Published online Oct 26, 2023. doi: 10.4330/wjc.v15.i10.469
Peer-review started: April 17, 2023
First decision: July 18, 2023
Revised: August 23, 2023
Accepted: September 6, 2023
Article in press: September 6, 2023
Published online: October 26, 2023
Processing time: 190 Days and 3.3 Hours
The rise in incidence rates of invasive candidiasis warrants an increase in atten
Core Tip: The incidence of Candida infective endocarditis has observed a noticeable rise. Despite the progress in medical understanding, Candida endocarditis (CE) continues to be linked with a notable increase in in-hospital mortality. This comprehensive review aims to elucidate the existing diagnostic modalities for identifying CE while emphasizing their inherent limitations. Furthermore, we clarify the prevailing standard treatment protocols, encompassing medical and surgical interventions. Additionally, we highlight the role of screening techniques in identifying high-risk patients and explore the discussion of prophylactic measures tailored to specific patient phenotypes.
- Citation: Jamil Y, Akinleye A, Mirzaei M, Lempel M, Farhat K, Pan S. Candida endocarditis: Update on management considerations. World J Cardiol 2023; 15(10): 469-478
- URL: https://www.wjgnet.com/1949-8462/full/v15/i10/469.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i10.469
Although our understanding of endocarditis has evolved since its first description by William Osler in the late 19th century, it remains a disease of high morbidity and mortality[1,2]. Candida endocarditis (CE) which accounts for only 1%-2% of all endocarditis cases, is associated with a mortality rate as high as 80%[3-6]. Given that CE is particularly rare, formal studies comparing various management approaches and subsequent outcomes are limited. Current guidelines recommend a treatment regimen of antifungal agents combined with surgical interventions, whether valves or indwelling prostheses removal[7,8]. However, individuals suffering from CE often have multiple comorbidities and are at high risk for reinfection, making conducting invasive interventions challenging.
Furthermore, the uptrend in fungemia incidence rates in recent years has led to an increased number of patients at risk for CE[9]. Nonetheless, CE studies have relatively small sample sizes, which make them insufficiently powered to get robust research evidence into use. Therefore, this review explores the current diagnostic techniques and treatment considerations when evaluating and managing patients with CE.
Per definition, community-onset candidemia occurs within 48 h of hospitalization, with most cases identified on the day of admission, whereas nosocomial candidiasis occurs after 48 h of hospitalization[10]. The incidence of invasive candidiasis in the community and nosocomial infections has increased. There has been a noticeable increase in the incidence of community-acquired cases since the 1970s due to the intravenous drug (IVD) use epidemic, the use of impure brown heroin, and poor harm reduction practices, which have been associated with Candida albicans (CA)[11]. With the implementation of harm reduction strategies in the 1990s, the causative organisms have shifted to non-albicans Candida (NAC) species. The increased likelihood of the latter among IVD users has led again to the recognition of IVD use as an important risk factor for candidemia[12].
Different factors were found to increase the incidence of nosocomial candidemia, including increased antibiotics use, prolonged fluconazole prophylaxis in immunocompromised patients, use of total parenteral nutrition, and use of long-term catheters and medical devices[13-16]. Additional risk factors include malignancies of the gastrointestinal tract, genitourinary tracts, and the breast associated with CA infection and hematologic malignancy associated with NAC infection. Lastly, immunosuppressive therapies, including chronic steroid therapy, have been found to increase the incidence of CE and mortality risk[17].
Different Candida species can lead to advanced clinical infections, yet the most common agent leading to CE remains CA despite distinct patient characteristics and underlying risk factors[18]. Moreover, Candida parapsilosis was linked to infected medical devices such as prosthetic valves and transmitted through direct contact[19-21]. Candida dubliniensis and Candida glabrata are predominantly found among human immunodeficiency virus/acquired immunodeficiency syndrome patients with oral thrush and patients who are immunosuppressed on broad-spectrum antibiotics, respectively[18,22]. Although CA is primarily sensitive to antifungals, there is an emergence of intrinsic resistance of various Candida species to antifungals. Thus, it is essential to conduct antifungal susceptibility to ensure appropriate coverage[23]. Candida auris has recently emerged as a pathogen of significant concern worldwide, especially among chronically hospitalized patients. It is intrinsically resistant to multiple currently available antifungal therapies with a high mortality rate, often due to delayed diagnosis and initiation of antifungal therapy[24].
Despite the availability of effective antifungal therapy in most cases, mortality rates related to candidemia remain elevated, ranging from 30%-80%[25]. The latter is likely attributed to patients predisposing factors such as immunocompromised status and malignancies, recent surgery, and prior infective endocarditis (IE). Early diagnosis and administration of appropriate antifungal therapies via a multidisciplinary approach may have improved mortality rates over the years[4,23,27-28].
Healthcare providers should be vigilant of an underlying CE when managing high-risk febrile patients with predisposing factors such as IVD users, prolonged antibiotic therapy, indwelling central venous catheter, prosthetic heart valve, history of endocarditis, parenteral nutrition, neutropenia, and diabetes mellitus[29]. Although the clinical presentation of CE can sometimes be indistinguishable from those of bacterial endocarditis, the loss of visual acuity, presence of cutaneous nodules, and cerebral embolization should raise concerns for a Candida infection[30,31]. Nevertheless, it remains a challenge to establish the diagnosis and differentiate CE from bacterial endocarditis.
In 1994, Durack et al[32] proposed the Duke Criteria, which are used to classify each case as definite, possible, or probable, and has been validated in several subsequent studies. Additionally, further trials emphasized Duke Criteria's high diagnostic sensitivity and specificity[33-35]. Furthermore, in 2000, Li et al[36] introduced the modified Duke Criteria, which have become the standard of care. Nevertheless, these criteria were developed to evaluate patients with suspected left-sided native valve IE, as its sensitivity is low in patients with cardiac device infection, prosthetic valves, and right-sided IE[37]. Duke Criteria were primarily implemented in the workup of bacterial endocarditis; however, to date, there are no specific diagnostic criteria for fungal endocarditis.
