Published online Aug 26, 2021. doi: 10.4330/wjc.v13.i8.243
Peer-review started: March 15, 2021
First decision: April 26, 2021
Revised: May 19, 2021
Accepted: July 26, 2021
Article in press: July 26, 2021
Published online: August 26, 2021
Processing time: 160 Days and 15.6 Hours
Percutaneous coronary intervention with stenting is followed by a duration of dual antiplatelet therapy (DAPT) to reduce stent thrombosis and avoid target lesion failure. The period of DAPT recommended in international guidelines following drug-eluting stent implantation is 12 mo for most patients with acute coronary syndrome, and 6 mo for patients with chronic coronary syndrome or high bleeding risk. The new generation of drug-eluting stents have metallic platforms with thinner struts, associated with significantly less stent thrombosis. Shortened DAPT has been investigated with these stents, with evidence from randomised clinical trials for some individual stents showing non-inferior safety and efficacy outcomes. This has to be balanced by the effect of DAPT on secondary prevention of systemic cardiovascular disease especially in high-risk populations. This review will outline the current evidence for individual stents with regards to DAPT duration for both acute coronary syndrome and chronic coronary syndrome and discuss further directions for research and personalised medicine in this contemporary percutaneous coronary intervention era.
Core Tip: The new generation of drug-eluting stents have different properties such as reduced strut thickness allowing lower level of local stent thrombosis, which may be feasible with shortened dual antiplatelet therapy (DAPT). Only a small number of individual stents have been validated for reduced DAPT, such as 1 mo for the BioFreedom stainless steel biolimus-eluting stent and the Onyx Resolute cobalt-chromium zotarolimus-eluting stent but in only certain populations. Future trials will compare DAPT durations within the same stent. Future research should also examine risk stratification and the parameters for patients to benefit the most from shortened DAPT.
- Citation: Han J, Attar N. Shortened dual antiplatelet therapy in contemporary percutaneous coronary intervention era. World J Cardiol 2021; 13(8): 243-253
- URL: https://www.wjgnet.com/1949-8462/full/v13/i8/243.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i8.243
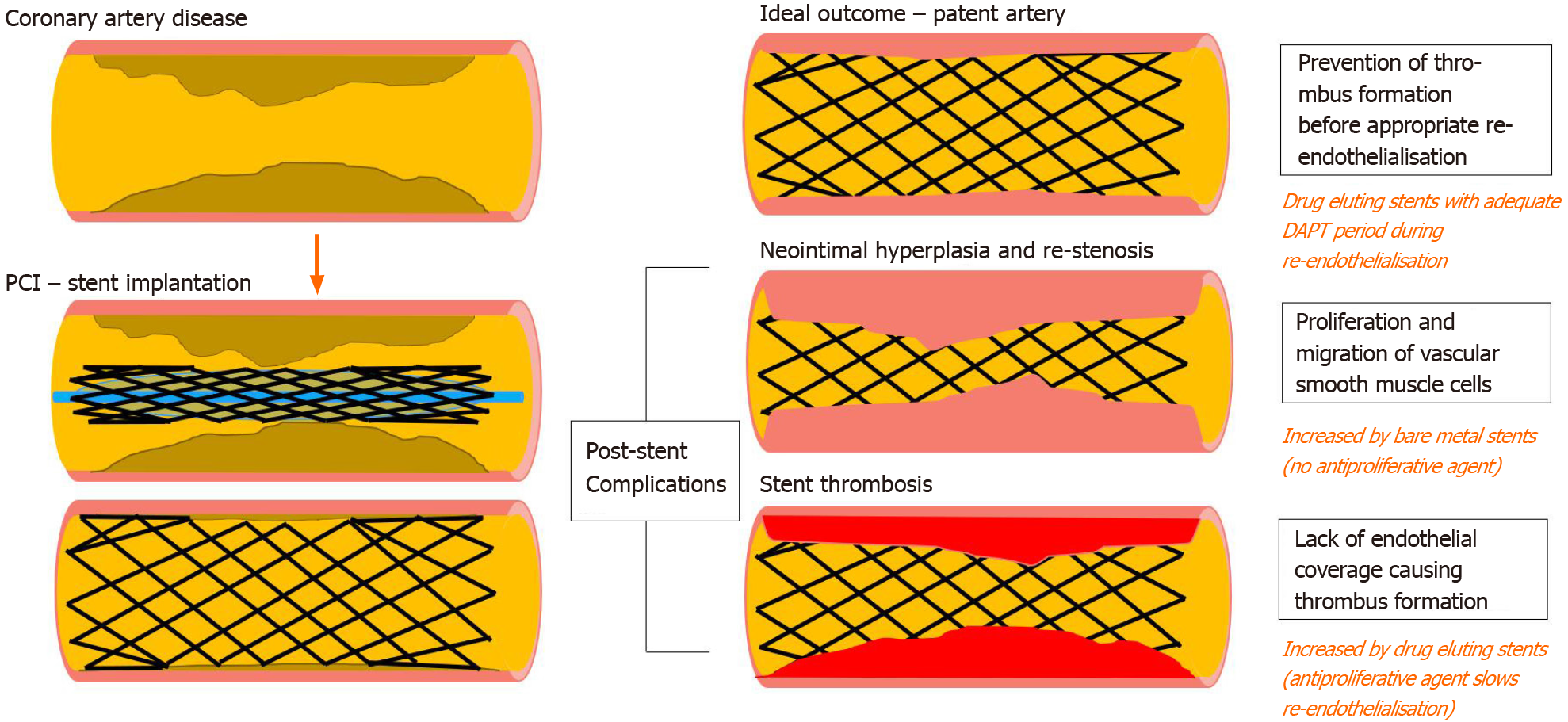
Percutaneous coronary intervention (PCI) is an interventional procedure which generally involves coronary angioplasty and stenting for individuals with coronary artery disease. Stent implantation is associated with greater lumen diameter increase in the acute period. The dimensions and the material of a stent are associated with different degrees of re-epithelialisation and risk of thrombosis. Intimal hyperplasia may cause restenosis of a vessel in bare metal stents (BMS), and metallic drug-eluting stents (DES) coated with anti-proliferative agents were developed to prevent this. However, delayed re-endothelialisation lengthens the duration of increased thro
Dual antiplatelet therapy (DAPT), which is a combination of aspirin and an oral inhibitor of the platelet P2Y12 inhibitor, is therefore recommended for a duration of time post stent implantation. Bare metal stents may only need 1 mo of DAPT, but the traditional duration for DAPT is at least 12 mo following drug-eluting stent insertion for low bleeding risk patients with acute coronary syndrome (ACS), which is Class I and Level I evidence in European Society of Cardiology and American College of Cardiology/American Heart Association Guidelines, and at least 6 mo for patients with stable coronary disease or with high bleeding risk[1,2]. High bleeding risk is defined as an increase of spontaneous bleeding during DAPT, which can be objectively calculated through risk scores such as PRECISE-DAPT[3,4].
