Published online Nov 26, 2023. doi: 10.4330/wjc.v15.i11.609
Peer-review started: August 23, 2023
First decision: October 9, 2023
Revised: October 19, 2023
Accepted: November 3, 2023
Article in press: November 3, 2023
Published online: November 26, 2023
Processing time: 91 Days and 18.2 Hours
Danon disease (DD), in which mutations in the X-linked lysosome-associated membrane protein-2 (LAMP-2) gene result in hypertrophic cardiomyopathy, is a rare disease, reported primarily in small samples or cases. However, with the development of cardiac magnetic resonance imaging and genetic technology in recent years, the number of reports has increased.
We report a case of DD in an adolescent male patient, confirmed by genetic testing. The patient was admitted to our hospital with complaints of a three-year history of chest tightness and shortness of breath. His preliminary clinical diagnosis is hypertrophic cardiomyopathy. Our report includes the patient’s clinical course from hospital admission to death, step-by-step diagnosis, treatment course, and noninvasive imaging features. We highlight how a noninvasive diagnostic approach, based solely on clinical and imaging “red flags” for DD, can be used to achieve a diagnosis of DD with a high degree of confidence.
DD is a very dangerous cardiomyopathy, and it is necessary to achieve early diagnosis and treatment.
Core Tip: Danon disease (DD) is a rare X-linked disorder caused by a deficiency of lysosome-associated membrane protein-2. DD is clinically characterized by severe cardiomyopathy, skeletal muscle disease, and intellectual disability. The most frequent high-risk form of DD is cardiomyopathy, which can result in arrhythmia(s), early-onset heart failure, and even sudden cardiac death. Our case report intents to raise the awareness of DD and improve the clinical suspicion of DD.
- Citation: Zhao YT, Cao XQ, Mu XL. Hypertrophic cardiomyopathy secondary to deficiency in lysosome-associated membrane protein-2: A case report. World J Cardiol 2023; 15(11): 609-614
- URL: https://www.wjgnet.com/1949-8462/full/v15/i11/609.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i11.609
Danon disease (DD) is a rare, hereditary, X-linked, and dominant lysosomal glycogen storage disease, caused by a deficiency in lysosome-associated membrane protein-2 (LAMP-2)[1]. The disease predominantly affects men, who usually exhibit more severe clinical manifestations and present at an earlier age than women. Clinical manifestations of DD include myocardial enlargement, skeletal myopathy, and mental aberrations[2], and the most frequent high-risk form of DD is cardiomyopathy, which can manifest as arrhythmia(s), early onset heart failure, or even sudden cardiac death. Young male patients with DD typically present with left ventricular hypertrophy, which, unfortunately, is frequently mistaken for hypertrophic cardiomyopathy or other secondary conditions related to left ventricular hypertrophy. Characterized by rapid progression and a propensity for high mortality at an early age, DD may be the most lethal cardiomyopathy in young male patients[3]. In the present case study, we describe the patient’s clinical course from hospital admission to death, including all diagnostic work-ups, his treatment course, and any notable noninvasive imaging features. Herein, we emphasize the importance of utilizing unique clinical signs and imaging features for the early recognition and diagnosis of DD.
An 18-year-old adolescent male patient was admitted to our hospital with complaints of a three-year history of chest tightness and shortness of breath.
The patient’s symptoms became noticeably worse one week before his admission, with increased chest tightness, an inability to lie supine, and anuria.
The patient was seen at a hospital in Beijing with complaints of chest tightness and was diagnosed with non-obstructive hypertrophic cardiomyopathy, a small pericardial effusion, and chronic bilateral lung inflammation with a few interstitial changes. Additionally, the patient suffered a cerebral infarction during the hospitalization, which improved slightly after treatment.
The patient denied any family history of genetic disorders or similar conditions.
Upon physical examination, the patient exhibited evidence of hypotension, with a blood pressure of 92/62 mmHg. He exhibited normal development, intelligence, consciousness, and retinal features. Limb muscle strength was graded as level 5 upon the first hospitalization, and muscle tone was normal; however, at the time of the final hospitalization, muscle strength in the right upper limb was grade 4, while muscle strength in the left lower, left upper, and right lower limbs were grade 1. Unfortunately, the hospital staff did not perform electromyography, skeletal muscle magnetic resonance imaging (MRI), or a pathological examination. Atrial/ventricular premature beats and ventricular blocks were visible on resting electrocardiography, although no pre-excitation was observed.
