Published online Sep 26, 2021. doi: 10.4330/wjc.v13.i9.514
Peer-review started: April 3, 2021
First decision: May 13, 2021
Revised: May 26, 2021
Accepted: July 23, 2021
Article in press: July 23, 2021
Published online: September 26, 2021
Processing time: 167 Days and 8.2 Hours
Chronic heart failure (CHF) is a complex syndrome characterized by a progressive reduction of the left ventricular (LV) contractility, low exercise tolerance, and increased mortality and morbidity. Diastolic dysfunction (DD) of the LV, is a keystone in the pathophysiology of CHF and plays a major role in the progression of most cardiac diseases. Also, it is well estimated that exercise training induces several beneficial effects on patients with CHF.
To evaluate the impact of a cardiac rehabilitation program on the DD and LV ejection fraction (EF) in patients with CHF.
Thirty-two stable patients with CHF (age: 56 ± 10 years, EF: 32% ± 8%, 88% men) participated in an exercise rehabilitation program. They were randomly assigned to aerobic exercise (AER) or combined aerobic and strength training (COM), based on age and peak oxygen uptake, as stratified randomization criteria. Before and after the program, they underwent a symptom-limited maximal cardiopulmonary exercise testing (CPET) and serial echocardiography evaluation to evaluate peak oxygen uptake (VO2peak), peak workload (Wpeak), DD grade, right ventricular systolic pressure (RVSP), and EF.
The whole cohort improved VO2peak, and Wpeak, as well as DD grade (P < 0.05). Overall, 9 patients (28.1%) improved DD grade, while 23 (71.9%) remained at the same DD grade; this was a significant difference, considering DD grade at baseline (P < 0.05). In addition, the whole cohort improved RVSP and EF (P < 0.05). Not any between-group differences were observed in the variables assessed (P > 0.05).
Exercise rehabilitation improves indices of diastolic and systolic dysfunction. Exercise protocol was not observed to affect outcomes. These results need to be further investigated in larger samples.
Core Tip: In this study, the exercise training rehabilitation (aerobic exercise with/ without strength training) effects on indices of diastolic and systolic cardiac function, were evaluated in stable chronic heart failure patients. Exercise training overall induced benefits on the diastolic dysfunction grade, the ejection fraction of the left ventricle, the right ventricular systolic pressure, as well as aerobic exercise capacity.
- Citation: Chaveles I, Papazachou O, Shamari MA, Delis D, Ntalianis A, Panagopoulou N, Nanas S, Karatzanos E. Effects of exercise training on diastolic and systolic dysfunction in patients with chronic heart failure. World J Cardiol 2021; 13(9): 514-525
- URL: https://www.wjgnet.com/1949-8462/full/v13/i9/514.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i9.514
Chronic heart failure (CHF) is a multisystem syndrome, characterized by an abnormality of the cardiac structure or function, condition, which leads to failure of the heart to deliver oxygen at a rate commensurate with the requirements of the metabo
Left ventricular diastolic dysfunction plays an essential role in the pathophysiology of the CHF. The term “diastolic dysfunction” (DD) refers to abnormalities in right or/and left ventricular relaxation[2-5]. Although DD most frequently refers to the context of HF with preserved ejection fraction (EF), due to its central role in its pathophysiology, impaired diastolic function often coexists with systolic dysfunction. HF patients may not accomplish the necessary increase in diastolic relaxation to accommodate the preload increase[1]. Severity of exercise intolerance is associated with left ventricular filling pressure and so the strong relationship between diastolic abnormality and exercise limitation should be underlined[6-8]. It is in that context, that exercise training is currently being extensively evaluated for additional benefits, over the classical medication, in the treatment of DD in patients with CHF[9].
As mentioned before, in patients with CHF, the exercise capacity may be limited by the number of frequently coexisting factors such as decreased contractility, DD, chronotropic incompetence, oxygen metabolism, or skeletal muscle mass disorders. This importance of skeletal muscle dysfunction provides part of the rationale for the use of cardiac rehabilitation[10]. It is well established that exercise training improves functional capacity, quality of life, and clinical outcomes in patients with stable CHF[10,11]. Specifically, in patients with reduced EF, exercise is beneficial in total and HF-related hospitalizations and relieves the symptoms of depression. Also, it decreases myocardial oxygen demands for the same level of external work performed, as demonstrated by the product of heart rate × systolic blood pressure, reducing in that way the likelihood of myocardial ischemia[12,13]. In major Cardiology Society Guidelines, exercise training is recommended in all patients with New York Heart Association functional class II to III, no matter of the EF[14-16].
Finally, aerobic regimes have been a major component of exercise rehabilitation to improve cardiorespiratory fitness and disease symptoms[16]. As skeletal muscle abnormalities are an important limitation to exercise intolerance and muscular strength impacts patients’ capacities to perform daily tasks, combined regimes of aerobic exercise (AER) and strength training have been employed to induce additional benefit[17,18]. However, there have not been any data on the effects of different regimes on diastolic dysfunction.
