Peer-review started: July 17, 2019
First decision: August 2, 2019
Revised: October 17, 2015
Accepted: November 26, 2019
Article in press: November 26, 2019
Published online: January 26, 2020
Processing time: 165 Days and 15.4 Hours
Nearly six million people in United States have heart failure. Fifty percent of these people have normal left ventricular (LV) systolic heart function but abnormal diastolic function due to increased LV myocardial stiffness. Most commonly, these patients are elderly women with hypertension, ischemic heart disease, atrial fibrillation, obesity, diabetes mellitus, renal disease, or obstructive lung disease. The annual mortality rate of these patients is 8%-12% per year. The diagnosis is based on the history, physical examination, laboratory data, echocardiography, and, when necessary, by cardiac catheterization. Patients with obesity, hypertension, atrial fibrillation, and volume overload require weight reduction, an exercise program, aggressive control of blood pressure and heart rate, and diuretics. Miniature devices inserted into patients for pulmonary artery pressure monitoring provide early warning of increased pulmonary pressure and congestion. If significant coronary heart disease is present, coronary revascularization should be considered.
Core tip: Three million people in United States have heart failure with normal left ventricular systolic function but abnormal diastolic function due to increased myocardial stiffness. These patients are often elderly women with hypertension, ischemic heart disease, atrial fibrillation, obesity, diabetes mellitus, renal disease, or obstructive lung disease. The annual mortality rate of these patients is 8%-12% per year. The diagnosis is based on history, physical examination, laboratory data, echocardiography, and, when necessary, by cardiac catheterization. These patients often require weight reduction, an exercise program, aggressive control of blood pressure and heart rate, and diuretics. If significant coronary heart disease is present, coronary revascularization should be considered.
- Citation: Henning RJ. Diagnosis and treatment of heart failure with preserved left ventricular ejection fraction. World J Cardiol 2020; 12(1): 7-25
- URL: https://www.wjgnet.com/1949-8462/full/v12/i1/7.htm
- DOI: https://dx.doi.org/10.4330/wjc.v12.i1.7
Heart failure is a clinical syndrome, characterized by symptoms of breathlessness and easy fatigability, and signs of fluid retention, such as elevated jugular venous pressure, pulmonary crackles, and ankle edema, that result from the impaired ability of the heart to maintain a cardiac output that meets the metabolic needs of the body. Currently, nearly six million people in the United States have heart failure and require 30.7 billion dollars per year for health care[1]. Moreover, the healthcare costs for treating patients with heart failure in the United States are projected to increase to $70 billion in 2030[2].
Approximately 50% of the patients with heart failure have normal, or near-normal left ventricular (LV) systolic heart function with a LV ejection fraction ≥ 50% and a LV end-diastolic volume index < 97 mL/m2. These patients are described as having heart failure with preserved ejection fraction (HFpEF). However, these patients have abnormal LV diastolic function with incomplete LV relaxation due to increased myocardial stiffness. This form of heart failure is becoming the dominant form of heart failure among older adults in the United States and in Europe due, in part, to the increasing longevity of the population. By the year 2020, 8% of the United States and European population older than 65 years of age will have HFpEF[3].
Although most physicians have only recently become aware of HFpEF, the first description of HFpEF was in 1966 when “presbycardia” with decreased elasticity of the heart, also known as “senile heart disease”, was first described in elderly individuals[4]. Currently, the most common patients with HFpEF are elderly women with comorbid conditions, such as hypertension with LV hypertrophy (60%-80%), ischemic heart disease (35%-70%) that may be occult, atrial fibrillation (15%-65%), obesity (32%-46%), diabetes mellitus (20%-45%), renal disease (51%-58%), and obstructive lung disease (24%-30%)[5,6]. In addition, women as they age develop greater systemic arterial and LV stiffness than men which results in concentric LV myocardial remodeling and HFpEF.
The systemic arterial and ventricular stiffness in HFpEF is amplified by the coexistence of hypertension, chronic renal disease, and diabetes mellitus. With an increase in arterial stiffness, the pressure wave ejected from the LV is reflected back to the heart, thereby increasing LV afterload, decreasing diastolic ventricular function, and increasing the hydraulic work and myocardial oxygen requirements of the heart. These hemodynamic effects lead to decreased ventricular diastolic function, increased ventricular diastolic filling pressures, decreased coronary flow reserve, and pulmonary vascular congestion. As a result, patients experience shortness of breath.
Heart disease with preserved ejection fraction is a heterogenous syndrome with multiple different conditions that can contribute to the syndrome. Understanding and targeting the pathological conditions that contribute to this syndrome may have greater patient benefit than targeting the final pathway of cardiac dysfunction alone. The specific conditions that can contribute to HFpEF are listed in Table 1 which is adapted in part from[7,8].
| Obesity |
| Hypertension |
| Coronary artery disease |
| Atrial fibrillation |
| Diabetes mellitus |
| Chronic obstructive pulmonary disease |
| Obstructive sleep apnea |
| Anemia |
Patients who are hospitalized because of HFpEF have complication rates that are similar to those patients with heart failure and reduced ejection fraction (HFrEF), including similar rates of cardiac arrest, acute coronary syndromes, renal insufficiency and failure, and admission to critical care units[9]. Despite similar rates of in-hospital complications in the two groups, patients with HFpEF are less likely to receive cardiology consultation while in the hospital than patients with HFrEF.
Table 2 compares the clinical characteristics of patients with HFpEF with patient with HFrEF and is adapted in part from[10,11].
| HFpEF | HFrEF | |
| Sex | Women (62%) | Men (60%) |
| Age (yr) | 74 | 70 |
| Obesity | 41.4% | 35.5% |
| Diabetes mellitus | 45% | 40% |
| Hypertension | 77% | 69% |
| Chronic kidney disease | 56% | 45% |
| Coronary artery disease | 50%` | 59% |
| Prior myocardial infarction | 24% | 36% |
| LV remodeling | Concentric | Eccentric |
| LV ejection fraction | ≥ 50% | < 40% |
| Atrial fibrillation in hospitalized patients | 65% | 53% |
| Ventricular dysrhythmias | 3% | 11% |
| Hospitalizations for heart failure | Increasing | Decreasing |
| Therapies that decrease mortality | None at present time | Beta-Blockers, ACE inhibitors, biventricular pacemakers, coronary revascularization |
The annual mortality rate of patients with HFpEF is approximately 8% per year but increases to approximately 10%-12% per year among patients older than 70 years of age[11-13]. The prognosis of patients after the first hospitalization for HFpEF is poor with one-year mortality rates as high as 25% among older patients and five-year mortality rates of 24% to 54%[14]. Thirty percent of the patients with HFpEF die of noncardiac causes, compared with 17% of patients with HFrEF, due to the presence of one or more comorbid conditions. In this regard, the predictors of death among patients with HFpEF include advanced age, increased systolic blood pressure, atrial fibrillation, a prior cerebral vascular event, renal insufficiency or other major organ dysfunction with sepsis[14,15].
LV diastolic dysfunction in individuals with HFpEF is characterized by increased diastolic ventricular stiffness, which slows LV relaxation, increases LV diastolic filling pressures and limits cardiac output[16,17]. Different pathophysiologic mechanisms are hypothesized to contribute individually or in combination to LV diastolic dysfunction in patients with HFpEF. The current hypotheses include: (1) Cardiomyocyte titin hypophosphorylation; (2) Vascular endothelial cell inflammation and dysfunction; (3) Abnormal calcium homeostasis; (4) Increased ventricular matrix formation; and (5) Obesity.
Resting tension in cardiomyocytes is highly dependent on titin, which is a large sarcomeric protein that functions as a “molecular spring” which stores energy during ventricular contraction and releases energy during ventricular relaxation. Consequently, titin contributes to early myocardial diastolic recoil and late myocardial diastolic distensability[18]. A shift in titin isoform expression from the complaint N2BA to the stiff N2B isoform, hypophosphorylation of the N2B isoform, and lower overall phosphorylation of titin increases the resting tension of cardiomyocytes and contributes to increased LV myocardial stiffness and diastolic dysfunction[18-21]. Consequently, manipulation of the phosphorylation state of titin and the titin isoforms represents a therapeutic target for the treatment of patients with HFpEF.