Laboratory testing revealed elevated levels of high-sensitivity cardiac troponin T (0.187 ng/mL) and N-terminal pro-B-type brain natriuretic peptide (NT-proBNP; 6,554 pg/mL), while liver function tests revealed elevated levels of creatine kinase (1075 U/L, 1545 U/L, and 936 U/L), creatine kinase-MB (63 U/L, 61 U/L, and 58 U/L ), lactate dehydrogenase (728 U/L), alanine transpeptidase (206 U/L), and aspartate aminotransferase (273 U/L). The patient’s troponin T and myocardial enzyme levels were also elevated, suggesting secondary myocardial damage.
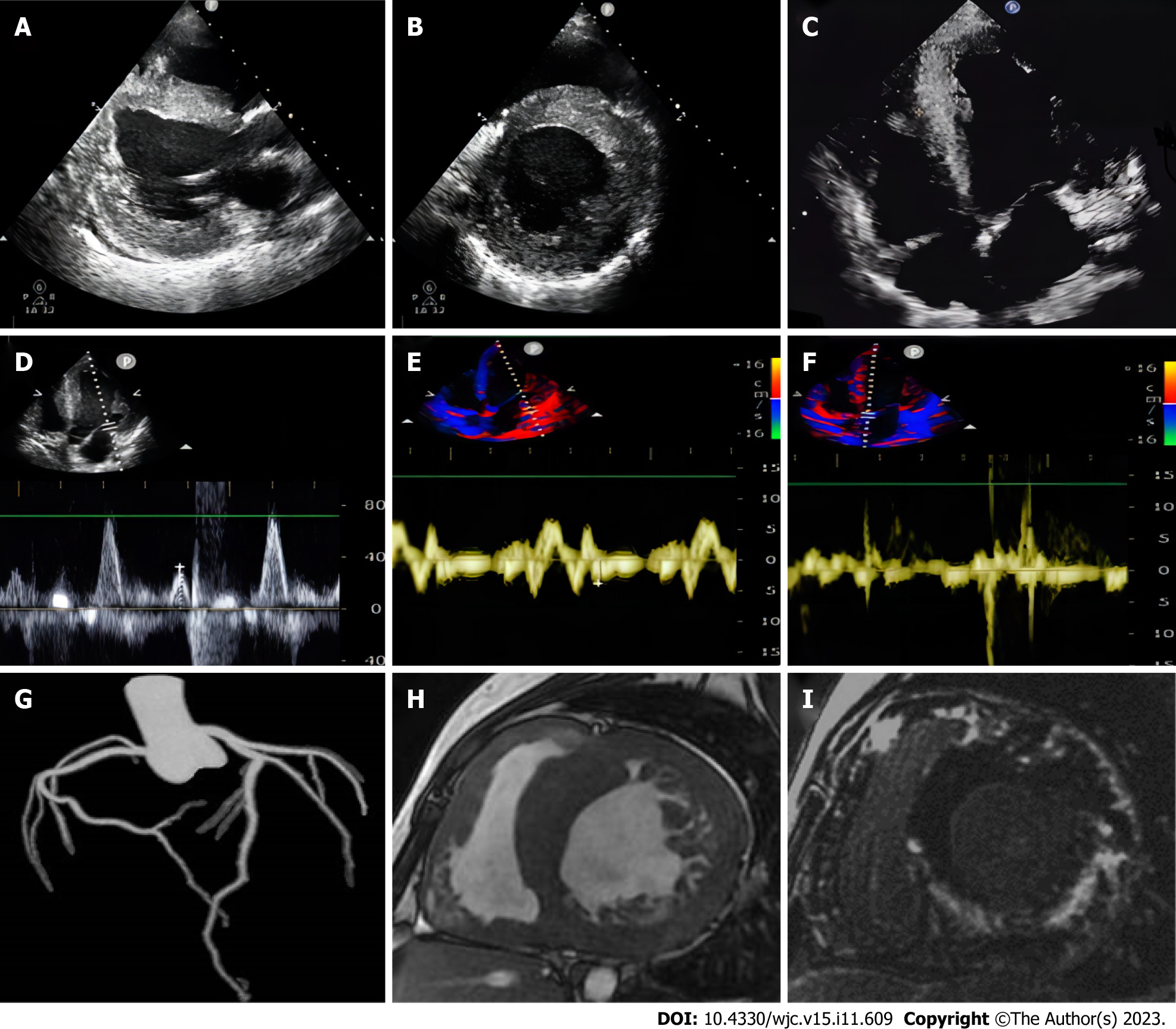
Left ventricular centripetal and right ventricular hypertrophy were observed on transthoracic echocardiography (ventricular septal thickness, 20-31 mm; lateral wall thickness, 26 mm) (Figures 1A-C). Tissue Doppler imaging revealed increased end-diastolic filling pressure, with an E value of 70 cm/s (Figure 1D), a ventricular septum e′ value of 3 cm/s, and a lateral wall e′ value of 4 cm/s (Figures 1E and F).