Isolation of the causative organism is critical in establishing the microbiologic diagnosis and selecting appropriate therapeutic agents. However, Candida traditionally does not grow well in standard bacterial blood culture media, if at all, requiring a longer time for the organism to grow. The sensitivity of detection of Candida in blood cultures is limited to 50%-75%[38,39]. In one case series from France, 14% of 620 IE cases had negative blood cultures, while another study showed that 31% had negative cultures[40]. Hence, this will delay the diagnosis and treatment, which could lead to drastic outcomes. Therefore, modifications to the original Duke Criteria have been suggested to include additional risk factors, such as CRP/ESR elevation, hematuria, central non-feeding venous lines, and peripheral lines, as part of the criteria. These, in turn, led to increased diagnostic sensitivity[42]. However, when the modified Duke Criteria was tested in blood culture-negative IE, it performed poorly, possibly due to the lack of serological criteria[43].
In light of earlier addressed issues related to fungal isolation and culture difficulties, which remain the gold standard of diagnostic testing, non-culture-based tests have been developed, and some have found clinical applications. 1, 3-β-D-glucan is a polysaccharide ubiquitous in the fungal cell wall. Its detection in the serum with a cutoff of 60 pg/mL has a sensitivity and specificity of 69.9% and 87.1%, respectively, in a patient with candidemia[31]. It carries several advantages, including improved sensitivity on serial testing, strong specificity, positive likelihood ratio, and most importantly, antifungal agents do not affect 1, 3 β-D-glucan serum levels[44,45]. In a patient with Candida glabrata IE, β-D-glucan was found to be a helpful tool in assessing treatment response, whereby after treatment, the assay became negative[46]. Hence, this could be used as a screening tool for CE and to monitor clinical response to therapy.
Mannan is an essential constituent of the Candida cell wall. Both mannan and anti-mannan antibodies have been found in the serum of patients with candidiasis. A meta-analysis by Mikulska et al[47] showed that combined testing using immunosorbent assays for detecting mannan antigen and anti-mannan antibodies have a sensitivity of 83% and specificity of 86% in patients with invasive candidiasis. However, its usefulness is limited due to rapid clearance from the bloodstream. Furthermore, immunosuppressed patients may not develop adequate antibody response against the mannan antigen, thus resulting in false negative tests[48].
The development of molecular diagnostic techniques has improved the ability to identify the causative pathogen and decreased the required time for microorganism identification from days to hours. Rice et al[49] found polymerase chain reaction (PCR) to have a threefold increase in sensitivity in detecting causative agents in the setting of IE compared to traditional bacterial Gram-stain and culture. Multiple cases of successful diagnosis of culture-negative fungal endo
These non-culture-based tests have several disadvantages, which have limited their widespread use. The yield of these tests is time-sensitive, and results might be altered with antifungal administration, which increases the possibility of false negative results. The accuracy of the test and its ability to make an identification depend on the quality of the database from which the test references, which is ever-expanding and improving. Thus, careful interpretation of test results is essential since sometimes the test may detect multiple or unusual organisms, which implies a critical determination of their clinical relevance. The availability of the test remains limited, and the turn-around time is significant, making them not a first-line diagnostic tool. Finally, these tests are costly, further limiting their widespread use[55,56].
The use of echocardiography in the diagnosis of endocarditis is well established. Transthoracic echocardiogram (TTE) has a sensitivity of approximately 70% in the diagnosis of native-valve endocarditis (NVE) and about 50% for prosthetic-valve endocarditis (PVE). The sensitivity for each will improve to more than 90% when a transesophageal echocardiogram (TEE) is used, suggesting the superiority of TEE. For fungal endocarditis, echocardiography has shown an overall sensitivity of 77%[6]. Indeed, for patients with candidemia, TTE was shown to detect vegetation in 2.9% of patients compared to 11.5% of patients with TEE[57]. Moreover, TTE had a sensitivity of 88.9% with NVE and 76.5% with PVE in the diagnosis of CE compared to TEE, which had an improved sensitivity of 92% with NVE but a worse sensitivity of 61.1% in the case of PVE, which could be related to the small sample of patients with PVE that underwent TEE[4,6].
Other imaging techniques have been developed that have shown promising applications in diagnosing endocarditis[38]. Positron emission tomography with 2-deoxy-2-(fluorine-18) fluoro-D-glucose integrated with computed tomography (18F-FDG-PET/CT), which identifies increased uptake of labeled glucose by cells in inflamed tissue, has improved sensitivity and specificity of the modified Duke criteria to 82% and 96%, respectively, even better if the NVE cases were excluded, up to 96% and 94% if only PVE and cardiac-device-related IE are considered. Limitations of this technique include potential myocardial and respiratory artifacts that need to be gated out, difficulty distinguishing between inflammatory from infectious lesions, and limited ability to detect small vegetations along the device leads[58]. 18F-FDG-PET/CT was reported to help diagnose CE and improve accuracy. Hence, it is promising in situations where TTE/TEE might not be diagnostic[59].
Single photon emission tomography with technetium 99m-hexamethyl propylene amine oxime (HMPAO)-labeled autologous leukocytes (99mTc-HMPAO-labeled SPECT/CT), on the other hand, takes advantage of the natural homing and recruitment of leukocytes to the site of inflammation/ infection to identify potential areas of abnormality. This technique does require additional time to prepare and complete compared to 18F-FDG-PET/CT. It takes time to obtain, isolate, and prepare autologous leukocytes from the host, and the images are acquired in multiple phases. Other imaging limitations include affectation by metallic artifacts, non-specific bowel activity due to hepatic HMPAO excretion, and its limited ability to detect small vegetations[60]. These techniques are technically challenging and require well-trained radiologists who are familiar with the technique. Therefore, they are not widely available and are usually implemented when other evaluation techniques, such as echocardiograms, are inconclusive. The yield of either study may be affected by antibiotic exposure; thus, finding the optimal time to utilize this imaging modality to achieve the maximal result in the course of patient evaluation remains an important question[61,62]. Nevertheless, these techniques have demonstrated very promising results in the diagnosis of PVE and cardiac device-related IE, with one study showing a sensitivity of 80%, specificity of 91%, negative predictive value of 80%, and positive predictive value of 91% using 18F-FDG PET/CT and 60%, 100%, 100%, and 85% for 99mTc-HMPAO-SPECT/CT, that the use of nuclear imaging study when appropriate has been included as a consideration in European IE guidelines[61-63].