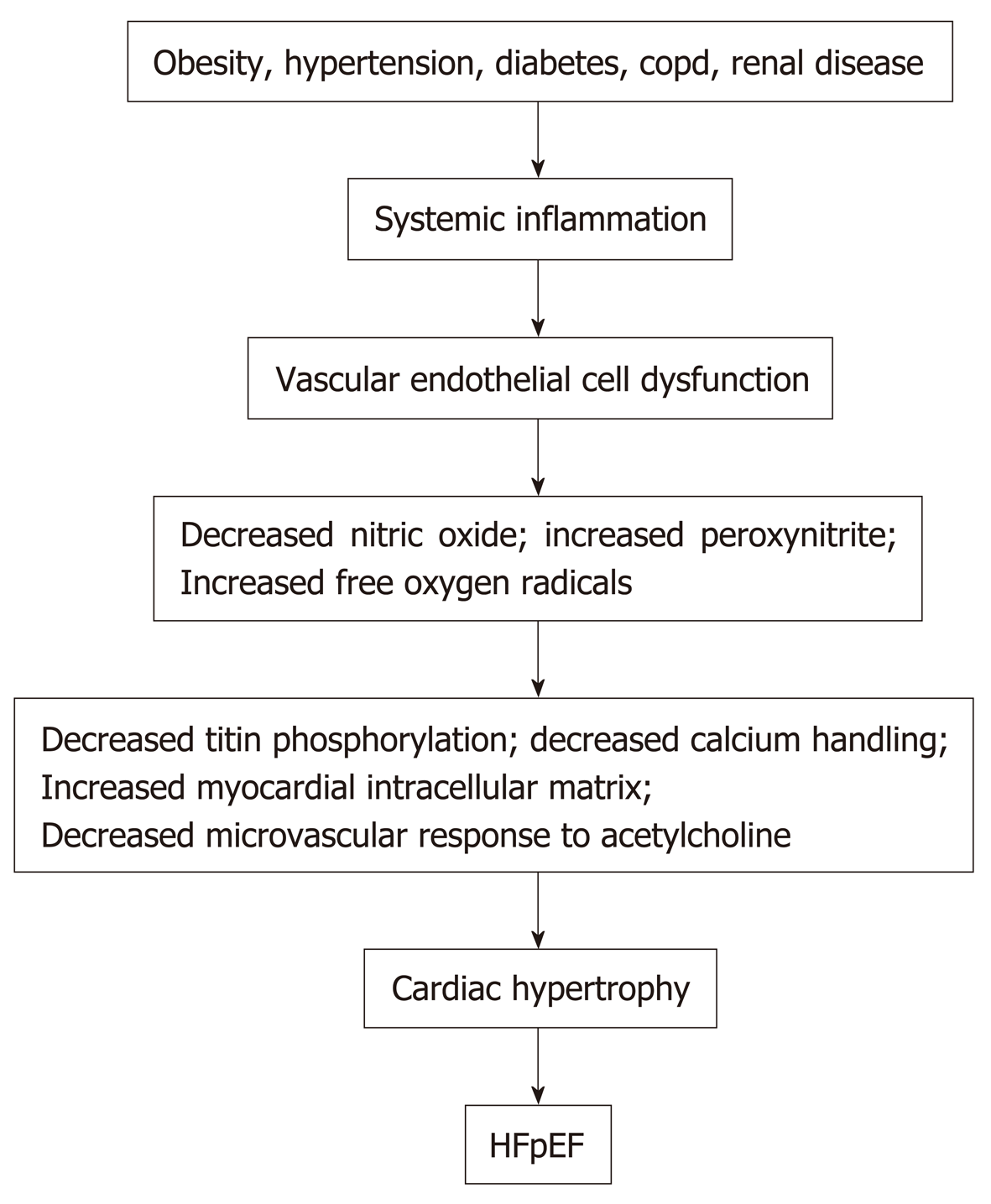
Obesity, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease, and anemia in patients can induce an inflammatory state with increased circulating C reactive protein, interleukin 1 (IL-1) receptor-like 1 protein, growth differentiation factor 15, IL-6, tumor necrosis factor-a, and pentraxin 3[22-24]. See Figure 1. Inflammation of the coronary microvascular endothelium produces free oxygen radicals and vascular cell adhesion molecules that attract circulating leukocytes to the microvascular which secrete transforming growth factor-β (TGF-β). TGF-β converts myocardial fibroblasts to myofibroblasts, which increase myocardial collagen deposition and cause interstitial fibrosis. Inflammatory free oxygen radicals also reduce nitric oxide (NO) bioavailability, and increase peroxynitrite (ONOO–), which reduces protein kinase G (PKG), which can increase cardiomyocyte stiffness, promote hypophosphorylation of titin, accelerate pro-hypertrophic signaling in cardiac myocytes and enhance diastolic dysfunction[17,21,25].
In addition, systemic inflammation decreases the vasodilator response of the coronary microvascular to acetylcholine and reduces renal blood flow and the ability of the kidneys to excrete sodium and water with resultant progressive expansion of intravascular volume[3]. Systemic inflammation also affects the lungs and skeletal muscle by contributing to pulmonary hypertension, muscle weakness and sarcopenia[3]. However, therapies to date that reduce systemic inflammation and/or improve or increase vascular endothelial function, such as statins, angiotensin receptor blockers, and phosphodiesterase-5 inhibitors have proven ineffective in improving the morbidity or the mortality of patients with HFpEF.
High circulating concentrations of the free oxygen radical peroxynitrite in patients with HFpEF increase cardiomyocyte protein phosphatase 2A activity, which decreases cardiomyocyte phospholambam phosphorylation, reduces sarcoplasmic reticulum Ca2+ uptake, and increases cardiomyocyte diastolic cytosolic Ca2+. In addition, abnormal cardiomyocyte sodium-calcium exchange and also calcium leak from the sarcoplasmic reticulum increase cardiomyocyte diastolic cytosolic calcium concentrations, which increases cardiomyocyte resting tension in HFPEF patients due to a delayed inactivation of actin-myosin crossbridges[26,27]. However, reductions in cardiomyocyte calcium overload with ranolazine to date have not significantly improved myocardial relaxation and diastolic dysfunction[28]. Nevertheless, abnormal calcium homeostasis is a potential therapeutic target for the treatment of patients with HFpEF.
Myocardial biopsies from patients with HFpEF, especially patients with hypertension and HFpEF, show an increase in the collagen volume fraction and an increase in myocardial fibrosis in comparison with patients without heart failure[29,30]. In this regard, Inflammation can cause the release of TGF-β from fibroblasts and monocytes/macrophages, which induce the differentiation of fibroblasts into collagen-producing myofibroblasts, while simultaneously decreasing matrix metalloproteinase (MMP)-1 and the tissue inhibitor of (MMP)-1[17,31,32]. In addition, endothelin-1, angiotensin II, platelet-derived growth factor, and connective tissue growth factor govern fibroblast activation/differentiation and represent potential therapeutic targets in the treatment of patients with HFpEF[33].
Myocardial collagen synthesis can also be increased during the female menopause where decreased circulating estrogen is associated with activation of the renin-angiotensin-aldosterone system[34]. Collagen expansion of the myocardial extracellular matrix, and especially an increase in the collagen type 1 fibers and the amount of crosslinked collagen, has an adverse effect on myocardial mechanical, electrical, and microvascular function and contributes to decreased diastolic function in HFpEF. Myocardial fibrosis also decreases myocardial capillary density, coronary perfusion reserve, and myocardial energy production[33]. Studies of anti-fibrotic agents, such as Pirfenidone and also valsartan/sacubitril, in the PIROUETTE trial, the Parallax and the PARAGON-HF trials will help to determine in patients with HFpEF whether significant regression of fibrosis in the myocardium can occur and can be associated with improvement in diastolic ventricular function.
Exercise studies of patients with HFpEF implicate LV stiffness and impaired exercise vasodilation and raise the possibility that the impaired diastolic reserve in these patients may be related to coronary microvascular dysfunction. In an autopsy study of 124 patients with HFpEF compared with 104 age-matched control patients, HFpEF patients had a median of 961 coronary microvessels/mm2vs 1316 vessels/mm2 in controls[31,35]. In addition, patients with HFpEF had more severe coronary artery disease with 65% of patients with ≥ 1 coronary vessel with > 50% diameter stenosis versus 13% in controls[34]. The vascular changes in the HFpEF patients were associated with heavier hearts with a median weight of 538 g with 9.6% myocardial fibrosis versus 335 g with 7.1% fibrosis in controls[35]. In addition, the myocardial fibrosis increased with decreasing microvascular density. However, cardiomyocyte size and LV hypertrophy are important determinants of the coronary microvascular density in adults and the role of cardiomyocyte size and LV hypertrophy in the determination of microvascular density in HFpEF requires further investigation.
Adipocytes synthesize cell-signaling molecules which include leptin, nerilysin and aldosterone which play a role in systemic inflammatory processes, cardiac fibrosis, and sodium and water retention[36]. In this regard, obesity can lead to cardiac fibrosis and increased ventricular diastolic stiffness through: (1) Leptin secretion from adipose tissue which induces cardiac fibrosis through galectin-3, TGF-β and connective tissue growth factors[37]; (2) Activation of beta2-adrenergic receptors which can enhance the synthesis of proinflammatory cytokines such as IL-6[38]; (3) Increased activity of neprilysin from adipocytes, which by degrading endogenous natriuretic peptides promotes plasma volume expansion, loss of the anti-aldosterone action of endogenous natriuretic peptides, and cardiac fibrosis[39]; and (4) Aldosterone secretion, in direct proportion to body mass, which increases collagen synthesis by cardiac fibroblasts[40].
Together these mechanisms can cause myocardial fibrosis and also cause sodium retention with plasma volume expansion. In addition, increased intramuscular fat decreases the supply of oxygen to working muscles and can impair oxidative metabolism in the skeletal musculature and, in this manner, contributes to the decreased functional capacity experienced by patients with HFpEF[5,41]. Figure 1 summarizes the different factors that can contribute to HFpEF.
