Peer-review started: October 20, 2020
First decision: November 16, 2020
Revised: November 25, 2020
Accepted: December 27, 2020
Article in press: December 27, 2020
Published online: January 26, 2021
Processing time: 86 Days and 20.5 Hours
Heart Failure (HF) in elderly patients is a systemic syndrome where advanced age, comorbidities with organ system deterioration, frailty and impaired cognition significantly impact outcome. Cardiac cachexia, sarcopenia and frailty despite overlap in definitions are different clinical entities that frequently coexist in HF patients. However, these co-factors often remain unaddressed, resulting in poor quality-of-life, prolonged physical disability and exercise intolerance and finally with higher rehospitalization rates and mortality. Strategy aim to increase muscle mass and muscle strength and delay the occurrence of frailty state appear essential in this regard. Common HF drugs therapy (b-blockers, angiotensin-converting enzyme inhibitors) and prescription of physical exercise program remain the cornerstone of therapeutic approach in HF patients with new promising data regarding nutritional supplementation. However, the treatment of all these conditions still remain debated and only a profound knowledge of the specific mechanisms and patterns of disease progression will allow to use the appropriate therapy in a given clinical setting. For all these reasons we briefly review current knowledge on frailty, sarcopenia and cachexia in HF patients with the attempt to define clinically significant degrees of multiorgan dysfunction, specific "red alert" thresholds in clinical practice and therapeutic approach.
Core Tip: The last heart failure (HF) guidelines of the European Society of Cardiology dedicate a chapter each for cachexia, sarcopenia and frailty and several studies regarding these topics are coming up. This wealth of information highlights the importance of these co-factors in HF management and are each uniquely relevant to evaluate older patients with HF. It is time to routinely assess cachexia, sarcopenia and frailty that could help in personalized care plan, improve outcomes and reduce hospitalization and institutionalization. However, definitions, pathophysiology and treatment of all these conditions still remain unclear and we briefly summarize the most recent knowledge available in literature.
- Citation: Beltrami M, Fumagalli C, Milli M. Frailty, sarcopenia and cachexia in heart failure patients: Different clinical entities of the same painting. World J Cardiol 2021; 13(1): 1-10
- URL: https://www.wjgnet.com/1949-8462/full/v13/i1/1.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i1.1
Heart failure (HF) in elderly patients is a systemic syndrome where advanced age, comorbidities with organ system deterioration, frailty and impaired cognition significantly impact outcome[1]. HF syndrome shows a significant impact on the health-care resources and its occurrence is ever increasing in the elderly[2]. Recently there are discussion regarding frailty and the common problems affecting skeletal muscle, both sarcopenia and cachexia. These comorbidities are crucial issue to plan health care resources for older patient with HF. After hospitalization for acute HF, these co-factors often remain unaddressed resulting in prolonged physical disability, poor quality-of-life, exercise intolerance and finally with higher rehospitalization rates and mortality. Besides, HF hospitalization independently and significantly increased the risk of limitations of Basic and Instrumental Activities of Daily Living (IADL)[3]. The complex relationship between frailty, muscle wasting and cachexia can coexist and different strategies and interventions need to be deeply investigated to improve outcome, quality of life and HF-related re-admissions. For all these reasons we briefly review current knowledge on frailty, sarcopenia and cachexia in HF patients with the attempt to define clinically significant degrees of multiorgan dysfunction, specific "red alert" thresholds in clinical practice and therapeutic approach.
Frailty recognize a biologic basis and it is characterized by a loss of strength and physical ability with a progressive decline of cognitive function. Incorrect definition includes this syndrome as a comorbidity or a disability, but comorbidity is a risk factor and disability is an outcome of frailty state[4]. Frailty patients show a strong susceptibility to several endogenous and exogenous stressors. This vulnerability state contributes to risk of falls, hospitalization and death. Frailty is a common finding in HF patients and the prevalence is higher in older age and correlates significantly with HF severity, however frailty must not be considered only in older individuals and all patients with HF deserve a frailty assessment[5]. FRAIL-HF study reveal the presence of frailty state in 70% of patients with HF and ≥ 80 years old. On the other hand frail patients show an higher risk to develop HF[6]. Several frailty score and pre-fail status assessment are now available; Clinical Frailty Scale, gait speed test, PRISMA/7 questionnaire, Fried Score, FRAIL Score and Short Physical Performance Battery (SPPB) are routinely used in clinical practice with good agreement between methods[7-9]. Recently the HF Association/European Society of Cardiology establish a new frailty score that is the first elaborated in HF setting. This new score consider a 4-domain framework such as clinical, functional, cognitive-psychological and social variables, extremely useful and practical to diagnose frailty in HF patients[10] (Table 1). Nowadays an overall geriatric assessment that consider not only the frailty state but also depression, cognitive impairment and muscle wasting are each uniquely relevant to evaluate older patients with HF and significantly impact prognosis and treatment success (i.e., transcatheter or surgical Aortic Valve Replacement)[11,12]. The SPPB is a set of objective measures of physical performance, highly predictive of disability, hospitalization, institutionalization, and mortality in community-dwelling older individuals[13,14]. The prognostic potential of the instrument has been proven in patients hospitalized for HF, pneumonia, chronic obstructive pulmonary disease (COPD) and minor stroke[15]. Furthermore, in a cohort of hospitalized elderly HF patients, a low physical performance status, as evaluated with SPPB, predicted mortality even after adjustment for left ventricle ejection fraction (LVEF), New York Heart Association (NYHA) Class and comorbidities[16]. Moreover, handgrip strength, serum albumin and IADL status are all associated with health outcome in elderly patients hospitalized for HF. Moreover, the pre-frailty state, a condition that is potentially reversible, identified by slow gait speed, exhaustion and low energy expenditure, is in the same way an independent predictor of new cardiovascular events in older adults[17]. For all these reasons closer follow up by HF team is highly necessary in frail patients. This approach could monitor HF symptoms, adjust medications and address reversible causes leading to worsening of frailty score and consequent HF decompensation.