The main aim of this study was to evaluate the impact of a cardiac exercise rehabilitation program, on the DD and the EF of the LV in patients with CHF. A secondary aim was the comparison of an aerobic and combined regimes to explore any potential difference on these indices.
The study population consisted of 32 consecutive CHF patients. The demographic, anthropometric, and clinical characteristics of these patients at baseline are described in Table 1. The patients were referred to our hospital's laboratory by HF outpatient clinics, screened for inclusion/exclusion criteria and consented to attend a rehabilitation program and undergo related evaluations including echocardiography assessment. They randomly assigned to AER (n = 17) or combined aerobic and strength training (COM, n = 15). Randomization process, based on age (50 years as cut-off value) and peak oxygen uptake (16 mL/kg/min as cut-off value) as stratified randomization criteria, was made by a researcher not involved in the rest of the tasks, such as exercise sessions and pre/post evaluations. Before and after the program, they underwent a symptom-limited maximal cardiopulmonary exercise testing (CPET) and serial echocardiography assessment. The researchers performed these evaluations were blinded to participants’ allocation.
CHF diagnosis was based on history forms, clinical evaluation, and laboratory testing. Patients were considered for inclusion in the study in case they were on stable systolic CHF, under optimal medication for at least 3 mo and had an EF value up to 49%. Exclusion criteria were severe valvulopathy, uncontrolled arterial hypertension, severe chronic obstructive pulmonary disease, severe peripheral angiopathy, neuromuscular diseases and contraindications for CPET. The patients were mainly treated with diuretics, b-blockers, aldosterone antagonists, angiotensin-converting enzyme inhibitors or sacubitril/valsartan. There were not any changes in treatment regimen during the study.
The study was conducted in accordance with the principles of the Helsinki Declaration and approved by the Administration Board and the Ethics Committee of our Hospital. Informed consent was provided by the participants.
Participants attended supervised exercise sessions at the laboratory three times per week for 12 wk in the early afternoon hours. If any sessions were missed, the duration of the program was extended so that the 36 sessions were accomplished. AER and COM protocols have been previously described in detail[19]. In short, the AER group performed 31 min of interval training (4 × 4–min at 80% VO2peak–5 × 3-min at 50% VO2peak) on a cycle ergometer (Ironman M3 Cycle) followed by balance and coordination exercises. The COM group performed 31 min of AER (in the same way as the AER group) followed by 14 min of strength training (2–3 sets, 10–12 repetitions, 60%-75% of 1 repetition maximum test-knee extension, knee flexion, chest press). Both regimes were of the same total duration.
Participants underwent a ramp incremental CPET on an electromagnetically braked cycle ergometer (Ergoline 800; Sensor Medics, Anaheim, CA, United States), before and after completion of the program. Individualized workload increments were estimated according to the equation of Hansen et al[20]. Gas exchange was measured with the patient breathing through a low resistance valve, with the nose clamped, using an ergospirometry system (Vmax229D; Sensor Medics) calibrated with a known gas mixture before each test. Respiratory indicators (breath-by-breath oxygen uptake [VO2], carbon dioxide output [VCO2] and ventilation [VE]) were measured. Peripheral O2 saturation was monitored continuously by pulse oximetry. Heart rate and rhythm were monitored by a MAX 1, 12-lead electrocardiographic system (Marquette Electronics, Milwaukee, WI, United States) and blood pressure was measured every 2 min with a mercury sphygmomanometer. All patients were verbally encouraged to exercise to intolerable leg fatigue or dyspnea. CPET variables employed in the study were VO2peak and peak workload (Wpeak). VO2peak was determined as the average value of VO2 data measured at the final 20-s period of the exercise phase, and Wpeak as the corresponding work rate[20].