The diagnosis of HFpEF in patients is based on the history, the physical examination, the laboratory data, the echocardiogram, and, when necessary, by cardiac catheterization.
The chest radiograph in patients with HFpEF often shows the presence of cardiomegaly and also evidence of pulmonary congestion. The electrocardiogram shows nonspecific ST-T wave changes although ECG evidence of myocardial ischemia or prior myocardial infarction may be present. Atrial fibrillation is frequently present on the ECG[42]. Plasma brain natriuretic peptide (BNP) or N-terminal pro-BNP (NT-proBNP) are often mildly increased with BNP > 100 pg/mL or NT-proBNP > 300 pg/mL. The concentrations of these peptides are less than the concentrations in patients with HFrEF[43]. Nevertheless, a BNP measurement > 100 pg/mL or a NT-proBNP > 300 pg/mL are independent predictors of adverse cardiovascular events in patients with HFpEF[44]. Currently, the measurements of natriuretic peptides and troponins are recommended for the diagnosis and the prognosis of patients with heart failure, and the measurements of suppression of tumorgenicity 2, a marker of systemic inflammation, and galectin-3, a marker of cardiac fibrosis, are recommended for additional patient risk stratification and prognosis[45,46].
Echocardiography is a very useful noninvasive technique in the diagnosis of patients with HFpEF and often demonstrates the presence of LV hypertrophy or concentric LV remodeling with a LVEF that is ≥ 50% and a LV volume index that is < 97 mL/m2. Left atrial enlargement is oftentimes present with a left atrial volume index > 34 mL/m2 in patients who are not in atrial fibrillation. Common echocardiographic indices of significant diastolic dysfunction are listed in Table 3 which is adapted in part from[47-49].
| 1. The ratio of the mitral blood flow velocity into the LV in early diastole (the E wave) to peak blood flow velocity in late diastole caused by atrial contraction (the A wave), or the E/A ratio, ≥ 2. The normal E/A is approximately 0.8. However, tachycardia, atrioventricular block, and left bundle branch block can lead to fusion of E and A waves, and ambiguity in diastolic function assessment. |
| 2. Increased left atrial pressure measured by early mitral blood flow velocity across the mitral valve (E wave) to the early diastolic velocity (e’) of the lateral mitral annulus, or E/e’ ratio. An E/e’ ratio 10 = mild and E/e’ ratio > 14 = significant LV dysfunction. If the lateral mitral annulus e' velocity is not quantifiable, the septal mitral annular e' velocity can be used. In this case, the E/e’ is increased if the ratio is > 15. |
| 3. Lateral mitral annular e’ velocity < 10 cm/s or septal e’ mitral annular velocity < 7 cm/s. |
| 4. Pulmonary artery systolic pressure > 35 mmHg indicative of pulmonary arterial hypertension. Pulmonary artery systolic pressure = 4 × (peak tricuspid regurgitation velocity)2 + estimated right atrial pressure. These criteria should not be used in patients with significant pulmonary disease. |
| 5. An echocardiographic determination of global longitudinal strain of -16.05 ± 2.16. This measurement can separate patients with HFpEF from patients with hypertension and normal controls in whom the global longitudinal strain measurements are -18.58 ± 2.84 and -19.59 ± 1.49, respectively. |
However, echocardiographic determined E/A and E/e’ ratios should not be used in isolation in assessing patients, but rather the ratios should be used in conjunction with the patient’s clinical characteristics and laboratory data to accurately diagnose LV diastolic dysfunction and HFpEF[50]. In this regard, echocardiographic studies of 745 patients with HFpEF in the I-PRESERVE study reported that the LV end-diastolic volume was within normal limits in > 95 percent of the patients, LV hypertrophy or concentric remodeling was present in 50 to 60 percent, LV diastolic dysfunction (mild to severe) was present in > 70 percent, and left atrial area enlargement was present in > 65 percent of patients[51].
Recently a scoring system has been developed to facilitate the diagnosis of HFpEF in patients with dyspnea and distinguish these patients from patients with non-cardiac causes of dyspnea[52]. The scoring system uses 6 clinical and echocardiographic characteristics (H2FPEF: Heavy, Hypertension, Atrial Fibrillation, Pulmonary Hypertension, Elderly, LV filling Pressure) that are obtained in the evaluation of patients with unexplained exertional dyspnea to determine the probability of HFpEF. Patients with score high s of 6 to 9 have a probability of HFpEF > 90%. Conversely, patients with low scores of 0 to 1 have a probability of HFpEF ≤ 23%. Patients at intermediate probability with H2FPEF scores of 2 to 5 require additional testing to determine the cause of dyspnea. Table 4 lists the H2FPEF scoring system and is adapted from[52].
| Clinical characteristic | Clinical measurement | Points awarded |
| Heavy | Body mass index > 30 kg/m2 | Two |
| Hypertension | Two or more hypertensive medications | One |
| Atrial fibrillation | Paroxysmal or persistent | Three |
| Pulmonary hypertension by echocardiogram | Pulmonary artery systolic pressure > 35 mmhg | One |
| Elderly | Age > 60 yr | One |
| LV filling pressure by echocardiogram | Echocardiographic e/e’ > 9 | One |
In patients with HFpEF, the myocardial extracellular matrix can be determined with CMR T1 mapping and is a predictor of LV myocardial stiffness and LV diastolic dysfunction. Excessive extracellular matrix deposition is a major contributor to the impaired cardiac relaxation and cardiac stiffness that are the hallmarks of HFpEF. Moreover, the CMR detection of myocardial extracellular matrix can precede the clinical diagnosis of HFpEF and correlates with patient myocardial biopsy specimens and hospitalizations for heart failure or death from cardiovascular causes[48,53-56].
In 410 patients with HFpEF or at risk for HFpEF, CMR determinations of increased myocardial extracellular volume (ECV) as an estimate of myocardial fibrosis were strongly associated with hospitalizations for heart failure or death during the subsequent four years[56]. In this investigation, myocardial ECV was more strongly associated with outcome than age, LV mass, atrial fibrillation, or previous myocardial infarction. See Table 5 which is adapted from[56] and information kindly provided by Erik Schelbert, MD.
| CMR ECV | Year one | Year two | Year three | Year four |
| ECV < 25% | 95.8% | 95.8% | 95.8% | 82.1% |
| 25% ≤ ECV < 30% | 95.5% | 90.5% | 87.6% | 81.1% |
| 30% ≤ ECV < 35% | 88.0% | 77.3% | 69.6% | 65% |
| 35% > ECV < 40% | 82.2% | 74.3% | 69.4% | 61.7% |
| ECV ≤ 40% | 40.0% | 40.0% | 40.0% | 40.0% |
| Cardiac amyloid | 47.1% | 23.5% | 0% | 0% |
CMR determined ECV measurements provide risk stratification for HFpEF during the ensuing four years. Patients with ECV > 30% had decreased event-free survival during the subsequent four years.
However, the ECV fraction can be normal in as many as one-third of patients with HFpEF, which demonstrates the pathophysiological variation in this syndrome and the necessity to utilize the patient’s history, the physical examination, laboratory data, echocardiography, and, if necessary, cardiac catheterization in order to establish the diagnosis.
For patients for whom the probability of HFpEF remains intermediate after history, physical examination, natriuretic peptide determinations, and echocardiography have been performed, invasive hemodynamic assessment of cardiac filling pressures, with provocative stress maneuvers such as exercise, is useful to make or exclude the diagnosis of HFpEF.
Many patients with HFPEF have normal right and LV end-diastolic pressures at rest, when measured either invasively or non-invasively. In these patients, right heart catheterization measurements of pulmonary artery wedge pressure, cardiac output, and oxygen extraction during exercise are valuable tools in the diagnosis of patients with dyspnea due to HFpEF. During submaximal exercise, patients with HFpEF display pulmonary artery wedge pressures > 25 mmHg or an increase of 7 ± 3 mm Hg above the resting measurement, and pulmonary artery systolic pressures ≥ 45 mmHg in contrast to patients with noncardiac dyspnea[57,58]. With exercise, older patients with HFpEF demonstrate a marked rise in arteriovenous oxygen content difference of 10.8 ± 1.8, driven by enhanced oxygen extraction, and lower increments in cardiac output in comparison with younger patients with HFpEF[5,59]. This is especially true in older women who have small LV chambers and rely on heart rate increases to meet the cardiac output demands of exercise.
Community-based studies show that coronary heart disease (CAD) is common in HFpEF, and is present in 40% to 60% of patients with HFpEF[60-62]. Patients with CAD are more likely to be men and to have CAD risk factors, including hypertension, diabetes, hyperlipidemia, and tobacco use[63]. However, symptoms of angina and heart failure are similar in patients with and without CAD, as are measures of cardiovascular structure, function, and hemodynamics. Nevertheless, HFpEF patients with CAD experience a fourfold greater decline in EF over time compared with patients without CAD[63]. The presence of CAD is associated with worse outcome in HFpEF, which appears to be independent of other predictors. In a study of elderly patients with a mean age of 72 years, patients with HFpEF and CAD have a 1-2-year mortality rate of 20%[63]. In these patients, coronary revascularization is associated with a decrease in mortality and with outcomes that are not different from patients with HFpEF without CAD[62,63]. Consequently, patients with HFpEF should be subcategorized according to the presence or absence of CAD. If significant coronary artery disease is present, the patients should be evaluated for coronary revascularization[62,63].