| Institution/authors, journal, year | Frailty | Institution/authors, journal, year | Cachexia | Institution/authors, journal, year | Sarcopenia |
| British Geriatrics Society, Age UK and Royal College of General Practitioners Report[7]. Age Aging 2014 | (1) A gait speed < 0.8 m/s; (2) Timed-up-and-go test > 10 s; (3) Score of ≥ 3 on the PRISMA 7 questionnaire. Falls, delirium and sudden immobility can used to indicate the presence of frailty | Argilés et al[19]. Consensus on cachexia definitions. J Am Med Dir Assoc 2010 | Weight loss > 5% of body weight (or BMI < 20 kg/m2) in ≤ 1 year in presence of chronic illness and three of five of these criteria. (1) Decreased muscle strength; (2) Fatigue; (3) Anorexia; (4) Low fat free mass index; (5) Anaemia (< 120 g/L), low serum albumin (< 32 g/L) and CRP > 5 mg/L, IL-6 > 4 pg/mL | Revised European consensus on definition and diagnosis[36]. Age Aging 2019 | (1) Low muscle strength; (2) Evidence of low muscle quantity or quality; (3) Detection of low physical performance. The combination of three represent severe sarcopenia |
| Heart Failure Association/European Society of Cardiology position paper on frailty in patients with heart failure[10]. Eur J Heart Fail 2019 | The frailty score in HF patients were built with the following variables: (1) Clinical: Comorbidities, weight loss, falls; (2) Psycho-cognitive: Cognitive impairment, dementia, depression; (3) Functional: ADL/IADL, mobility, balance; (4) Social: Living alone, no social support, institutionalisation | International Consensus on Cancer Cachexia Classification[65]. Lancet 2003 | The agreed diagnostic criterion for cachexia was weight loss greater than 5%, or weight loss greater than 2% in individuals already showing depletion according to current bodyweight and height (BMI < 20 kg/m2) or skeletal muscle mass reduction | Society of Sarcopenia, Cachexia and Wasting Disorders[37]. J Am Med Dir Assoc 2011 | Muscle loss with (1) walking speed equal or less than 1 m/s; (2) walks less than 400 m during a 6-minute walk. Appendicular lean mass/height2 > 2 SD below the mean mass of a healthy person (aged 20–30 yr) |
| Fried et al[4]. J Gerontol A Biol Sci Med Sci 2001 | Frailty was defined with three or more of the following criteria: (1) Unintentional weight loss (4, 5 kgs in past year); (2) Self-reported exhaustion; (3) Weakness (reduced grip strength); (4) Slow walking speed; (5) Low physical activity. A pre-frail status is accordingly whit one or two criteria | SCRINIO working group[66]. JPEN J Parenter Enteral Nutr 2009 | The patients were divided in 4 groups based on combinations of body weight loss < 10% in precachexia and ≥ 10% in cachexia; associated to the presence/absence of at least 1 symptom of anorexia, fatigue or early satiation | International working group on sarcopenia[39]. J Am Med Dir Assoc 2011 | Gait speed of less than 1 ms (-1) and low muscle mass (appendicular mass relative to ht (2) that is ≤ 7.23 kg/m2 in men and ≤ 5.67 kg/m2 in women) |
| Canadian Study of Health and Aging[9]. CMAJ 2005 | Clinical frailty scale is based on IADL, activity, mobility, energy, and symptoms all associated with clinical judgement | Special Interest Groups "Cachexia-Anorexia in Chronic wasting diseases" and "Nutrition in Geriatrics“[21]. Clin Nutr 2008 | Reduced muscle mass, strength and function (low handgrip strength (men < 26 kg, women < 16 kg), gait speed ≤ 0.8 m/s, low appendicular lean mass/BMI) |
Cachexia is a serious but underrecognized consequence of many chronic diseases such as cancers, COPD, malnutrition state, neurological disease, rheumatoid arthritis and kidney disease. It is considered a wasting process at multiorgan levels (skeletal muscle, fat and bone tissue)[18]. Cachexia is defined by a weight loss > 5% of body weight [or body mass index (BMI) < 20 kg/m2] in ≤ 1 year in presence of chronic illness and three of five of these criteria (1) decreased muscle strength; (2) fatigue; (3) anorexia; (4) low fat free mass index; and (5) abnormal biochemistry [anaemia (< 120 g/L), low serum albumin (< 32 g/L), increased inflammatory markers (C-reactive protein > 5 mg/L), interleukin-6 (IL-6) > 4 pg/mL][19] (Table 1). The overall prevalence in Western World is still growing and affects around 1% of patients population[20,21]. Cachexia is more frequently common in end-stage HF, its prevalence ranges from 5%-15%[22], but surprisingly it is not clear a close relationship with LVEF, in particular the prevalence of the disease is the same in various HF phenotype[23]. In a prospective study that enrolled outpatients with LVEF ≤ 40%, 18% are cachectic and cardiac cachexia is associated with intestinal congestion irrespective of HF stage and cardiac function. Inflammation state, gastrointestinal discomfort, appetite loss provide probable mechanisms, by which intestinal congestion may trigger cardiac cachexia[24]. Instead right ventricular dysfunction and weight loss may have a pathophysiological linked (venous congestion, malabsorption, anorexia, gut bacteria translocation)[25]; improvement of right ventricular function may delay the occurrence of cachexia. Moreover, tricuspid regurgitation and pulmonary hypertension are associated with low BMI and they are accentuating risk factor for cachexia, together with hypoalbuminemia and hyponatremia due to protein-losing enteropathy[26,27].