However, the duration of this period has constantly been a subject of research, which has been influenced by the development of different P2Y12 inhibitors, and evolution of drug eluting stents with different materials and anti-proliferative agent. The newer generation of stents have reduced the risk of late and very late stent thrombosis. This yields the question of whether the full period for DAPT is required and/or could it be shortened. Shortening DAPT will reduce the risk of bleeding, reduce patient noncompliance, and will be more cost effective.
This clinical review will examine the current generation of stents, and evidence for the shortened length of DAPT post stent implantation for acute coronary syndrome and stable coronary disease in the contemporary PCI era.
A DES comprises 3 main components: the metallic platform, the polymer, and the anti-proliferative agent (Table 1)[5-19].
| Stent | Metallic platform | Polymer | Anti-proliferative agent | DAPT duration validated for | Ref. |
| Onyx Resolute | Cobalt-chromium (has a platinum iridium core) | Permanent | Zotarolimus | 1 mo | [5,6] |
| BioFreedom | Stainless steel | Polymer-free | Biolimus | 1 mo1 | [7,8] |
| Biomatrix; Biomatrix Flex | Cobalt-chromium | Bioresorbable | Biolimus | Standard/no short DAPT | [9,10] |
| Nobori | Cobalt-chromium | Bioresorbable | Biolimus | 6 mo | [11] |
| Xience | Cobalt-chromium | Permanent | Everolimus | 1-3 mo2 | [12,13] |
| EluNIR | Cobalt-chromium | Permanent | Ridaforolimus | Standard/no short DAPT | [14] |
| Ultimaster; Orsino | Cobalt-chromium | Permanent | Sirolimus | Standard/no short DAPT | [15,16] |
| Cre8 | Cobalt-chromium | Polymer-free | Amphilimus | Standard/no short DAPT | [17] |
| Xposition S (self-apposing) | Nickel-titanium | Permanent | Silolimus | Standard/no short DAPT | [18] |
| Promus | Platinium-chromium | Bioresorbable | Everolimus | 6 mo | [12] |
| Synergy | Platinium-chromium | Bioresorbable | Everolimus | 3 mo1 | [19] |
The metallic platform has a direct relationship with the dimensions of the stent. First-generation stents were constructed from stainless steel, but alloys such as cobalt-chromium, nickel-titanium, and platinum-chromium allow greater tensile strength with similar or reduced elasticity, allowing thinner struts[20]. Stainless steel stents normally have a strut of > 100 μm, but some of the newer second-generation stents such as the Orsino stent (60 μm), MiStent (64 μm), and BioMime (65 μm) are associated with 16% reduction in target lesion failure, and lower rates of any stent thrombosis in a meta-analysis of 10 trials[19]. Another meta-analysis of 69 trials comparing ultrathin (60-80 μm), thin (81-100 μm), intermediate (101-120 μm), and thick (≥ 120 μm) strut-thickness DES found that ultrathin devices had significantly less stent thrombosis (OR = 0.43, 95%CI: 0.27-0.68)[21].
Some stents have permanent or bioresorbable polymers which incorporate the anti-proliferative drug. Permanent polymers are non-biodegradable and allow continuous release of the drug. Bioresorbable polymers allow the effect of the drug to occur in the early phase of stent deployment and reverts to a bare metal stent once the polymer has become degraded, aiming to reduce very late stent thrombosis. The polymers used in first-generation stents were historically associated with delayed vascular healing and more very late stent thrombosis, but newer polymers are biocompatible. To avoid polymers entirely, some stents have the drug applied over the metallic platform of the DES in micropores. Many network meta-analyses have found no significant difference in stent thrombosis, target lesion revascularisation, very-late stent thrombosis (defined as ≥ 1 year after implantation), or clinical outcomes between the use of different polymers independent of struct thickness and metallic platform[22,23].
The anti-proliferative drugs tend to be immunosuppressive agents, and the majority comprise sirolimus, everolimus, biolimus, and zotarolimus. Other drugs also used include amphilimus and ridaforolimus. A meta-analysis showed no difference in stent thrombosis between zotarolimus and everolimus stent[24]. However, the cobalt-chromium sirolimus stent Orsino appeared to have less stent thrombosis than the stainless-steel biolimus stent Nobori, though this may have been due to other confounding factors such as metallic platform and strut thickness, which has been previously shown to affect thrombosis[25].