Patients with HFpEF and major obstructive coronary artery disease should be treated with coronary artery revascularization. In addition, since many hospitalizations and deaths in patients with HFpEF are due to noncardiovascular causes such as chronic obstructive lung, chronic kidney disease, and diabetes, these disorders must be identified early in the clinical course and aggressively treated. In patients with NYHA class II and III heart failure and iron deficiency (ferritin < 100 ng/mL or 100 to 300 ng/mL if transferrin saturation is < 20%), intravenous iron replacement is reasonable to improve functional status and quality of life[46].
Medical treatment in HFpEF patients with non-obstructive coronary artery disease includes weight reduction and control of blood pressure, heart rate, and fluid status. More than 80% of patients with heart failure with HFPEF, are overweight or obese and deconditioned. A caloric restriction diet is feasible and safe in older, obese patients with HFpEFs, and significantly improves patient dyspnea, peak oxygen consumption, and quality of life[64]. Caloric restriction combined with endurance exercise, such as walking exercise for one hour three or more times per week, is additive and the combination can produce a 2.5 mL/kg/min increase in peak oxygen consumption and an increase in exercise capacity[64]. Exercise training, such as can occur with a cardiac rehabilitation program, can also improve the maximum heart rate and the diastolic function of the LV as measured by the echocardiographic ratio of early to late mitral valve filling (E/A ratio) and the LV filling pressure E/e′ ratio[65]. Moreover, a decrease in body weight and an increase in peak oxygen consumption is strongly correlated with a decrease in the systemic biomarkers of inflammation in the body and is primarily attributable to increased peripheral microvascular and skeletal muscle function[66].
Fifty-one percent of patients with HFpEF have hypertension, whereas among patients with HFrEF, hypertension is present in 41%[67]. In a meta-analysis of 123 studies with 613815 hypertensive patients, a 10 mmHg decrease in systolic BP reduced the risk of heart failure complications by 28%, independently of the baseline BP or co-morbidity status[68]. In this regard, diuretic drugs are effective for blood pressure control and for the prevention of volume overload. Analysis from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack (Allhat) Trial found that the diuretic chlorthalidone reduced the risk of HFpEF and the incidence of HFpEF hospitalizations among high-risk hypertensive patients when compared with lisinopril, amlodipine, and doxazosine[69]. For patients whom are persistently hypertensive despite diuretic therapy, renin-angiotensin-aldosterone inhibition with an angiotensin converting enzyme inhibitor or an angiotensin receptor blocker are recommended for reduction in the blood pressure to ≤ 130/80 mmHg[46]. In addition, mineralocorticoid antagonists, such as spironolactone or eplerenone, can play an important role in treatment of patients with HFpEF by limiting aldosterone, which is a volume retention and profibrotic hormone, and blood pressure. In the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial, spironolactone reduced patient hospitalizations for heart failure, reduced the composite end-point of cardiovascular death, heart failure hospitalization, and aborted cardiac arrest in patients from the Americas but not patients from Russia and Georgia[70]. Furthermore, two recent retrospective studies report that the long-term treatment with a mineraloreceptor antagonist, such as spironolactone or eplerenone, and a beta-adrenergic receptor blocker drugs is associated with a reduction in the incidence of new-onset HFpEF in patients with hypertension[9,71,72]. Consequently, the effective control of vascular volume and blood pressure in hypertensive patients can prevent patient progression to symptomatic HFpEF. However, while diuretics and antihpertensive medications can be effective in bringing about symptomatic relief, care must be taken to avoid hypotension and azotemia in patients with HFpEF.
Atrial fibrillation is present in 15% to as many as 41% of patients with HFPEF[73]. The arrhythmia may be associated with increased fatigue and exertional intolerance, natriuretic peptide elevation, left atrial remodeling and an increase in the risk of death[74,75]. The treatment includes identification and treatment of the causes of atrial fibrillation, such as poorly controlled hypertension, obstructive sleep apnea, diabetes mellitus, or thyrotoxicosis. Patients with atrial fibrillation, HFpEF, and sleep apnea shoud undergo a sleep study in order to determine whether the sleep apnea is predominantly obstructive or central in nature and patients with obstructive sleep apnea treated with continuous positive airway pressure[46,76]. Anticoagulation is recommended for patients with a CHA2DS2 VAS score ≥ 2. Beta-adrenergic receptor blocking drugs or non-dihydropyridine calcium channel blocking drugs, such as verapamil or diltiazem, are suggested for rate control. Experience with atrial catheter ablation of atrial fibrillation in patients with HFpEF is limited. In a small, single center study, catheter ablation of atrial fibrillation improved diastolic function in patients who maintained sinus rhythm[77]. Additional investigations of catheter ablation of atrial fibrillation in patients with HFpEF are necessary.
Recent clinical trials of sodium glucose cotransporter-2 inhibitors have shown promising effects on heart failure outcomes in patients with heart failure and diabetes mellitus. In the EMPA-REG Cardiovascular Outcome Event Trial in patients with type 2 diabetes mellitus, the sodium-glucose cotransporter-2 (SGLT2) inhibitor empagliflozin was associated with a reduction in major adverse cardiovascular endpoints and a significant reduction in heart failure hospitalizations[78]. SGLT2 inhibitors, such as empagliflozin, promote gylcosuria and diuresis by reducing glucose and sodium absorption in the proximal renal tubule, without activating the sympathetic nervous system. SGLT2 inhibitors also appear to decrease markers of inflammation[79]. Additional studies of SGLT2 inhibitors in patients with HFpEF and diabetes mellitus are in progress.
In summary, inhibitors of the sympathetic nervous and renin-angiotensin-aldosterone systems should be considered in patients with HFpEF who have coronary artery disease, hypertension, and atrial fibrillation[46]. Sodium glucose cotransporter-2 inhibitors should be considered in patients with HFpEF and type 2 diabetes mellitus[78]. Patients with major obstructive coronary artery disease that contributes to heart failure should be evaluated for coronary artery revascularization[62,63].
The pharmacologic therapy trials for LV dysfunction in patients with HFpEF with beta-adrenergic receptor blockers, calcium channel blocking agents, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and nitrates are not consistent and, in general, have been neutral in decreasing patient mortality. This is, in part, due to differences in trial design and patient population heterogeneity with differences in heart failure etiologies or stages of disease. Recently, patients with LV ejection fractions between 41% and 49% have been categorized as heart failure with mid-range ejection fraction. Patients in this intermediate category match a phenotype that is closer to the clinical profile of HFrEF and have a higher risk of sudden cardiac death and cardiovascular death than patients with HFpEF. Table 6 lists the major pharmacologic studies that have been performed in patients with HFpEF and patients with heart failure with mid-range ejection fraction. Table 6 is adapted, in part, from[80].