Cachectic patients show an impaired functional capacity, more severe symptoms and low quality of life[28]. This serious complication is associated with frequent hospitalization increased length of in hospital stay and health care cost[29]. Weight loss displays an additional prognostic information beyond clinical features of HF severity with significant association with morbidity and mortality[30]. Wasting disorders is and independent risk factor for impaired survival in chronic HF patients (adjusting for age, sex, NYHA class, LVEF, and VO2 consumption); mortality at 18 months of followup is around 50% in patients with cachexia[31]. In hospitalized HF patients with cardiac cachexia factors such renal function, age, and haemoglobin are pivotal prognostic markers[32].
As opposite, obesity is an independent risk factor associated with HF, but recently it is well demonstrated that obesity in patients that have already developed HF (across a wide range of BMI) is related to lower mortality; highlighting the concept of “obesity paradox” also common in other chronic disease[33]. However, BMI does not reflect the body composition regarding the percentage of fat mass, fat-free mass and lean mass. Inside this different large spectrum of body composition, patients with preserved skeletal muscle mass show a better prognosis compared with patients with reduced lean mass due to increased stroke volume and consequent better tissue perfusion[34]. Thus, fat mass loss but not lean mass has a prognostic impact and it is a good indicator of enhanced catabolism and has a role of cardioprotection in advanced HF.
Recently the 12th Cachexia Conference held in Berlin in December 2019 highlights preclinical and clinical studies in the field of wasting disorders[35]. The definition of sarcopenia remains a matter of discussion, however for the first time it is recognized that strength is better than mass to evaluate adverse outcomes and actually the European consensus on definition and diagnosis Sarcopenia define the disease with three criterion: (1) Low muscle strength, that is considered the most accurate parameter to evaluate sarcopenia; (2) Evidence of low muscle quantity or quality; and (3) The detection of low physical performance (Table 1). The presence of all three criteria permit to define ad advance sarcopenia status. Several tests and different tools are widely described to assess sarcopenia in practice and in research with specific description of each method used[36-39]. Sarcopenia is age related and old muscle mass is reduced by 1%-2% annually after 50 years old with a contemporary decline in muscle strength by about 1.5%[40]. Its prevalence increases around 3% annually after the 60 years old[41]. For the first time the European Society of Cardiology in the guidelines of 2016 dedicate a chapter to cachexia and sarcopenia recognizing as important comorbidities of HF[42]. The prevalence of sarcopenia is around 20% in HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction patients without clear difference in prevalence across different HF phenotype. A monotonic association existed between increasing sarcopenia prevalence and other comorbidities, in particular sarcopenia correlates with the decline of glomerular filtration rate[43,44]. Six-minutes-walk test and spiroergometry show lower Vitamin E/VCO2 (VO2) and shorter exercise time in sarcopenic HF patients, highlighting the impact of muscle wasting in lower muscle strength and lower physical performance in HF population. Bekfani et al[45] find better quality of life in patients with higher values of muscle strength/muscle mass ratio (evaluated by Visual Analogue Scale derived from the EQ-5D)[45]. Emami et al[46] demonstrate a percentage of overlap (6.7% in HF population studied) of both sarcopenia and cachexia. The lowest values of muscle strength and function, as assessed by handgrip and quadriceps strength, 6 min walking test, SPPB score and peak VO2, are observed only in sarcopenic groups[46]. Moreover, sarcopenia significantly impact the functional capacity of HF patients; and it is associated with increased likelihood of adverse events including falls, fractures, worst neurocognitive profile and low physical performance that may precipitate a relative clinical HF stable condition. In older patients low physical activity is strictly related with the occurrence of sarcopenia and it is an independent factor in prolonging hospital stay among patients admitted to hospital care[47]. However, muscle wasting might be present also in younger patients with HF and non-ischemic dilated cardiomyopathy, particularly in those with advance clinical status[48]. In literature, some discordant opinions are evident regarding the recovery of skeletal muscle after heart transplantation (HT) or left ventricle assistant device implantation[49]. Skeletal muscle impairment seems to persist after months from HT and contribute to the impaired exercise capacity[50], however a recent study demonstrates the recovery of muscle mass and strength after 1.5-3 years of follow up after HT. Moreover, HT and ventricular assist device therapy lead to an improvement in frailty score during follow up highlighting that sarcopenia and frailty are both dynamic and not a fixed entity[51].
