Published online Jan 26, 2022. doi: 10.4330/wjc.v14.i1.40
Peer-review started: May 27, 2021
First decision: July 30, 2021
Revised: August 22, 2021
Accepted: January 13, 2022
Article in press: January 13, 2022
Published online: January 26, 2022
Processing time: 236 Days and 11.9 Hours
In acute coronary syndrome (ACS), the use of anticoagulants in conjunction with antiplatelet agents in the acute phase has resulted in reduced ischemic events and is more effective than either class of drug used alone. Though parenteral anticoagulation is essential at the time of diagnosis, a balance must be made between ischemic benefit and the increased risk of bleeding when prescribing anticoagulants. Adverse events associated with anticoagulants, such as heparin-induced thrombocytopenia, bleeding problems, and the need for close monitoring of anticoagulant activity, have contributed to finding agents that reduce these limitations. Studies like the Organization to Assess Strategies in Ischemic Syndromes 5 and 6 and their meta-analysis have proven the efficacy of Fonda
Core Tip: The simultaneous use of antithrombotic therapy and anti-platelet therapy in the acute coronary syndrome acute phase is associated with reduced ischemic events and is more effective than either class of drug used alone. The physicians must maintain a balance while prescribing these drugs to maintain an overall benefit-risk ratio. Fondaparinux is one of the simple and effective anti-coagulant for the management of acute coronary syndrome.
- Citation: Khan MY, Ponde CK, Kumar V, Gaurav K. Fondaparinux: A cornerstone drug in acute coronary syndromes. World J Cardiol 2022; 14(1): 40-53
- URL: https://www.wjgnet.com/1949-8462/full/v14/i1/40.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i1.40
Acute coronary syndrome (ACS) is one of the main causes of fatality worldwide. When compared to other countries, South Asians have a higher rate of myocardial infarction (MI) at a younger age (average age 53 years vs average age 58.8 years)[1-4]. In the Indian population as well, ACS presents a decade earlier in comparison to the West. Furthermore, India has the greatest ACS load in the world, with a burden 3 to 4 times that of White Americans, 6 times that of Chinese, and 20 times that of Japanese people. ACS is responsible for 3 million fatalities each year in India, accounting for 25% of all deaths[5]. Anti-coagulant therapies form the cornerstone in the management of ACS.
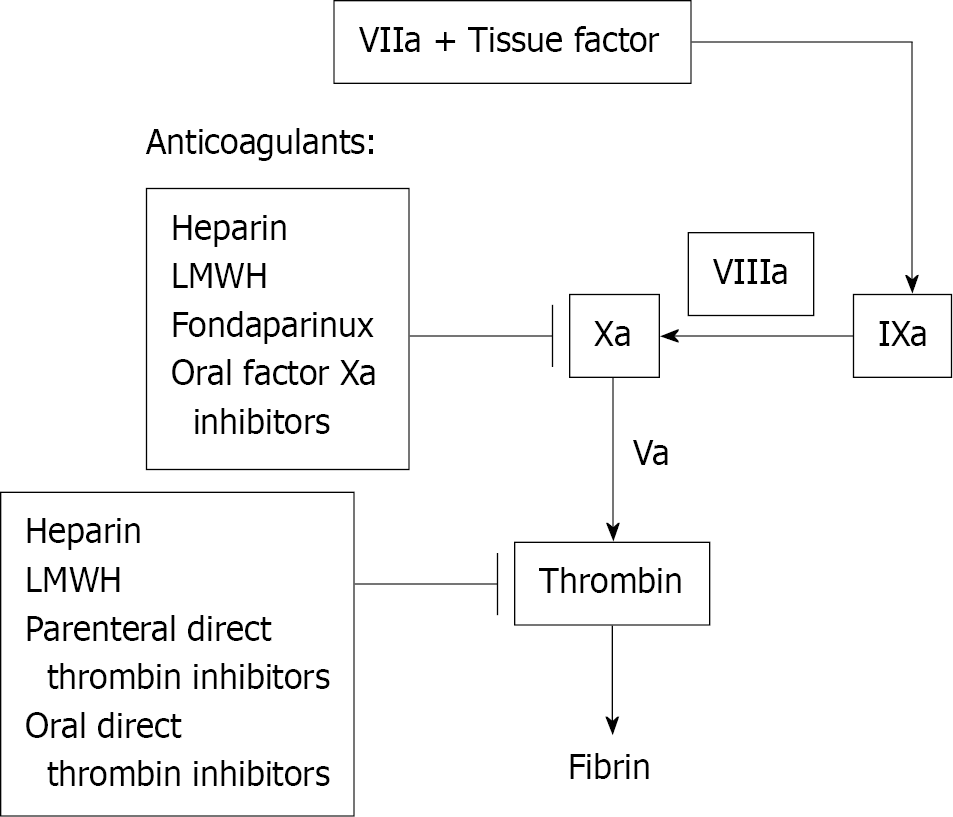
The mechanism of action of various anti-coagulant therapies is described in Figure 1. Anticoagulant medication is an important part of the treatment of ACS[6], while being only one step of the treatment pathway. In the past, unfractionated heparin was the most commonly used parenteral anticoagulant. However, as compared to other treatments, unfractionated heparin (UFH) has a variable dose-response and a small therapeutic window that necessitates frequent monitoring and is linked with a higher risk of side effects [e.g., heparin-induced thrombocytopenia (HIT), hemorrhage, and osteoporosis]. Other systemic anticoagulants that do not require frequent monitoring or dose adjustment are more commonly used these days than UFH[7]. Maintaining a fine balance between reducing cardiovascular mortality and increased risk of bleeding is the main concern for the available treatments for ACS. The main objective of this review is to identify the current gaps of using anti-coagulant therapies in ACS and thus to bring in the evaluated clinical benefits of Fondaparinux in ACS patients.
Fondaparinux, a synthetic anticoagulant, consists of a highly sulfated penta saccharide derived from the minimal antithrombin (AT)-binding region of heparin. It works as an indirect factor Xa inhibitor by binding to AT and producing a conformational change in AT that improves AT's ability to inactivate factor Xa (Figure 1)[8].
Unfractionated heparin and low molecular weight heparins (LMWHs) such as Enoxaparin act by inhibiting both factor Xa and thrombin. LMWHs have a lower effect on thrombin than the effect of unfractionated heparin. Fondaparinux specifically inhibits factor Xa coagulation factors[8].
Fondaparinux can be administered through subcutaneous (s.c.) or intravenous (i.v.) routes. The bioavailability is 100% after s.c. administration of fondaparinux in healthy volunteers and mean maximum plasma concentration is achieved at 1.7 h. The mean maximum plasma concentration with i.v. dose is achieved at an even faster rate without affecting half-life[9].
When compared to LMWHs like Enoxaparin and UFHs, fondaparinux sodium had no effect on fibrinolytic activity or bleeding time at the prescribed dose, promoting hemostasis and a favorable bleeding risk profile. Fondaparinux does not bind to or interact with other plasma proteins or cellular elements such as platelets or platelet factor 4, and thus, unlike Enoxaparin and UFH, it does not cause heparin-induced thrombocytopenia-like syndrome[10].
Fondaparinux is 100% bioavailable after s.c. injection. The half-life of fondaparinux is 17.2 h, which is quite long and allows once-daily dosing[11].