| Ref. | Drug vs control | Drug half-life hours | Number patients | Duration | LVEF | Results |
| Beta-blockers | ||||||
| Swedish Heart Failure Registry[81] | All BBs (prescribed at discharge | 6-h Atenolol; 12-19 h Nevibolol | 8244 | 755 d | LVEF 49%-50% and LVEF > 50% | β-blockers decreased mortality but not combined all-cause mortality or hospitalizations |
| SWEDIC Trial[82] | Carvedilol | 6-10 h | 97 | 6 mo | LVEF ≥ 40% | E/A ratio improved but no other measures of diastolic function |
| J-DHF Trial[83] | Carvedilol | 6-10 h | 245 | 38 mo | LVEF ≥ 40% | Standard dose, but not low dose, carvedilol reduced; CV mortality and hospitalizations |
| COHERE Registry[84] | Carvedilol | 6-10 h | 4280 | 12 mo | LVEF > 40% | Carvedilol had no mortality benefit but decreased hospitalization |
| SENIORS[85,86] | Nebivolol | 2.5-20 h | 643 | 21 mo | LVEF > 35% | Nebivolol did not decrease CV hospitalizations or mortality |
| ELANDD Trial[87] | Nebivolol | 2.5-20 h | 116 | 21 mo | LVEF ≥ 45% | Nebivolol did not increase exercise capacity |
| CIBIS-ELD Trial[88] | Bisoprolol vs Carvedilol | 9-12 vs 6-10 h | 250 | 3 mo | LVEF ≥ 45% | Bisoprolol and carvedilol had no effect on established and prognostic markers of diastolic function |
| El-Refai et al[89] | Beta Blocker (bisoprolol, carvedilol, metoprolol, labetalol, and atenolol) | 6-7 h (Atenolol); 9-12 h (Bisoprolol) | 741 | 25 mo | LVEF ≥ 50% | Beta blockers decreased mortality and HF rehospitalizations |
| β-PRESERVE[90] | Metoprolol succinate vs control | 3-9 h | 1200 | 24 mo | LVEF ≥ 50% | Trial Results not available |
| OPTIMIZE-HF[91] | All BBs (prescribed at discharge) | 6-7 h Atenolol 12-19 h Nebivolol | 21149 | 3 mo | LVEF 40%-49% and ≥ 50% | Beta blockers had no effect on mortality and rehospitalization |
| Calcium channel blockers | ||||||
| Setaro et al[92] | Verapamil vs placebo | 4.5-12 h | 20 | 1 mo | LVEF ≥ 45% | Verapamil increased exercise capacity clinicoradio-graphic score. No change in LVEF. |
| Hung et al[93] | Verapamil vs placebo | 4.5-12 h | 15 | 3 mo | Normal LVEF | Verapamil increased exercise time and LV diastolic function |
| ACE inhibitors | ||||||
| Aronow et al[94] | Enalapril vs control (diuretics alone) | 11 h | 21 | 3 mo | LVEF ≥ 50% | Enalapril increased exercise time and LVEF |
| PEP-CHF trial[95] | ACE inhibitor (perindopril) vs placebo | 3-10 h with prolonged terminal elimination | 207 | 12 mo | LVEF ≥ 45% | Perindopril increased 6 min walk distance but did not decrease mortality |
| Angiotensin II receptor blockers | ||||||
| I-PRESERVE[96] | Irbesartan vs placebo | 11-15 h | 4563 | 24 mo | LVEF ≥ 45% | No decrease in hospitalization or mortality |
| CHARM-Preserved[97] | Candesartan vs control medication (ACE Inhibitor, BB, CCB) | 9 h | 3023 | 37 mo | LVEF ≥ 40% | Candesartan slightly decreased hospitalizations but did not decrease mortality |
| Angiotensin receptor blocker/nephrilysin inhibitors | ||||||
| PARAMOUNT Trial[98] | Sacubitril/valsartan vs valsartan | 11.5 h | 301 | 3 and 8-9 mo | LVEF ≥ 45% | Sacubiril Valsartan reduced NT-proBNP |
| PARAGON-HF Governmental Trial NCT01920711 | Sacubitril/valsartan vs valsartan | 11.5 | 4300 | 57 mo | LVEF ≥ 45% | Sacubitril/valsartan not superior to valsartan alone in decreasing hospitalization or cardiovascular mortality |
| Ivabradine | ||||||
| Kosmala et al[99] | Ivabradine vs placebo | 11 h | 61 | 7 d | LVEF ≥ 50% | Ivabradine increased exercise time, peak oxygen uptake, and decreased E/e’ |
| EDIFY trial[100] | Ivabradine vs placebo | 11 h | 179 | 8 mo | LVEF ≥ 45% | No improvement in 6 min walk, E/e’, or NT-proBNP |
| Statins | ||||||
| Fukuta et al[101] | Standard HF therapy with a statin vs without a statin | 2 h (lovastatin)-19 h (rosuvastatin) | 137 | 21 mo | LVEF ≥ 50% | Statin therapy associated with reduced mortality |
| Ouzounian et al[102] | Standard HF therapy with a statin vs without a statin | 2 h (lovastatin)-19 h (rosuvastatin) | 6451 | 38 mo | LVEF ≥ 50% | Statins did not decrease morbidity or mortality in patients with HF without CAD |
| Animal model of heart ailure (rats)[103] | Standard HF therapy with rosuvastatin vs without rosuvastatin | 19 h | 46 | 19 mo | Preserved EF | Statins had no benefit |
| Digoxin | ||||||
| (DIG) trial[104] | Digoxin vs placebo | 36-48 h | 988 | 37 mo | LVEF ≥ 45% | Digoxin had no effect on all-cause and CV mortality, heart failure hospitalizations |
| Phosphodiesterase-5 inhibitors | ||||||
| RELAX trial[105] | Sildenafil vs placebo | 3-4 h | 216 | 24 mo | LVEF ≥ 50% | No improvement in 6 min walk distance, clinical status, or peak O2 consumption |
| Nitrates | ||||||
| NEAT-HFpEF trial[106] | Isosorbide mononitrate vs placebo | 2.5-5.1 h | 110 | 22 mo | LVEF ≥ 50% | No improvement in 6 min walk distance or NT-proBNP |
| INDIE-HFpEF[107,108] | Inhaled inorganic nitrite vs placebo | 0.7 h | 105 | 4 wk | LVEF ≥ 50% | No significant improvement in exercise tolerance, NY Heart Association Class, E/e’, NT-proBNP |
| Governmental trial NCT02840799 | Oral KNO3 vs KCL | 1.2 h | 26 | 1 mo | LVEF ≥ 50% | KNO3 trial is in progress |
Patients with HFpEF and chronotropic incompetence are currently being tested with rate-adaptive atrial pacing (government trial NCT02145351). Patients with HFpEF can have normal resting left atrial pressures but develop marked increases in left atrial pressures and pulmonary hypertension with exercise due to a decrease in LV diastolic compliance. This is typically manifested as exertional dyspnea. Innovative medical devices, listed below, inserted into patients with HFpEF in clinical trials have shown some promise in improving patient symptomatology.
An 8 mm unidirectional interatrial left to right shunt device in patients with HFpEF has been investigated to reduce left atrial pressure by 3 to 11 mmHg and provide symptomatic patient relief from dyspnea that is due to increased pulmonary venous pressure. In a randomized Reduce-Left Atrial Pressure in Heart Failure Trial I in 39 patients, the decrease in pulmonary wedge pressure during exercise and the improvement in workload corrected pulmonary capillary wedge pressure, exercise duration, and peak exercise workload compared to sham controls were numerically better in the treatment group but the differences from controls did not achieve statistical significance[109,110], A larger trial (REDUCE LAP-HF II Governmental Trial NCT03088033) is currently examining the effects of an interatrial septal device on clinical outcomes and quality of life. The long term effects of volume loading with this device and similar devices, such as the V Wave device (Governmental Trial NCT02511912) and the Atrial Flow Regulator (Governmental Trial NCT03030274), on right heart chambers and function, the pulmonary circulation, the cardiac rhythm, and the potential for paradoxical embolism in older patients who typically have HFpEF, require further investigation.
The CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients) Trial was a prospective, randomized controlled trial that investigated whether medical treatment based on daily pulmonary artery pressure monitoring would significantly reduce hospitalizations for heart failure treatment[111]. A 15 mm electromechanical pressure sensor was permanently implanted by right heart catheterization into a branch of the right pulmonary artery of each patient and transmitted pulmonary artery pressures by radio signals to an internet web-based system that notified the patient’s physician if the daily pulmonary pressure measurements were outside of a defined range. After 17.6 mo of follow-up, the hospitalization rate was 50% lower in patients where medical treatment decisions were made based on the pulmonary artery pressure measurements[111]. Hemodynamic-guided management using PA pressure measurements appears to be a successful strategy to improve the outcome of patients with HFpEF. More recently, the HEMODYNAMICALLY GUIDED MANAGEMENT OF HEART FAILURE (Guide-HF Governmental Trial NCT03387813) Trial is examining the effects of pulmonary artery pressure monitoring on patient mortality from heart failure.
For patients with severe refractory HFpEF in whom medical therapies have provided no benefit, mechanical circulatory support is being investigated to decrease LV filling pressures and pulmonary congestion and increase cardiac output. In this regard, a partial mechanical circulatory microdevice, which is inserted with a minimally invasive approach, directs blood from the left atrium into the subclavian artery[112]. This system is reported to decrease pulmonary and left atrial pressures in patients with HFpEF. However, patients with HFpEF have small LV dimensions and further decreases in LV stroke volume may limit patient coronary and cerebral blood flow. Additional investigations are needed to evaluate whether this invasive therapy decreases patient morbidity and mortality.
The specific etiologies of HFpEF, the mechanisms, the clinical manifestations, and the contributions of comorbidities to the morbidity and mortality of HFpEF must be fully identified in patients. Such identifications will be facilitated by the pursuit of clinical registries and large clinical trials that focus on collecting clinical, imaging, laboratory, and outcome data and treatments. Successful pursuit of these goals will ultimately permit the development of specific drugs and medical devices that will decrease the morbidity and mortality of HFpEF in patients.
The American College of Cardiology, the American Heart Association and the Heart Failure Society recommend the following treatments for patients with HFpEF and symptoms and signs of heart failure (adapted from[46,113]): (1) Treatment of hypertension in all HFpEF patients with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers or beta-adrenergic receptor blocking drugs; (2) Treatment of patients with HFpEF with volume overload with diuretics; (3) In patients with HFpEF with increased BNP, creatinine < 2.5 mg/dL, glomerular filtration rate > 30 mL/min and potassium < 5 mEq/L, treatment with an aldosterone antagonist; (4) Control of heart rate with medications in patients with atrial fibrillation; (5) In patients with HfpEF and type 2 diabetes mellitus, treatment with a SGLT-2 inhibitor such as Empaglifozin should be considered; and (6) Treatment of patients with symptomatic obstructive coronary artery disease and myocardial ischemia that contributes to heart failure with coronary revascularization.