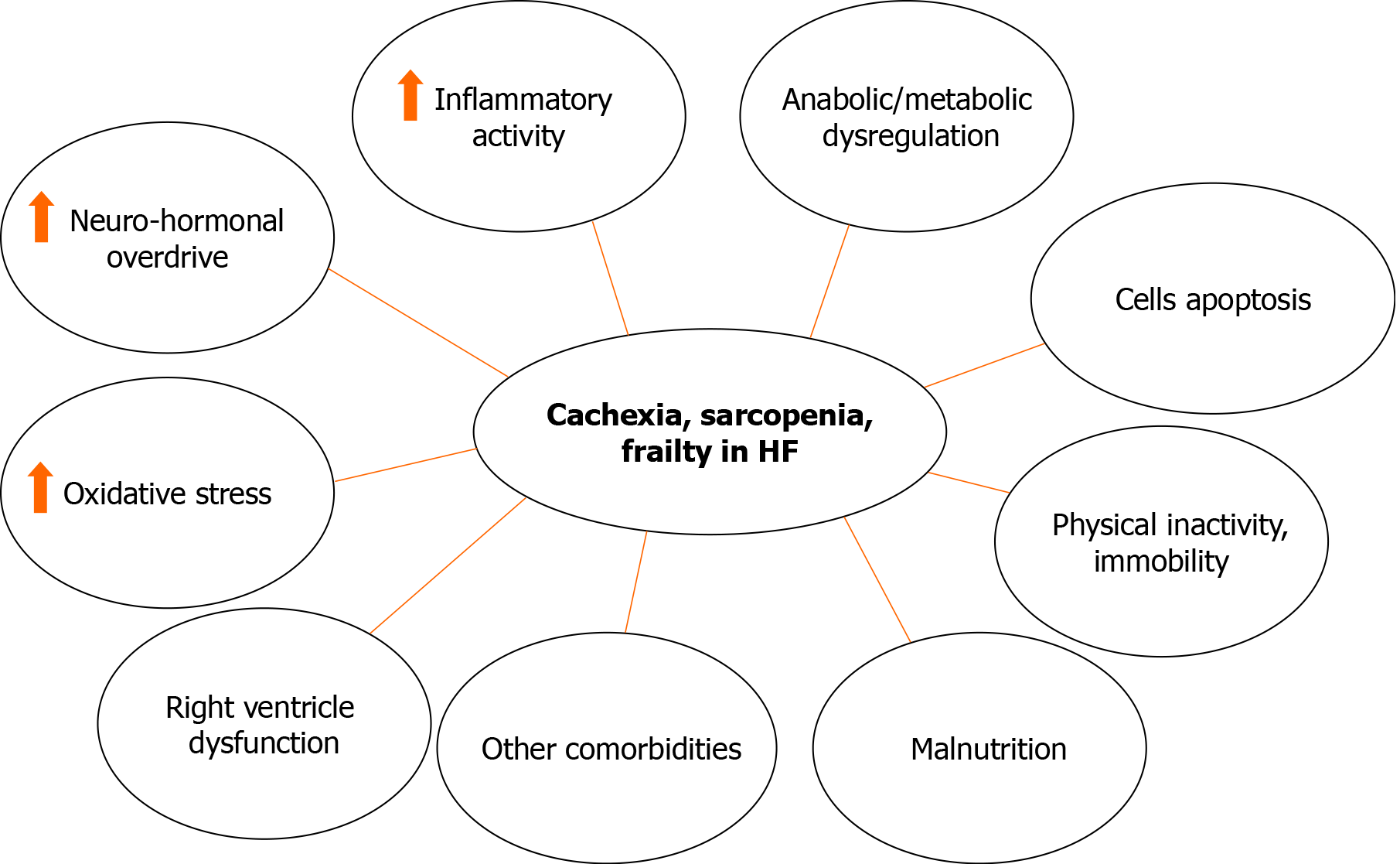
Cardiac cachexia, sarcopenia and frailty in HF patients recognize similar pathophysiological features (Figure 1). Systemic inflammation and hypermetabolism play a pivotal role; cachectic patients display an increased cortisol/dehydroepiand-rosterone ratio and higher cytokine levels such as tumor necrosis factor (TNF)-alpha, soluble TNF-receptor 1 and IL-6. Higher levels of hormones and cytokines activities are both associated with muscle wasting, reduced fat tissue and bone mass. Few hormones are implicated in the pathophysiology in cachexia and sarcopenia. Growth hormone, insulin resistance and insulin-like growth factor-1 levels are all associated with muscle mass loss and consequently with a significant reduction of physical performance[52]. Triiodothyronine in cachectic oncologic patients is increased compared to non-cachectic cancer patients and it is also normal in patients with benign weight loss. Ghrelin significantly inhibits the production of cytokines with inflammatory pathway and exhibits anti-cachectic activity both with growth hormone dependent and independent mechanisms. Low testosterone levels are usually common in all HF patients and contribute to the progression of cardiac cachexia, sarcopenia and frailty through altered peripheral vascular resistance, increased cardiac afterload, and decreased cardiac output. Nutritional alterations and gastrointestinal malabsorption lead to an abnormal calorie uptake, protein balance and insulin resistance[53]. Advanced HF status is linked to gastroenteropathy secondary to intestinal edema that result in protein losing enteropathy which causes malabsorption and anorexia[54]. Catabolic/anabolic imbalance change the substrate utilization in tissues. Moreover, cachexia in HF is associated with an increase in adiponectin concentration strictly related to type B natriuretic peptide levels[55]. All these reasons exacerbate the wasting process and muscle cells apoptosis, and therefore result in muscle atrophy and lower muscular strength favoring the occurrence of frailty[56].
Physical inactivity due to the progression of HF syndrome are common in frailty patients. Sarcopenic, cachectic and frailty patients show impaired exercise capacity and limitations in common activity such as food preparation and eating, contributing to disability and muscle loss. Skeletal muscle improvement after exercise training explain the reduction of maximal oxygen consumption demonstrating how