Fondaparinux does not require monitoring in routine clinical use. The anticoagulant effect of fondaparinux can be assessed with dedicated anti-Xa assays in high-risk patient populations (renal insufficiency, bodyweight less than 50 kg)[6].
Fondaparinux is mainly cleared through the kidney and is excreted unchanged in the urine. Clearance of this drug is reduced in individuals with reduced creatinine clearance and is therefore not recommended for use in individuals with creatinine clearance < 30 mL/min[10]. Table 1 compares pharmacokinetics, dosage frequency, and indications between UFH, LMWHs (Enoxaparin), and Fondaparinux.
| UFH | Enoxaparin | Fondaparinux | |
| Source | Biological | Biological | Synthetic |
| Bioavailability | 30% | 90% | 100% |
| Mechanism | Augments AT effects on Factor Xa and thrombin. Binds to plasma proteins not specifically → unpredictable dose-response | Augments AT effects more on Factor Xa than on thrombin. Low binding to plasma proteins → more predictable dose-response, low inter-patient variability | Augments anti-Xa activity of AT, no direct effect on thrombin. Specific for AT → no binding to other plasma proteins, predictable dose-response |
| Plasma half-life | 1-2 h | 4.5-7 h | 17-21 h |
| Reversal agents | Protamine sulfate | Protamine sulfate | Irreversible by protamine factor VII- limited data |
| Routine monitoring | Yes | No | No |
| Dosing frequency in ACS | Treatment - Continuous i.v. infusion | BID | Once daily |
| Clearance | Hepatic & Reticuloendothelial clearance. No renal adjustments | Renal | Renal |
| Adjustment needed for CrCl < 30 mL/min | Contraindication: CrCl < 30 mL/min | ||
| Ability to cause HIT | Yes | Yes | No cases in major trials |
| Bleeding risk | Increased | Increased | Lesser |
The effectiveness and safety profile of fondaparinux has been studied in ACS. An enormous reduction in bleeding complications in ACS has been seen in various studies where fondaparinux is utilized as an anticoagulant in ACS. Here, we present evidence on the clinical efficacy and safety of fondaparinux in patients with ACS.
Organization to Assess Strategies in Ischemic Syndromes (OASIS) 5 was a randomized, double-dummy, randomized, parallel-group, controlled trial to see if Fondaparinux, when used as an anticoagulant in unstable angina and non-ST elevation ACS (NSTE-ACS), would preserve the anti-ischemic benefits of Enoxaparin while reducing bleeding. Following randomization, the study medication was administered subcutaneously. Enoxaparin 1 mg/kg twice daily s.c. for 2 to 8 d or until clinically stable, or fondaparinux 2.5 mg once daily s.c. for 8 d or until discharge, whichever came first. In patients with a creatinine clearance of 20 mL/min to 30 mL/min, Enoxaparin was given once daily at a dose of 1 mg/kg. Subjects received regular medical care, including interventions, in addition to the study medicine [percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery][12].