Detailed echocardiography assessment was performed in all patients. A Philips E 33 Doppler analyzer equipped with tissue Doppler imaging (TDI) was used. The period between echocardiography assessment and cardiopulmonary exercise testing was less than 2 wk. Each patient was examined, according to the guidelines of the European Society of Echocardiography (2016 update[21]), in the left lateral and supine position. The EF was calculated using the modified Simpson method from apical two- and four- chambers view (2D and 4D). Analysis of pulsed Doppler mitral flow velocity was attained, and three consecutive cardiac cycles were analyzed and averaged for each patient. Transmitral inflow velocities (E, A, deceleration time of E [DTe] and E/A ratio) were assessed by pulsed-wave Doppler, with the sample volume placed between the mitral leaflet tips in the apical four-chamber view during diastole. When from the Echo–TDI the septal e' was less than 8 and the lateral e' less than 10, the Echo-Doppler transmitral flow was examined. Based on this, three grades of diastolic dysfunction (DD) are described: Grade I (impaired relaxation) is characterized by E/A ratio less than 0.8 and DTe more than 200 ms. Grade II (pseudonormal) is characterized by elevated left atrial pressures. The E/A ratio is 0.8–2 and the DTe is more than 200 ms. Grade III (restrictive pattern), is characterized by a marked decrease in left ventricular compliance (E/A ratio more than 2, DTe less than 160 ms[15]). Another grade, grade 0, refers to normal diastolic function. The E/A ratio was considered as normal if it was 0.78–1.78 and the DTe 150–200 ms. Valsalva maneuver was used to discriminate pseudo normal from true normal pattern. From the apical four-chamber view, a 10 mm3 sample volume was placed at the septal and lateral mitral annulus, and spectral TDI was recorded, calculating septal e', lateral e' and the mean value (E'). Left atrial volume was measured at end-systole and it was normalized to body surface area (LAVI, mL/m2). Finally, the right ventricular systolic pressure (RVSP) was calculated using the Bernoulli equation RVSP = 4(V)2 of peak tricuspid regurgitation velocity (V).