physical activity is the major treatment of these patients[57]. Physical exercise program and nutritional supplementation are more effective in individuals with low functional level and increased number of frailty criteria[58]. Growing evidence show a potential benefit from oral supplementation in terms of protein and energy intake in HF patients with the aim to avoid the loss of lean mass[59,60], this personalized dietary intervention results in a potential benefit with significant impact in reducing mortality and hospital readmission. In sarcopenic patients potential treatments may include appetite stimulants and anabolic agents, including testosterone, in combination with the application of nutritional supplements and anti-catabolic interventions, although none is of proven benefit and their safety is unknown[61]. However, scientific data are scarce and the quality of the evidence is low, and no strong recommendations can be currently made in HF setting[62].
In patients with HFrEF and sinus rhythm, b-blockers significantly improve the outcome due its effect on weight gain counterpoising the sympathetic activation that it is an important determinant of cardiac cachexia[63]. Moreover treatment with an angiotensin-converting enzyme (ACE) inhibitor (enalapril) reduces the risk of weight loss in patients with HFrEF[64-66].
Cardiac cachexia, sarcopenia and frailty despite overlap in definitions are different clinical entities that frequently coexist in HF patients. All these conditions are serious complications in HF patients and are associated with increasing hospitalization and mortality rates[1]. It is time to produce an important effort to include a routinely assessment in clinical practice of cachexia, sarcopenia and frailty for HF patients that could help in earlier therapeutic decision, personalized care plan, improve outcomes and reduce hospitalization and institutionalization. Strategy aim to increase muscle mass and muscle strength and delay the occurrence of frailty state appear essential in this regard. Common HF drugs therapy (b-blockers, ACE inhibitors) and prescription of physical exercise program remain the cornerstone of therapeutic approach in HF patients with new promising data regarding nutritional supplementation. However, the treatment of all these conditions still remain debated and only a profound knowledge of the specific mechanisms and patterns of disease progression will allow to use the appropriate therapy in a given clinical setting.