Currently, 5.7 million people in the United States have heart failure and require 30.7 billion dollars per year for health care and medications.
50% of patients with heart failure have normal, or near-normal LV systolic heart function but abnormal LV diastolic function with incomplete LV relaxation due to increased myocardial stiffness. These patients have HFpEF.
The most common patients are elderly women with hypertension, ischemic heart disease, atrial fibrillation, obesity, diabetes mellitus, renal disease, or obstructive lung disease.
Patients who are hospitalized because of HFpEF have complication rates that are similar to those patients with HFrEF.
The annual mortality rate of patients is approximately 8% per year but increases to approximately 10%-12% per year among patients older than 70 years of age.
Current hypotheses include: (1) Cardiomyocyte titin hypophosphorylation; (2) Vascular endothelial cell inflammation and dysfunction; (3) Abnormal calcium homeostasis; (4) Increased ventricular matrix formation; and (5) Obesity.
The diagnosis is based on the patient’s history and physical examination with the assistance of laboratory data, echocardiography, and, when necessary, by cardiac catheterization.
A scoring system facilitates the diagnosis of HFpEF in patient with dyspnea and distinguish these patients from patients with non-cardiac causes of dyspnea.
In patients with HFpEF, the myocardial extracellular matrix can be determined with CMR T1 mapping and is a predictor of LV myocardial stiffness, LV diastolic dysfunction, and patient outcome.
Since many hospitalizations and deaths in patients with HFpEF are due to non- cardiovascular causes such as chronic obstructive lung, chronic kidney disease, and diabetes, these disorders must be identified early and aggressively treated.
Patients with obesity, hypertension, and atrial fibrillation require weight reduction, an exercise program, and aggressive control of blood pressure and heart rate.
Patients with volume overload should be treated with diuretics.
Pharmacologic therapy trials with beta-adrenergic receptor blockers, calcium channel blocking agents, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and nitrates have, in general, been neutral in decreasing patient mortality.
Medical treatment based on pulmonary artery pressure monitoring with a permanently implanted right pulmonary artery microsensor significantly reduces hospitalizations for treatment of heart failure. A clinical trial is currently examining the effects on patient mortality from heart failure.
If significant coronary artery disease is present and contributes to heart failure, the patient with HFpEF should be evaluated for coronary revascularization.
| 1. | Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67-e492. [PubMed] |
| 2. | Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2162] [Cited by in RCA: 2312] [Article Influence: 154.1] [Reference Citation Analysis (0)] |
| 3. | van Heerebeek L, Paulus WJ. Understanding heart failure with preserved ejection fraction: where are we today? Neth Heart J. 2016;24:227-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Dock W. How some hearts age. JAMA. 1966;195:442-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Nanayakkara S, Patel HC, Kaye DM. Hospitalisation in Patients With Heart Failure With Preserved Ejection Fraction. Clin Med Insights Cardiol. 2018;12:1179546817751609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254-2262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 613] [Article Influence: 29.2] [Reference Citation Analysis (8)] |
| 7. | Gazewood JD, Turner PL. Heart Failure with Preserved Ejection Fraction: Diagnosis and Management. Am Fam Physician. 2017;96:582-588. [PubMed] |
| 8. | Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070-3077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 579] [Cited by in RCA: 577] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 9. | Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1449] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 10. | Rogers FJ, Gundala T, Ramos JE, Serajian A. Heart Failure With Preserved Ejection Fraction. J Am Osteopath Assoc. 2015;115:432-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Quiroz R, Doros G, Shaw P, Liang CS, Gauthier DF, Sam F. Comparison of characteristics and outcomes of patients with heart failure preserved ejection fraction versus reduced left ventricular ejection fraction in an urban cohort. Am J Cardiol. 2014;113:691-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105:1387-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 748] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 13. | Sherazi S, Zaręba W. Diastolic heart failure: predictors of mortality. Cardiol J. 2011;18:222-232. [PubMed] |
| 14. | Doury P. Autochthonous anguilluliasis in France. Bull Soc Pathol Exot. 1993;86:116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Krittanawong C, Yue B, Ul H, Virk H, Zhang HJ, Haider S, Kitai T, Tang W. A Contemporary Analysis of Predictors of Mortality in Patients With Heart Failure and Preserved Ejection Fraction From the National Inpatient sample. Circulation. 2018;136:A12243. |
| 16. | Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1116] [Cited by in RCA: 1128] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 17. | Westermann D, Kasner M, Steendijk P, Spillmann F, Riad A, Weitmann K, Hoffmann W, Poller W, Pauschinger M, Schultheiss HP, Tschöpe C. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117:2051-2060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 364] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 18. | Lewis GA, Schelbert EB, Williams SG, Cunnington C, Ahmed F, McDonagh TA, Miller CA. Biological Phenotypes of Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2017;70:2186-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 19. | Linke WA, Hamdani N. Gigantic business: titin properties and function through thick and thin. Circ Res. 2014;114:1052-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 20. | Borbély A, Falcao-Pires I, van Heerebeek L, Hamdani N, Edes I, Gavina C, Leite-Moreira AF, Bronzwaer JG, Papp Z, van der Velden J, Stienen GJ, Paulus WJ. Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104:780-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 279] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 21. | van Heerebeek L, Hamdani N, Falcão-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW, Paulus WJ. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 415] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 22. | Collier P, Watson CJ, Voon V, Phelan D, Jan A, Mak G, Martos R, Baugh JA, Ledwidge MT, McDonald KM. Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? Eur J Heart Fail. 2011;13:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 23. | Shah KB, Kop WJ, Christenson RH, Diercks DB, Henderson S, Hanson K, Li SY, deFilippi CR. Prognostic utility of ST2 in patients with acute dyspnea and preserved left ventricular ejection fraction. Clin Chem. 2011;57:874-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Matsubara J, Sugiyama S, Nozaki T, Sugamura K, Konishi M, Ohba K, Matsuzawa Y, Akiyama E, Yamamoto E, Sakamoto K, Nagayoshi Y, Kaikita K, Sumida H, Kim-Mitsuyama S, Ogawa H. Pentraxin 3 is a new inflammatory marker correlated with left ventricular diastolic dysfunction and heart failure with normal ejection fraction. J Am Coll Cardiol. 2011;57:861-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Zuo L, Chuang CC, Hemmelgarn BT, Best TM. Heart failure with preserved ejection fraction: Defining the function of ROS and NO. J Appl Physiol (1985). 2015;119:944-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Runte KE, Bell SP, Selby DE, Häußler TN, Ashikaga T, LeWinter MM, Palmer BM, Meyer M. Relaxation and the Role of Calcium in Isolated Contracting Myocardium From Patients With Hypertensive Heart Disease and Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2017;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 27. | Selby DE, Palmer BM, LeWinter MM, Meyer M. Tachycardia-induced diastolic dysfunction and resting tone in myocardium from patients with a normal ejection fraction. J Am Coll Cardiol. 2011;58:147-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Maier LS, Layug B, Karwatowska-Prokopczuk E, Belardinelli L, Lee S, Sander J, Lang C, Wachter R, Edelmann F, Hasenfuss G, Jacobshagen C. RAnoLazIne for the treatment of diastolic heart failure in patients with preserved ejection fraction: the RALI-DHF proof-of-concept study. JACC Heart Fail. 2013;1:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 29. | Borbély A, van der Velden J, Papp Z, Bronzwaer JG, Edes I, Stienen GJ, Paulus WJ. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 413] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 30. | Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, Bradshaw AD, Slater R, Palmer BM, Van Buren P, Meyer M, Redfield MM, Bull DA, Granzier HL, LeWinter MM. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation. 2015;131:1247-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 546] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 31. | Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 717] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 32. | Kapur NK. Transforming growth factor-β: governing the transition from inflammation to fibrosis in heart failure with preserved left ventricular function. Circ Heart Fail. 2011;4:5-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Schelbert EB, Fonarow GC, Bonow RO, Butler J, Gheorghiade M. Therapeutic targets in heart failure: refocusing on the myocardial interstitium. J Am Coll Cardiol. 2014;63:2188-2198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 34. | Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM. Sex Differences in Cardiovascular Pathophysiology: Why Women Are Overrepresented in Heart Failure With Preserved Ejection Fraction. Circulation. 2018;138:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 370] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 35. | Zeng H, Chen JX. Microvascular Rarefaction and Heart Failure With Preserved Ejection Fraction. Front Cardiovasc Med. 2019;6:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 36. | Packer M. Derangements in adrenergic-adipokine signalling establish a neurohormonal basis for obesity-related heart failure with a preserved ejection fraction. Eur J Heart Fail. 2018;20:873-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 37. | Martínez-Martínez E, Jurado-López R, Valero-Muñoz M, Bartolomé MV, Ballesteros S, Luaces M, Briones AM, López-Andrés N, Miana M, Cachofeiro V. Leptin induces cardiac fibrosis through galectin-3, mTOR and oxidative stress: potential role in obesity. J Hypertens. 2014;32:1104-14; discussion 1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 38. | Tanaka S, Momose Y, Tsutsui M, Kishida T, Kuroda J, Shibata N, Yoshida T, Yamagishi R. Quantitative estimation of myocardial fibrosis based on receptor occupancy for beta2-adrenergic receptor agonists in rats. J Toxicol Sci. 2004;29:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Miura SI, Suematsu Y, Matsuo Y, Tomita S, Nakayama A, Goto M, Arimura T, Kuwano T, Yahiro E, Saku K. The angiotensin II type 1 receptor-neprilysin inhibitor LCZ696 blocked aldosterone synthesis in a human adrenocortical cell line. Hypertens Res. 2016;39:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Lijnen P, Petrov V. Induction of cardiac fibrosis by aldosterone. J Mol Cell Cardiol. 2000;32:865-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 191] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 41. | Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14:591-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 1096] [Article Influence: 121.8] [Reference Citation Analysis (0)] |