Primary efficacy outcome: In terms of the primary efficacy outcome, Fondaparinux was shown to be non-inferior to Enoxaparin [Fondaparinux 5.8% vs Enoxaparin EX 5.7%, hazard ratio (HR), 1.01; 95% confidence interval (CI), 0.90 to 1.13] (Table 2)[12].
Primary safety outcome: When compared to Enoxaparin, Fondaparinux reduced serious bleeding rates by nearly half (2.2% vs 4.1%; HR, 0.52; 95%CI, 0.44 to 0.61; P = 0.001) (Table 2)[12].
| Outcomes | Fondaparinux | Enoxaparin | HR (95%CI) | P value |
| Primary efficacy outcome: Cumulative event rate-Death, MI, refractory ischemia at 9 d | ||||
| Cumulative event rate | 5.80% | 5.70% | 1.01 (0.90-1.13) | 0.007 |
| Primary safety outcome: Major bleeding at 9 d | ||||
| Major bleeding | 2.20% | 4.10% | 0.52 (0.44-0.61) | P < 0.001 |
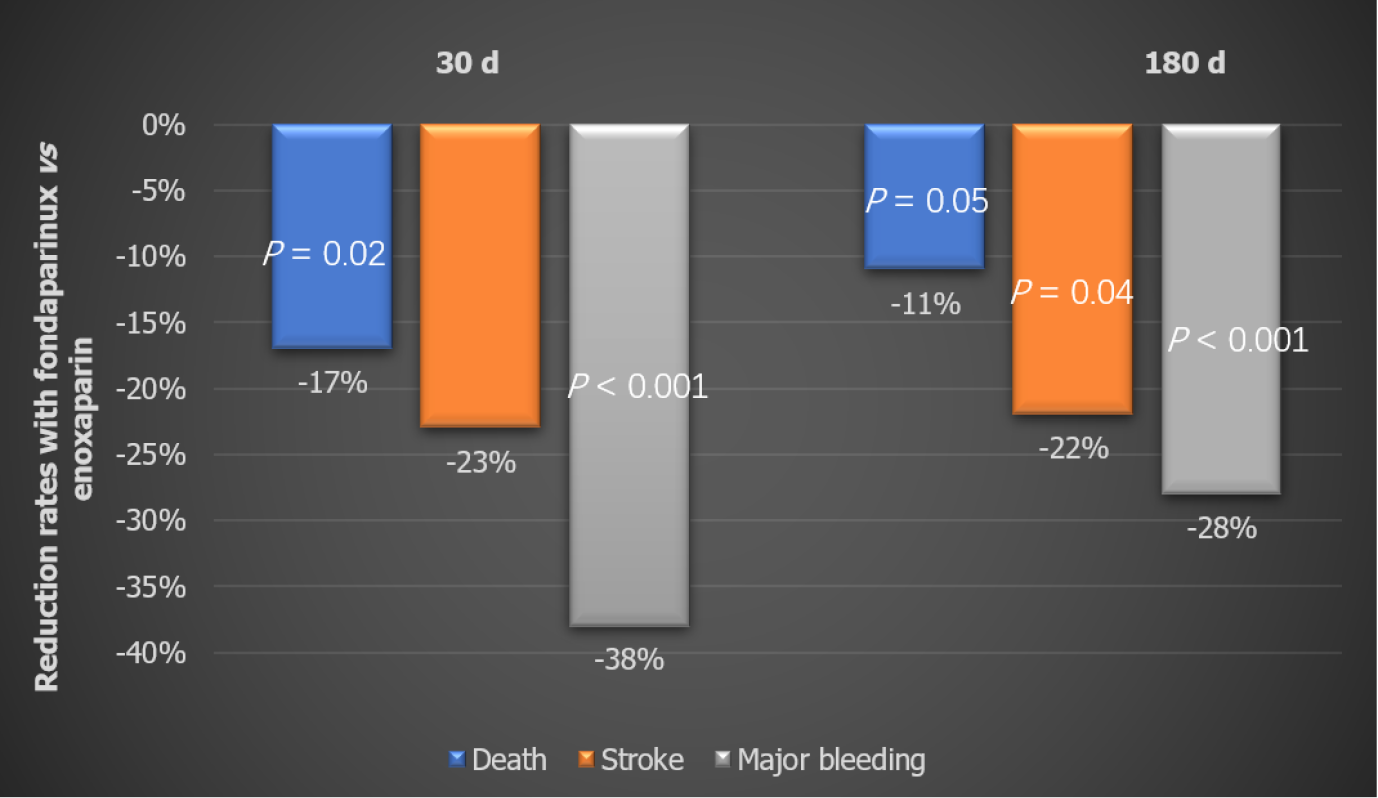
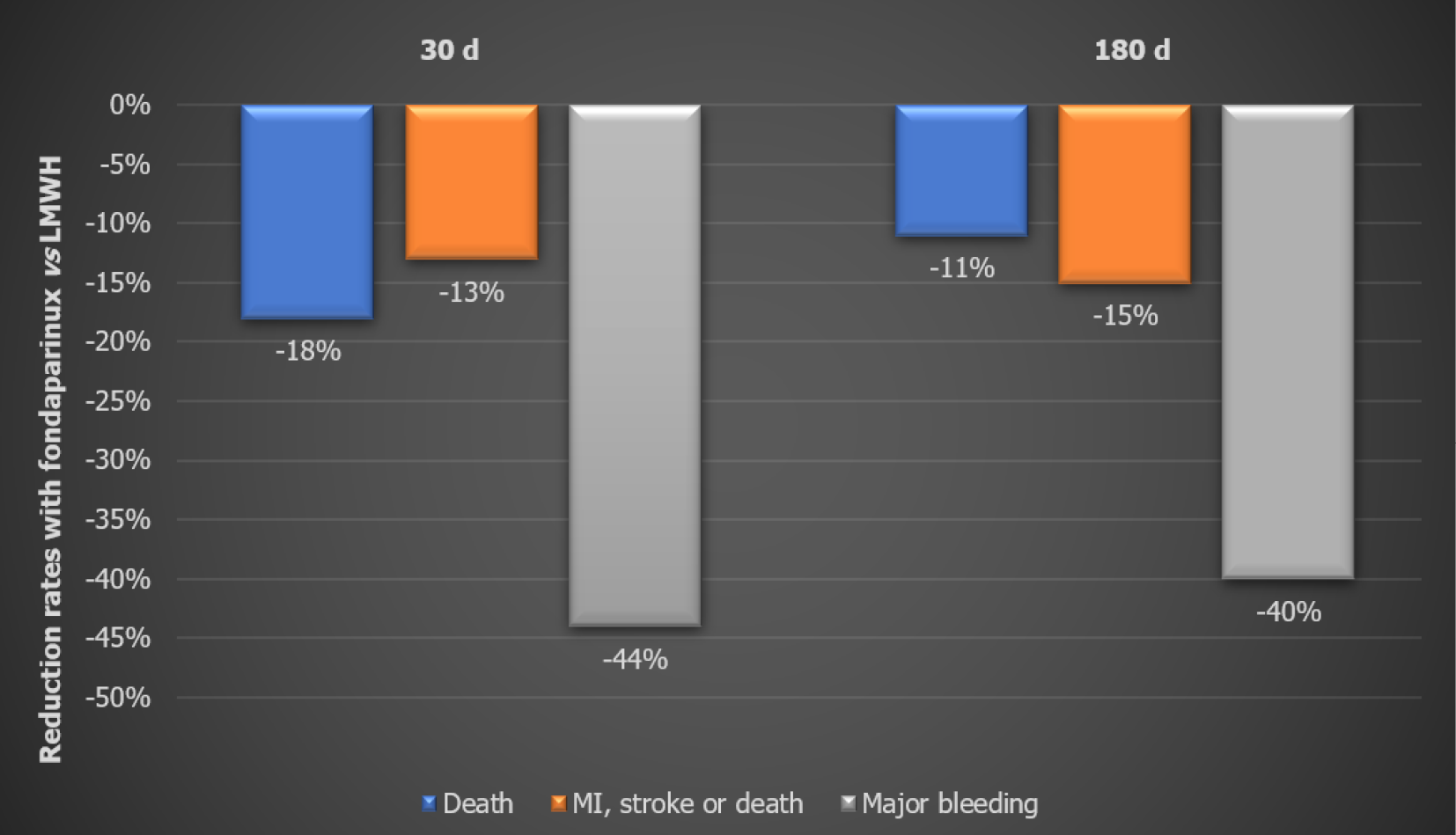
Secondary outcomes: At the end of 30 d, the Fondaparinux group showed 17% lower mortality (P < 0.02) and 23% lower stroke rate vs the Enoxaparin group. Bleeding rates were significantly reduced too. The effect on death, stroke, and major bleeding was maintained till the end of the study (Figure 2). Fondaparinux also resulted in significant reductions in the combined endpoints of death/MI/stroke at 30 d (-11%) and 180 d (-11%). There was also a significant reduction in fatal and severe bleeding rates for Fondaparinux as compared to Enoxaparin both at 30 d and 180 d. The difference in mortality favoring Fondaparinux in this study was almost completely attributed to the reduced bleeding rates[12].
Balance of benefit and risk: The major effectiveness and safety outcomes were combined to determine the balance of benefit and risk. At 9 d, patients in the Fondaparinux group had a considerably reduced rate of death, MI, refractory ischemia, and severe bleeding than those in the Enoxaparin group. This disparity continued until the study's conclusion. The benefits and risk were consistent across subgroups, including age and gender, renal function spectrum, whether unfractionated heparin was provided before randomization, and whether revascularization was conducted within 9 d[12].
The safety of Fondaparinux over Enoxaparin was also validated in three key subgroups: Those with a wide range of renal impairment in the OASIS 5 trial, those who underwent PCI within 24 h, and those who took glycoprotein (GP) IIb/IIIa inhibitors.