| 1. | van Deursen VM, Urso R, Laroche C, Damman K, Dahlström U, Tavazzi L, Maggioni AP, Voors AA. Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail. 2014;16:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 341] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 2. | Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O'Connor CM, She L, Stough WG, Yancy CW, Young JB, Fonarow GC; OPTIMIZE-HF Investigators and Coordinators. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296:2217-2226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 727] [Cited by in RCA: 761] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 3. | Skalska A, Wizner B, Więcek A, Zdrojewski T, Chudek J, Klich-Rączka A, Piotrowicz K, Błędowski P, Mossakowska M, Michel JP, Grodzicki T. Reduced functionality in everyday activities of patients with self-reported heart failure hospitalization--population-based study results. Int J Cardiol. 2014;176:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13384] [Cited by in RCA: 17005] [Article Influence: 680.2] [Reference Citation Analysis (2)] |
| 5. | Sze S, Pellicori P, Zhang J, Weston J, Clark AL. Identification of Frailty in Chronic Heart Failure. JACC Heart Fail. 2019;7:291-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 6. | Vidán MT, Sánchez E, Fernández-Avilés F, Serra-Rexach JA, Ortiz J, Bueno H. FRAIL-HF, a study to evaluate the clinical complexity of heart failure in nondependent older patients: rationale, methods and baseline characteristics. Clin Cardiol. 2014;37:725-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Turner G, Clegg A; British Geriatrics Society; Age UK; Royal College of General Practioners. Best practice guidelines for the management of frailty: a British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age Ageing. 2014;43:744-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 452] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 8. | Woo J, Leung J, Morley JE. Comparison of frailty indicators based on clinical phenotype and the multiple deficit approach in predicting mortality and physical limitation. J Am Geriatr Soc. 2012;60:1478-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 301] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 9. | Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4103] [Cited by in RCA: 6267] [Article Influence: 298.4] [Reference Citation Analysis (18)] |
| 10. | Vitale C, Jankowska E, Hill L, Piepoli M, Doehner W, Anker SD, Lainscak M, Jaarsma T, Ponikowski P, Rosano GMC, Seferovic P, Coats AJ. Heart Failure Association/European Society of Cardiology position paper on frailty in patients with heart failure. Eur J Heart Fail. 2019;21:1299-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 206] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 11. | Afilalo J, Lauck S, Kim DH, Lefèvre T, Piazza N, Lachapelle K, Martucci G, Lamy A, Labinaz M, Peterson MD, Arora RC, Noiseux N, Rassi A, Palacios IF, Généreux P, Lindman BR, Asgar AW, Kim CA, Trnkus A, Morais JA, Langlois Y, Rudski LG, Morin JF, Popma JJ, Webb JG, Perrault LP. Frailty in Older Adults Undergoing Aortic Valve Replacement: The FRAILTY-AVR Study. J Am Coll Cardiol. 2017;70:689-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 631] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 12. | Gorodeski EZ, Goyal P, Hummel SL, Krishnaswami A, Goodlin SJ, Hart LL, Forman DE, Wenger NK, Kirkpatrick JN, Alexander KP; Geriatric Cardiology Section Leadership Council; American College of Cardiology. Domain Management Approach to Heart Failure in the Geriatric Patient: Present and Future. J Am Coll Cardiol. 2018;71:1921-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 205] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 13. | Pritchard JM, Kennedy CC, Karampatos S, Ioannidis G, Misiaszek B, Marr S, Patterson C, Woo T, Papaioannou A. Measuring frailty in clinical practice: a comparison of physical frailty assessment methods in a geriatric out-patient clinic. BMC Geriatr. 2017;17:264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 14. | Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85-M94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5576] [Cited by in RCA: 7110] [Article Influence: 222.2] [Reference Citation Analysis (0)] |
| 15. | Volpato S, Cavalieri M, Sioulis F, Guerra G, Maraldi C, Zuliani G, Fellin R, Guralnik JM. Predictive value of the Short Physical Performance Battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2011;66:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 343] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 16. | Chiarantini D, Volpato S, Sioulis F, Bartalucci F, Del Bianco L, Mangani I, Pepe G, Tarantini F, Berni A, Marchionni N, Di Bari M. Lower extremity performance measures predict long-term prognosis in older patients hospitalized for heart failure. J Card Fail. 2010;16:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Sergi G, Veronese N, Fontana L, De Rui M, Bolzetta F, Zambon S, Corti MC, Baggio G, Toffanello ED, Crepaldi G, Perissinotto E, Manzato E. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol. 2015;65:976-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 211] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 18. | Doehner W, Anker SD. Cardiac cachexia in early literature: a review of research prior to Medline. Int J Cardiol. 2002;85:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Argilés JM, Anker SD, Evans WJ, Morley JE, Fearon KC, Strasser F, Muscaritoli M, Baracos VE. Consensus on cachexia definitions. J Am Med Dir Assoc. 2010;11:229-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Farkas J, von Haehling S, Kalantar-Zadeh K, Morley JE, Anker SD, Lainscak M. Cachexia as a major public health problem: frequent, costly, and deadly. J Cachexia Sarcopenia Muscle. 2013;4:173-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr. 2008;27:793-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1496] [Cited by in RCA: 1710] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 22. | von Haehling S, Ebner N, Dos Santos MR, Springer J, Anker SD. Muscle wasting and cachexia in heart failure: mechanisms and therapies. Nat Rev Cardiol. 