| 42. | Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation. 2013;128:1085-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 295] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 43. | Iwanaga Y, Nishi I, Furuichi S, Noguchi T, Sase K, Kihara Y, Goto Y, Nonogi H. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol. 2006;47:742-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 404] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 44. | Grewal J, McKelvie RS, Persson H, Tait P, Carlsson J, Swedberg K, Ostergren J, Lonn E. Usefulness of N-terminal pro-brain natriuretic Peptide and brain natriuretic peptide to predict cardiovascular outcomes in patients with heart failure and preserved left ventricular ejection fraction. Am J Cardiol. 2008;102:733-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Zile MR, Baicu CF. Biomarkers of diastolic dysfunction and myocardial fibrosis: application to heart failure with a preserved ejection fraction. J Cardiovasc Transl Res. 2013;6:501-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 46. | Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail. 2017;23:628-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 454] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 47. | Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2879] [Cited by in RCA: 4098] [Article Influence: 409.8] [Reference Citation Analysis (9)] |
| 48. | Mordi IR, Singh S, Rudd A, Srinivasan J, Frenneaux M, Tzemos N, Dawson DK. Comprehensive Echocardiographic and Cardiac Magnetic Resonance Evaluation Differentiates Among Heart Failure With Preserved Ejection Fraction Patients, Hypertensive Patients, and Healthy Control Subjects. JACC Cardiovasc Imaging. 2018;11:577-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 49. | Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539-2550. [PubMed] [DOI] [Full Text] |
| 50. | Mitter SS, Shah SJ, Thomas JD. A Test in Context: E/A and E/e' to Assess Diastolic Dysfunction and LV Filling Pressure. J Am Coll Cardiol. 2017;69:1451-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 283] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 51. | Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE; I-PRESERVE Investigators. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491-2501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 408] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 52. | Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure With Preserved Ejection Fraction. Circulation. 2018;138:861-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 872] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 53. | Mascherbauer J, Marzluf BA, Tufaro C, Pfaffenberger S, Graf A, Wexberg P, Panzenböck A, Jakowitsch J, Bangert C, Laimer D, Schreiber C, Karakus G, Hülsmann M, Pacher R, Lang IM, Maurer G, Bonderman D. Cardiac magnetic resonance postcontrast T1 time is associated with outcome in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging. 2013;6:1056-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 54. | Su MY, Lin LY, Tseng YH, Chang CC, Wu CK, Lin JL, Tseng WY. CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. JACC Cardiovasc Imaging. 2014;7:991-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 55. | Rommel KP, von Roeder M, Latuscynski K, Oberueck C, Blazek S, Fengler K, Besler C, Sandri M, Lücke C, Gutberlet M, Linke A, Schuler G, Lurz P. Extracellular Volume Fraction for Characterization of Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol. 2016;67:1815-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 56. | Schelbert EB, Fridman Y, Wong TC, Abu Daya H, Piehler KM, Kadakkal A, Miller CA, Ugander M, Maanja M, Kellman P, Shah DJ, Abebe KZ, Simon MA, Quarta G, Senni M, Butler J, Diez J, Redfield MM, Gheorghiade M. Temporal Relation Between Myocardial Fibrosis and Heart Failure With Preserved Ejection Fraction: Association With Baseline Disease Severity and Subsequent Outcome. JAMA Cardiol. 2017;2:995-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 183] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 57. | Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588-595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 913] [Cited by in RCA: 875] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 58. | Nanayakkara S, Haykowsky M, Mariani J, Van Empel V, Maeder MT, Vizi D, Kaye DM. Hemodynamic Profile of Patients With Heart Failure and Preserved Ejection Fraction Vary by Age. J Am Heart Assoc. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 374] [Cited by in RCA: 360] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 60. | Trevisan L, Cautela J, Resseguier N, Laine M, Arques S, Pinto J, Orabona M, Barraud J, Peyrol M, Paganelli F, Bonello L, Thuny F. Prevalence and characteristics of coronary artery disease in heart failure with preserved and mid-range ejection fractions: A systematic angiography approach. Arch Cardiovasc Dis. 2018;111:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Choudhury L, Gheorghiade M, Bonow RO. Coronary artery disease in patients with heart failure and preserved systolic function. Am J Cardiol. 2002;89:719-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Pfeffer MA, Shah AM, Borlaug BA. Heart Failure With Preserved Ejection Fraction In Perspective. Circ Res. 2019;124:1598-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 610] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 63. | Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:2817-2827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 64. | Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA. 2016;315:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 699] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 65. | Chan E, Giallauria F, Vigorito C, Smart NA. Exercise training in heart failure patients with preserved ejection fraction: a systematic review and meta-analysis. Monaldi Arch Chest Dis. 2016;86:759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 66. | Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 282] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 67. | Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012;33:1750-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 548] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 68. | Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2732] [Cited by in RCA: 2490] [Article Influence: 249.0] [Reference Citation Analysis (1)] |
| 69. | Collicott PE. 1991: an "intense" year for the house of medicine. Nebr Med J. 1991;76:17-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 70. | Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1656] [Cited by in RCA: 2063] [Article Influence: 171.9] [Reference Citation Analysis (0)] |
| 71. | Gu J, Fan YQ, Han ZH, Fan L, Bian L, Zhang HL, Xu ZJ, Yin ZF, Xie YS, Zhang JF, Wang CQ. Association between long-term prescription of aldosterone antagonist and the progression of heart failure with preserved ejection fraction in hypertensive patients. Int J Cardiol. 2016;220:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | Gu J, Fan YQ, Bian L, Zhang HL, Xu ZJ, Zhang Y, Chen QZ, Yin ZF, Xie YS, Wang CQ. Long-term prescription of beta-blocker delays the progression of heart failure with preserved ejection fraction in patients with hypertension: A retrospective observational cohort study. Eur J Prev Cardiol. 2016;23:1421-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 73. | Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M. Heart Failure With Preserved Ejection Fraction and Atrial Fibrillation: Vicious Twins. J Am Coll Cardiol. 2016;68:2217-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 363] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 74. | Lam CS, Rienstra M, Tay WT, Liu LC, Hummel YM, van der Meer P, de Boer RA, Van Gelder IC, van Veldhuisen DJ, Voors AA, Hoendermis ES. Atrial Fibrillation in Heart Failure With Preserved Ejection Fraction: Association With Exercise Capacity, Left Ventricular Filling Pressures, Natriuretic Peptides, and Left Atrial Volume. JACC Heart Fail. 2017;5:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 75. | Mamas MA, Caldwell JC, Chacko S, Garratt CJ, Fath-Ordoubadi F, Neyses L. A meta-analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur J Heart Fail. 2009;11:676-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 312] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 76. | Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JGF, de Boer RA, Drexel H, Ben Gal T, Hill L, Jaarsma T, Jankowska EA, Anker MS, Lainscak M, Lewis BS, McDonagh T, Metra M, Milicic D, Mullens W, Piepoli MF, Rosano G, Ruschitzka F, Volterrani M, Voors AA, Filippatos G, Coats AJS. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21:1169-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 77. | Machino-Ohtsuka T, Seo Y, Ishizu T, Sugano A, Atsumi A, Yamamoto M, Kawamura R, Machino T, Kuroki K, Yamasaki H, Igarashi M, Sekiguchi Y, Aonuma K. Efficacy, safety, and outcomes of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013;62:1857-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 78. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7124] [Cited by in RCA: 8705] [Article Influence: 791.4] [Reference Citation Analysis (2)] |