Bioresorbable vascular scaffolds (BVS) are an alternative option which fully resorbs over time. However, they are less favoured in PCI currently due to association with increased risk of stent thrombosis and target lesion failure during the first 3 years after implantation and would therefore be less suitable for consideration for shortened DAPT duration[26].
As reducing strut thickness appears to be associated with less stent thrombosis, the development of the current generation of ultrathin DES with smaller strut thickness raises the question of whether shortened DAPT may be suitable in these patients.
The success of a PCI, stent and DAPT regimen is measured by outcomes including target lesion failure, target lesion revascularisation, major adverse cardiovascular events including stroke, myocardial infarction (MI) and cardiac death, stent thrombosis, and bleeding events measured through Thrombolysis in Myocardial Infarction (TIMI) or Bleeding Academic Research Consortium (BARC).
A systematic review and network meta-analysis analysed 17 randomised control trials comparing at least 2 of the 3 durations of DAPT (short term < 6 mo, standard term 12 mo, and long term > 12 mo) post PCI for both ACS and chronic coronary syndrome with DES deployment, and determined that there were higher rates of major bleeding (OR = 1.78, 95%CI: 1.27-2.49) and non-cardiac death (OR = 1.63, 95%CI: 1.03-2.59) in those on long-term treatment, and higher rates of any bleeding (OR = 1.39, 95%CI: 1.01-1,92), and no significant difference in other primary endpoints[27]. Additionally, assessing the subgroup of patients with newer-second generation DES found longer term DAPT had higher bleeding events and all-cause mortality compared to short-term DAPT (OR = 1.99, 95%CI: 1.04-3.81), but had similar efficacy and safety[27].
It appears that shortened DAPT may be suitable for some of the new second-generation DES. The STOPDAPT single-arm study was the first prospective study to examine DAPT shorter than 6 mo for the everolimus-eluting cobalt-chromium stent (CoCr-EES). The study enrolled 1525 patients to 3 mo of DAPT followed by aspirin monotherapy after the XIENCE CoCr-EES and compared this to the CoCr-EES group in the RESET trial as a historical control, where 90% had DAPT at 12 mo. At 1 year, the primary endpoint of cardiovascular death, myocardial infarction, stroke, stent thrombosis, and TIMI major/minor bleeding was less in the STOPDAPT than the RESET group (2.8% vs 4.0%, P = 0.06), and the incidence of stent thrombosis was lower in the STOPDAPT than in the RESET group (0% vs 0.3%, P = 0.03), suggesting that 3 mo was non-inferior to the historical control group[28].
The EVOLVE Short DAPT trial prospectively assessed the safety of 3-mo DAPT followed by aspirin monotherapy in high bleeding risk patients with the SYNERGY stent and compared this to a historical 12-mo DAPT control[19]. The primary results showed adjusted rate of death or MI in the following 3 to 15 mo was 5.6% in the 3-mo DAPT group, and 5.7% in the 12-mo control[19]. Unlike the other studies, this trial excluded acute myocardial infarction and complex lesions.
The STOPDAPT-2 randomised clinical trial examined patients undergoing PCI with the Xience CoCr-EES and randomised them to 1-mo DAPT followed by clopidogrel against 12 mo of DAPT for 3045 patients. 1-mo DAPT was superior to 12-mo DAPT (2.36% vs 3.70%, hazard ratio 0.64, P = 0.04 for superiority), for the primary endpoint, which was a composite of cardiovascular death, myocardial infarction, stroke, definite stent thrombosis, or TIMI major/minor bleeding at 12 mo[29]. There was slightly increased stent thrombosis for 1-mo DAPT compared to 12-mo DAPT (0.3% vs 0.07%, P = 0.21 for superiority), but this was not statistically significant. 1-mo DAPT was shown to be superior with statistical significance in secondary outcomes such as TIMI major/minor bleeding and BARC 3 or 5 bleeding at 1 year.
Further studies have examined reducing this time further and using aspirin as monotherapy. The two DES with most evidence for 1-mo DAPT are the BioFreedom DES and the Resolute Onyx DES.
The LEADERS FREE I double-blinded randomised control trial examined 2466 patients with a high risk of bleeding and randomised them to the BioFreedom Biolimus polymer-free stent or the Gazelle uncoated BMS with only one month of DAPT. The BioFreedom DES was shown to be superior for the primary safety endpoint of cardiac death, myocardial infarction, or stent thrombosis, and superior for the efficacy endpoint, defined as clinically driven target-lesion revascularization[7]. This was the first time 1-mo DAPT was shown to be feasible in a DES and favourably compared to a BMS, though limitations are that BMS only has a small indication possibly in patients who need urgent surgery in < 1 mo after stenting[30].
A randomised control trial of 3020 patients compared the BioFreedom DES with short 1-mo DAPT followed by aspirin monotherapy against other DES (BioMatrix and Ultimaster) with 6-12 mo DAPT was presented at the American Heart Association Scientific Sessions 2020[8]. They examined a composite primary outcome of cardiac death, nonfatal MI, target vessel revascularisation, cerebrovascular attack, or major bleeding. There was no difference in the BioFreedom short DAPT arm compared to the contemporary DES treatment arm (5.9% vs 6.5%, p for noninferiority < 0.001, P for superiority 0.475). There was no statistical difference in the individual components of the composite. This included patients with and without a high bleeding risk, but did not include patients with myocardial infarction. However, an intention-to-treat analysis was used for this study, and 17% of patient remained on DAPT at 1.5 mo. Additionally, the DES that BioFreedom was compared to had not been given FDA approval for use, so the comparison group was not the current best available therapy.
