Published online Jan 26, 2020. doi: 10.4330/wjc.v12.i1.55
Peer-review started: May 14, 2019
First decision: June 6, 2019
Revised: August 28, 2019
Accepted: October 14, 2019
Article in press: October 15, 2019
Published online: January 26, 2020
Processing time: 227 Days and 15.1 Hours
Phrenic nerve (PN) injury is one of the recognized possible complications following epicardial ablation of ventricular tachycardia (VT). High-output pacing is a widely used maneuver to establish a relationship between the PN and the ablation catheter tip. An absence of PN capture is usually considered an indication that it is safe to ablate, and that successful ablation may be performed at adjacent sites. However, PN capture may impact the procedural outcome. Only a few cases have been reported in the literature that avoid PN injury by using different techniques.
Three patients with a previous history of myocarditis and one patient with ischemic cardiomyopathy underwent epicardial ablation for drug-refractory VT. Before the procedure, transthoracic echocardiogram, coronary angiogram, and cardiac magnetic resonance imaging were performed on all patients. Under general anesthesia, endo/epicardial three-dimensional anatomical and substrate maps of the left ventricle were accomplished. Before radiofrequency delivery, the course of the PN was identified by provoking diaphragmatic stimulation with high-output pacing from the distal electrode of the ablation catheter. In every case, a scar region with late potentials was mapped along the PN course. After obtaining another epicardial access, a second introducer sheath was placed, and a vascular balloon catheter was inserted into the epicardial space and inflated with saline solution to separate the PN from the epicardium. Once the absence of PN capture had been proven, radiofrequency was applied to aim for complete late potential elimination and avoid VT induction.
PN injury can occur as one of the complications following epicardial VT ablation procedures, and may prevent successful ablation of these arrhythmias. PN displacement by using large balloon catheters into the epicardial space seems to be feasible and reproducible, avoid procedure-related morbidity, and improve ablation success when performed in selected centers and by experienced operators.
Core tip: Epicardial ventricular tachycardia ablation procedures are constantly increasing in number. Among the complications potentially carried by this approach, Phrenic nerve (PN) injury can be prevented using certain precautions. However, when ablation is at risk of being unsuccessful due to PN proximity, there are some helpful tips and tricks available. We herein present a case series of epicardial ablation of ventricular tachycardia, in which PN displacement was necessary to successfully eliminate the arrhythmia. This case series highlights the importance of an accurate definition of PN course, and reports upon the feasibility of PN displacement through use of a vascular balloon placed into the epicardial space.
- Citation: Conti S, Bonomo V, Taormina A, Giordano U, Sgarito G. Phrenic nerve displacement by intrapericardial balloon inflation during epicardial ablation of ventricular tachycardia: Four case reports. World J Cardiol 2020; 12(1): 55-66
- URL: https://www.wjgnet.com/1949-8462/full/v12/i1/55.htm
- DOI: https://dx.doi.org/10.4330/wjc.v12.i1.55