2017;14:323-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 296] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 23. | Florea VG, Henein MY, Rauchhaus M, Koloczek V, Sharma R, Doehner W, Poole-Wilson PA, Coats AJ, Anker SD. The cardiac component of cardiac cachexia. Am Heart J. 2002;144:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Valentova M, von Haehling S, Bauditz J, Doehner W, Ebner N, Bekfani T, Elsner S, Sliziuk V, Scherbakov N, Murín J, Anker SD, Sandek A. Intestinal congestion and right ventricular dysfunction: a link with appetite loss, inflammation, and cachexia in chronic heart failure. Eur Heart J. 2016;37:1684-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 177] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 25. | Pittman JG, Cohen P. The Pathogenesis of Cardiac Cachexia. N Engl J Med. 1964;271:453-460 Concl. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 638] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 27. | Ajayi AA, Adigun AQ, Ojofeitimi EO, Yusuph H, Ajayi OE. Anthropometric evaluation of cachexia in chronic congestive heart failure: the role of tricuspid regurgitation. Int J Cardiol. 1999;71:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Saitoh M, Dos Santos MR, Emami A, Ishida J, Ebner N, Valentova M, Bekfani T, Sandek A, Lainscak M, Doehner W, Anker SD, von Haehling S. Anorexia, functional capacity, and clinical outcome in patients with chronic heart failure: results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF). ESC Heart Fail. 2017;4:448-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 29. | Arthur ST, Noone JM, Van Doren BA, Roy D, Blanchette CM. One-year prevalence, comorbidities and cost of cachexia-related inpatient admissions in the USA. Drugs Context. 2014;3:212265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Rossignol P, Masson S, Barlera S, Girerd N, Castelnovo A, Zannad F, Clemenza F, Tognoni G, Anand IS, Cohn JN, Anker SD, Tavazzi L, Latini R; GISSI-HF and Val-HeFT Investigators. Loss in body weight is an independent prognostic factor for mortality in chronic heart failure: insights from the GISSI-HF and Val-HeFT trials. Eur J Heart Fail. 2015;17:424-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 31. | Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 986] [Cited by in RCA: 1054] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 32. | Sato Y, Yoshihisa A, Kimishima Y, Yokokawa T, Abe S, Shimizu T, Misaka T, Yamada S, Sato T, Kaneshiro T, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Takeishi Y. Prognostic factors in heart failure patients with cardiac cachexia. J Geriatr Cardiol. 2020;17:26-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 33. | von Haehling S, Anker SD. Prevalence, incidence and clinical impact of cachexia: facts and numbers-update 2014. J Cachexia Sarcopenia Muscle. 2014;5:261-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 298] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 34. | Carbone S, Lavie CJ, Arena R. Obesity and Heart Failure: Focus on the Obesity Paradox. Mayo Clin Proc. 2017;92:266-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 234] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 35. | Ebner N, Anker SD, von Haehling S. Recent developments in the field of cachexia, sarcopenia, and muscle wasting: highlights from the 12th Cachexia Conference. J Cachexia Sarcopenia Muscle. 2020;11:274-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2); and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 599] [Cited by in RCA: 1745] [Article Influence: 249.3] [Reference Citation Analysis (0)] |
| 37. | Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, Cederholm T, Coats AJ, Cummings SR, Evans WJ, Fearon K, Ferrucci L, Fielding RA, Guralnik JM, Harris TB, Inui A, Kalantar-Zadeh K, Kirwan BA, Mantovani G, Muscaritoli M, Newman AB, Rossi-Fanelli F, Rosano GM, Roubenoff R, Schambelan M, Sokol GH, Storer TW, Vellas B, von Haehling S, Yeh SS, Anker SD; Society on Sarcopenia; Cachexia and Wasting Disorders Trialist Workshop. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 931] [Cited by in RCA: 810] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 38. | Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6987] [Cited by in RCA: 8788] [Article Influence: 549.3] [Reference Citation Analysis (4)] |
| 39. | Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2197] [Cited by in RCA: 2410] [Article Influence: 160.7] [Reference Citation Analysis (0)] |
| 40. | Dodds RM, Granic A, Davies K, Kirkwood TB, Jagger C, Sayer AA. Prevalence and incidence of sarcopenia in the very old: findings from the Newcastle 85+ Study. J Cachexia Sarcopenia Muscle. 2017;8:229-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 41. | Evans W. Functional and metabolic consequences of sarcopenia. J Nutr. 1997;127:998S-1003S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 65] [Reference Citation Analysis (0)] |
| 42. | Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Rev Esp Cardiol (Engl Ed). 2016;69:1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 230] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 43. | Foley RN, Wang C, Ishani A, Collins AJ, Murray AM. Kidney function and sarcopenia in the United States general population: NHANES III. Am J Nephrol. 2007;27:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 222] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 44. | Fülster S, Tacke M, Sandek A, Ebner N, Tschöpe C, Doehner W, Anker SD, von Haehling S. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur Heart J. 2013;34:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 381] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 45. | Bekfani T, Pellicori P, Morris DA, Ebner N, Valentova M, Steinbeck L, Wachter R, Elsner S, Sliziuk V, Schefold JC, Sandek A, Doehner W, Cleland JG, Lainscak M, Anker SD, von Haehling S. Sarcopenia in patients with heart failure with preserved ejection fraction: Impact on muscle strength, exercise capacity and quality of life. Int J Cardiol. 