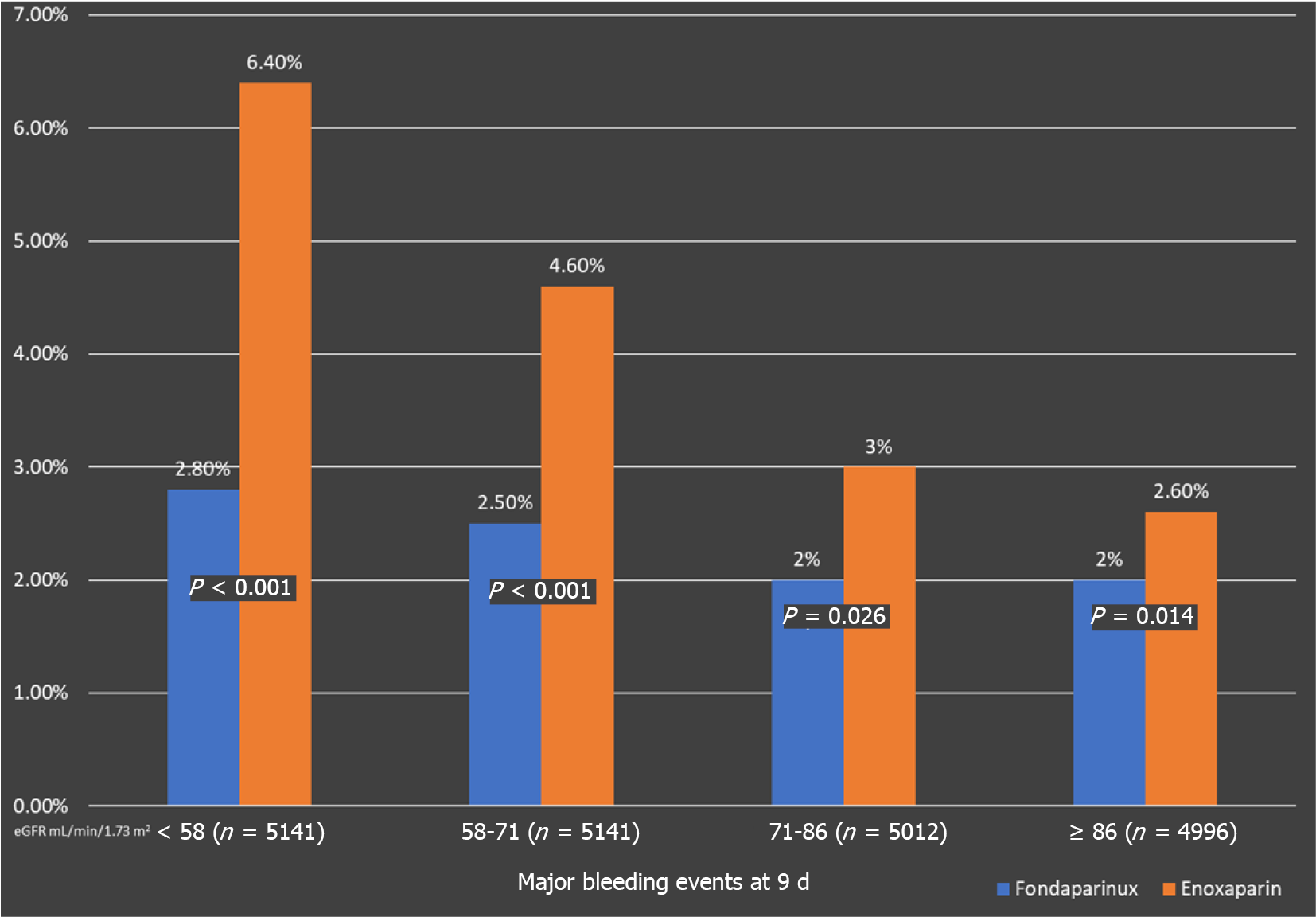
This subgroup analysis assessed if the enhanced bleeding risk with Enoxaparin was related to the level of kidney function in study participants. Efficacy and safety data have been grouped into quartiles according to estimated glomerular filtration rate [(eGFR): < 58 (n = 5141); 58 to 71 (n = 4845); 71 to 86 (n = 5012); and ≥ 86 mL/min/1.73 m2 (n = 4996), respectively][13].
When compared to Enoxaparin, Fondaparinux was associated with significantly fewer bleeding episodes. Across all eGFR quartiles, Fondaparinux treatment was linked with decreased severe bleeding on day 9 (Figure 3). This pattern maintained for the next 30 d and 180 d. Those with an eGFR of less than 58 mL/min/1.73 m2 had the most noticeable bleeding disparities[13].
Only individuals with an eGFR of less than 58 mL/min/1.73 m2 saw a substantial reduction in the composite outcome of mortality, MI, and refractory ischemia at day 30. In comparison to Enoxaparin, the other eGFR groups had similar endpoint rates[13]. Therefore, the benefits of Fondaparinux over Enoxaparin in NSTE- ACS are most marked among patients with renal dysfunction and are largely due to a better safety profile of Fondaparinux due to lower rates of major bleeding with Fondaparinux.
More than 60% of patients had catheterization and more than 30% had PCI In OASIS 5. When the last dose of Enoxaparin was more than 6 h before the procedure, patients were given weight adjusted UFH during PCI. An additional dosage of i.v. Fonda
When Fondaparinux was used instead of Enoxaparin on day 9, serious bleeding was considerably reduced (2.4% vs 5.1%, HR 0.46; P < 0.00001). When compared to Enoxaparin, Fondaparinux was associated with a slight increase in the rate of catheter-related thrombi (in patients having PCI) (0.9% vs 0.4%) (Table 3). The findings show that upstream Fondaparinux therapy in NSTE-ACS patients undergoing early PCI was superior to Enoxaparin in lowering severe bleeding by 50% while maintaining the same effectiveness, resulting in a superior net therapeutic benefit[14]. Patients who received open-label UFH before the treatment had a considerably lower rate of catheter-related thrombi in both groups. The Fondaparinux with Unfractionated Heparin (FUTURA) trial, which is addressed later in this article, looked at this evidence further.
| Outcome day 9 | Enoxaparin (n = 3072) | Fondaparinux (n = 3106) | Hazard ratio | P value |
| Death, MI, or stroke | 6.2 | 6.3 | 1.03 | 0.79 |
| Major bleeding | 5.1 | 2.4 | 0.46 | < 0.00001 |
| Catheter thrombosis | 0.4 | 0.9 | 3.59 | 0.001 |
In patients with ACS treated with GP IIb/IIIa inhibitors or thienopyridines, the efficacy and safety of Fondaparinux were compared to Enoxaparin. Patients with ACS (n = 20078) were randomized to either Fondaparinux or Enoxaparin as part of the OASIS 5 study. The treating physician selected whether or not to utilize GP IIb/IIIa inhibitors or thienopyridines. Fondaparinux reduced significant bleeding and improved net clinical outcome in individuals using GP IIb/IIIa inhibitors or thienopyridines when compared to Enoxaparin.
While Fondaparinux and Enoxaparin both reduce the risk of ischemic events, the rate of serious bleeding with Fondaparinux was much lower than with Enoxaparin. In addition, Fondaparinux has a lower rate of combined mortality, MI, refractory ischemia, and severe bleeding than Enoxaparin. As a result, Fondaparinux at a daily dose of 2.5 mg is an appealing option for preventing ischemic events in patients with acute coronary syndromes without ST-segment elevation in the short term, and because it is associated with significantly less bleeding–this effect translates to lower long-term mortality and morbidity compared to Enoxaparin[12-14].
FUTURA during revascularization in ACS (FUTURA/OASIS 8): The trial compared the safety of two UFH treatment regimens during PCI in high-risk patients with NSTE initially treated with Fondaparinux in a double-blind, randomized, parallel-group, multicenter trial with 2026 NSTE-ACS patients undergoing PCI within 72 h treated with Fondaparinux[15].
UFH regimens: The regimens were divided into low dose group and standard-dose group. All patients received 50 IU/kg UFH irrespective of the use of GP IIb IIIa in the low dose group. In the standard UFH group, the mean dose was 85 IU/kg to maintain activated clotting time (ACT) between 300-350 s and was reduced to 60 IU/g in those receiving GP IIb IIIa. The primary goal of the FUTURA trial was to see if low fixed-dose unfractionated heparin vs standard ACT guided unfractionated heparin during PCI reduces the composite of peri-PCI major, minor bleeding, and vascular access site complications in ACS patients treated with Fondaparinux. The secondary goal was to see if major bleeding rates in FUTURA were higher than in OASIS 5 PCI (with Fondaparinux used alone)[15].