Phrenic nerve (PN) injury is one of the recognized possible complications following catheter ablation procedures. Although uncommon, PN injury can result in permanent paralysis of the diaphragm. The symptoms of diaphragmatic palsies can vary from asymptomatic to cough, dyspnea, recurrent pneumonia, and severe respiratory dysfunction requiring mechanical ventilation. The severity of clinical presentation primarily depends upon the degree of PN injury and underlying lung capacity. PN injury has been reported after endocardial catheter ablation of atrial fibrillation[1], atrial tachycardia[2], inappropriate sinus tachycardia[3], left-sided accessory pathways[4], and epicardial ablation of ventricular tachycardia (VT)[5-7]. In particular, the increasing number of epicardial VT ablations[8] could increase the risk of PN injury, given that the left PN is in direct contact with the epicardial surface. The course of the PN is usually determined by eliciting diaphragmatic contraction through high-voltage output pacing, and which can be viewed using one of the available 3D electroanatomical mapping (3D-EAM) systems[9]. Different techniques for avoiding PN injury have been reported, including introduction of air and/or saline into the pericardial space, or introduction of a deflectable sheath as well as different types of large balloons[10]. We have reported our single-center experience of epicardial VT ablation procedures, where the use of a valvuloplasty balloon catheter successfully prevented PN injury.
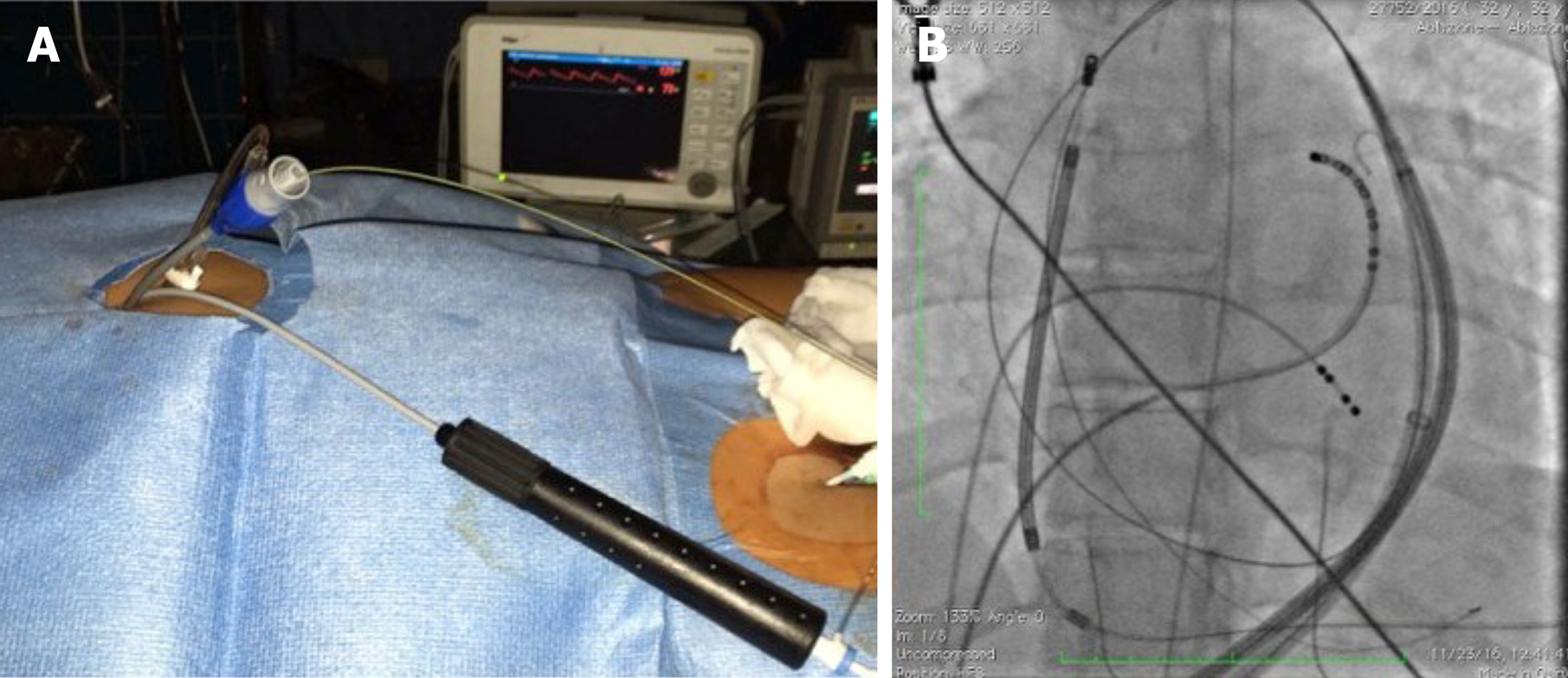
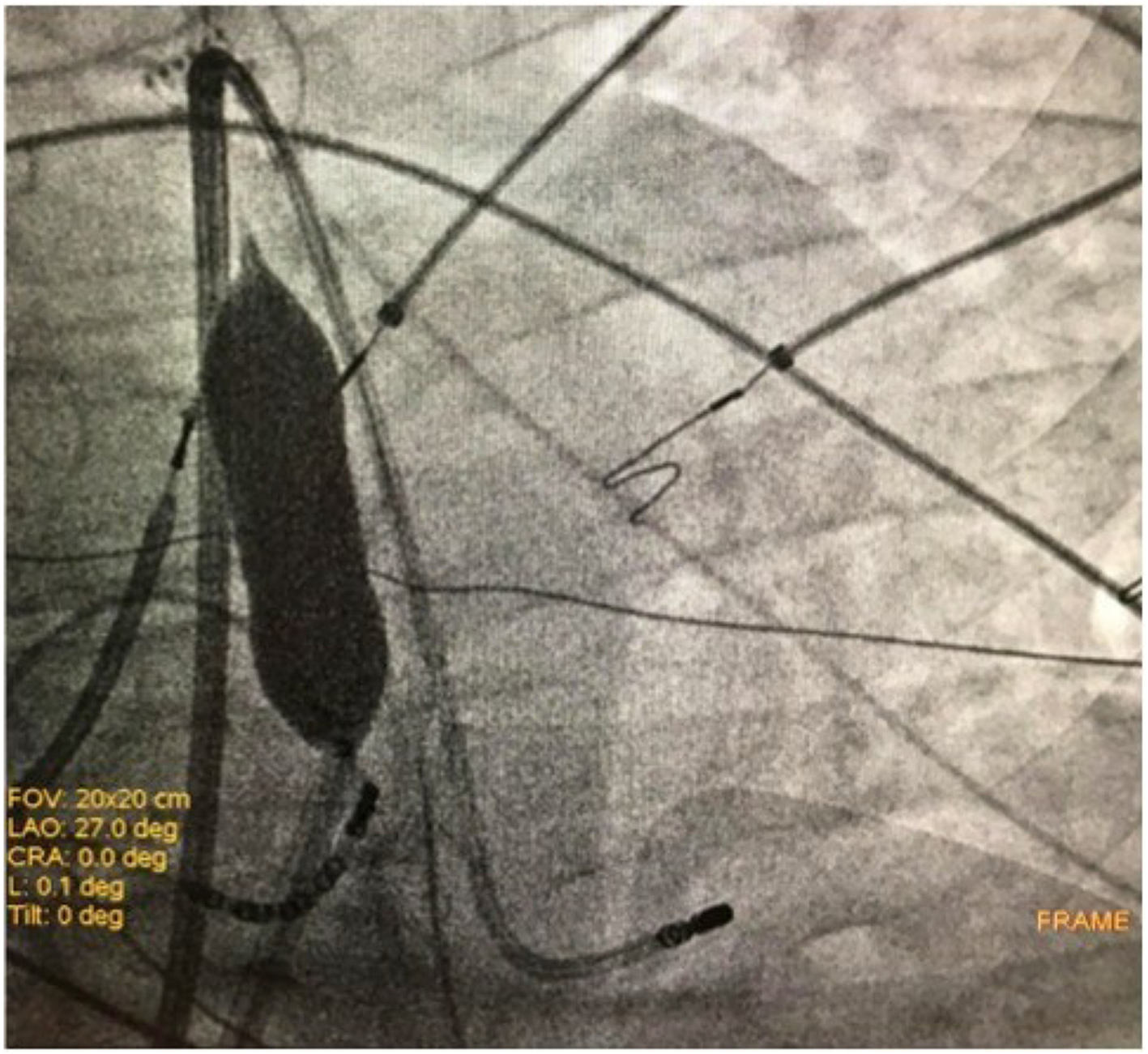
After informed consent was obtained, three patients underwent epicardial ablation for drug-refractory VT under general anesthesia. Intracardiac catheters were inserted via the right and left femoral veins, and included a 6F decapolar catheter placed into the coronary sinus, a 6F quadripolar catheter placed into the right ventricular apex, a standard multipolar or a high-definition mapping catheter (LiveWire 2-2-2 or Advisor HD Grid, Abbott, Medical, United States), an ablation catheter, and an intracardiac echo probe (AcuNav, Siemens). Invasive blood pressure was monitored from the right femoral artery. The EnSite PrecisionTM electroanatomical mapping system (Abbott Medical, United States) was used to create endo/epicardial geometries, substrates, and activation maps. Epicardial access was obtained by subxiphoid pericardial puncture, as described elsewhere[11]. Once an epicardial map was obtained and the area of interest identified, a coronary angiogram was performed upon all patients in order to show the safety distance between the coronary arteries and the scar region. Then, in order to prevent PN injury, the course of the PN was identified by provoking diaphragmatic stimulation with high-output pacing from the distal electrode of the ablation catheter. The locations of PN capture were marked on the 3D-EAM. Considering the close relationship between the area of interest and the PN, we decided to displace the PN in order to increase its distance from the ablation catheter. We then obtained another epicardial access in order to insert a 12F introducer sheath with a hydrophilic coating (Sentrant, Medtronic) (Figure 1A and B). In every case, an 18 mm × 40 mm percutaneous transluminal balloon aortic and pulmonic valvuloplasty catheter (Nucleus-X, Braun Medical) was inserted and inflated with saline solution to separate the PN from the epicardium (Figure 2A and B). Finally, high-output pacing was repeated to reassess PN capture. Radiofrequency (RF) was delivered using a 3.5 mm open irrigated tip ablation catheter (FlexAbility SE, Abbott Medical, United States) and an Agilis EPI steerable epicardial sheath (Abbott Medical, United States). The energy setting was 40-50 w, 43 °C maximum temperature, 17 mL/min ablation catheter flow rate. Successful RF ablation was defined as the complete elimination of all LPs, and the inability to induce VTs with programmed stimulation. A final remap was performed to assess the complete elimination of LPs. After completion of RF delivery, the valvuloplasty balloon catheter was deflated and removed. PN capture was tested after ablation. Following the procedure, the absence of fluid in the pericardial space was confirmed by the intracardiac echo.