Current Infectious Diseases Society of America (IDSA) guidelines recommend treating native or prosthetic valve CE with either a lipid formulation of amphotericin with or without flucytosine or a high dose echinocandin for initial therapy, with step-down therapy to fluconazole for patients who have susceptible isolates, that have demonstrated clinical improvement, and have cleared Candida from their bloodstream. Step-down therapy to oral voriconazole can be used for susceptible isolates that are not susceptible to fluconazole. Valve replacement is recommended if there is no contraindication, followed by continued antifungal treatment for six weeks after surgery. Finally, long-term therapy with fluconazole is recommended for patients who cannot undergo valve replacement[8]. Despite this seemingly straightforward algorithm, this infection has seen little or no improvement in patient outcomes. While this may indicate an inherent defect in our approach to treatment, one must further analyze this sophisticated pathogen and the hosts most likely to become infected.
As Candida is known to form biofilms that result in decreased cell membrane ergosterol content through reduced expression of ergosterol biosynthetic genes while upregulating the expression of genes involved in amino acid and nucleotide metabolism and efflux pumps[64,65], a combination antifungal regimen would theoretically be more effective in the treatment of fungal infection. However, using an in vitro model, Pai et al[66] compared the activities of flucytosine, micafungin, and voriconazole as either single agents or in combination against several Candida species and found no difference in the reduction of fungal burden between triple vs single agent antifungal therapy. Conversely, a 2011 meta-analysis of 64 cases of CE who received fluconazole alone, concurrently, or in sequence with other antifungals without surgical intervention suggested that multiple-agent therapy is preferable. In this study, Smego and Ahmad[67] reported that combination regimens, including fluconazole cured or improved 86% and 68% of patients with native and prosthetic valve infections, respectively. Furthermore, fluconazole administered alone was associated with a 42% rate of relapse or death. At the same time, the best outcomes were found in patients maintained on chronic suppressive fluconazole therapy following an initial amphotericin or echinocandin regimen for a minimum of six months. Although antifungal agents tend to have significant side effects and drug-drug interaction profiles, prolonged fluconazole use is relatively benign. In a retrospective study of individuals receiving chronic fluconazole for suppression of artificial implant infection, Penk and Pittrow[68] found no significant adverse events. Of note, this study's maximum duration of treatment was 4.5 years, and the maximum daily dose was 750 mg.
A study compared amphotericin B and echinocandin-based therapy directly. While there was a higher percentage of older patients in the echinocandin group and the majority of infections in the amphotericin B group were community-acquired, the rates of utilization of combination antifungal therapy, suppressive antifungal therapy and adjunctive surgery were statistically equivalent. Mortality rates measured in-hospital, at 42 d and 1 year, did not differ between the two groups. Based on this study, the echinocandin group could be a better choice of initial therapy given similar clinical outcomes and a better side effect profile than amphotericin[69].
Combination antifungal therapy of amphotericin B and flucytosine was shown to have similar clinical outcomes compared to an antifungal followed by adjunctive surgical intervention[70]. Different antifungal combinations have been tried, including azoles plus echinocandins, 5-FC-combination therapies, and polyenes plus azoles[71]. Amphotericin B and flucytosine have been found to work synergistically, albeit with nephrotoxic side effects[67]. Furthermore, the combination of amphotericin B and fluconazole demonstrated antagonism[69]. IDSA guidelines recommend echinocandins with or without fluconazole as first-line therapy, which was found to be non-inferior to amphotericin B in managing endocarditis[8,69]. Additional challenges are encountered with NAC species, such as Candida lusitaniae and Candida krusei, which are intrinsically resistant to polyenes and fluconazole, respectively[72,73].
Significant differences in clinical outcomes have been observed between right and left-sided disease in bacterial and fungal infections[74-6]. In a 2018 retrospective study, Siciliano et al[77] found that patients with isolated right-sided CE had a 32% mortality rate vs 61% for left-sided disease. Furthermore, individuals with right-sided disease have lower rates of acute heart failure and perivalvular complications. While right-sided CE still portends a poor prognosis, isolated valve involvement should be a characteristic considered when discussing outcomes and treatment options.
Literature on the surgical approach in CE is limited to small prospective studies with conflicting evidence and weak recommendations. For instance, 15 case reports from patients with CE showed that a combination of surgical and medical, when carried out early on admission, had lower mortality than single therapy[27]. A meta-analysis of 879 cases of CE found that patients who underwent adjunctive surgery had lower mortality. However, higher mortality was seen in surgical repairs before 1980, fungal monotherapy, and left-sided endocarditis. Although, this did not meet statistical significance[70]. In contrast, an observational cohort study in 2015 failed to show mortality benefits between those undergoing surgical therapy and those receiving medical treatment alone[69]. The European Society of Clinical Microbiology and Infectious Diseases recommends earlier surgery in the setting of prosthetic valves as opposed to infection involving native valves[38]. The need for surgical intervention may differ among cases caused by different Candida species. A recent retrospective study showed that surgery was performed earlier in cases of CE caused by Candida parapsilosis compared to CA endocarditis[78]. Rivoisy et al[79] demonstrated that in patients with prosthetic valve CE, early surgery was not associated with better survival at six months compared to medical management alone with liposomal amphotericin B induction and long-term suppression with fluconazole.
A newer approach with minimally invasive surgical intervention with angioVac has been used in right-sided IE with vegetation debulking. A meta-analysis of AngioVac-assisted vegetation debulking demonstrated procedural and clinical success of 89.2% and 79.1%, respectively. Also, greater than 50% vegetation removal was achieved in 90% and bacteremia clearance of 82.5% with procedure-related complications of 10.1%. However, documentation of this approach for left-sided endocarditis is not yet available[80]. It has been proposed that debulking in CE can lead to the resolution of fungemia similar to bacteremia in bacterial endocarditis[81].