2016;222:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 191] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 46. | Emami A, Saitoh M, Valentova M, Sandek A, Evertz R, Ebner N, Loncar G, Springer J, Doehner W, Lainscak M, Hasenfuß G, Anker SD, von Haehling S. Comparison of sarcopenia and cachexia in men with chronic heart failure: results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF). Eur J Heart Fail. 2018;20:1580-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 172] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 47. | Yang M, Hu X, Wang H, Zhang L, Hao Q, Dong B. Sarcopenia predicts readmission and mortality in elderly patients in acute care wards: a prospective study. J Cachexia Sarcopenia Muscle. 2017;8:251-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 200] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 48. | Hajahmadi M, Shemshadi S, Khalilipur E, Amin A, Taghavi S, Maleki M, Malek H, Naderi N. Muscle wasting in young patients with dilated cardiomyopathy. J Cachexia Sarcopenia Muscle. 2017;8:542-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 49. | Fernandes LCBC, de Oliveira IM, Fernandes PFCBC, de Souza Neto JD, Farias MDSQ, de Freitas NA, Magalhães NC, Bacal F. Impact of Heart Transplantation on the Recovery of Peripheral and Respiratory Muscle Mass and Strength in Patients With Chronic Heart Failure. Transplant Direct. 2018;4:e395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Schaufelberger M, Eriksson BO, Lönn L, Rundqvist B, Sunnerhagen KS, Swedberg K. Skeletal muscle characteristics, muscle strength and thigh muscle area in patients before and after cardiac transplantation. Eur J Heart Fail. 2001;3:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Jha SR, Hannu MK, Newton PJ, Wilhelm K, Hayward CS, Jabbour A, Kotlyar E, Keogh A, Dhital K, Granger E, Connellan M, Jansz P, Spratt PM, Montgomery E, Smith A, Harkess M, Tunicliff P, Davidson PM, Macdonald PS. Reversibility of Frailty After Bridge-to-Transplant Ventricular Assist Device Implantation or Heart Transplantation. Transplant Direct. 2017;3:e167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 52. | Anker SD, Ponikowski PP, Clark AL, Leyva F, Rauchhaus M, Kemp M, Teixeira MM, Hellewell PG, Hooper J, Poole-Wilson PA, Coats AJ. Cytokines and neurohormones relating to body composition alterations in the wasting syndrome of chronic heart failure. Eur Heart J. 1999;20:683-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 278] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 53. | Anker SD, Chua TP, Ponikowski P, Harrington D, Swan JW, Kox WJ, Poole-Wilson PA, Coats AJ. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96:526-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 571] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 54. | Curcio F, Testa G, Liguori I, Papillo M, Flocco V, Panicara V, Galizia G, Della-Morte D, Gargiulo G, Cacciatore F, Bonaduce D, Landi F, Abete P. Sarcopenia and Heart Failure. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 177] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 55. | McEntegart MB, Awede B, Petrie MC, Sattar N, Dunn FG, MacFarlane NG, McMurray JJ. Increase in serum adiponectin concentration in patients with heart failure and cachexia: relationship with leptin, other cytokines, and B-type natriuretic peptide. Eur Heart J. 2007;28:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 56. | Kinugawa S, Takada S, Matsushima S, Okita K, Tsutsui H. Skeletal Muscle Abnormalities in Heart Failure. Int Heart J. 2015;56:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 57. | Haykowsky MJ, Brubaker PH, Morgan TM, Kritchevsky S, Eggebeen J, Kitzman DW. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci. 2013;68:968-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 58. | Abizanda P, López MD, García VP, Estrella Jde D, da Silva González Á, Vilardell NB, Torres KA. Effects of an Oral Nutritional Supplementation Plus Physical Exercise Intervention on the Physical Function, Nutritional Status, and Quality of Life in Frail Institutionalized Older Adults: The ACTIVNES Study. J Am Med Dir Assoc 2015; 16: 439.e9-439. e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 59. | Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB; Health ABC Study. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 800] [Cited by in RCA: 854] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 60. | Kalantar-Zadeh K, Anker SD, Horwich TB, Fonarow GC. Nutritional and anti-inflammatory interventions in chronic heart failure. Am J Cardiol. 2008;101:89E-103E. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 61. | von Haehling S, Anker SD. Treatment of cachexia: an overview of recent developments. J Am Med Dir Assoc. 2014;15:866-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 62. | Habaybeh D, de Moraes MB, Slee A, Avgerinou C. Nutritional interventions for heart failure patients who are malnourished or at risk of malnutrition or cachexia: a systematic review and meta-analysis. Heart Fail Rev. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 63. | Rienstra M, Damman K, Mulder BA, Van Gelder IC, McMurray JJ, Van Veldhuisen DJ. Beta-blockers and outcome in heart failure and atrial fibrillation: a meta-analysis. JACC Heart Fail. 2013;1:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 64. | Loncar G, Springer J, Anker M, Doehner W, Lainscak M. Cardiac cachexia: hic et nunc: "hic et nunc" - here and now. Int J Cardiol. 2015;201:e1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 65. | Anker SD, Negassa A, Coats AJ, Afzal R, Poole-Wilson PA, Cohn JN, Yusuf S. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet. 2003;361:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 526] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 66. | Bozzetti F, Mariani L. Defining and classifying cancer cachexia: a proposal by the SCRINIO Working Group. JPEN J Parenter Enteral Nutr. 2009;33:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Čulić V, Li T S-Editor: Fan JR L-Editor: A P-Editor: Li JH