Continuous variables were tested for normality of distribution with Shapiro-Wilk test. Within-group differences were assessed with paired sample Student t-test or Wilcoxon signed-rank test, based on normality of distribution. Chi-square test was employed to check for between-group differences on categorical variables. Between-group differences of ordinal variables were assessed with Mann-Whitney U test. McNemar-Bowker test was also used to check for differences on diastolic dysfunction grade before and after exercise intervention. Time by group interactions were assessed with factorial 2 × 2 analysis of variance. Correlations between variables were tested with Pearson or Spearman coefficient. Continuous variables were presented as mean ± SD. Level of statistical significance P was set at 0.05. Statistical computations were made with IBM SPSS 26 statistics.
The whole cohort improved indices of AER capacity, namely VO2peak (from 19.4 ± 4.5 to 21.3 ± 6.0 mL/kg/min, P = 0.03) and Wpeak (from 109 ± 39 to 130 ± 43 watts, P < 0.01).
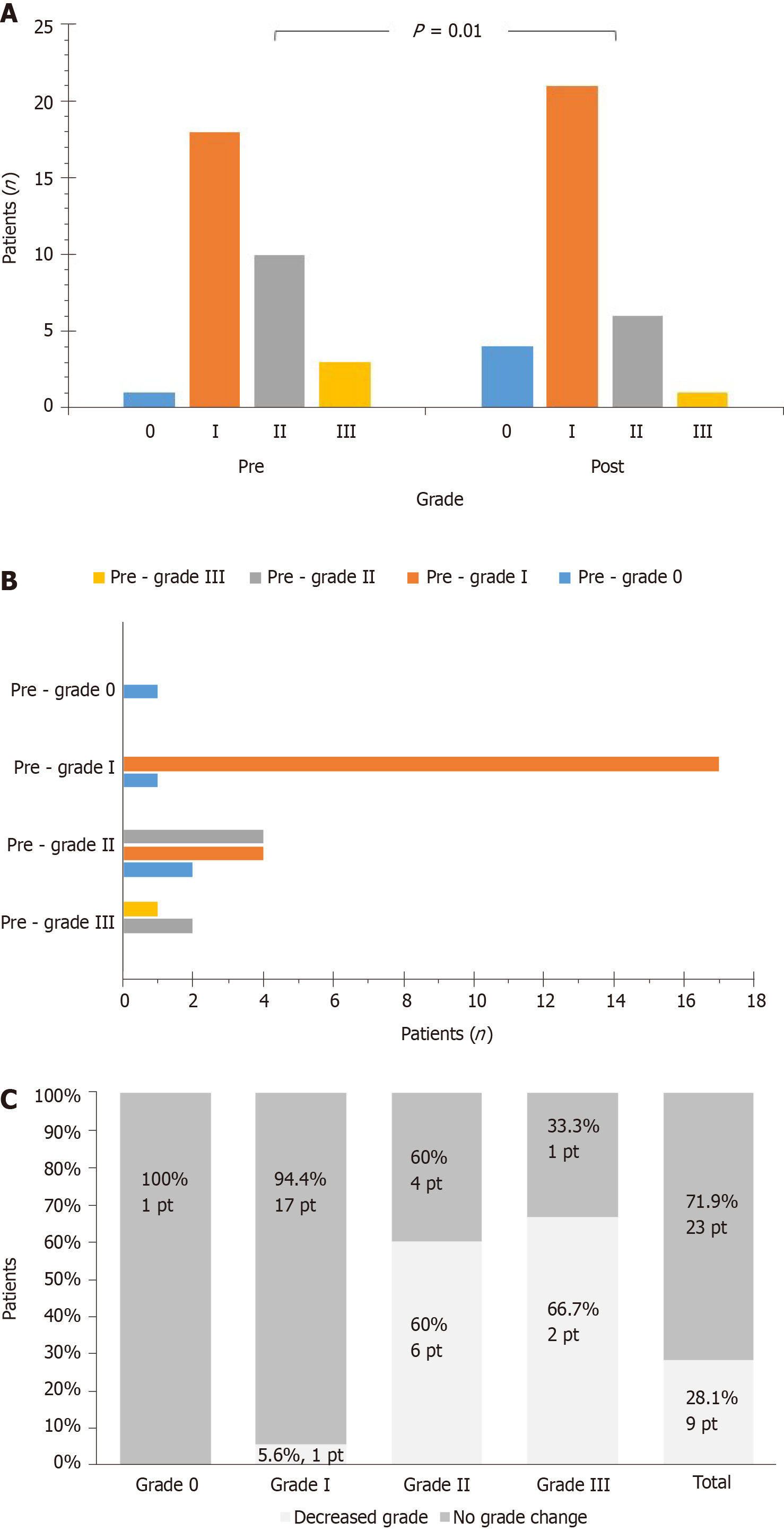
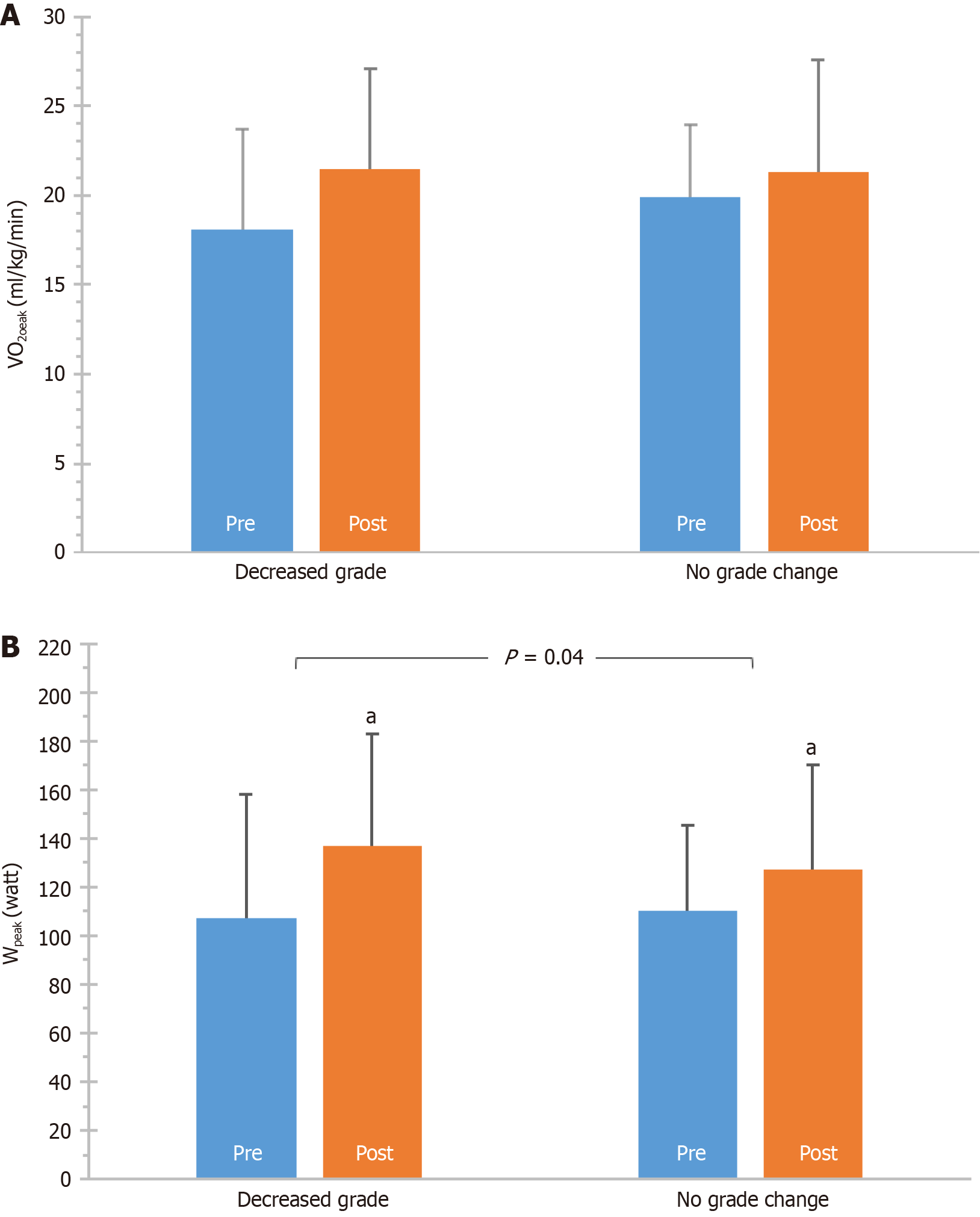
The whole group improved DD, as assessed with grades. Before the exercise program, the number of patients categorized as grade -0, -I, -II, or -III were 1 (3.1%), 18 (56.3%), 10 (31.2%), 3 (9.4%) respectively. After the program, the respective patients were 4 (12.5%), 21 (65.6%), 6 (18.8%), 1 (3.1%). A significant difference was found between total pre- and post-values (P = 0.01) (Figure 1A). That was also the case when analysis was performed based on change of DD grade (P = 0.06) (Figure 1B). Overall, 9 patients (28.1%) improved DD grade, while 23 ones (71.9%) remained at the same DD grade; this was a significant difference, considering DD grade at baseline (P < 0.01) (Figure 1C). In addition, VO2peak, tended to improve more in patients that also improved grade (P = 0.09), while Wpeak was improved more in these patients (P = 0.04) (Figure 2).