Prophylactic measures have been given to selected patients at high risk of complications from candidemia. Several studies have explored high-risk populations, and many were found to have similar underlying genetic alterations leading to candidemia. Some generic modifications include Toll-like receptor signaling influence and certain single nucleotide polymorphisms[82-84]. A meta-analysis by Shorr et al[85] showed that prophylactic fluconazole reduced the risk of infection but failed to show improved mortality. The latter was also demonstrated in another randomized controlled trial between 1995-2000 in 26 intensive care units (ICU), where patients with central line catheters were randomized to fluconazole 800 mg vs placebo. It also failed to improve primary composite outcomes, including fever resolution, absence of invasive fungal infection, and discontinuation of prophylaxis due to toxicity and the need for additional systemic antifungal medication[86]. On the other hand, prophylaxis might increase the risk of resistance to antifungal drugs, which was described in the SNETRY Antifungal surveillance program that underlined both intrinsic and acquired resistance to fluconazole based on differences in species distribution among the geographic areas, variation in antifungal usage and infection control practice. Other disadvantages include cost and side effects[87]. IDSA guidelines report that prophylaxis with fluconazole (12 mg/kg) loading dose and then 6 mg/kg daily gained weak recommendation when administered to high-risk ICU patients[8]. This points to the need for a new screening modality to identify patients at high risk for severe candidemia and its complications.
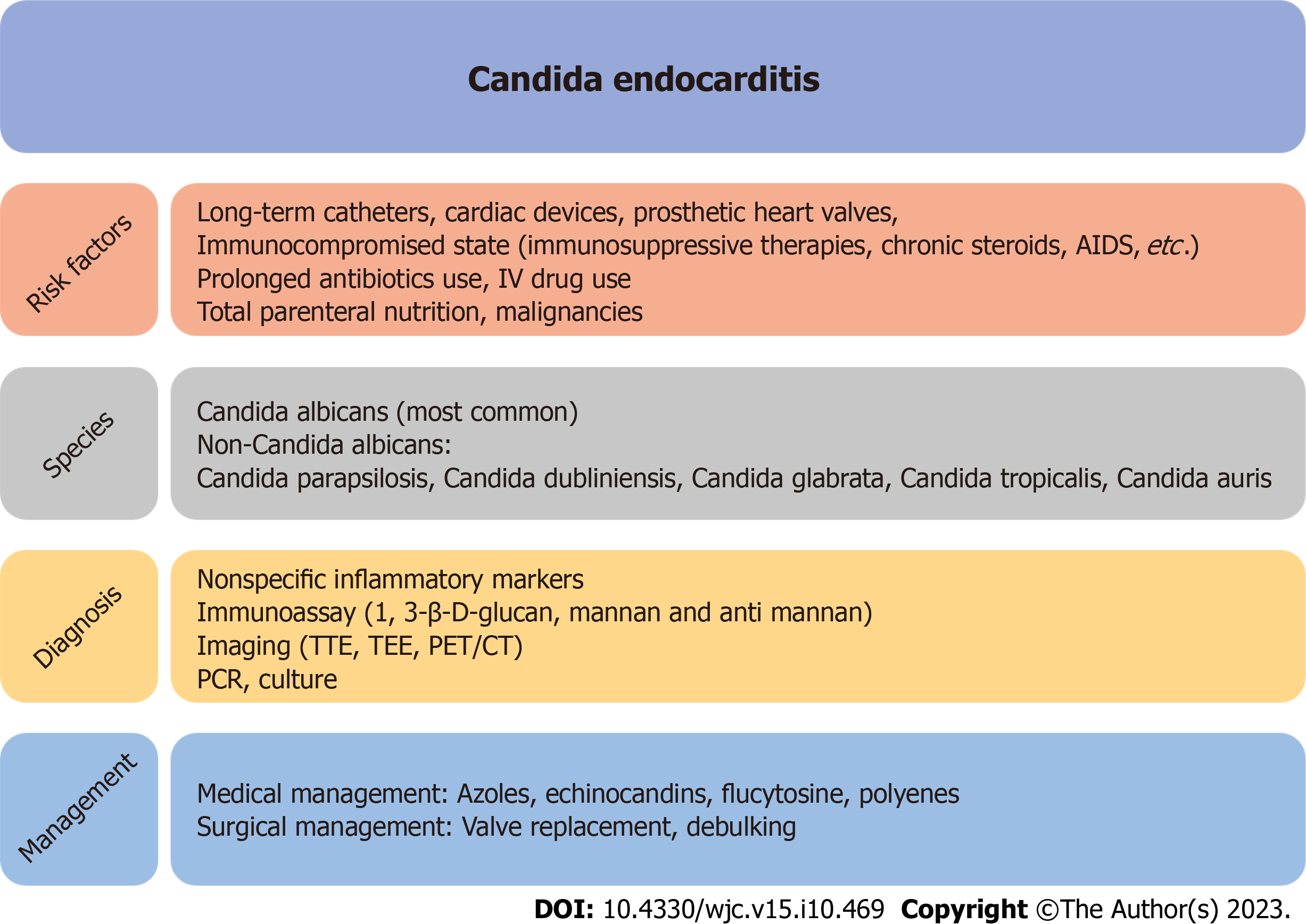
Despite advances in cardiac surgery and antifungal therapy, overall mortality and morbidity due to CE have not improved significantly. Given the increasing incidence of candidemia and the emergence of multi-drug resistant Candida species, there is an urgent need for further development of fungal-specific diagnostic criteria, including novel diagnostic tests, and management guidelines, both therapeutic and surgical. While the modified Duke criteria have been applied to diagnose fungal endocarditis, one of the major criteria requires positive blood cultures, which can be a source of delay in diagnosing CE. As there are limited data regarding some of the newer diagnostic techniques, criteria specific for fungal endocarditis utilizing fungal antigens and PCR technology would lead to earlier diagnosis and treatment (Figure 1).
