries and Types of Coronary Spasm. J Clin Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, Horio T, Hoshide S, Ikeda S, Ishimitsu T, Ito M, Ito S, Iwashima Y, Kai H, Kamide K, Kanno Y, Kashihara N, Kawano Y, Kikuchi T, Kitamura K, Kitazono T, Kohara K, Kudo M, Kumagai H, Matsumura K, Matsuura H, Miura K, Mukoyama M, Nakamura S, Ohkubo T, Ohya Y, Okura T, Rakugi H, Saitoh S, Shibata H, Shimosawa T, Suzuki H, Takahashi S, Tamura K, Tomiyama H, Tsuchihashi T, Ueda S, Uehara Y, Urata H, Hirawa N. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res. 2019;42:1235-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 1421] [Article Influence: 236.8] [Reference Citation Analysis (0)] |
| 20. | Hallan SI, Matsushita K, Sang Y, Mahmoodi BK, Black C, Ishani A, Kleefstra N, Naimark D, Roderick P, Tonelli M, Wetzels JF, Astor BC, Gansevoort RT, Levin A, Wen CP, Coresh J; Chronic Kidney Disease Prognosis Consortium. Age and association of kidney measures with mortality and end-stage renal disease. JAMA. 2012;308:2349-2360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 479] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 21. | Caralis DG, Deligonul U, Kern MJ, Cohen JD. Smoking is a risk factor for coronary spasm in young women. Circulation. 1992;85:905-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Hiroki J, Shimokawa H, Mukai Y, Ichiki T, Takeshita A. Divergent effects of estrogen and nicotine on Rho-kinase expression in human coronary vascular smooth muscle cells. Biochem Biophys Res Commun. 2005;326:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009;73:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 569] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 24. | Kawano H, Motoyama T, Ohgushi M, Kugiyama K, Ogawa H, Yasue H. Menstrual cyclic variation of myocardial ischemia in premenopausal women with variant angina. Ann Intern Med. 2001;135:977-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Yoshimura M, Yasue H, Nakayama M, Shimasaki Y, Ogawa H, Kugiyama K, Saito Y, Miyamoto Y, Ogawa Y, Kaneshige T, Hiramatsu H, Yoshioka T, Kamitani S, Teraoka H, Nakao K. Genetic risk factors for coronary artery spasm: significance of endothelial nitric oxide synthase gene T-786-->C and missense Glu298Asp variants. J Investig Med. 2000;48:367-374. [PubMed] |
| 26. | Kitano D, Takayama T, Sudo M, Kogo T, Kojima K, Akutsu N, Nishida T, Haruta H, Fukamachi D, Kawano T, Kanai T, Hiro T, Saito S, Hirayma A. Angioscopic differences of coronary intima between diffuse and focal coronary vasospasm: Comparison of optical coherence tomography findings. J Cardiol. 2018;72:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Nishimiya K, Suda A, Fukui K, Hao K, Takahashi J, Matsumoto Y, Mitsuishi K, Watanabe T, Ohyama K, Sugisawa J, Tsuchiya S, Satoh K, Shindo T, Godo S, Kikuchi Y, Shiroto T, Yasuda S, Shimokawa H. Prognostic Links Between OCT-Delineated Coronary Morphologies and Coronary Functional Abnormalities in Patients With INOCA. JACC Cardiovasc Interv. 2021;14:606-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 28. | Sueda S, Kohno H. Differential incidence and type of spasm according to coronary arterial location. Coron Artery Dis. 2016;27:273-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sangani V, United States; Zhang S, United States S-Editor: Liu JH L-Editor: A P-Editor: Yu HG