The whole sample did not improve any of the other DD variables examined individually. These were E/A ratio (from 1.00 ± 0.64 to 0.88 ± 0.35, P = 0.27), LAVI (from 38.70 ± 13.74 to 38.44 ± 17.03 mL/m2, P = 0.14), E΄ (from 7.74 ± 2.31 to 7.55 ± 1.85 cm/s, P = 0.62), E/E΄ ratio (from 9.15 ± 3.41 to 8.48 ± 3.45, P = 0.15), and DTe (from 213.34 ± 41.60 to 212.38 ± 32.99 m/s, P = 0.59). In addition, the whole cohort improved RVSP (from 28.92 ± 7.75 to 27.75 ± 6.46 mmHg, P = 0.05) and EF (from 32% ± 8% to 36% ± 8%, P < 0.01), after completion of the exercise rehabilitation program.
Pre- and post-values of the variables examined in relation to AER and COM groups comparison are presented in Table 2. The AER group improved DD grades (P = 0.02) and tended to improve LAVI (P = 0.10). The COM group improved RVSP (P = 0.01) and tended to improve grades (P = 0.10). Both groups improved EF (P < 0.01). Not any between-group differences were observed in the variables observed (P > 0.05).
| Aerobic group | Combined group | |||
| Pre | Post | Pre | Post | |
| VO2peak (ml/kg/min) | 19.0 ± 5.5 | 22.2 ± 7.8a | 19.8 ± 3.1 | 20.3 ± 2.9 |
| Wpeak (watt) | 111 ± 46 | 129 ± 48a | 108 ± 31 | 131 ± 39a |
| E/A | 1.15 ± 0.80 | 1.00 ± 0.41 | 0.83 ± 0.37 | 0.74 ± 0.20 |
| LAVI (ml/m2) | 38.83 ± 12.75 | 37.23 ± 11.78 | 38.55 ± 15.23 | 39.81 ± 21.91 |
| E’ (cm/s) | 8.00 ± 2.81 | 7.74 ± 1.97 | 7.45 ± 1.61 | 7.33 ± 1.74 |
| E/E’ | 9.09 ± 4.06 | 8.76 ± 4.57 | 9.22 ± 2.63 | 8.15 ± 1.51 |
| DTe (m/s) | 220.65 ± 51.59 | 213.00 ± 38.08 | 205.07 ± 25.54 | 211.67 ± 27.41 |
| RVSP (mmHg) | 28.56 ± 7.65 | 28.23 ± 6.36 | 29.33 ± 8.10 | 27.20 ± 6.74a |
| EF (%) | 34 ± 8 | 38 ± 9a | 29 ± 6 | 33 ± 6a |
| DD grade 0/I/II/III (n) | 1/9/4/3 | 3/10/3/1a | 0/9/6/0 | 1/11/3/0 |
The exercise rehabilitation effects on parameters of diastolic and systolic dysfunction were explored among CHF patients in this study. Exercise training overall beneficially affected DD stage, RVSP, EF of the left ventricle and AER capacity. Not any differences between the aerobic and combined group were observed.
HF is currently considered a pathophysiological syndrome of multifactorial origin and not just a disease. DD is a common characteristic of the HF patients[22]. Hamlin et al[4] showed that CHF patients present with reduced ability to augment the diastolic relaxation, accountable for the inability to accommodate the increase in estimated preload during exercise, resulting in turn in higher filling pressure. Also, HF patients have generally shorter diastolic periods, situation that lead to inability of the myocardium to relax and accept the large volume of blood[23]. The inability to perform exercise without discomfort may be one of the first symptoms experienced by patients with HF and is often the principal reason for seeking medical care[14,24]. Therefore, exercise intolerance is inextricably linked to the diagnosis of HF. Exercise training has been an important means of rehabilitation, with a class IA recommen
The RVSP improvement observed in this study is also in line with previous findings. Mehani et al[32] after a 5-mo training program, showed a significant decrease of RVSP by 12.05 mmHg in a group of patients suffering from pulmonary hyper
In our study, there was not any decrease in the LAVI, an important index in determination of DD. Previous studies showed that left atrial enlargement is an independent marker of adverse outcome in both primary and secondary cardiovascular prevention[33]. Limited data, however, have been reported on the exercise training effects on LAVI in CHF, suggesting conflicting results. Edelmann et al[34] reported a significant decrease in LAVI in the training group, while Palau et al[35] reported no change in LAVI after interval training. Sandri et al[36] also, failed to find a significant change in LA size after 4 wk of training.