The patient underwent coronary computed tomography (CT) angiography with maximum intensity projection, excluding acute coronary syndrome, which revealed the patency of the lumen of the coronary trunk and its branches (Figure 1G). To further characterize the cardiac phenotype, a cardiac MRI (CMRI) was performed, which revealed bilateral ventricular hypertrophy, severe trabecularization of the biventricular wall, and minor pericardial effusion. Additionally, myocardial strain analyses of the entire left ventricle (Figure 2A), as well as each segment, were performed. The 16-segment bullseye diagram and longitudinal strain map revealed varying degrees of strain reduction in each segment of the patient’s left ventricle, indicating myocardial injury (Figures 2B and C). The ventricular myocardium exhibited substantial late gadolinium enhancement (LGE) on MRI, sparing the mid-basal septum but involving the apex (Figures 1H and I), which is a unique imaging characteristic of DD[4].
After reviewing the patient’s gene sequencing, in conjunction with the aforementioned data, the patient’s final diagnosis was DD.
The patient experienced ventricular thrombosis, pulmonary thrombosis, pulmonary hypertension, and cerebral infarction in previous hospitalization, which required the administration of the cardiotonic medication digoxin and the anticoagulant warfarin. The patient and his family approved a therapeutic regimen; however, after undergoing treatment with the medications for > 1 mo, the patient’s chest tightness worsened, anuria developed, and his lungs started to crackle. Additionally, paroxysmal atrial fibrillation was detected on resting electrocardiography and laboratory results showed an additional elevation in high-sensitivity cardiac troponin T (0.527 ng/mL) and NT-proBNP (84, 072.43 pg/mL) concentrations, as well as hyperkalemia (potassium level, 6.4 mmol/L). Multislice chest CT revealed serious infection in both lungs. The patient was treated with diuretics, nutritional supplementation for the myocardium, anticoagulants, and anti-infection therapy for one week. However, he did not show any significant improvement.
The patient’s prognosis continued to be poor, and he subsequently exhibited signs of entering a deep coma and died, despite attempts at active resuscitation.
DD is a highly penetrant vacuolar myopathy caused by a primary deficiency in LAMP-2[5]. This condition usually involves the skeletal muscle and myocardium and can be definitively diagnosed through a biopsy. The characteristic pathological features of DD include intracytoplasmic vacuoles harboring autophagic components and glycogen[1]. The classic “trifecta,” which includes cardiac enlargement, skeletal myopathy, and abnormal liver function, is used to definitively diagnose patients with suspected DD[5]. Other red flags, such as pre-excitation on resting electrocardiography, are not always present in patients with DD. Although the patient presented herein did not undergo a skeletal muscle biopsy, his decreased muscle strength, in combination with an increase in creatine kinase concentrations, indicated the presence of skeletal muscle damage.