New techniques, such as minimally invasive suction thrombectomy, can remove and debulk vegetations[88,89] without the risk and complications associated with conventional surgery and may provide alternative solutions. Current and future innovations in diagnostic tests and medical and surgical management of CE will permit earlier recognition of infection, reduce rates of potential complications, and improve long-term outcomes. The most successful strategy will likely require increased attention to the prophylactic reduction of indwelling foreign devices, antibiotic stewardship, and dedicating resources to the IVD use epidemic.
| 1. | Osler W. The Gulstonian Lectures, on Malignant Endocarditis. Br Med J. 1885;1:467-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 446] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 2. | Holland TL, Baddour LM, Bayer AS, Hoen B, Miro JM, Fowler VG Jr. Infective endocarditis. Nat Rev Dis Primers. 2016;2:16059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 289] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 3. | Benjamin DK Jr, Miro JM, Hoen B, Steinbach WJ, Fowler VG Jr, Olaison L, Habib G, Abrutyn E, Perfect J, Zass A, Corey GR, Eykyn S, Thuny F, Jiménez-Expósito MJ, Cabell CH; ICE-MD Study Group. Candida endocarditis: contemporary cases from the International Collaboration of Infectious Endocarditis Merged Database (ICE-mD). Scand J Infect Dis. 2004;36:453-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Ellis ME, Al-Abdely H, Sandridge A, Greer W, Ventura W. Fungal endocarditis: evidence in the world literature, 1965-1995. Clin Infect Dis. 2001;32:50-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 333] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Lefort A, Chartier L, Sendid B, Wolff M, Mainardi JL, Podglajen I, Desnos-Ollivier M, Fontanet A, Bretagne S, Lortholary O; French Mycosis Study Group. Diagnosis, management and outcome of Candida endocarditis. Clin Microbiol Infect. 2012;18:E99-E109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Pierrotti LC, Baddour LM. Fungal endocarditis, 1995-2000. Chest. 2002;122:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 216] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD; ACC/AHA Task Force Members. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:2440-2492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 1105] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 8. | Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1860] [Cited by in RCA: 2432] [Article Influence: 243.2] [Reference Citation Analysis (1)] |
| 9. | Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4208] [Cited by in RCA: 4341] [Article Influence: 188.7] [Reference Citation Analysis (0)] |
| 10. | Sofair AN, Lyon GM, Huie-White S, Reiss E, Harrison LH, Sanza LT, Arthington-Skaggs BA, Fridkin SK. Epidemiology of community-onset candidemia in Connecticut and Maryland. Clin Infect Dis. 2006;43:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Bisbe J, Miro JM, Latorre X, Moreno A, Mallolas J, Gatell JM, de la Bellacasa JP, Soriano E. Disseminated candidiasis in addicts who use brown heroin: report of 83 cases and review. Clin Infect Dis. 1992;15:910-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 80] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Poowanawittayakom N, Dutta A, Stock S, Touray S, Ellison RT 3rd, Levitz SM. Reemergence of Intravenous Drug Use as Risk Factor for Candidemia, Massachusetts, USA. Emerg Infect Dis. 2018;24:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Ericson JE, Benjamin DK Jr. Fluconazole prophylaxis for prevention of invasive candidiasis in infants. Curr Opin Pediatr. 2014;26:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP. Risk factors for hospital-acquired candidemia. A matched case-control study. Arch Intern Med. 1989;149:2349-2353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 307] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Zilberberg MD, Shorr AF, Kollef MH. Secular trends in candidemia-related hospitalization in the United States, 2000-2005. Infect Control Hosp Epidemiol. 2008;29:978-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Labchuk A, Hamwi M, Han A, Khan M, Stone A. Fungal Endocarditis With Severe Vegetations of the Aortic Valve and Septic Emboli Secondary to Total Parenteral Nutrition. Cureus. 2022;14:e32357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Ding X, Yan D, Sun W, Zeng Z, Su R, Su J. Epidemiology and risk factors for nosocomial Non-Candida albicans candidemia in adult patients at a tertiary care hospital in North China. Med Mycol. 2015;53:684-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Mamtani S, Aljanabi NM, Gupta Rauniyar RP, Acharya A, Malik BH. Candida Endocarditis: A Review of the Pathogenesis, Morphology, Risk Factors, and Management of an Emerging and Serious Condition. Cureus. 2020;12:e6695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Sharma S, Samantaray S, Kumar D, Meena DS, Chaudhary R, Jain V, Bohra GK, Garg MK. Prosthetic valve endocarditis due to Candida parapsilosis - a rare case report. Access Microbiol. 2023;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Sakakibara K, Nakajima H. A case of Candida parapsilosis bioprosthetic valve endocarditis. Clin Case Rep. 2023;11:e6950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Chen CB, Jarrett H, Goldman SM. Candida endocarditis in a transcatheter aortic valve. Eur Heart J. 2023;44:1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Mohammadi S, Mohammadi J, Forrest GN. Epidemiology of Candida Endocarditis. Curr Fungal Infect Rep. 2013;7:306-310. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Calvo B, Melo AS, Perozo-Mena A, Hernandez M, Francisco EC, Hagen F, Meis JF, Colombo AL. First report of Candida auris in America: Clinical and microbiological aspects of 18 episodes of candidemia. J Infect. 2016;73:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 325] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 24. | Sears D, Schwartz BS. Candida auris: An emerging multidrug-resistant pathogen. Int J Infect Dis. 2017;63:95-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 25. | Garbino J, Kolarova L, Rohner P, Lew D, Pichna P, Pittet D. Secular trends of candidemia over 12 years in adult patients at a tertiary care hospital. Medicine (Baltimore). 