A significant improvement in the left ventricular EF (LVEF) was also found in this study. This finding is in line with a recent metanalysis[37], which included trials reporting on LVEF, LV end-diastolic and end-systolic volumes. Overall, AER training improved these parameters. Both continuous and interval regimes were able to induce similar benefits, which may be also affected by the program duration.
Finally, considering the regimes of the exercise applied in this study, overall aerobic and combined regimes improved EF, RVSP, and DD grades. Not any between-group differences were observed. Aerobic training can induce beneficial effects also on skeletal muscles, and continuous aerobic training has been found to reverse partially skeletal myopathy of the HF[38]. Interval exercise training, which was also employed in the present study, can be an effective regime, as it can apply higher exercise stimuli on skeletal muscles via a higher exercise intensity[39,40]. Furthermore, strength training has been shown to induce muscle hypertrophy, while the combination of resistance training with aerobic training has the potential to induces greater benefits in vascular endothelium[19], muscle strength and aerobic improvement of CHF patients, than AER alone[17,18,41]. Interestingly, in a CHF study that employed a combined exercise protocol vs control, ventricular stroke volume and left ventricular diastolic indices were improved[28]. The effects of different exercise regimes on the diastolic dysfunction need to be further investigated.
This study had some limitations. First, there is no single non-invasive measure that quantifies the LV diastolic function. Instead, a number of indices are utilized, and current recommendations use a combination of conversional and TDI parameters to determine the diastolic function of the LV[21]. In our study, there was a statistically significant improvement on the DD grades, but other parameters, such as E/A and E/E', were not found to improve. This may be related to the method used to determine the diastolic stage and the sample size. In fact, some results were underpowered to reach definite conclusion. Also, LV diastolic filling patterns as evaluated with transmitral Echo-Doppler are influenced by a variety of factors including valvular insufficiency, myocardium viscoelastic properties, ventricular compliance and loading conditions[21]. However, all patients were under constant medication during the study and all of them had mild degree of mitral regurgitation. Finally, considering that the exercise benefits on CHF have already been well documented, a control group was not employed, and patients randomized in two groups, AER and COM, to explore any potential differences on the diastolic dysfunction.
In conclusion, DD plays an essential role in the pathophysiology of the HF syndrome and interventions that improve it can be beneficial in terms of symptoms and outcome. In this study, the effects of an exercise training rehabilitation program (AER with/ without strength training), were evaluated on the indices of diastolic and systolic cardiac function, in stable CHF patients. Exercise training overall induced benefits on the DD as assessed with grades, the EF of the LV, the RVSP and the AER capacity. The exercise protocol was not observed to affect outcomes.
Diastolic dysfunction (DD) of the left ventricular (LV) is a keystone in the patho
Exercise training induces several beneficial effects on CHF patients. However, the effects of exercise training on diastolic DD have not been adequately studied. This is also the case for the effects of different exercise regimes on DD.
The main aim of the study was to evaluate the impact of a cardiac exercise rehabilitation program, on the DD and the ejection fraction (EF) of the LV in patients with CHF. A secondary aim was the comparison of an aerobic and combined regimes to explore any potential difference on these indices.
In this randomized clinical trial study, 32 patients with CHF were screened for inclusion/exclusion criteria and consented to attend a rehabilitation program and undergo related evaluations. They randomly assigned to aerobic exercise (AER) or combined aerobic and strength training, by a researcher not involved in the rest of the tasks. Before and after the program, they underwent a symptom-limited maximal cardiopulmonary exercise testing (CPET) and serial echocardiography assessment. The researchers performed these evaluations were blinded to participants’ allocation.
Exercise training overall beneficially affected DD grade, right ventricular systolic pressure (RVSP), EF of the LV and AER capacity. No differences between the aerobic and combined group were observed.
In this study, the effects of an exercise training rehabilitation program (AER with/without strength training) were evaluated on the indices of diastolic and systolic cardiac function, in stable CHF patients. Exercise training overall induced benefits on the DD as assessed with grades, the EF of the LV, the RVSP and the AER capacity. The exercise protocol was not observed to affect outcomes.