The ONYX ONE Global trial randomised 1996 patients to the BioFreedom DES and the Resolute zotarolimus-eluting Onyx DES, both with 1-mo DAPT followed by single antiplatelet therapy with aspirin or P2Y12 inhibitor. Inclusion criteria were those with high bleeding risk (adjunctive oral anticoagulation to continue after PCI, age ≥ 75, baseline haemoglobin < 110 g/L, previous intracerebral bleed, stroke in the last 12 mo, hospital admission for bleeding during the last 12 mo, nonskin cancer diagnosed or treated ≤ 3 years, planned daily NSAID or steroids for ≥ 30 d after PCI, planned surgery that would require interruption of DAPT within the next 12 mo, renal failure CrCl < 40 mL/min, thrombocytopenia < 100000/mm3, severe chronic liver disease, expected noncompliance to prolonged DAPT). 51% of these had acute coronary syndrome, and 32% had atrial fibrillation. They found no significant difference between Resolute and BioFreedom in primary outcomes of composite cardiac death/myocardial infarction/stent thrombosis (17.1% vs 16.9% P = 0.011 nonin
There appear to be some observational studies with evidence for shortened DAPT. The Ultimaster stent had similar rates of a composite measure of target lesion vascularisation, cardiac death, and MI for 6-mo and 12-mo DAPT in an observational study[15]. Similarly, for the Cre8 stent, an observational study showed no significant difference between patients discharged with ≤ 3-mo DAPT due to high bleeding risk or urgent non-cardiac surgery and regular DAPT duration ≥ 6 mo for a composite endpoint of target vessel revascularisation, cardiac death, and MI[17]. Although this does show the real-world feasibility of shortened DAPT for this population of patients, the lack of a randomised control means that there is limited information for the direct comparison of DAPT duration.
EXCELLENT was a randomised control trial examining 6-mo DAPT compared to standard 12-mo DAPT in 1443 patients with the XIENCE/Promus everolimus stent which demonstrated non-inferiority for target vessel failure[11]. However, stent thrombosis appeared to occur more, and the study appeared underpowered for death and myocardial infarction. The XIENCE Short DAPT Program was announced in 2020, consisting of a series of 3 single-arm trials investigating 3-mo and 1-mo duration in patients undergoing PCI with XIENCE compared with historical controls[12].
Similarly, the prospective multicentre REIWA registry was announced in 2020 to investigate very short DAPT for patients with biodegradable polymer DES, such as the Synergy, Ultimaster, or Orsino. It will investigate the safety and feasibility of 1-mo DAPT followed by P2Y12 inhibitor monotherapy, much like the STOPDAPT-2 trial, but this time on bioresorbable-polymer DES rather than permanent-polymer DES[31].
Further focus could examine the use of P2Y12 inhibitor monotherapy for these stents, as this has been proved useful in the STOPDAPT-2 randomised clinical trial, or trials focusing on purely aspirin, unlike the ONYX ONE global trial. As well as clopidogrel monotherapy, ticagrelor monotherapy has also been investigated, where high-risk patients with PCI and 3-mo of DAPT continued with ticagrelor and were randomised to aspirin or placebo for one year. This showed death from any cause, MI, or stroke was 3.9% in both groups, but the 3-mo group had a lower incidence of clinically significant bleeding, warrant this as an area for further research[32]. A direct comparison of DAPT lengths between groups of 1-mo vs 3-mo and 6-mo DAPT can be carried out and keeping the stent type constant. Very short DAPT can be assessed in specific non-high bleeding risk patient groups to broaden the population validated for (Table 2).
| Population | Study | Intervention | Control | Primary endpoint | |
| Single-arm study | ACS (32%) and CCS (68%) | STOPDAPT[28] | 1525 patients, XIENCE CoCr-EES with 3-mo DAPT followed by aspirin monotherapy | 1559 patients, Endeavor CoCr-EES approximately 90% with 1-yr DAPT followed by aspirin monotherapy (RESET trial) | 12-mo cardiovascular death, MI, stroke, thrombosis, bleeding; 2.8% vs 4.0% (P = 0.06) |
| No acute myocardial infarction, High bleeding risk | EVOLVE Short DAPT[19] | 1487 patients, SYNERGY stent with 3-mo DAPT followed by aspirin monotherapy | 1493 patients, 1-year DAPT | 3-15 mo death or MI; 5.6% vs 5.7% (P = 0.0016 non-inferiority) | |
| RCT | ACS (38%) and CCS (62%) | STOPDAPT-2[29] | 1523 patients, XIENCE CoCr-EES with 1-mo DAPT followed by clopidogrel monotherapy | 1522 patients, XIENCE CoCr-EES with 12-mo DAPT | 12-mo Cardiovascular death, MI, stroke, thrombosis, bleeding; 2.36% vs 3.70% (superiority P = 0.04) |
| ACS (42%) and CCS (58%); High bleeding risk | LEADERS FREE I[7] | 1196 patients, BioFreedom DES with 1-month DAPT followed by one antiplatelet | 1189 patients, Gazelle uncoated BMS with 1-month DAPT followed by one antiplatelet | 390 d cardiovascular death, MI, stent thrombosis 9.4% vs 12.9% (P = 0.005 superiority) | |
| No acute myocardial infarction | One-month DAPT trial[8] | 1507 patients, BioFreedom DES with 1-mo DAPT followed by aspirin monotherapy | 1513 patients, BioMatrix and Ultimaster DES with 6-12 mo DAPT | 12-mo cardiovascular death, MI, target vessel revasculariation, stroke, major bleeding; 5.9% vs 6.5% (noninferority P < 0.001) | |
| ACS (62%) and CCS (38%)High bleeding risk | ONXY ONE Global[5] | 1003 patients, Resolute Onyx DES with 1-mo DAPT followed by one antiplatelet | 993 patients, BioFreedom DES with 1-mo DAPT followed by one antiplatelet | Cardiac death, MI, thrombosis 17.1% vs 16.9% (P = 0.011 noninferiority) |
Although the characteristics of the newer generation of stents may be associated with the feasibility of shortened DAPT to reduce local stent thrombosis, it may be balanced out by the effect of DAPT on secondary prevention of systemic thrombosis and major adverse cardiovascular events. The PEGASUS-TIMI 54 randomised control trial examined patients ≥ 50 years old with at least one additional high-risk feature and spontaneous MI 1-3 years before enrolment. These included ≥ 65 years, diabetes mellitus, second myocardial infarction, multivessel disease, or chronic renal dysfunction. 60mg ticagrelor twice a day had marginally higher absolute benefit in terms of the primary efficacy endpoint than the absolute harm in the primary safety endpoint[33]. The DAPT trial compared 12 mo of DAPT with 30 mo of DAPT and showed an absolute risk reduction in the primary efficacy end point of the composite of death, MI, and stroke by 1.6%, and stent thrombosis by 1%[34].