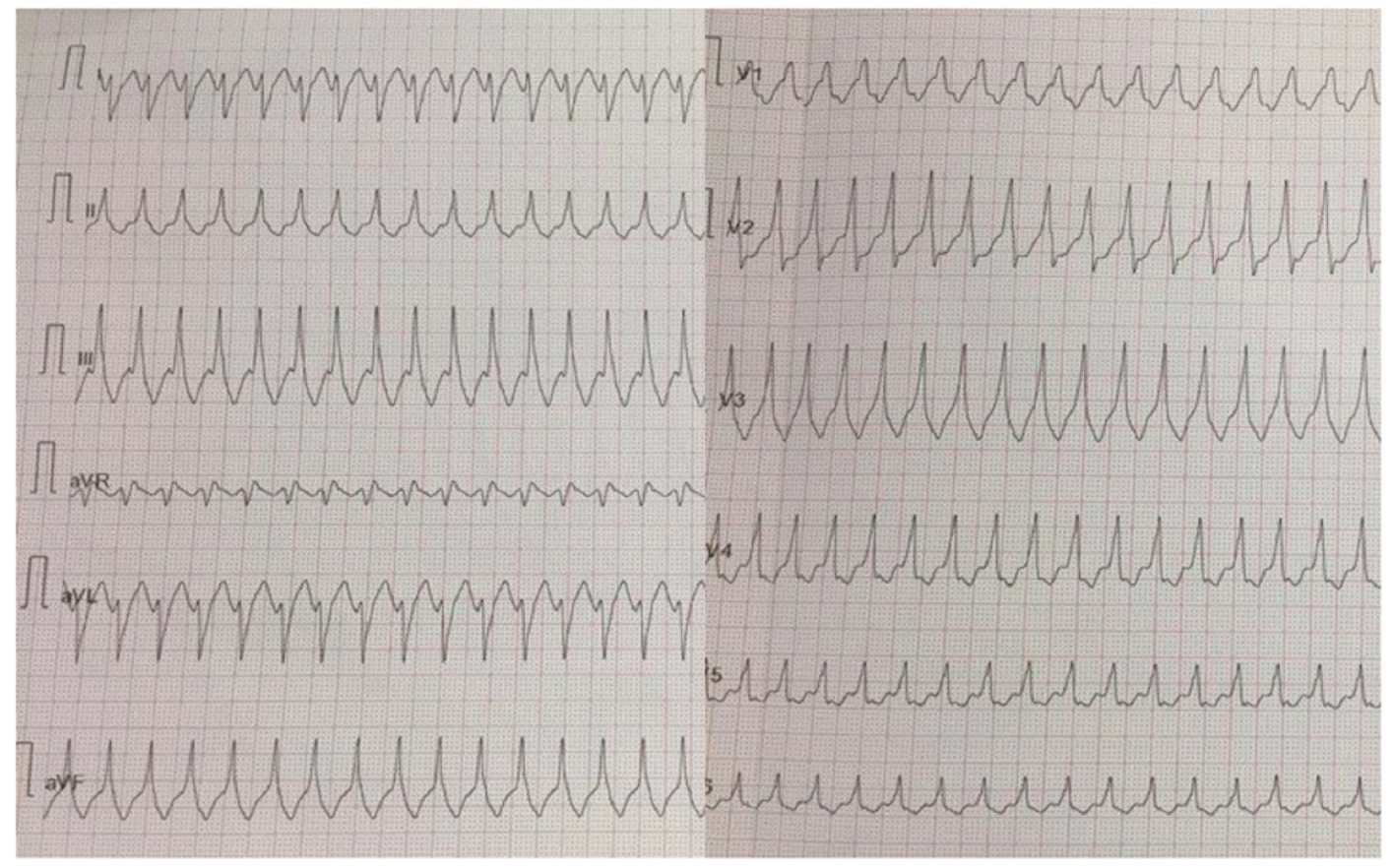
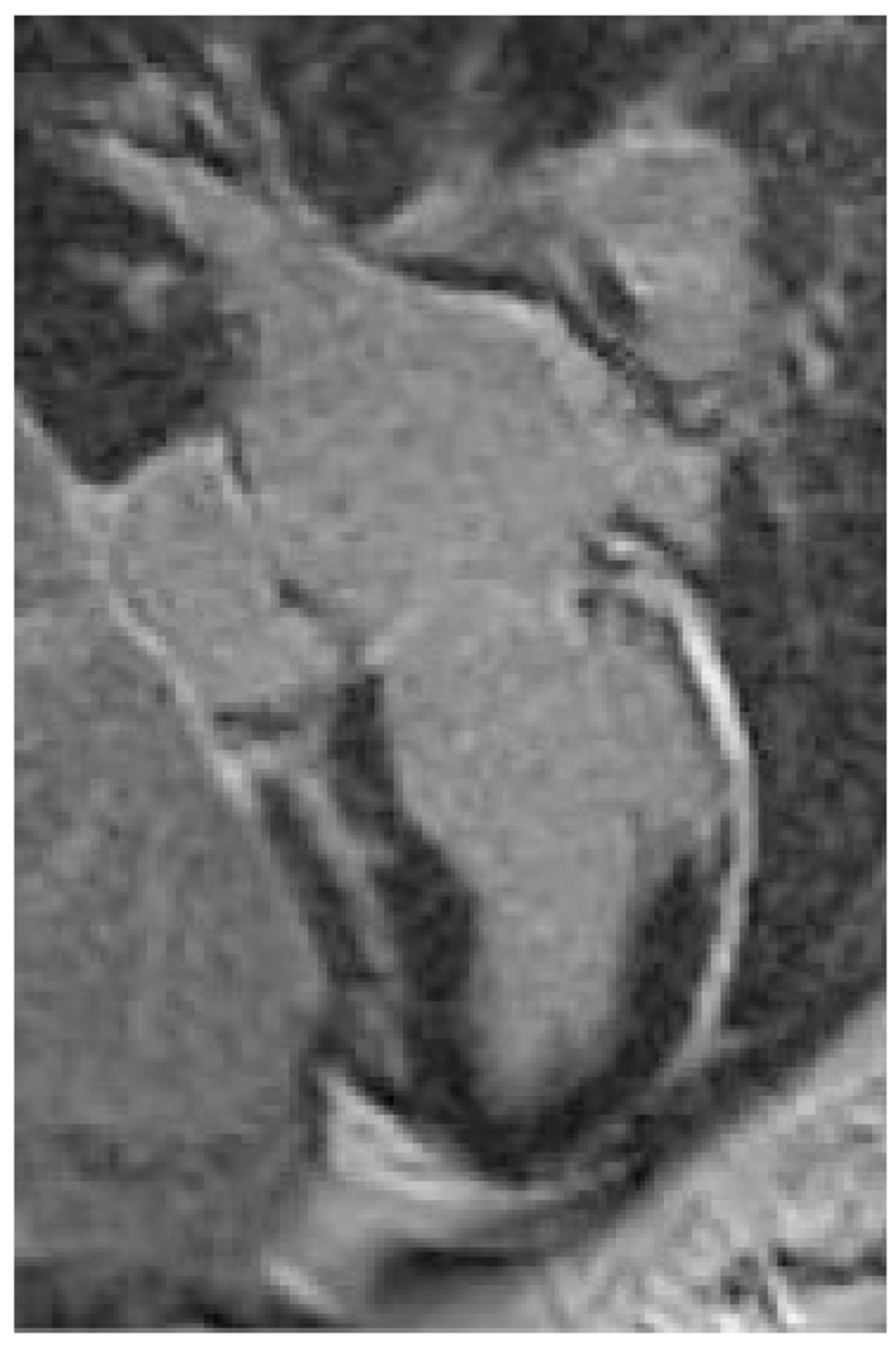
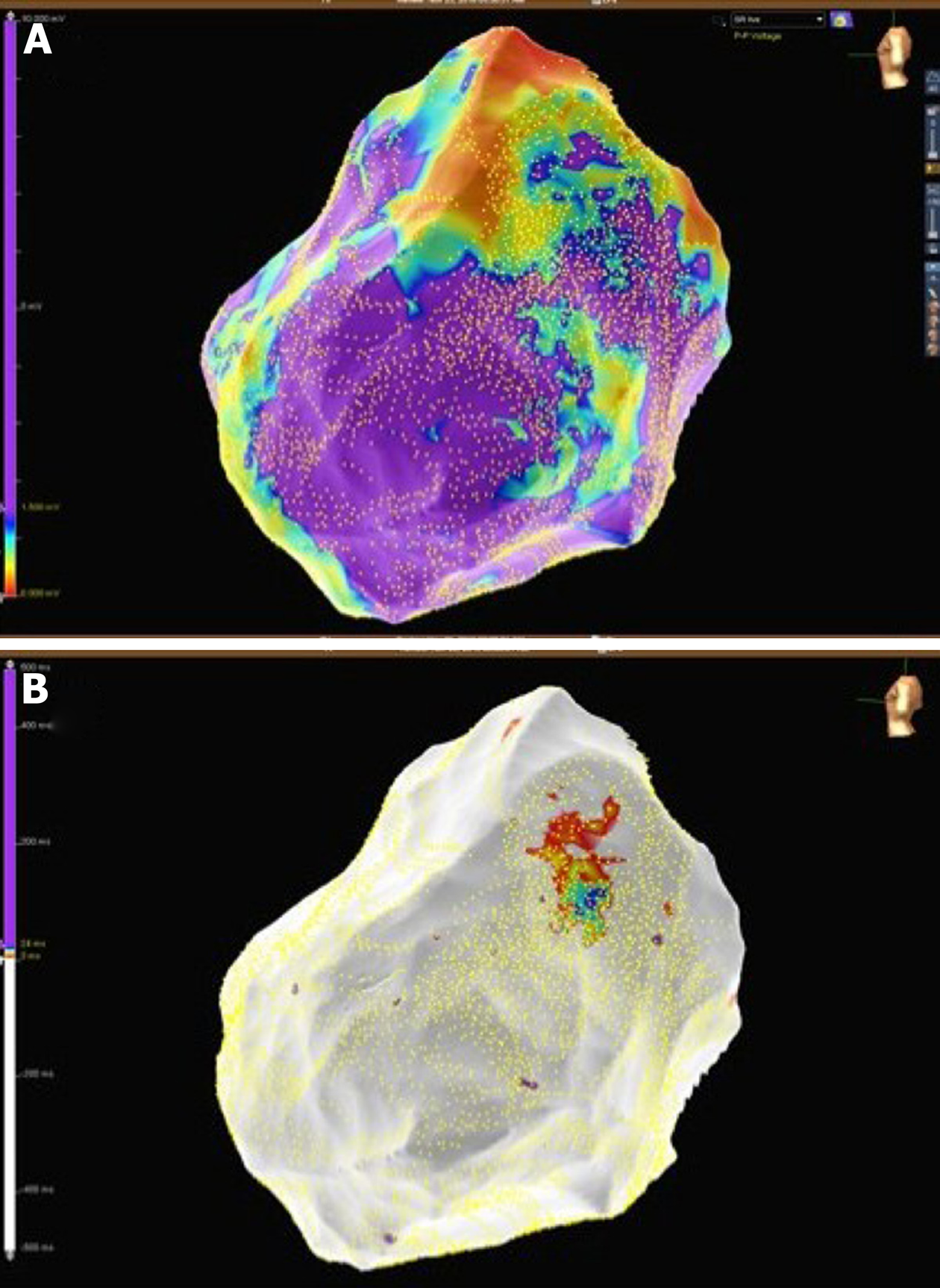
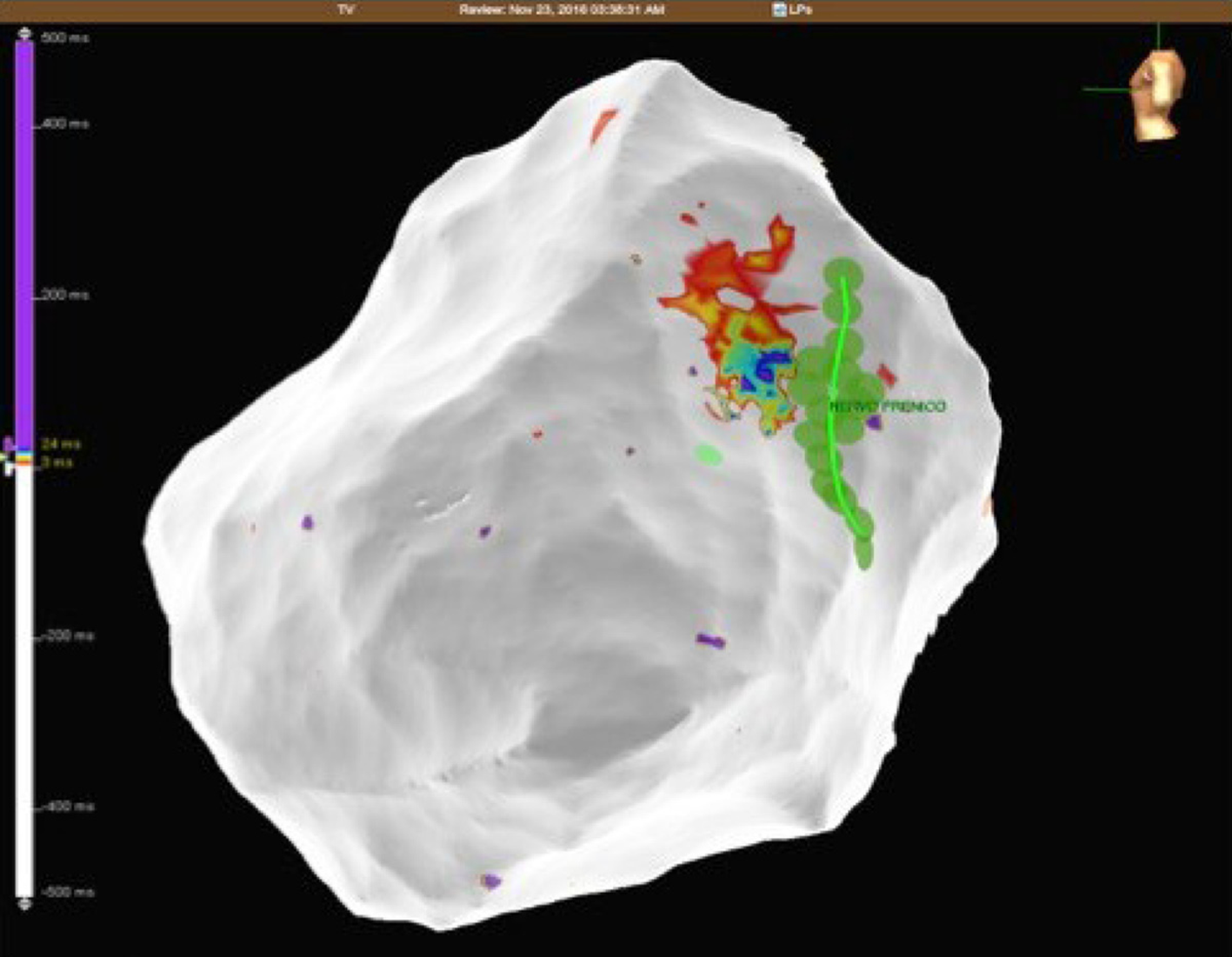
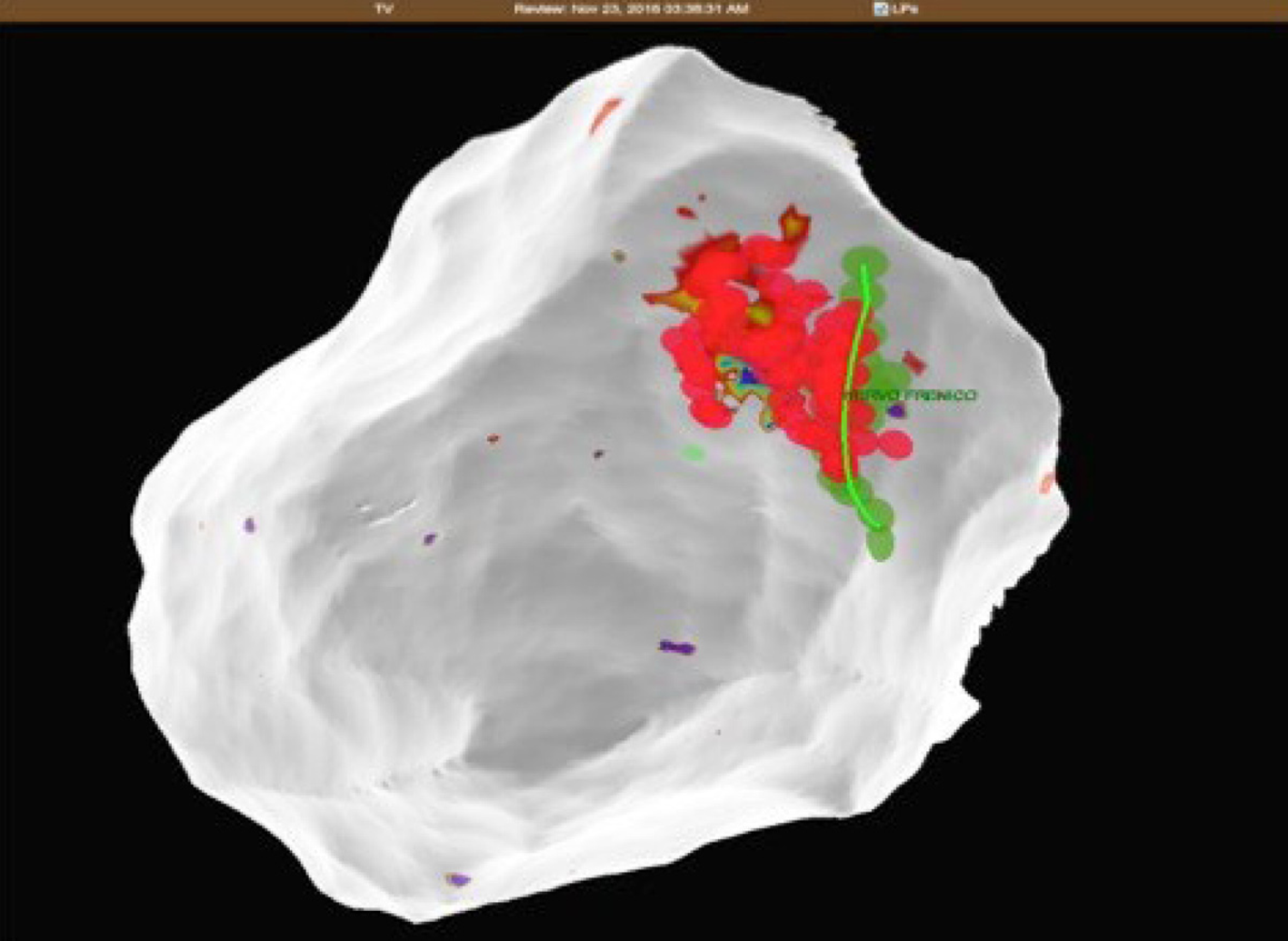
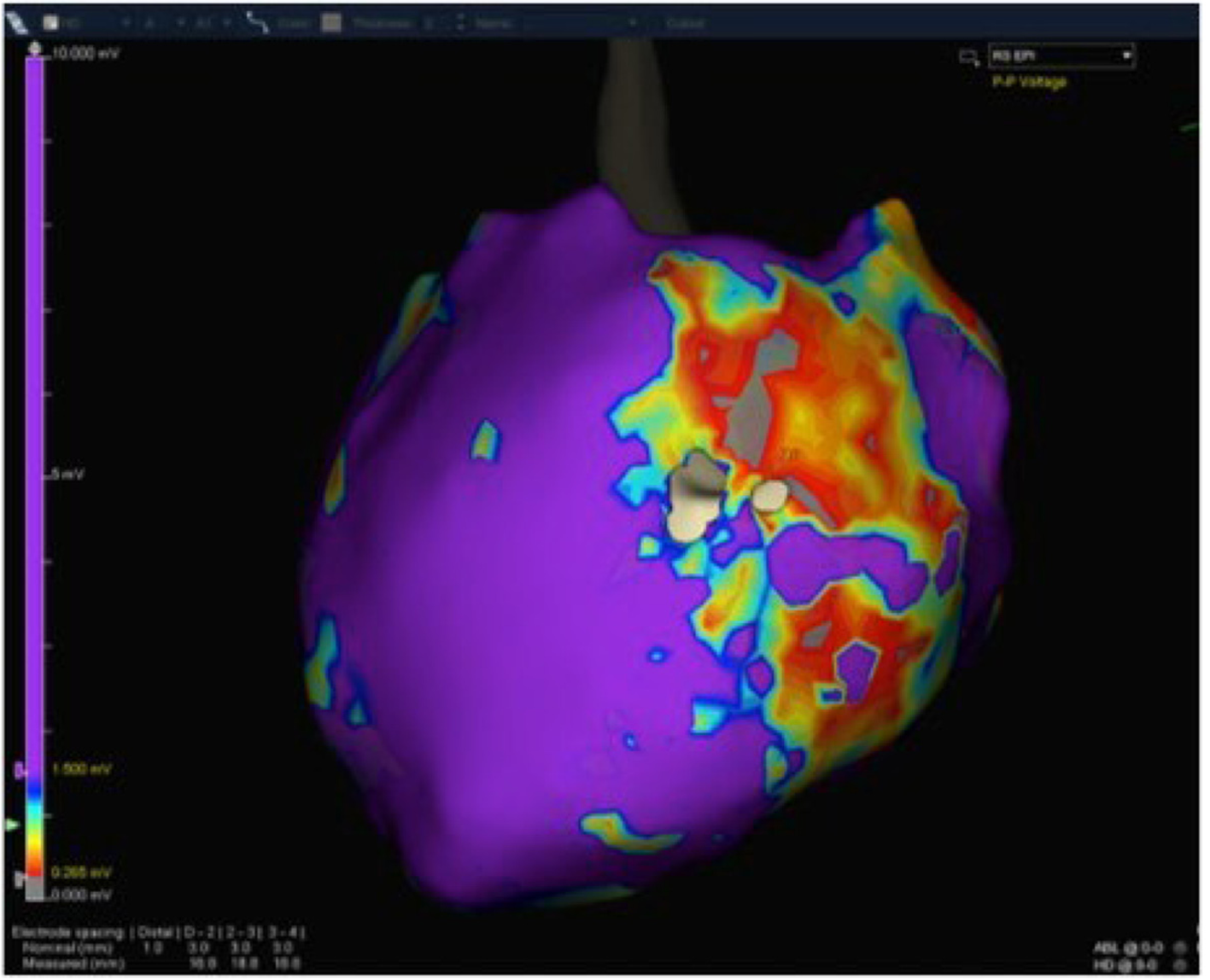
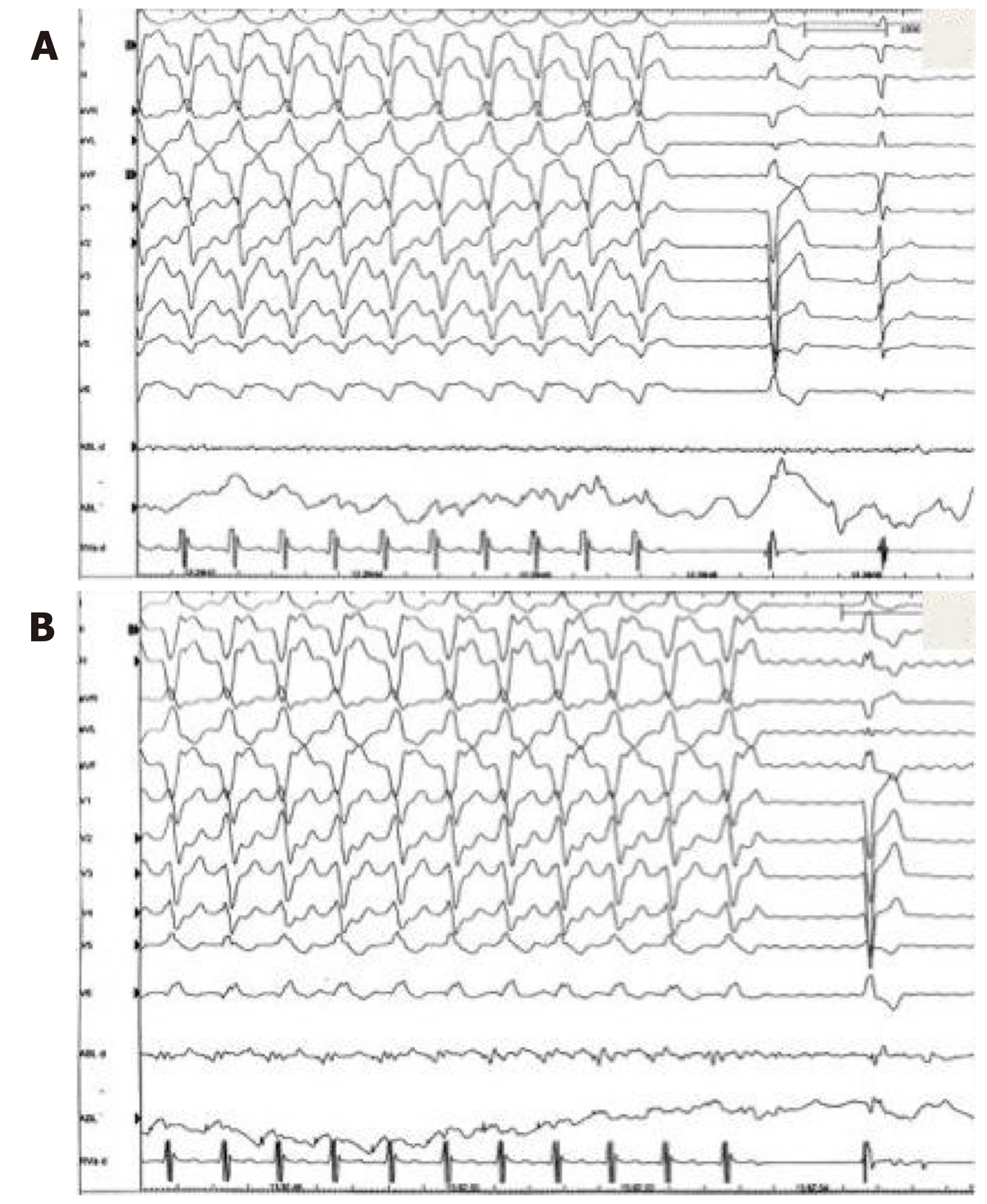
A 32-year-old woman with a prior history of myocarditis and a subcutaneous implantable cardioverter defibrillator (S-ICD) presented at the Emergency Department of our hospital after three syncope and S-ICD shocks. The 12-lead ECG showed a VT with a CL of 230 ms and right bundle inferior axis (RBIA) morphology (Figure 3). Echocardiogram showed a normal LV ejection fraction (LVEF) of 55%. Coronary