2002;81:425-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Baddley JW, Benjamin DK Jr, Patel M, Miró J, Athan E, Barsic B, Bouza E, Clara L, Elliott T, Kanafani Z, Klein J, Lerakis S, Levine D, Spelman D, Rubinstein E, Tornos P, Morris AJ, Pappas P, Fowler VG Jr, Chu VH, Cabell C; International Collaboration on Endocarditis-Prospective Cohort Study Group (ICE-PCS). Candida infective endocarditis. Eur J Clin Microbiol Infect Dis. 2008;27:519-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 27. | Falcone M, Barzaghi N, Carosi G, Grossi P, Minoli L, Ravasio V, Rizzi M, Suter F, Utili R, Viscoli C, Venditti M; Italian Study on Endocarditis (SEI). Candida infective endocarditis: report of 15 cases from a prospective multicenter study. Medicine (Baltimore). 2009;88:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Ishikane M, Hayakawa K, Kutsuna S, Takeshita N, Ohmagari N. The impact of infectious disease consultation in candidemia in a tertiary care hospital in Japan over 12 years. PLoS One. 2019;14:e0215996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Pasha AK, Lee JZ, Low SW, Desai H, Lee KS, Al Mohajer M. Fungal Endocarditis: Update on Diagnosis and Management. Am J Med. 2016;129:1037-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | Johnson MD, Johnson CD. Neurologic presentations of infective endocarditis. Neurol Clin. 2010;28:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Varghese GM, Sobel JD. Fungal endocarditis. Curr Infect Dis Rep. 2008;10:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96:200-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1650] [Cited by in RCA: 1562] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 33. | Cecchi E, Parrini I, Chinaglia A, Pomari F, Brusasco G, Bobbio M, Trinchero R, Brusca A. New diagnostic criteria for infective endocarditis. A study of sensitivity and specificity. Eur Heart J. 1997;18:1149-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Hoen B, Béguinot I, Rabaud C, Jaussaud R, Selton-Suty C, May T, Canton P. The Duke criteria for diagnosing infective endocarditis are specific: analysis of 100 patients with acute fever or fever of unknown origin. Clin Infect Dis. 1996;23:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Hoen B, Selton-Suty C, Danchin N, Weber M, Villemot JP, Mathieu P, Floquet J, Canton P. Evaluation of the Duke criteria vs the Beth Israel criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 1995;21:905-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, Bashore T, Corey GR. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2699] [Cited by in RCA: 2890] [Article Influence: 111.2] [Reference Citation Analysis (1)] |
| 37. | Prendergast BD. Diagnostic criteria and problems in infective endocarditis. Heart. 2004;90:611-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, Bille J, Castagnola E, Cuenca-Estrella M, Donnelly JP, Groll AH, Herbrecht R, Hope WW, Jensen HE, Lass-Flörl C, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ; ESCMID Fungal Infection Study Group. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18 Suppl 7:19-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 917] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 39. | Cuenca-Estrella M, Verweij PE, Arendrup MC, Arikan-Akdagli S, Bille J, Donnelly JP, Jensen HE, Lass-Flörl C, Richardson MD, Akova M, Bassetti M, Calandra T, Castagnola E, Cornely OA, Garbino J, Groll AH, Herbrecht R, Hope WW, Kullberg BJ, Lortholary O, Meersseman W, Petrikkos G, Roilides E, Viscoli C, Ullmann AJ; ESCMID Fungal Infection Study Group. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: diagnostic procedures. Clin Microbiol Infect. 2012;18 Suppl 7:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 281] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 40. | Hoen B, Selton-Suty C, Lacassin F, Etienne J, Briançon S, Leport C, Canton P. Infective endocarditis in patients with negative blood cultures: analysis of 88 cases from a one-year nationwide survey in France. Clin Infect Dis. 1995;20:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 132] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 41. | Fournier PE, Thuny F, Richet H, Lepidi H, Casalta JP, Arzouni JP, Maurin M, Célard M, Mainardi JL, Caus T, Collart F, Habib G, Raoult D. Comprehensive diagnostic strategy for blood culture-negative endocarditis: a prospective study of 819 new cases. Clin Infect Dis. 2010;51:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 309] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 42. | Lamas CC, Eykyn SJ. Suggested modifications to the Duke criteria for the clinical diagnosis of native valve and prosthetic valve endocarditis: analysis of 118 pathologically proven cases. Clin Infect Dis. 1997;25:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 112] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Lamas CC, Eykyn SJ. Blood culture negative endocarditis: analysis of 63 cases presenting over 25 years. Heart. 2003;89:258-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 172] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 44. | Koo S, Bryar JM, Page JH, Baden LR, Marty FM. Diagnostic performance of the (1-->3)-beta-D-glucan assay for invasive fungal disease. Clin Infect Dis. 2009;49:1650-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 45. | Marr KA, Laverdiere M, Gugel A, Leisenring W. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin Infect Dis. 2005;40:1762-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 369] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 46. | Slim J, Saling C, Szabela M, Brown M, Johnson T, Goldfarb I. (1→3)-β-D-Glucan Assay in Monitoring Response to Anti-Fungal Therapy in Fungal Endocarditis. J Heart Valve Dis. 2017;26:208-210. [PubMed] |