A meta-analysis which included PEGASUS along with other studies with clopidogrel and prasugrel found prolonged DAPT decreased major adverse cardiac events and cardiovascular deaths compared with aspirin, but it was associated with a significant risk of bleeding, and no difference in all-cause mortality[35]. However, the efficacy may have been underestimated by PEGASUS, which allowed patients who had been off DAPT for several years to restart therapy. European and American guidelines therefore recommend consideration of prolonged DAPT in patients at low risk of bleeding with class IIb evidence[1-3].
With the development of risk stratification and personalised medicine, DAPT duration will also be influenced by patient factors. The PRECISE-DAPT score is used at the time of stenting to determine whether the patient may benefit from 3-6 mo short DAPT or 12-24 mo standard/DAPT, and the DAPT score is used after 12 mo of DAPT to determine whether DAPT can be stopped, or if the patient should continue it to 30 mo. The PRECISE-DAPT score uses factors such as Hb, WBC, age, creatinine clearance, and prior bleeding[4]. The DAPT score uses patient factors such as age, smoking, diabetes mellitus, previous stenting or myocardial infarction, but also disease factors such as stent characteristics (diameter, paclitaxel, vein graft stent), left ventricular ejection faction, myocardial infarction at presentation[36]. A further risk scoring model was generated from the Patterns of Non-Adherence to Anti-Platelet Regimen in Stented Patients (PARIS) registry, which calculates a risk score for both ischaemia and major bleeding at 24 mo post PCI to inform decision-making[37]. The coronary thrombotic events score used the factors of diabetes mellitus, ACS, smoking, renal function, previous PCI, and previous CABG. The bleeding events score used the factors of age, body mass index, smoking, anaemia, renal function, and triple therapy on discharge[37]. However, these have not been prospectively validated in a randomised control trial. The approval of the Onyx DES indicated for high bleeding risk patients is a step towards the personalised assessment of DAPT duration, and future research will focus on further parameters and patient stratification to identify individuals which may benefit from very short DAPT.
The period of DAPT following PCI is an area of research which has been increasingly focused on with the current cohort of contemporary DES. New stents such as the Orsino, MiStent, and BioMime are associated with thinner struts which are generally associated with less late thrombosis and may be suitable for shortened DAPT. This has been supported by a network meta-analysis which demonstrated less all-cause mortality for shortened DAPT duration (3-6 mo) compared to standard DAPT duration in the subgroup of patients with newer second-generation DES.
The STOPDAPT and STOPDAPT-2 trials directly examined the feasibility of shortened DAPT duration of less than 6 mo with CoCr-EES. STOPDAPT showed no significant difference in primary outcomes of bleeding, stent thrombosis, cardio
Some patients may benefit from shortened DAPT more due to their high bleeding risk or need to be on monotherapy by a certain point. These are patients who may have coagulation abnormalities, previous significant bleeding, recent malignancy, severe kidney or liver dysfunction, or are due to undergo timely surgical procedures. Assessing this patient subgroup for shortened DAPT trials may show greater benefit in terms of composite outcome of stent thrombosis, bleeding, cardiovascular death or myocardial infarction. The Resolute Onyx DES was tested in this population and has subsequently received a 1-mo DAPT indication in Europe and the United States. The BioFreedom DES was tested in patients both with and without a high bleeding risk. However, the limitation of these trials was the comparison against DES which were not currently licensed or standard management. Future research should focus on comparison against contemporary DES representative of current therapy, and direct comparison of DAPT duration for the same stent. There are currently registries and trials undergoing for the XIENCE, Synergy, Orsino, and Ultimaster stent, allowing direct comparison of DAPT duration in the same stent.
In addition to local effects to prevent stent thrombosis, recent evidence suggests that DAPT is useful for secondary prevention of systemic thrombosis and preventing future major adverse cardiovascular events, especially in patients with high-risk features such as diabetes mellitus, a second myocardial infarction, or multivessel disease. European Society of Cardiology and American College of Cardiology/ American Heart Association guidelines support the consideration of extended DAPT for these patients.
Therefore, we are gradually moving from a “one size fits all approach” towards an era of personalised medicine where different parameters are identified to guide DAPT duration, which is reflected in current international guidelines. The development of risk scores to guide DAPT duration reflects this shift, but these remain to be clinically validated.
The contemporary PCI era brings a new generation of stents which may be beneficial, some of which are validated for shortened DAPT especially in patients with high bleeding risk. Further research is required to assess which other patient groups may benefit from this shortened DAPT approach, and balance this against the patient groups which may benefit from systemic antiplatelet therapy.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: British Cardiovascular Intervention Society, No. 4312; British Cardiovascular Society, No. 2069; Royal College of Physicians, London, No. 86511; European Society of Cardiology, No. 240517.