Primary outcome: There were no significant differences between low fixed-dose and standard-dose unfractionated heparin in the primary outcomes of peri-PCI major bleeding or major vascular access site problems (P = 0.27) (See Table 4).
| Primary outcomes at 48 h | Standard dose UFH (n = 1002) | Low dose UFH (n = 1024) | Odds ratio | 95%CI | P value |
| Peri-PCI major, minor, bleeds and vascular access complications | 5.80% | 4.70% | 0.80 | 0.54-1.19 | 0.27 |
| Components | |||||
| Major bleeds | 1.20% | 1.40% | 1.14 | 0.53-2.49 | 0.73 |
| Minor bleeds | 1.70% | 0.70% | 0.40 | 0.16-0.97 | 0.04 |
| Major vascular access site complications | 4.30% | 3.20% | 0.74 | 0.47-1.18 | 0.21 |
Key secondary outcome: The primary secondary outcomes of Peri-PCI major bleeding, mortality, MI, and target vessel revascularization had a nominally significant difference. The low-dose UFH group had a rate of 5.8% at 30 d compared to 3.9% in the standard-dose UFH group (odds ratio, 1.51; 95%CI, 1.00-2.28; P = 0.05). There were very little catheter thrombus rates (0.5% in the low-dose UFH group and 0.1% in the standard-dose UFH group, P = 0.15). The low-dose regimen was linked to a numerically higher rate of definite stent thrombosis (n = 12 vs 5) but no reduction in severe bleeding (Table 5)[15].
| Secondary outcomes at 30 d | Standard dose UFH (n = 1002) | Low dose UFH (n = 1024) | Odds ratio | 95%CI | P value |
| Peri-PCI major bleeding, Death, MI, TVR | 3.90% | 5.80% | 1.51 | 1.00-2.28 | 0.05 |
| Death, MI, TVR | 2.90% | 4.50% | 1.58 | 0.98-2.53 | 0.06 |
| Death | 0.60% | 0.80% | 1.31 | 0.45-3.78 | |
| MI | 2.50% | 3.00% | 1.22 | 0.72-2.08 | |
| TVR | 0.30% | 0.90% | 2.95 | 0.80-10.9 | |
| Stent thrombosis | 0.50% | 1.20% | 2.36 | 0.83-6.73 | 0.11 |
| Catheter thrombosis | 0.10% | 0.5% | 4.91 | 0.57-42.1 | 0.15 |
The risk of major bleeding within 48 h was 1.1% in the standard-dose unfractionated heparin arm and 1.2% with low-dose heparin in FUTURA/OASIS 8, compared with 3.6% with Enoxaparin in OASIS 5. As a result, UFH + Fondaparinux has no effect on peri-PCI severe bleeding, and rates appear to be lower than when Enoxaparin is administered (Table 6)[15].
| Adjusted major bleeding rate | OASIS 5 PCI | OASIS 5 PCI |
| Fondaparinux | Enoxaparin | |
| Major bleeding | Major bleeding | |
| FUTURA | 1.5% | 3.6% |
| Standard dose UFH 1.1% (0.6-2.1) | ||
| FUTURA | ||
| Low dose UFH 1.2% (0.6-2.2) |
When UFH was used for PCI on a Fondaparinux background, severe bleeding was not increased when compared to Fondaparinux alone, and it was lower than when Enoxaparin was used for PCI prior. Adding UFH to Fondaparinux during PCI preserves Fondaparinux's advantages and safety (e.g., reduced bleeding) while reducing catheter thrombus. The finding that augmenting ACT-guided conventional UFH to Fondaparinux during PCI does not increase severe bleeding in patients with non-ST segment elevation MI (NSTEMI) is significant for interventional cardiologists. As a result, NSTE-ACS patients who have been treated with Fondaparinux can safely undergo PCI with UFH. There is no need to deviate from the UFH regular dose regimen advised by the guidelines[15].
Fondaparinux vs control in patients with ST-elevated MI (STEMI) was randomized within 24 h after the start of symptoms in a randomized, double-blind, controlled, parallel-group, multi-center, global trial. For the use of UFH, patients with confirmed STEMI were randomized to one of two strata based on the indication[16].
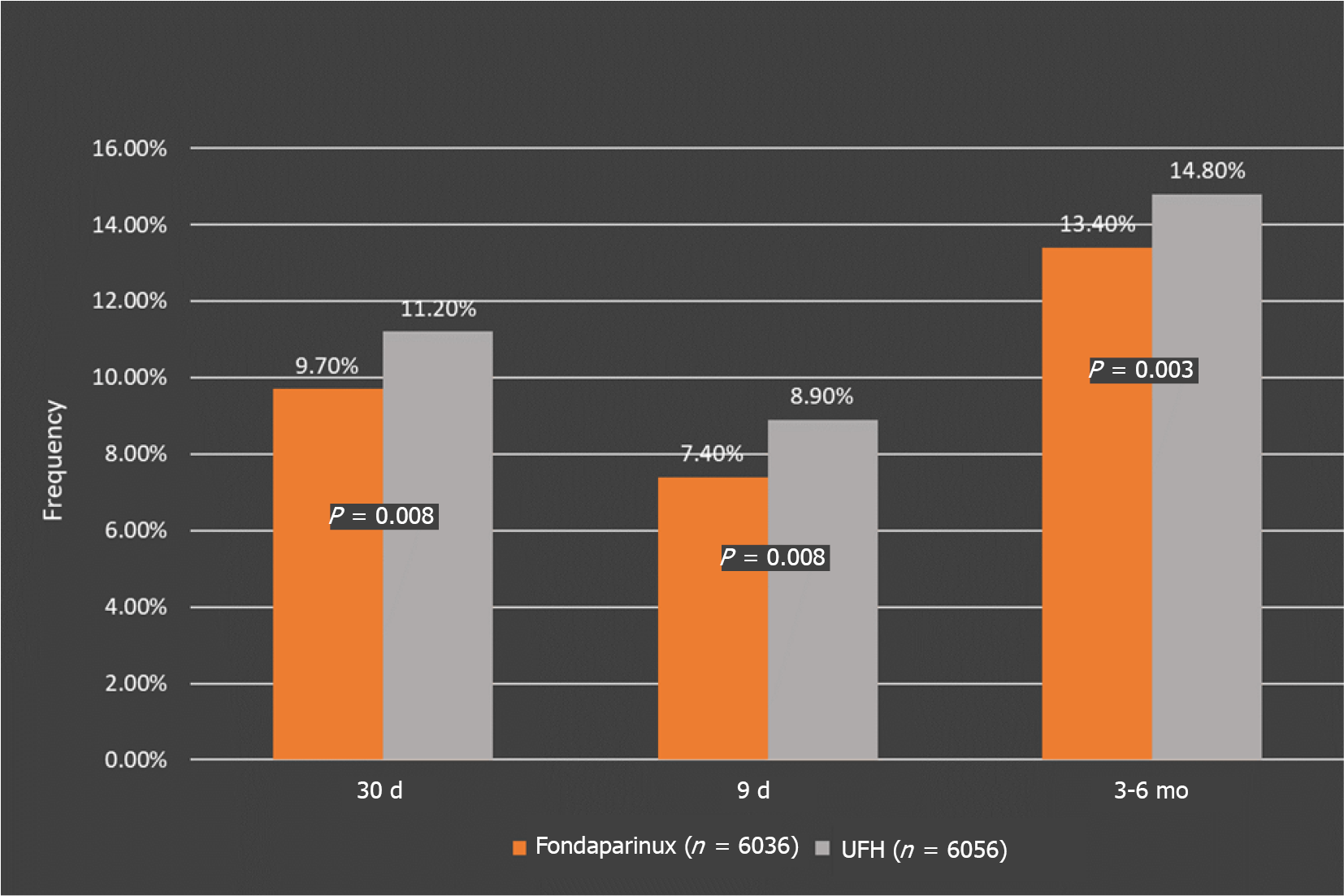
Primary efficacy outcomes: At 30 d, the Fondaparinux group had a significantly reduced risk of death or reinfarction than the control group (9.7% vs 11.2%, HR, 0.86; P = 0.008), and the results were similar at 9 d (HR, 0.83, P = 0.003) and at the end of the study (HR, 0.88; P = 0.008) (Figure 4). At 9 d, the relative risk reduction was 17%, 14% at 30 d, and 12% at the end of the study. This difference remained throughout the study, showing that treatment benefits accrue quickly and are sustained over time (Table 7).