| 79. | Butler J, Hamo CE, Filippatos G, Pocock SJ, Bernstein RA, Brueckmann M, Cheung AK, George JT, Green JB, Januzzi JL, Kaul S, Lam CSP, Lip GYH, Marx N, McCullough PA, Mehta CR, Ponikowski P, Rosenstock J, Sattar N, Salsali A, Scirica BM, Shah SJ, Tsutsui H, Verma S, Wanner C, Woerle HJ, Zannad F, Anker SD; EMPEROR Trials Program. The potential role and rationale for treatment of heart failure with sodium-glucose co-transporter 2 inhibitors. Eur J Heart Fail. 2017;19:1390-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 80. | Krittanawong C, Kukin ML. Current Management and Future Directions of Heart Failure With Preserved Ejection Fraction: a Contemporary Review. Curr Treat Options Cardiovasc Med. 2018;20:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 81. | Lund LH, Benson L, Dahlström U, Edner M, Friberg L. Association between use of β-blockers and outcomes in patients with heart failure and preserved ejection fraction. JAMA. 2014;312:2008-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 82. | Bergström A, Andersson B, Edner M, Nylander E, Persson H, Dahlström U. Effect of carvedilol on diastolic function in patients with diastolic heart failure and preserved systolic function. Results of the Swedish Doppler-echocardiographic study (SWEDIC). Eur J Heart Fail. 2004;6:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 163] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 83. | Yamamoto K, Origasa H, Hori M; J-DHF Investigators. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure Study (J-DHF). Eur J Heart Fail. 2013;15:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 280] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 84. | Massie BM, Nelson JJ, Lukas MA, Greenberg B, Fowler MB, Gilbert EM, Abraham WT, Lottes SR, Franciosa JA; COHERE Participant Physicians. Comparison of outcomes and usefulness of carvedilol across a spectrum of left ventricular ejection fractions in patients with heart failure in clinical practice. Am J Cardiol. 2007;99:1263-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 85. | Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, Soler-Soler J, Tavazzi L, Spinarova L, Toman J, Böhm M, Anker SD, Thompson SG, Poole-Wilson PA; SENIORS Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1208] [Cited by in RCA: 1042] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 86. | van Veldhuisen DJ, Cohen-Solal A, Böhm M, Anker SD, Babalis D, Roughton M, Coats AJ, Poole-Wilson PA, Flather MD; SENIORS Investigators. Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: Data From SENIORS (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure). J Am Coll Cardiol. 2009;53:2150-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 396] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 87. | Conraads VM, Metra M, Kamp O, De Keulenaer GW, Pieske B, Zamorano J, Vardas PE, Böhm M, Dei Cas L. Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: results of the ELANDD study. Eur J Heart Fail. 2012;14:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 88. | Edelmann F, Musial-Bright L, Gelbrich G, Trippel T, Radenovic S, Wachter R, Inkrot S, Loncar G, Tahirovic E, Celic V, Veskovic J, Zdravkovic M, Lainscak M, Apostolović S, Neskovic AN, Pieske B, Düngen HD; CIBIS-ELD Investigators and Project Multicenter Trials in the Competence Network Heart Failure. Tolerability and Feasibility of Beta-Blocker Titration in HFpEF Versus HFrEF: Insights From the CIBIS-ELD Trial. JACC Heart Fail. 2016;4:140-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 89. | El-Refai M, Peterson EL, Wells K, Swadia T, Sabbah HN, Spertus JA, Williams LK, Lanfear DE. Comparison of β-blocker effectiveness in heart failure patients with preserved ejection fraction versus those with reduced ejection fraction. J Card Fail. 2013;19:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 90. | Zhou J, Shi H, Zhang J, Lu Y, Fu M, Ge J; beta-PRESERVE Study Investigators. Rationale and design of the beta-blocker in heart failure with normal left ventricular ejection fraction (beta-PRESERVE) study. Eur J Heart Fail. 2010;12:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 91. | Hernandez AF, Hammill BG, O'Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. J Am Coll Cardiol. 2009;53:184-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 249] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 92. | Setaro JF, Zaret BL, Schulman DS, Black HR, Soufer R. Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic filling and normal left ventricular systolic performance. Am J Cardiol. 1990;66:981-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 284] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 93. | Hung MJ, Cherng WJ, Kuo LT, Wang CH. Effect of verapamil in elderly patients with left ventricular diastolic dysfunction as a cause of congestive heart failure. Int J Clin Pract. 2002;56:57-62. [PubMed] |
| 94. | Aronow WS, Kronzon I. Effect of enalapril on congestive heart failure treated with diuretics in elderly patients with prior myocardial infarction and normal left ventricular ejection fraction. Am J Cardiol. 1993;71:602-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 159] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 95. | Cleland JG, Tendera M, Adamus J, Freemantle N, Gray CS, Lye M, O'Mahony D, Polonski L, Taylor J. Perindopril for elderly people with chronic heart failure: the PEP-CHF study. The PEP investigators. Eur J Heart Fail. 1999;1:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 96. | Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A; I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456-2467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1395] [Cited by in RCA: 1471] [Article Influence: 81.7] [Reference Citation Analysis (0)] |
| 97. | Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2143] [Cited by in RCA: 2138] [Article Influence: 93.0] [Reference Citation Analysis (6)] |
| 98. | Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ; Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 913] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 99. | Kosmala W, Holland DJ, Rojek A, Wright L, Przewlocka-Kosmala M, Marwick TH. Effect of If-channel inhibition on hemodynamic status and exercise tolerance in heart failure with preserved ejection fraction: a randomized trial. J Am Coll Cardiol. 2013;62:1330-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 100. | Komajda M, Isnard R, Cohen-Solal A, Metra M, Pieske B, Ponikowski P, Voors AA, Dominjon F, Henon-Goburdhun C, Pannaux M, Böhm M; prEserveD left ventricular ejectIon fraction chronic heart Failure with ivabradine studY (EDIFY) Investigators. Effect of ivabradine in patients with heart failure with preserved ejection fraction: the EDIFY randomized placebo-controlled trial. Eur J Heart Fail. 2017;19:1495-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 101. | Fukuta H, Sane DC, Brucks S, Little WC. Statin therapy may be associated with lower mortality in patients with diastolic heart failure: a preliminary report. Circulation. 2005;112:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 213] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 102. | Ouzounian M, Tu JV, Austin PC, Chong A, Liu PP, Lee DS. Statin therapy and clinical outcomes in heart failure: a propensity-matched analysis. J Card Fail. 2009;15:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 103. | Gómez-Garre D, González-Rubio ML, Muñoz-Pacheco P, Caro-Vadillo A, Aragoncillo P, Fernández-Cruz A. Rosuvastatin added to standard heart failure therapy improves cardiac remodelling in heart failure rats with preserved ejection fraction. Eur J Heart Fail. 2010;12:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 104. | Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, Love TE, Aronow WS, Adams KF, Gheorghiade M. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114:397-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 482] [Cited by in RCA: 445] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 105. | Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E; RELAX Trial. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 959] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 106. | Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WH, McNulty SE, Velazquez EJ, Shah MR, Braunwald E; NHLBI Heart Failure Clinical Research Network. Isosorbide Mononitrate in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2015;373:2314-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 451] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 107. | Borlaug BA, Anstrom KJ, Lewis GD, Shah SJ, Levine JA, Koepp GA, Givertz MM, Felker GM, LeWinter MM, Mann DL, Margulies KB, Smith AL, Tang WHW, Whellan DJ, Chen HH, Davila-Roman VG, McNulty S, Desvigne-Nickens P, Hernandez AF, Braunwald E, Redfield MM; National Heart, Lung, and Blood Institute Heart Failure Clinical Research Network. Effect of Inorganic Nitrite vs Placebo on Exercise Capacity Among Patients With Heart Failure With Preserved Ejection Fraction: The INDIE-HFpEF Randomized Clinical Trial. JAMA. 2018;320:1764-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 108. | Borlaug BA, Melenovsky V, Koepp KE. Inhaled Sodium Nitrite Improves Rest and Exercise Hemodynamics in Heart Failure With Preserved Ejection Fraction. Circ Res. 2016;119:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 109. | Feldman T, Mauri L, Kahwash R, Litwin S, Ricciardi MJ, van der Harst P, Penicka M, Fail PS, Kaye DM, Petrie MC, Basuray A, Hummel SL, Forde-McLean R, Nielsen CD, Lilly S, Massaro JM, Burkhoff D, Shah SJ; REDUCE LAP-HF I Investigators and Study Coordinators. Transcatheter Interatrial Shunt Device for the Treatment of Heart Failure With Preserved Ejection Fraction (REDUCE LAP-HF I [Reduce Elevated Left Atrial Pressure in Patients With Heart Failure]): A Phase 2, Randomized, Sham-Controlled Trial. Circulation. 2018;137:364-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 211] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 110. | Feldman T. A Transcatheter InterAtrial Shunt Device for the Treatment of Heart Failure With Preserved or Mid-Range Ejection Fraction (REDUCE LAP-HF I): 1-Year Results from the Phase 2, Randomized, Blinded, Sham-Controlled Trial. Paper presented at: The European Society of Cardiology; 2018; Aug 27; Munich, Germany. |
| 111. | Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, Henderson J, Cowart P, Stevenson LW. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7:935-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 326] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 112. | Burkhoff D, Maurer MS, Joseph SM, Rogers JG, Birati EY, Rame JE, Shah SJ. Left atrial decompression pump for severe heart failure with preserved ejection fraction: theoretical and clinical considerations. JACC Heart Fail. 2015;3:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 113. | van der Meer P, Gaggin HK, Dec GW. ACC/AHA Versus ESC Guidelines on Heart Failure: JACC Guideline Comparison. J Am Coll Cardiol. 2019;73:2756-2768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 173] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Nurzynska D, Ueda H S-Editor: Dou Y L-Editor: A E-Editor: Zhang YL