Future research is warranted to further explore the effects of different exercise training regimes on diastolic dysfunction.
| 1. | Piepoli MF, Guazzi M, Boriani G, Cicoira M, Corrà U, Dalla Libera L, Emdin M, Mele D, Passino C, Vescovo G, Vigorito C, Villani GQ, Agostoni P; Working Group 'Exercise Physiology, Sport Cardiology and Cardiac Rehabilitation', Italian Society of Cardiology. Exercise intolerance in chronic heart failure: mechanisms and therapies. Part I. Eur J Cardiovasc Prev Rehabil. 2010;17:637-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Yu CM, Lin H, Yang H, Kong SL, Zhang Q, Lee SW. Progression of systolic abnormalities in patients with "isolated" diastolic heart failure and diastolic dysfunction. Circulation. 2002;105:1195-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 359] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 3. | Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105:1387-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 749] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 4. | Hamlin SK, Villars PS, Kanusky JT, Shaw AD. Role of diastole in left ventricular function, II: diagnosis and treatment. Am J Crit Care. 2004;13:453-66; quiz 467. [PubMed] |
| 5. | Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2270] [Cited by in RCA: 2363] [Article Influence: 139.0] [Reference Citation Analysis (2)] |
| 6. | Genovesi-Ebert A, Marabotti C, Palombo C, Giaconi S, Rossi G, Ghione S. Echo Doppler diastolic function and exercise tolerance. Int J Cardiol. 1994;43:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Rovner A, Greenberg NL, Thomas JD, Garcia MJ. Relationship of diastolic intraventricular pressure gradients and aerobic capacity in patients with diastolic heart failure. Am J Physiol Heart Circ Physiol. 2005;289:H2081-H2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Yip GW, Frenneaux M, Sanderson JE. Heart failure with a normal ejection fraction: new developments. Heart. 2009;95:1549-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | van Tol BA, Huijsmans RJ, Kroon DW, Schothorst M, Kwakkel G. Effects of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure: a meta-analysis. Eur J Heart Fail. 2006;8:841-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 202] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 10. | Kokkinos PF, Choucair W, Graves P, Papademetriou V, Ellahham S. Chronic heart failure and exercise. Am Heart J. 2000;140:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Ades PA, Keteyian SJ, Balady GJ, Houston-Miller N, Kitzman DW, Mancini DM, Rich MW. Cardiac rehabilitation exercise and self-care for chronic heart failure. JACC Heart Fail. 2013;1:540-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 12. | Pandey A, Parashar A, Kumbhani D, Agarwal S, Garg J, Kitzman D, Levine B, Drazner M, Berry J. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail. 2015;8:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 405] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 13. | Keteyian SJ, Isaac D, Thadani U, Roy BA, Bensimhon DR, McKelvie R, Russell SD, Hellkamp AS, Kraus WE; HF-ACTION Investigators. Safety of symptom-limited cardiopulmonary exercise testing in patients with chronic heart failure due to severe left ventricular systolic dysfunction. Am Heart J. 2009;158:S72-S77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Piña IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Myers JN, Sullivan MJ; American Heart Association Committee on exercise, rehabilitation, and prevention. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107:1210-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 750] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 15. | Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147-e239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4116] [Cited by in RCA: 4720] [Article Influence: 363.1] [Reference Citation Analysis (1)] |
| 16. | Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4368] [Cited by in RCA: 5007] [Article Influence: 500.7] [Reference Citation Analysis (4)] |
| 17. | Georgantas A, Dimopoulos S, Tasoulis A, Karatzanos E, Pantsios C, Agapitou V, Ntalianis A, Roditis P, Terrovitis J, Nanas S. Beneficial effects of combined exercise training on early recovery cardiopulmonary exercise testing indices in patients with chronic heart failure. J Cardiopulm Rehabil Prev. 2014;34:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Bouchla A, Karatzanos E, Dimopoulos S, Tasoulis A, Agapitou V, Diakos N, Tseliou E, Terrovitis J, Nanas S. The addition of strength training to aerobic interval training: effects on muscle strength and body composition in CHF patients. J Cardiopulm Rehabil Prev. 2011;31:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Kourek C, Alshamari M, Mitsiou G, Psarra K, Delis D, Linardatou V, Pittaras T, Ntalianis A, Papadopoulos C, Panagopoulou N, Vasileiadis I, Nanas S, Karatzanos E. The acute and long-term effects of a cardiac rehabilitation program on endothelial progenitor cells in chronic heart failure patients: Comparing two different exercise training protocols. Int J Cardiol Heart Vasc. 2021;32:100702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129:S49-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 675] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 21. | Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2879] [Cited by in RCA: 4123] [Article Influence: 412.3] [Reference Citation Analysis (9)] |