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Papafaklis M S-Editor: Ma YJ L-Editor: A P-Editor: Liu JH
| 1. | Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann FJ, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN; ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2299] [Cited by in RCA: 2140] [Article Influence: 267.5] [Reference Citation Analysis (0)] |
| 2. | Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, Mehran R, Mukherjee D, Newby LK, O'Gara PT, Sabatine MS, Smith PK, Smith SC Jr. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134:e123-e155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 1067] [Article Influence: 106.7] [Reference Citation Analysis (0)] |
| 3. | Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2791] [Cited by in RCA: 4801] [Article Influence: 800.2] [Reference Citation Analysis (0)] |
| 4. | Costa F, van Klaveren D, James S, Heg D, Räber L, Feres F, Pilgrim T, Hong MK, Kim HS, Colombo A, Steg PG, Zanchin T, Palmerini T, Wallentin L, Bhatt DL, Stone GW, Windecker S, Steyerberg EW, Valgimigli M; PRECISE-DAPT Study Investigators. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 870] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 5. | Windecker S, Latib A, Kedhi E, Kirtane AJ, Kandzari DE, Mehran R, Price MJ, Abizaid A, Simon DI, Worthley SG, Zaman A, Hudec M, Poliacikova P, Abdul Ghapar AKB, Selvaraj K, Petrov I, Mylotte D, Pinar E, Moreno R, Fabbiocchi F, Pasupati S, Kim HS, Aminian A, Tie C, Wlodarczak A, Hur SH, Marx SO, Jankovic I, Brar S, Bousquette L, Liu M, Stone GW; ONYX ONE Investigators. Polymer-based or Polymer-free Stents in Patients at High Bleeding Risk. N Engl J Med. 2020;382:1208-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 231] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 6. | Kandzari DE, Kirtane AJ, Windecker S, Latib A, Kedhi E, Mehran R, Price MJ, Abizaid A, Simon DI, Worthley SG, Zaman A, Choi JW, Caputo R, Kanitkar M, McLaurin B, Potluri S, Smith T, Spriggs D, Tolleson T, Nazif T, Parke M, Lee LC, Lung TH, Stone GW; Onyx ONE US/Japan Investigators. One-Month Dual Antiplatelet Therapy Following Percutaneous Coronary Intervention With Zotarolimus-Eluting Stents in High-Bleeding-Risk Patients. Circ Cardiovasc Interv. 2020;13:e009565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrié D, Naber C, Lipiecki J, Richardt G, Iñiguez A, Brunel P, Valdes-Chavarri M, Garot P, Talwar S, Berland J, Abdellaoui M, Eberli F, Oldroyd K, Zambahari R, Gregson J, Greene S, Stoll HP, Morice MC; LEADERS FREE Investigators. Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. N Engl J Med. 2015;373:2038-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 649] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 8. | Hong MK. Presented at the American Heart Association Virtual Scientific Sessions. November 15, 2020. |
| 9. | Menown IBA, Mamas MA, Cotton JM, Hildick-Smith D, Eberli FR, Leibundgut G, Tresukosol D, Macaya C, Copt S, Sadozai Slama S, Stoll HP. First clinical evidence characterizing safety and efficacy of the new CoCr Biolimus-A9 eluting stent: The Biomatrix Alpha™ registry. Int J Cardiol Heart Vasc. 2020;26:100472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Urban P, Valdés M, Menown I, Eberli F, Alhaddad I, Hildick-Smith D, Iosseliani D, Roffi M, Oldroyd K, Kalloudi E, Eerdmans P, Berland J, Kleber FX; e-Biomatrix investigators. Outcomes following implantation of the Biolimus A9-eluting BioMatrix coronary stent: Primary analysis of the e-BioMatrix registry. Catheter Cardiovasc Interv. 2015;86:1151-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Nakamura M, Iijima R, Ako J, Shinke T, Okada H, Ito Y, Ando K, Anzai H, Tanaka H, Ueda Y, Takiuchi S, Nishida Y, Ohira H, Kawaguchi K, Kadotani M, Niinuma H, Omiya K, Morita T, Zen K, Yasaka Y, Inoue K, Ishiwata S, Ochiai M, Hamasaki T, Yokoi H; NIPPON Investigators. Dual Antiplatelet Therapy for 6 Versus 18 Months After Biodegradable Polymer Drug-Eluting Stent Implantation. JACC Cardiovasc Interv. 2017;10:1189-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | Gwon HC, Hahn JY, Park KW, Song YB, Chae IH, Lim DS, Han KR, Choi JH, Choi SH, Kang HJ, Koo BK, Ahn T, Yoon JH, Jeong MH, Hong TJ, Chung WY, Choi YJ, Hur SH, Kwon HM, Jeon DW, Kim BO, Park SH, Lee NH, Jeon HK, Jang Y, Kim HS. Six-month vs 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation. 2012;125:505-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 501] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 13. | Valgimigli M, Cao D, Makkar RR, Bangalore S, Bhatt DL, Angiolillo DJ, Saito S, Ge J, Neumann FJ, Hermiller J, Picon H, Toelg R, Maksoud A, Chehab BM, Wang LJ, Wang J, Mehran R. Design and rationale of the XIENCE short DAPT clinical program: An assessment of the safety of 3-month and 1-month DAPT in patients at high bleeding risk undergoing PCI with an everolimus-eluting stent. Am Heart J. 2021;231:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Kandzari DE, Smits PC, Love MP, Ben-Yehuda O, Banai S, Robinson SD, Jonas M, Kornowski R, Bagur R, Iniguez A, Danenberg H, Feldman R, Jauhar R, Chandna H, Parikh M, Perlman GY, Balcells M, Markham P, Ozan MO, Genereux P, Edelman ER, Leon MB, Stone GW. Randomized Comparison of Ridaforolimus- and Zotarolimus-Eluting Coronary Stents in Patients With Coronary Artery Disease: Primary Results From the BIONICS Trial (BioNIR Ridaforolimus-Eluting Coronary Stent System in Coronary Stenosis). Circulation. 