A distinctive LGE pattern and basal septum preservation, seen on CMRI, may help distinguish DD from sarcomeric or other hypertrophic cardiomyopathy phenotypes[4,6] and strengthen a suspected diagnosis of DD. Physicians should confirm cardiac hypertrophy, impaired liver function, and a genuine pattern of progressive gadolinium enhancement on CMRI to diagnose DD. To date, CMRI findings in patients with DD have been described in only a few case reports[4]. CMRI provides accurate imaging of morphological structures and tissue characteristics, such as unusual LGE patterns, which may have practical utility in the identification and differential diagnosis of cardiomyopathy in patients with DD. Additionally, adding myocardial fibrosis to the list of indicators of DD may be appropriate. For the first time, we added feature-tracking technology to investigate cases of DD. The analysis of global and segmental longitudinal myocardial strain using CMRI-feature tracking revealed that both were damaged; however, no other unique features were observed. We speculate that the patient presented herein was in the late stage of heart failure, although he had left ventricular non-compaction, which differs from the preservation of normal apical strain patterns described in previous reports. We hope that more patients with DD will undergo feature-tracking analysis in the future, to further characterize the unique features of cardiac strain seen in these patients.
The clinical results of treating DD depend on the severity of cardiomyopathy, which can be difficult to identify, frequently being discovered only in the presence of clinically significant symptoms. In the case presented herein, substantial myocardial injury, cardiac insufficiency, and arrhythmia were the secondary causes of numerous organ disorders that ultimately led to the patient’s death. Physicians should be aware that young males exhibiting myocardial hypertrophic changes, elevated plasma creatine kinase levels, pre-excitation syndrome on electrocardiography, and/or distinctive LGE on CMRI may have DD; therefore, abnormal clinical or laboratory results and CMRI features can be used to diagnose and distinguish DD from other diseases. Furthermore, the aforementioned data allow a diagnosis to be made in a noninvasive manner, providing an alternative to the current gold standard for the diagnosis of DD – genetic testing for LAMP-2 deficiency/mutations. Although there is currently no specific gene therapy for DD, the adenovirus-mediated delivery of a functional LAMP2B transgene has been achieved in a mice model, the efficacy of which is being tested in adolescent male patients with DD.
The Dalian Municipal Central Hospital Affiliated to Dalian Medical University Ethics Committee approved the present case study (No. YN2023-003-04). The patient’s parents consented to the publication of anonymized case details.
In summary, the authors would like to emphasize the importance of the early diagnosis of DD, which facilitates advanced treatments, such as targeted gene therapy, cardiac transplantation, or the installation of an implantable cardioverter defibrillator. A deeper understanding of the unique clinical signs and imaging features of DD is needed, particularly with the use of CMRI. Genetic testing should also be performed as early as possible to increase diagnostic accuracy.
The authors gratefully acknowledge the patient and his family for their participation in the present case study.
| 1. | Sugie K, Noguchi S, Kozuka Y, Arikawa-Hirasawa E, Tanaka M, Yan C, Saftig P, von Figura K, Hirano M, Ueno S, Nonaka I, Nishino I. Autophagic vacuoles with sarcolemmal features delineate Danon disease and related myopathies. J Neuropathol Exp Neurol. 2005;64:513-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | D'Souza RS, Law L. Danon Disease. 2023 Jul 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. [PubMed] |
| 3. | Xu J, Li Z, Liu Y, Zhang X, Niu F, Zheng H, Wang L, Kang L, Wang K, Xu B. Danon disease: a case report and literature review. Diagn Pathol. 2021;16:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Wei X, Zhao L, Xie J, Liu Y, Du Z, Zhong X, Ye W, Wang Y, Chen Y, Lu M, Liu H. Cardiac Phenotype Characterization at MRI in Patients with Danon Disease: A Retrospective Multicenter Case Series. Radiology. 2021;299:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Miliou A, Antonopoulos AS, Kouris N, Lazaros G, Tsioufis K, Vlachopoulos C. Danon Cardiomyopathy: Specific Imaging Signs. JACC Case Rep. 2022;4:1496-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Rigolli M, Kahn AM, Brambatti M, Contijoch FJ, Adler ED. Cardiac Magnetic Resonance Imaging in Danon Disease Cardiomyopathy. JACC Cardiovasc Imaging. 2021;14:514-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sharma D, India; Batta A, India S-Editor: Liu JH L-Editor: A P-Editor: Yuan YY