| Measures | Fondaparinux | Control (UFH) |
| Primary composite outcome: Death or reinfarction | ||
| Frequency at 30 d | 9.70% | 11.20% |
| P value | P = 0.008 | |
| Relative risk reduction | 14% | |
| Frequency at 9 d | 7.40% | 8.90% |
| P value | P = 0.003 | |
| Relative risk reduction | 17% | |
| Frequency at 3-6 mo | 13.40% | 14.80% |
| P value | P = 0.008 | |
| Relative risk reduction | 12% | |
Primary safety outcomes: At 9 d, the risk of significant bleeding in patients receiving Fondaparinux was 1.8% (107/6036), compared to 2.1% (130/6056) in patients given placebo or UFH. At 9 d, Fondaparinux was associated with significantly fewer serious bleeds (79 for placebo/UFH vs 61 for Fondaparinux) as well as significantly fewer cardiac tamponade episodes (48 vs 28; P = 0.02)[16].
In subgroups defined by the time from symptom onset to randomization in men and women, in those older or younger than the median age (61 years), with the use of various concurrent therapies or various types of thrombolytic medicines, the outcomes on death or MI were not statistically diverse.
A subgroup analysis of the OASIS 6 trial looked at the role of Fondaparinux as an addition to thrombolytic treatment in acute MI. The major goal of this subgroup analysis was to compare the efficacy and safety of Fondaparinux to a control (placebo or UFH) in thrombolytic treatment patients. Streptokinase was the most used thrombolytic agent (73%). Streptokinase and urokinase were non-fibrin specific thrombolytic medicines. Tissue plasminogen activator, reteplase, and tenecteplase were fibrin-specific thrombolytic drugs (See Table 8). In this study, stratum 1 included 4415 patients who did not have an indication for unfractionated heparin, and stratum 2 included 1021 patients who did have such an indication.
| OASIS 6 | Stratum I | Stratum II | Total | ||
| Placebo | Fondaparinux | UFH | Fondaparinux | ||
| 2835 | 2823 | 3221 | 3213 | 12092 | |
| Non-fibrin specific thrombolytic | 2216 | 2179 | 83 | 83 | 4561 |
| Fibrin specific thrombolytic | 9 | 11 | 436 | 419 | 875 |
| Any thrombolytic | 2225 | 2190 | 519 | 502 | 5436 |
The main trial's major outcomes were employed, namely the 30-d rates of mortality, MI, and serious bleeding. Fondaparinux dramatically reduced the risk of death, re-MI, and serious bleeding in STEMI patients treated with thrombolytic drugs (mostly streptokinase). The results were consistent in both strata, over varied time periods from symptom start to treatment, and across different types of thrombolytics[16].
In the OASIS 6 trial, this sub-study assessed the efficacy and safety of Fondaparinux to placebo or UF heparin in a pre-specified subset of 2867 patients who were not undergoing any sort of reperfusion therapy. When compared to conventional care (UF heparin infusion or placebo), a treatment plan with Fondaparinux 2.5 mg s.c. once daily reduced the composite of mortality or cardiac re-infarction without increasing severe bleeding or strokes in STEMI patients who were not getting reperfusion therapy[16].
In patients with STEMI, Fondaparinux significantly reduced death and reinfarction vs UFH/placebo at 30 d. There is a trend to a lower rate of severe bleeding with Fonda
Fondaparinux lowers mortality and reinfarction in STEMI patients, particularly those who are not undergoing primary percutaneous coronary intervention, without increasing bleeding or strokes. Fondaparinux, on the other hand, showed no benefit in patients receiving PCI[16].
The Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART) was a prospective, multicenter cohort study of NSTEMI patients treated with Fondaparinux or LMWH (n = 40616). The outcome measures were severe bleeding events and mortality (in-hospital), 30- and 180-d major bleeding, stroke, death, and recurrent MI. In-hospital bleeding rates and death were lowered by 46% and 25% with Fondaparinux as compared to LMWH. The positive impact on bleeding was maintained over the 30-d and 180-d periods. Similarly, the composite outcome of MI, death and bleeding, also showed a reduction over the longer time intervals (Figure 5). The decrease in mortality was significant for both the 30-d and 180-d periods.
However, treatment with Fondaparinux was associated with lower severe in-hospital bleeding rates within each renal function strata, an effect that was maintained over the long term (30 and 180 d). Similarly, in-hospital mortality rates were also reduced with Fondaparinux vs LMWH in almost all categories of renal function. Since the SWEDEHEART analysis was conducted in a routine clinical care setting across patient subgroups, the short and long-term benefits of Fondaparinux in NSTEMI were reinforced by this analysis[17].