angiogram showed no significant coronary artery disease. Cardiac magnetic resonance imaging (MRI) showed an LVEF of 50%. Moreover, MRI showed intramural and transmural late-gadolinium enhancement (LGE) in the basal anterolateral LV wall and sub-epicardial LGE in the mid-basal lateral wall (Figure 4). Electrophysiological (EP) study with 3D-EAM of the epicardium was performed as described above. In concordance with the MRI findings, we found a mid-basal lateral epicardial scar, and LPs were identified within the epicardial scar (Figure 5A and B). After the angiogram, high-output pacing was performed to assess PN capture. The scar region was along the PN course (Figure 6). Through the second epicardial access, we inserted another sheath and the valvuloplasty balloon in the epicardial space. Once the balloon was inflated in the epicardial space, high-output pacing was repeated without PN capture. RF was delivered along the scar region, aiming for complete LP elimination with no complications (Figure 7). Once the lesion set was considered complete, the valvuloplasty balloon was deflated and removed. Programmed ventricular stimulation was performed up to three extrastimuli without induction of ventricular arrhythmia. No VT were reported to have recurred within the 2 years of follow-up.
A 56-year-old woman with no previous cardiac history presented to our Centre with a hemodynamically-tolerated VT with an RBIA morphology and CL 320 ms (Figure 8). The VT was converted to sinus rhythm after an intravenous bolus of amiodarone. Transthoracic echocardiogram and coronary angiogram were both normal. However, the MRI showed a subepicardial scar in the basal lateral-posterolateral LV wall with LGE, which was compatible with myocarditis. During the EP study, the clinical VT was induced by programmed ventricular stimulation. At first, an endocardial ablation was attempted in the basal posterolateral LV, but it was unsuccessful. Therefore, we obtained epicardial access and, in concordance with the MRI findings, we found a basal posterolateral epicardial scar with LPs (Figure 9). As per the protocol, we performed a coronary angiogram that showed enough distance between the coronary arteries and the scar. We observed PN capture along the scar, and LPs were located along its course. Following the same procedural steps, we inserted the second epicardial sheath and the balloon facing the PN. Once the balloon was inflated, an absence of PN was noted and all LPs could be targeted and eliminated. After successful RF delivery, the balloon was deflated and removed. Programmed ventricular stimulation demonstrated no acute VT inducibility, and no VT recurrences have been documented within the 1-year follow-up.