2017;136:1304-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Godino C, Beneduce A, Ferrante G, Ielasi A, Pivato CA, Chiarito M, Cappelletti A, Perfetti G, Magni V, Prati E, Falcone S, Pierri A, De Martini S, Montorfano M, Parisi R, Rutigliano D, Locuratolo N, Anzuini A, Tespili M, Margonato A, Benassi A, Briguori C, Fabbiocchi F, Reimers B, Bartorelli A, Colombo A. One-year clinical outcome of biodegradable polymer sirolimus-eluting stent in patients needing short dual antiplatelet therapy. Insight from the ULISSE registry (ULtimaster Italian multicenter all comerS Stent rEgistry). Int J Cardiol. 2019;290:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Kandzari DE, Mauri L, Koolen JJ, Massaro JM, Doros G, Garcia-Garcia HM, Bennett J, Roguin A, Gharib EG, Cutlip DE, Waksman R; BIOFLOW V Investigators. Ultrathin, bioresorbable polymer sirolimus-eluting stents vs thin, durable polymer everolimus-eluting stents in patients undergoing coronary revascularisation (BIOFLOW V): a randomised trial. Lancet. 2017;390:1843-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 229] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 17. | Godino C, Chiarito M, Donahue M, Testa L, Colantonio R, Cappelletti A, Monello A, Magni V, Milazzo D, Parisi R, Nicolino A, Moshiri S, Fattori R, Aprigliano G, Palloshi A, Caramanno G, Montorfano M, Bedogni F, Briguori C, Margonato A, Colombo A. Midterm and one-year outcome of amphilimus polymer free drug eluting stent in patients needing short dual antiplatelet therapy. Insight from the ASTUTE registry (AmphilimuS iTalian mUlticenTer rEgistry). Int J Cardiol. 2017;231:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Pellegrini D, Cortese B. Focus on STENTYS® Xposition S Self-Apposing® stent: a review of available literature. Future Cardiol. 2019;15:145-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Kirtane AJ, Stoler R, Feldman R, Neumann FJ, Boutis L, Tahirkheli N, Toelg R, Othman I, Stein B, Choi JW, Windecker S, Yeh RW, Dauerman HL, Price MJ, Underwood P, Allocco D, Meredith I, Kereiakes DJ. Primary Results of the EVOLVE Short DAPT Study: Evaluation of 3-Month Dual Antiplatelet Therapy in High Bleeding Risk Patients Treated With a Bioabsorbable Polymer-Coated Everolimus-Eluting Stent. Circ Cardiovasc Interv. 2021;14:e010144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 20. | Bangalore S, Toklu B, Patel N, Feit F, Stone GW. Newer-Generation Ultrathin Strut Drug-Eluting Stents Versus Older Second-Generation Thicker Strut Drug-Eluting Stents for Coronary Artery Disease. Circulation. 2018;138:2216-2226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 237] [Article Influence: 33.9] [Reference Citation Analysis (1)] |
| 21. | Iantorno M, Lipinski MJ, Garcia-Garcia HM, Forrestal BJ, Rogers T, Gajanana D, Buchanan KD, Torguson R, Weintraub WS, Waksman R. Meta-Analysis of the Impact of Strut Thickness on Outcomes in Patients With Drug-Eluting Stents in a Coronary Artery. Am J Cardiol. 2018;122:1652-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | El-Hayek G, Bangalore S, Casso Dominguez A, Devireddy C, Jaber W, Kumar G, Mavromatis K, Tamis-Holland J, Samady H. Meta-Analysis of Randomized Clinical Trials Comparing Biodegradable Polymer Drug-Eluting Stent to Second-Generation Durable Polymer Drug-Eluting Stents. JACC Cardiovasc Interv. 2017;10:462-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 23. | Kobayashi T, Sotomi Y, Suzuki S, Suwannasom P, Nakatani S, Morino Y, Ako J, Kozuma K, Hirayama A, Sakata Y, Higuchi Y. Five-year clinical efficacy and safety of contemporary thin-strut biodegradable polymer vs durable polymer drug-eluting stents: a systematic review and meta-analysis of 9 randomized controlled trials. Cardiovasc Interv Ther. 2020;35:250-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Piccolo R, Stefanini GG, Franzone A, Spitzer E, Blöchlinger S, Heg D, Jüni P, Windecker S. Safety and efficacy of resolute zotarolimus-eluting stents compared with everolimus-eluting stents: a meta-analysis. Circ Cardiovasc Interv. 2015;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Jensen LO, Thayssen P, Maeng M, Ravkilde J, Krusell LR, Raungaard B, Junker A, Terkelsen CJ, Veien KT, Villadsen AB, Kaltoft A, Tilsted HH, Hansen KN, Aaroe J, Kristensen SD, Hansen HS, Jensen SE, Madsen M, Bøtker HE, Berencsi K, Lassen JF, Christiansen EH. Randomized Comparison of a Biodegradable Polymer Ultrathin Strut Sirolimus-Eluting Stent With a Biodegradable Polymer Biolimus-Eluting Stent in Patients Treated With Percutaneous Coronary Intervention: The SORT OUT VII Trial. Circ Cardiovasc Interv. 2016;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 26. | Stone GW, Kimura T, Gao R, Kereiakes DJ, Ellis SG, Onuma Y, Chevalier B, Simonton C, Dressler O, Crowley A, Ali ZA, Serruys PW. Time-Varying Outcomes With the Absorb Bioresorbable Vascular Scaffold During 5-Year Follow-up: A Systematic Meta-analysis and Individual Patient Data Pooled Study. JAMA Cardiol. 