| 47. | Mikulska M, Calandra T, Sanguinetti M, Poulain D, Viscoli C; Third European Conference on Infections in Leukemia Group. The use of mannan antigen and anti-mannan antibodies in the diagnosis of invasive candidiasis: recommendations from the Third European Conference on Infections in Leukemia. Crit Care. 2010;14:R222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 227] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 48. | Sendid B, Poirot JL, Tabouret M, Bonnin A, Caillot D, Camus D, Poulain D. Combined detection of mannanaemia and antimannan antibodies as a strategy for the diagnosis of systemic infection caused by pathogenic Candida species. J Med Microbiol. 2002;51:433-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 111] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 49. | Rice PA, Madico GE. Polymerase chain reaction to diagnose infective endocarditis: will it replace blood cultures? Circulation. 2005;111:1352-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Grijalva M, Horváth R, Dendis M, Erný J, Benedík J. Molecular diagnosis of culture negative infective endocarditis: clinical validation in a group of surgically treated patients. Heart. 2003;89:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Posteraro B, Valentini P, Delogu A, De RG, Boccacci S, Sanguinetti M, Nacci A, Sopo SM, Ranno O, Morace G, Fadda G. Candida albicans endocarditis diagnosed by PCR-based molecular assay in a critically ill pediatric patient. Scand J Infect Dis. 2002;34:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Asghar W, Sher M, Khan NS, Vyas JM, Demirci U. Microfluidic Chip for Detection of Fungal Infections. ACS Omega. 2019;4:7474-7481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 53. | Xie TA, Liu YL, Liang C, Huang YY, Li JW, Li ZW, Fan SJ, Chen JT, Xia Y, Li XY, Ouyang S, Ji TX, Guo XG. Accuracy of matrix-assisted LASER desorption ionization-time of flight mass spectrometry for identification of Candida. Biosci Rep. 2019;39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 54. | Yaman G, Akyar I, Can S. Evaluation of the MALDI TOF-MS method for identification of Candida strains isolated from blood cultures. Diagn Microbiol Infect Dis. 2012;73:65-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 55. | Avni T, Leibovici L, Paul M. PCR diagnosis of invasive candidiasis: systematic review and meta-analysis. J Clin Microbiol. 2011;49:665-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 287] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 56. | Eghtedar Nejad E, Ghasemi Nejad Almani P, Mohammadi MA, Salari S. Molecular identification of Candida isolates by Real-time PCR-high-resolution melting analysis and investigation of the genetic diversity of Candida species. J Clin Lab Anal. 2020;34:e23444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 57. | Fernández-Cruz A, Cruz Menárguez M, Muñoz P, Pedromingo M, Peláez T, Solís J, Rodríguez-Créixems M, Bouza E; GAME Study Group (Grupo de Apoyo al Manejo de la Endocarditis). The search for endocarditis in patients with candidemia: a systematic recommendation for echocardiography? A prospective cohort. Eur J Clin Microbiol Infect Dis. 2015;34:1543-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Granados U, Fuster D, Pericas JM, Llopis JL, Ninot S, Quintana E, Almela M, Paré C, Tolosana JM, Falces C, Moreno A, Pons F, Lomeña F, Miro JM; Hospital Clinic Endocarditis Study Group. Diagnostic Accuracy of 18F-FDG PET/CT in Infective Endocarditis and Implantable Cardiac Electronic Device Infection: A Cross-Sectional Study. J Nucl Med. 2016;57:1726-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 59. | Salomäki SP, Saraste A, Jalava-Karvinen P, Pirilä L, Hohenthal U. Prosthetic Valve Candida Endocarditis: A Case Report with 18F-FDG-PET/CT as Part of the Diagnostic Workup. Case Rep Cardiol. 2020;2020:4921380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 60. | Erba PA, Conti U, Lazzeri E, Sollini M, Doria R, De Tommasi SM, Bandera F, Tascini C, Menichetti F, Dierckx RA, Signore A, Mariani G. Added value of 99mTc-HMPAO-labeled leukocyte SPECT/CT in the characterization and management of patients with infectious endocarditis. J Nucl Med. 2012;53:1235-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 61. | Jasińska A, Teresińska A, Hryniewiecki T. Diagnosis of infective endocarditis with nuclear medicine techniques: use of SPECT/CT with [99mTc]Tc-HMPAO-labelled leukocytes. Folia Cardiologica. 2022;17:100-108. [RCA] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 62. | Rouzet F, Chequer R, Benali K, Lepage L, Ghodbane W, Duval X, Iung B, Vahanian A, Le Guludec D, Hyafil F. Respective performance of 18F-FDG PET and radiolabeled leukocyte scintigraphy for the diagnosis of prosthetic valve endocarditis. J Nucl Med. 2014;55:1980-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 63. | Calais J, Touati A, Grall N, Laouénan C, Benali K, Mahida B, Vigne J, Hyafil F, Iung B, Duval X, Lepage L, Le Guludec D, Rouzet F. Diagnostic Impact of (18)F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography and White Blood Cell SPECT/Computed Tomography in Patients With Suspected Cardiac Implantable Electronic Device Chronic Infection. Circ Cardiovasc Imaging. 2019;12:e007188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 64. | d'Enfert C. Biofilms and their role in the resistance of pathogenic Candida to antifungal agents. Curr Drug Targets. 2006;7:465-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 65. | Mukherjee PK, Chandra J. Candida biofilm resistance. Drug Resist Updat. 2004;7:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 172] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 66. | Pai MP, Samples ML, Mercier RC, Spilde MN. Activities and ultrastructural effects of antifungal combinations against simulated Candida endocardial vegetations. Antimicrob Agents Chemother. 2008;52:2367-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 67. | Smego RA Jr, Ahmad H. The role of fluconazole in the treatment of Candida endocarditis: a meta-analysis. Medicine (Baltimore). 2011;90:237-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 68. | Penk A, Pittrow L. Role of fluconazole in the long-term suppressive therapy of fungal infections in patients with artificial implants. Mycoses. 1999;42 Suppl 2:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Arnold CJ, Johnson M, Bayer AS, Bradley S, Giannitsioti E, Miró JM, Tornos P, Tattevin P, Strahilevitz J, Spelman D, Athan E, Nacinovich F, Fortes CQ, Lamas C, Barsic B, Fernández-Hidalgo N, Muñoz P, Chu VH. Candida infective endocarditis: an observational cohort study with a focus on therapy. Antimicrob Agents Chemother. 