| 22. | Brubaker PH, Peter H. Exercise therapy for the failing heart harmful or helpful? ACSM Health Fit J. 2010;14:9-15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Lisauskas JB, Singh J, Bowman AW, Kovács SJ. Chamber properties from transmitral flow: prediction of average and passive left ventricular diastolic stiffness. J Appl Physiol (1985). 2001;91:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Myers J, Zaheer N, Quaglietti S, Madhavan R, Froelicher V, Heidenreich P. Association of functional and health status measures in heart failure. J Card Fail. 2006;12:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Lewinter C, Doherty P, Gale CP, Crouch S, Stirk L, Lewin RJ, LeWinter MM, Ades PA, Køber L, Bland JM. Exercise-based cardiac rehabilitation in patients with heart failure: a meta-analysis of randomised controlled trials between 1999 and 2013. Eur J Prev Cardiol. 2015;22:1504-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Belardinelli R, Georgiou D, Cianci G, Purcaro A. Effects of exercise training on left ventricular filling at rest and during exercise in patients with ischemic cardiomyopathy and severe left ventricular systolic dysfunction. Am Heart J. 1996;132:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999;99:1173-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 720] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 28. | Malfatto G, Branzi G, Osculati G, Valli P, Cuoccio P, Ciambellotti F, Parati G, Facchini M. Improvement in left ventricular diastolic stiffness induced by physical training in patients with dilated cardiomyopathy. J Card Fail. 2009;15:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen Ø, Skjaerpe T. Superior cardiovascular effect of aerobic interval training vs moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086-3094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1422] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 30. | Pearson MJ, Mungovan SF, Smart NA. Effect of exercise on diastolic function in heart failure patients: a systematic review and meta-analysis. Heart Fail Rev. 2017;22:229-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Mehani SH. Correlation between changes in diastolic dysfunction and health-related quality of life after cardiac rehabilitation program in dilated cardiomyopathy. J Adv Res. 2013;4:189-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Mehani SHM, Abdeen HAA. Cardiopulmonary rehabilitation program impact on prognostic markers in selected patients with resting and exercise-induced ventilatory inefficiency: a clinical trial. J Phys Ther Sci. 2017;29:1803-1810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Lazzeroni D, Gaibazzi N, Bini M, Bussolati G, Camaiora U, Cassi R, Geroldi S, Ugolotti PT, Brambilla L, Brambilla V, Castiglioni P, Coruzzi P. Prognostic value of new left atrial volume index severity partition cutoffs after cardiac rehabilitation program in patients undergoing cardiac surgery. Cardiovasc Ultrasound. 2016;14:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Edelmann F, Gelbrich G, Düngen HD, Fröhling S, Wachter R, Stahrenberg R, Binder L, Töpper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Löffler M, Hasenfuss G, Halle M, Pieske B. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58:1780-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 516] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 35. | Palau P, Domínguez E, Núñez E, Schmid JP, Vergara P, Ramón JM, Mascarell B, Sanchis J, Chorro FJ, Núñez J. Effects of inspiratory muscle training in patients with heart failure with preserved ejection fraction. Eur J Prev Cardiol. 2014;21:1465-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Sandri M, Kozarez I, Adams V, Mangner N, Höllriegel R, Erbs S, Linke A, Möbius-Winkler S, Thiery J, Kratzsch J, Teupser D, Mende M, Hambrecht R, Schuler G, Gielen S. Age-related effects of exercise training on diastolic function in heart failure with reduced ejection fraction: the Leipzig Exercise Intervention in Chronic Heart Failure and Aging (LEICA) Diastolic Dysfunction Study. Eur Heart J. 2012;33:1758-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Tucker WJ, Beaudry RI, Liang Y, Clark AM, Tomczak CR, Nelson MD, Ellingsen O, Haykowsky MJ. Meta-analysis of Exercise Training on Left Ventricular Ejection Fraction in Heart Failure with Reduced Ejection Fraction: A 10-year Update. Prog Cardiovasc Dis. 2019;62:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 38. | Levy WC, Cerqueira MD, Abrass IB, Schwartz RS, Stratton JR. Endurance exercise training augments diastolic filling at rest and during exercise in healthy young and older men. Circulation. 1993;88:116-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 164] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Hambrecht R, Fiehn E, Yu J, Niebauer J, Weigl C, Hilbrich L, Adams V, Riede U, Schuler G. Effects of endurance training on mitochondrial ultrastructure and fiber type distribution in skeletal muscle of patients with stable chronic heart failure. J Am Coll Cardiol. 1997;29:1067-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 261] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 40. | Meyer K, Samek L, Schwaibold M, Westbrook S, Hajric R, Beneke R, Lehmann M, Roskamm H. Interval training in patients with severe chronic heart failure: analysis and recommendations for exercise procedures. Med Sci Sports Exerc. 1997;29:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 103] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Karatzanos L, Dimopoulos S, Tasoulis A. The addition of strength training to high-intensity interval exercise training in chronic heart failure patients. Proc 16th Congress of the European College of Sports Medicine, 259, Liverpool, 2011. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Rehabilitation
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Osailan A S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Wang LYT