2019;4:1261-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 27. | Yin SH, Xu P, Wang B, Lu Y, Wu QY, Zhou ML, Wu JR, Cai JJ, Sun X, Yuan H. Duration of dual antiplatelet therapy after percutaneous coronary intervention with drug-eluting stent: systematic review and network meta-analysis. BMJ. 2019;365:l2222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 28. | Natsuaki M, Morimoto T, Yamamoto E, Shiomi H, Furukawa Y, Abe M, Nakao K, Ishikawa T, Kawai K, Yunoki K, Shimizu S, Akao M, Miki S, Yamamoto M, Okada H, Hoshino K, Kadota K, Morino Y, Igarashi K, Tanabe K, Kozuma K, Kimura T. One-year outcome of a prospective trial stopping dual antiplatelet therapy at 3 months after everolimus-eluting cobalt-chromium stent implantation: ShortT and OPtimal duration of Dual AntiPlatelet Therapy after everolimus-eluting cobalt-chromium stent (STOPDAPT) trial. Cardiovasc Interv Ther. 2016;31:196-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Watanabe H, Domei T, Morimoto T, Natsuaki M, Shiomi H, Toyota T, Ohya M, Suwa S, Takagi K, Nanasato M, Hata Y, Yagi M, Suematsu N, Yokomatsu T, Takamisawa I, Doi M, Noda T, Okayama H, Seino Y, Tada T, Sakamoto H, Hibi K, Abe M, Kawai K, Nakao K, Ando K, Tanabe K, Ikari Y, Hanaoka KI, Morino Y, Kozuma K, Kadota K, Furukawa Y, Nakagawa Y, Kimura T; STOPDAPT-2 Investigators. Effect of 1-Month Dual Antiplatelet Therapy Followed by Clopidogrel vs 12-Month Dual Antiplatelet Therapy on Cardiovascular and Bleeding Events in Patients Receiving PCI: The STOPDAPT-2 Randomized Clinical Trial. JAMA. 2019;321:2414-2427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 676] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 30. | Colombo A, Giannini F, Briguori C. Should We Still Have Bare-Metal Stents Available in Our Catheterization Laboratory? J Am Coll Cardiol. 2017;70:607-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Ishida M, Takahashi F, Goto I, Niiyama M, Saitoh H, Sakamoto T, Maegawa Y, Osaki T, Nishiyama O, Endo H, Sakamoto R, Kojima T, Koeda Y, Kimura T, Itoh T, Morino Y; REIWA investigators. Clinical outcomes of patients treated using very short duration dual antiplatelet therapy after implantation of biodegradable-polymer drug-eluting stents: rationale and design of a prospective multicenter REIWA registry. Cardiovasc Interv Ther. 2020;35:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, Cha JY, Collier T, Dangas G, Dudek D, Džavík V, Escaned J, Gil R, Gurbel P, Hamm CW, Henry T, Huber K, Kastrati A, Kaul U, Kornowski R, Krucoff M, Kunadian V, Marx SO, Mehta SR, Moliterno D, Ohman EM, Oldroyd K, Sardella G, Sartori S, Shlofmitz R, Steg PG, Weisz G, Witzenbichler B, Han YL, Pocock S, Gibson CM. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N Engl J Med. 2019;381:2032-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 738] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 33. | Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, Bengtsson O, Oude Ophuis T, Budaj A, Theroux P, Ruda M, Hamm C, Goto S, Spinar J, Nicolau JC, Kiss RG, Murphy SA, Wiviott SD, Held P, Braunwald E, Sabatine MS; PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1314] [Cited by in RCA: 1430] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 34. | Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR Jr, Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Ver Lee P, Rinaldi MJ, Massaro JM; DAPT Study Investigators. Twelve or 30 mo of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371:2155-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1401] [Cited by in RCA: 1530] [Article Influence: 127.5] [Reference Citation Analysis (0)] |
| 35. | Udell JA, Bonaca MP, Collet JP, Lincoff AM, Kereiakes DJ, Costa F, Lee CW, Mauri L, Valgimigli M, Park SJ, Montalescot G, Sabatine MS, Braunwald E, Bhatt DL. Long-term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: a collaborative meta-analysis of randomized trials. Eur Heart J. 2016;37:390-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 36. | Yeh RW, Secemsky EA, Kereiakes DJ, Normand SL, Gershlick AH, Cohen DJ, Spertus JA, Steg PG, Cutlip DE, Rinaldi MJ, Camenzind E, Wijns W, Apruzzese PK, Song Y, Massaro JM, Mauri L; DAPT Study Investigators. Development and Validation of a Prediction Rule for Benefit and Harm of Dual Antiplatelet Therapy Beyond 1 Year After Percutaneous Coronary Intervention. JAMA. 2016;315:1735-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 718] [Cited by in RCA: 748] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 37. | Baber U, Mehran R, Giustino G, Cohen DJ, Henry TD, Sartori S, Ariti C, Litherland C, Dangas G, Gibson CM, Krucoff MW, Moliterno DJ, Kirtane AJ, Stone GW, Colombo A, Chieffo A, Kini AS, Witzenbichler B, Weisz G, Steg PG, Pocock S. Coronary Thrombosis and Major Bleeding After PCI With Drug-Eluting Stents: Risk Scores From PARIS. J Am Coll Cardiol. 2016;67:2224-2234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 466] [Article Influence: 46.6] [Reference Citation Analysis (0)] |