2015;59:2365-2373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 70. | Steinbach WJ, Perfect JR, Cabell CH, Fowler VG, Corey GR, Li JS, Zaas AK, Benjamin DK Jr. A meta-analysis of medical vs surgical therapy for Candida endocarditis. J Infect. 2005;51:230-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 71. | Fioriti S, Brescini L, Pallotta F, Canovari B, Morroni G, Barchiesi F. Antifungal Combinations against Candida Species: From Bench to Bedside. J Fungi (Basel). 2022;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 72. | Stripeli F, Tsolia M, Trapali Ch, Papaevangelou V, Vlachos E, Pasparakis D, Constantopoulos A. Successful medical treatment of Candida endocarditis with liposomal amphotericin B without surgical intervention. Eur J Pediatr. 2008;167:469-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 73. | Tattevin P, Revest M, Lefort A, Michelet C, Lortholary O. Fungal endocarditis: current challenges. Int J Antimicrob Agents. 2014;44:290-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 74. | Kamaledeen A, Young C, Attia RQ. What are the differences in outcomes between right-sided active infective endocarditis with and without left-sided infection? Interact Cardiovasc Thorac Surg. 2012;14:205-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 75. | Ortiz C, López J, García H, Sevilla T, Revilla A, Vilacosta I, Sarriá C, Olmos C, Ferrera C, García PE, Sáez C, Gómez I, San Román JA. Clinical classification and prognosis of isolated right-sided infective endocarditis. Medicine (Baltimore). 2014;93:e137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 76. | Revilla A, López J, Villacorta E, Gómez I, Sevilla T, del Pozo MA, de la Fuente L, Manzano Mdel C, Mota P, Flórez S, Vilacosta I, Sarriá C, Sánchez M, San Román JA. Isolated right-sided valvular endocarditis in non-intravenous drug users. Rev Esp Cardiol. 2008;61:1253-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 77. | Siciliano RF, Gualandro DM, Sejas ONE, Ignoto BG, Caramelli B, Mansur AJ, Sampaio RO, Pierrotti LC, Barbosa G, Golebiovski W, Weksler C, Lamas C, Fortes NRQ, Fortes CQ, Tarasoutchi F, Strabelli TMV. Outcomes in patients with fungal endocarditis: A multicenter observational cohort study. Int J Infect Dis. 2018;77:48-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 78. | Jerónimo A, Olmos C, Vilacosta I, Sáez C, López J, Sanz M, Cabezón G, Pérez-Serrano JB, Zulet P, San Román JA. Contemporary comparison of infective endocarditis caused by Candida albicans and Candida parapsilosis: a cohort study. Eur J Clin Microbiol Infect Dis. 2022;41:981-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 79. | Rivoisy C, Vena A, Schaeffer L, Charlier C, Fontanet A, Delahaye F, Bouza E, Lortholary O, Munoz P, Lefort A; French Mycoses Study Group and Grupo de Apoyo al Manejo de las Endocarditis en España (GAMES). Prosthetic Valve Candida spp. Endocarditis: New Insights Into Long-term Prognosis-The ESCAPE Study. Clin Infect Dis. 2018;66:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 80. | Mhanna M, Beran A, Al-Abdouh A, Jabri A, Sajdeya O, Al-Aaraj A, Alharbi A, Khuder SA, Eltahawy EA. AngioVac for Vegetation Debulking in Right-sided Infective Endocarditis: A Systematic Review and Meta-Analysis. Curr Probl Cardiol. 2022;47:101353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 81. | Morton K, Heindl B, McElwee SK, Litovsky S, Ahmed MI, Clarkson S. Percutaneous debulking of tricuspid valve endocarditis in severe COVID-19 pneumonia after prolonged venovenous extracorporeal membrane oxygenation with right-ventricular support: a case series. Eur Heart J Case Rep. 2023;7:ytac409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (1)] |
| 82. | Johnson MD, Plantinga TS, van de Vosse E, Velez Edwards DR, Smith PB, Alexander BD, Yang JC, Kremer D, Laird GM, Oosting M, Joosten LA, van der Meer JW, van Dissel JT, Walsh TJ, Perfect JR, Kullberg BJ, Scott WK, Netea MG. Cytokine gene polymorphisms and the outcome of invasive candidiasis: a prospective cohort study. Clin Infect Dis. 2012;54:502-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 83. | Kumar V, Cheng SC, Johnson MD, Smeekens SP, Wojtowicz A, Giamarellos-Bourboulis E, Karjalainen J, Franke L, Withoff S, Plantinga TS, van de Veerdonk FL, van der Meer JWM, Joosten LAB, Bochud PY, Marchetti O, Perfect JR, Xavier R, Kullberg BJ, Wijmenga C, Netea MG. Immunochip SNP array identifies novel genetic variants conferring susceptibility to candidaemia. Nat Commun. 2014;5:4675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 84. | Plantinga TS, Johnson MD, Scott WK, van de Vosse E, Velez Edwards DR, Smith PB, Alexander BD, Yang JC, Kremer D, Laird GM, Oosting M, Joosten LA, van der Meer JW, van Dissel JT, Walsh TJ, Perfect JR, Kullberg BJ, Netea MG. Toll-like receptor 1 polymorphisms increase susceptibility to candidemia. J Infect Dis. 2012;205:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 85. | Shorr AF, Chung K, Jackson WL, Waterman PE, Kollef MH. Fluconazole prophylaxis in critically ill surgical patients: a meta-analysis. Crit Care Med. 2005;33:1928-35; quiz 1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 86. | Schuster MG, Edwards JE Jr, Sobel JD, Darouiche RO, Karchmer AW, Hadley S, Slotman G, Panzer H, Biswas P, Rex JH. Empirical fluconazole vs placebo for intensive care unit patients: a randomized trial. Ann Intern Med. 2008;149:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 87. | Pfaller MA, Diekema DJ, Turnidge JD, Castanheira M, Jones RN. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species From 1997-2016. Open Forum Infect Dis. 2019;6:S79-S94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 586] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 88. | Makdisi G, Casciani T, Wozniak TC, Roe DW, Hashmi ZA. A successful percutaneous mechanical vegetation debulking used as a bridge to surgery in acute tricuspid valve endocarditis. J Thorac Dis. 2016;8:E137-E139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 89. | Talebi S, Tan BEX, Gazali RM, Herzog E. Last resort: successful AngioVac of fungal tricuspid valve vegetation. QJM. 2017;110:673-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Teragawa H, Japan S-Editor: Lin C L-Editor: Webster JR P-Editor: Xu ZH