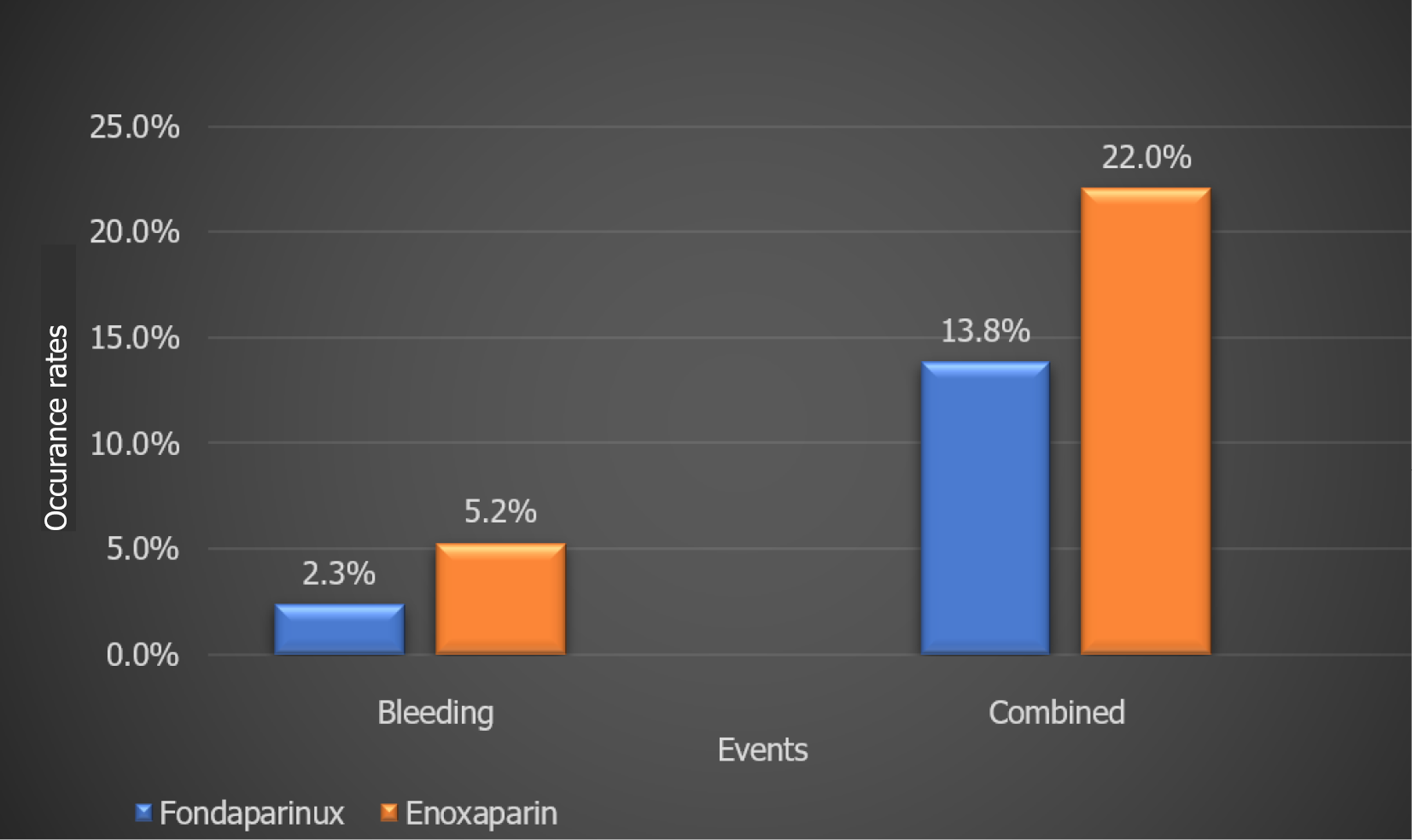
A retrospective analysis of Brazilian NSTE-ACS patients (n = 2282) treated with Fondaparinux or Enoxaparin with in-hospital all-cause mortality as the primary outcome and secondary outcome being combined events (cardiogenic shock, reinfarction, death, stroke, and bleeding) showed a significant impact of Fondaprinux therapy on bleeding as well as combined events (Figure 6). This study further established the superiority of Fondaparinux over Enoxaparin in NSTE-ACS patients. Several additional studies provide clinical evidence favoring the use of Fondaparinux in ACS patients (Table 9)[17].
| Name of study | Type of study | No of patients | Endpoints | Results | Conclusions |
| Comparative efficacy and safety of anticoagulant strategies for acute coronary syndromes | Network metanalysis of 42 randomized controlled trials | 117353 | Death, MI, revascularization, bleeding | Death and MI rates with Fondaparinux were lower than that with 5 other anticoagulant regimens. [UFH + glycoprotein IIb/IIIa inhibitor (GPI), UFH ± GPI, Bivalirudin, LMWH, and Otamixaban (a direct Factor Xa inhibitor)]. | Fondaparinux had the most balanced profile compared to other evaluated strategies, ranking high for both efficacy and safety. |
| Comparison between Fondaparinux and low molecular-weight heparin in patients with acute coronary syndrome | Meta-analysis | 62900 | MACE, mortality, major bleeding events | Fondaparinux had significantly lower rates of MACE and major bleeding events. Lower all-cause mortality (-16%) vs LMWH. | In this meta-analysis of head-to-head comparisons, fondaparinux-based regimens presented advantages in MACE and major bleeding, as well as a net clinical benefit, compared with LMWH. |
| Choosing between Enoxaparin and Fondaparinux for the management of patients with acute coronary syndrome: A systematic review and meta-analysis | Meta-analysis | 9618 | Mortality, MI, Stroke, Minor/Major and all bleeding | Fondaparinux resulted in significantly lower bleeding rates during short-term (10 d) and long-term (30 d or 6 mo to 1 yr) intervals. | Fondaparinux could be a better option vs Enoxaparin, especially in NSTEMI patients, in terms of short to mid-term bleeding risk. |
| Comparison of Efficacy, Safety and Hemostatic Parameters of Enoxaparin and Fondaparinux in unstable coronary artery disease | Prospective, comparative study | 180 | Recovery, recurrence, major and minor bleeding | Recurrent MI or angina numerically more in the Enoxaparin group. At 30 d, enoxaparin showed a higher incidence of hemorrhage than fondaparinux (P < 0.05). | Fondaparinux appears to be better than enoxaparin in efficacy. Fondaparinux also has a better safety profile. Therefore, Fondaparinux is an attractive option compared to Enoxaparin in NSTE-ACS patients. |
Fondaparinux is recommended by several guidelines for use in ACS. Its use is recommended in case of both invasive and conservative management strategies. The latest ESC guidelines[18] recommend Fondaparinux in NSTE-ACS as having the most favorable efficacy–safety profile with respect to anticoagulation. ACC/AHA 2014[19] and National Institute for Health and Care Excellence 2010 guidelines also recommend its use in NSTE-ACS management (Table 10). Various Studies like the OASIS 5 and 6 also showed the efficacy of Fondaparinux in ACS. The convenience of once-daily administration, lack of monitoring, reduction in mortality, and better safety profile make Fondaparinux a simple and effective anti-coagulant agent. Fondaparinux is a reasonable choice in NSTE-ACS where patients are managed with invasive approaches like angiography and possible revascularization and thus are at increased risk of the bleeding[6].
| AHA/ACC 2014 | SC Fondaparinux for the duration of hospitalization or until PCI is performed. | 2.5 mg s.c. daily | IB |
| ESC 2015 | Fondaparinux is recommended as having the most favorable efficacy – safety profile regardless of the management strategy. In patients on Fondaparinux (2.5 mg s.c. daily) undergoing PCI, a single i.v. bolus of UFH (70-85 IU/kg, or 50-60 IU/kg in the case of concomitant use of glycoprotein IIb/IIIa inhibitors) is recommended during the procedure. | 2.5 mg s.c. once daily | IB |
| NICE 2010 | Fondaparinux is offered to patients who do not have a high bleeding risk (unless coronary angiography is planned within 24 h of admission). It should not be used in patients with significant renal dysfunction (those with a serum creatinine > 265 μmol/L were excluded from the trial). | 2.5 mg s.c. once daily | NA |
| SIGN 2016 | When there are ischemic electrocardiograph changes or elevation of cardiac markers, treat immediately with Fondaparinux. Continue for 8 d, or until hospital discharge or coronary revascularization. | 2.5 mg s.c. once daily | 1++ |
| CPG Malaysian guidelines 2011 | Fondaparinux for 8 d or duration of hospitalization. | 2.5 mg s.c. daily | IA |
| SBC Brazilian guidelines 2015 | Fondaparinux once a day for 8 d or until hospital discharge. | 2.5 mg s.c. daily | IB |
Compared to Fondaparinux, Enoxaparin is a twice-daily formulation with the dose tailored to the bodyweight of the patient. This increases the cost of the medication and the time invested by the clinician in calculating and administering the appropriate dose. The cost-effectiveness of Fondaparinux has been proven in economic analyses carried out in several countries such as Brazil, Thailand, United States of America (US), and Canada.
In Thailand, when compared to Enoxaparin, Fondaparinux was 99% more cost-effective [threshold of 160000 Thai Baht (THB), i.e. 4857.3 USD/ quality-adjusted life-year (QALY)], especially in NSTEMI patients. The benefit was 2-fold, from both the provider and societal perspectives. In another analysis, the economic benefit of Fondaparinux was observed across all the subgroups studied, with maximum impact seen in younger patients, in those at high cardiac risk, and those with the greatest risk of bleeding. Avoidance of the costs associated with managing major bleeding also makes Fondaparinux an attractive option.
Fondaparinux was also more cost-effective than Enoxaparin in the Brazilian registry for NSTE-ACS patients. In a Canadian study, data from the OASIS 5 trial were used to evaluate short-term (180 d) and life-term costs with Fondaparinux vs Enoxaparin. Fondaparinux was found to be more cost-effective with a saving of $439 for 180 d and a lifetime incremental cost-effectiveness ratio of $4293/QALY. This was determined by not only its lower acquisition cost but also due to the decrease in clinical event rates over 6 mo post-treatment. In a US study evaluating the OASIS 5 data, the 180-d cost saving with Fondaparinux was found to be $547 per patient. This resulted in long-term cost-effectiveness both in terms of cost as well as QALYs across the range of event risks observed. These analyses add yet another positive aspect to the treatment of ACS with Fondaparinux, making it one of the best options available to clinicians today.
In India, registry data have shown that the prevalence of ACS is quite varied, and the time taken to reach the hospital after symptom onset is more than in the western world and it is an area of concern. Hence, a patient who is presented later than 6 h (i.e. has been suffering for an extended period) especially needs prompt and effective treatment. Effective antithrombotic treatment in the form of antiplatelet agents and anticoagulants has been accepted as the cornerstone of therapy for ACS. However, reducing ischemic events without increasing bleeding risk with matchless anticoagulant therapy is the need of the hour. This requirement is remarkably fulfilled by the novel anticoagulant Fondaparinux, with its unique mode of action, once-daily administration, efficacy across patient groups, and consistent effectiveness in reducing bleeding risk. Moreover, several studies conducted in ACS patients have compared Fondaparinux to Enoxaparin and found it to be a safer and equally effective option. Its proven clinical efficacy has resulted in several reputed organizations recommending its use in ACS patients. Fondaparinux also scores over Enoxaparin in terms of cost-effectiveness, both by way of actual costs and QALYs. All these aspects reaffirm that Fondaparinux is one of the best choices in the treatment of ACS.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kharlamov AN S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ
| 1. | Kumar A, Cannon CP. Acute coronary syndromes: diagnosis and management, part I. Mayo Clin Proc. 2009;84:917-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 111] [Reference Citation Analysis (0)] |
| 2. | Sharma R, Bhairappa S et. al. Clinical characteristics, angiographic profile and in hospital mortality in acute coronary syndrome patients in south Indian population. Heart India, 2014; 2: 65-69.. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Xavier D, Pais P, Devereaux PJ, Xie C, Prabhakaran D, Reddy KS, Gupta R, Joshi P, Kerkar P, Thanikachalam S, Haridas KK, Jaison TM, Naik S, Maity AK, Yusuf S; CREATE registry investigators. Treatment and outcomes of acute coronary syndromes in India (CREATE): a prospective analysis of registry data. Lancet. 2008;371:1435-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 370] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 4. | Kaul U, Sethi KK, Dalal J, Parikh K, Hiremath MS, Mullasari AS, Nair T, Sandhu PS, Kotak B. A multicentre retrospective study to understand anti-platelet treatment patterns and outcomes of acute coronary syndrome patients in India (TRACE). Indian Heart J. 2014;66:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Ralapanawa U, Kumarasiri PVR, Jayawickreme KP, Kumarihamy P, Wijeratne Y, Ekanayake M, Dissanayake C. Epidemiology and risk factors of patients with types of acute coronary syndrome presenting to a tertiary care hospital in Sri Lanka. BMC Cardiovasc Disord. 2019;19:229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Onwordi EN, Gamal A, Zaman A. Anticoagulant Therapy for Acute Coronary Syndromes. Interv Cardiol. 2018;13:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Keam SJ, Goa KL. Fondaparinux sodium. Drugs. 2002;62:1673-85; discussion 1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Donat F, Duret JP, Santoni A, Cariou R, Necciari J, Magnani H, de Greef R. The pharmacokinetics of fondaparinux sodium in healthy volunteers. Clin Pharmacokinet. 2002;41 Suppl 2:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Fondaparinux Prescribing Information. 2006. |
| 10. | Bauer KA. Fondaparinux sodium: a selective inhibitor of factor Xa. Am J Health Syst Pharm. 2001;58 Suppl 2:S14-S17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Switaj TL, Christensen SR, Brewer DM. Acute Coronary Syndrome: Current Treatment. Am Fam Physician. 2017;95:232-240. [PubMed] |
| 12. | Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators; Yusuf S, Mehta SR, Chrolavicius S, Afzal R, Pogue J, Granger CB, Budaj A, Peters RJ, Bassand JP, Wallentin L, Joyner C, Fox KA. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med. 2006;354:1464-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 758] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 13. | Fox KA, Bassand JP, Mehta SR, Wallentin L, Theroux P, Piegas LS, Valentin V, Moccetti T, Chrolavicius S, Afzal R, Yusuf S; OASIS 5 Investigators. Influence of renal function on the efficacy and safety of fondaparinux relative to enoxaparin in non ST-segment elevation acute coronary syndromes. Ann Intern Med. 2007;147:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Mehta SR, Granger CB, Eikelboom JW, Bassand JP, Wallentin L, Faxon DP, Peters RJ, Budaj A, Afzal R, Chrolavicius S, Fox KA, Yusuf S. Efficacy and safety of fondaparinux vs enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: results from the OASIS-5 trial. J Am Coll Cardiol. 2007;50:1742-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 15. | FUTURA/OASIS-8 Trial Group, Steg PG, Jolly SS, Mehta SR, Afzal R, Xavier D, Rupprecht HJ, López-Sendón JL, Budaj A, Diaz R, Avezum A, Widimsky P, Rao SV, Chrolavicius S, Meeks B, Joyner C, Pogue J, Yusuf S.. Low-dose vs standard-dose unfractionated heparin for percutaneous coronary intervention in acute coronary syndromes treated with fondaparinux: the FUTURA/OASIS-8 randomized trial. JAMA. 2010;304:1339-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Yusuf S, Mehta SR, Chrolavicius S, Afzal R, Pogue J, Granger CB, Budaj A, Peters RJ, Bassand JP, Wallentin L, Joyner C, Fox KA; OASIS-6 Trial Group. Effects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction: the OASIS-6 randomized trial. JAMA. 2006;295:1519-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 499] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 17. | Turpie AG, Bauer KA, Eriksson BI, Lassen MR. Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: a meta-analysis of 4 randomized double-blind studies. Arch Intern Med. 2002;162:1833-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 482] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 18. | 2020 Acute Coronary Syndromes (ACS) in Patients Presenting without Persistent ST-Segment Elevation (Management of) Guidelines. ESC Clinical Practice Guidelines. |
| 19. | Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139-e228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1840] [Cited by in RCA: 2252] [Article Influence: 187.7] [Reference Citation Analysis (0)] |