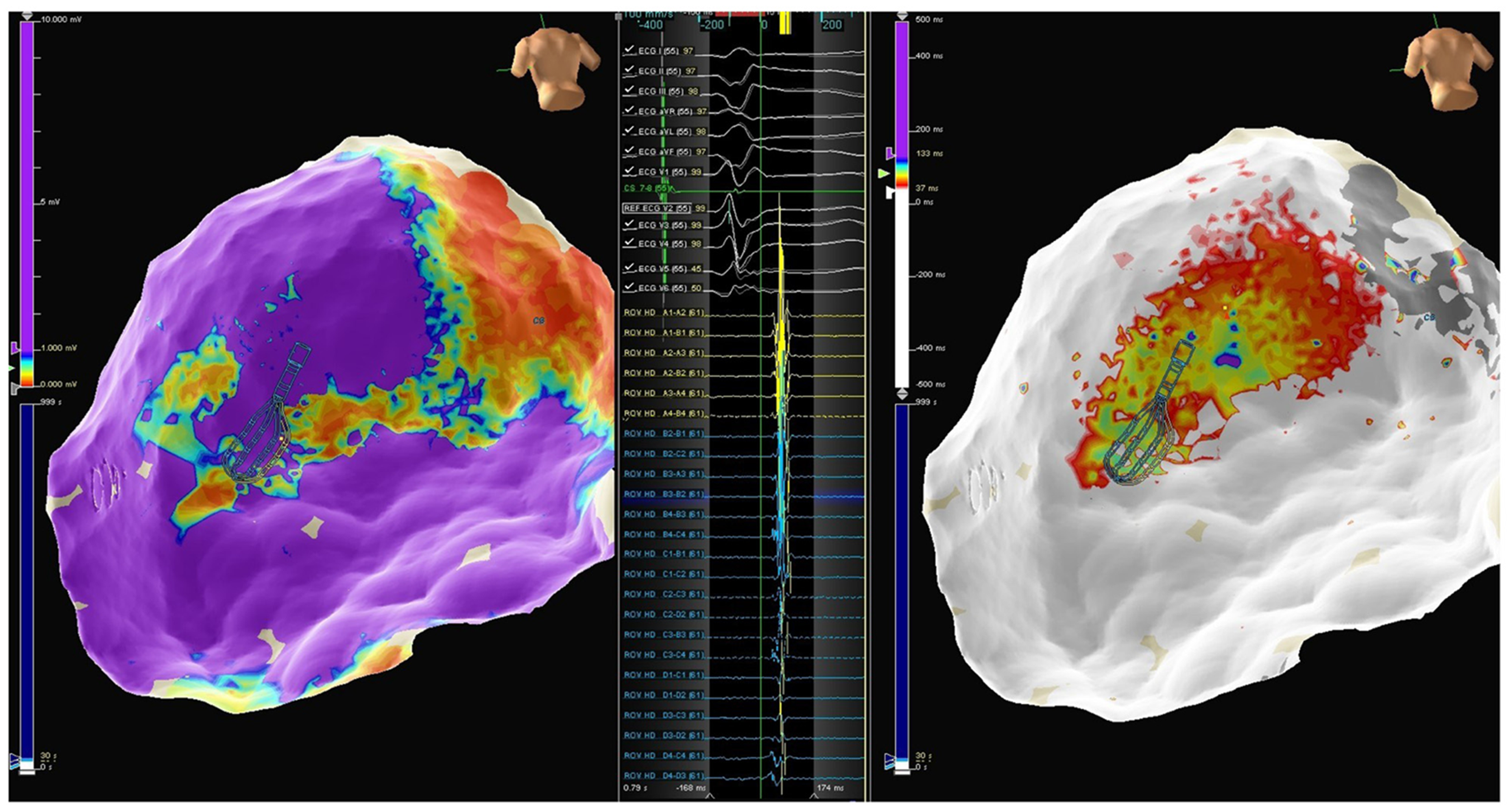
A 46-year-old man with a previous history of myocarditis and implantable cardioverter defibrillator implantation was admitted to our Emergency Department because of intense palpitations lasting up to 2 h. The 12-lead ECG showed a hemodynamically-tolerated VT with an RBIA morphology and CL 300 ms. After ineffective intravenous amiodarone, VT was converted to sinus rhythm through the use of a 200J DC shock. Echocardiography showed an LVEF of 50%, and a coronary angiogram showed normal coronary arteries. Cardiac MRI showed a subepicardial scar in the basal lateral LV wall with sub-epicardial LGE in the mid-basal anterolateral wall. An endo-epicardial substrate map revealed a scar in the mid-basal lateral and posterolateral LV wall, with LPs along the border zone of the mid-basal posterolateral scar and along the mid-basal lateral wall (Figure 10). PN capture was documented along the scar and in the LP region. Once again, after obtaining secondary epicardial access, the balloon was inserted and positioned to avoid PN capture, and RF was delivered along the entire extension of the scar (Figure 11). The final remap showed no more signs of LPs, and programmed ventricular stimulation showed no VT inducibility. The patient had not reported any VT recurrences at the time of the 9-mo follow-up.
A 66-year-old man with a previous history of ischemic cardiomyopathy, implantable cardioverter defibrillator, and previous endocardial VT ablation was admitted to our Centre because of several ICD therapies noted during the remote monitoring follow-up. The 12-lead ECG showed normal sinus rhythm. Echocardiography showed an LVEF of 30%, and a coronary angiogram did not show new coronary artery lesions. An epicardial substrate map revealed a scar in the infero-postero-lateral mid-basal LV wall, with LPs along the border zone of the scar (Figure 12). PN capture was documented along the scar and in the LP region. The same approach was used to avoid PN injury (Figure 13). After obtaining secondary epicardial access, the balloon was inserted and positioned in order to avoid PN capture. Two slightly different morphologies of VT were induced by ventricular programmed stimulation, and RF was delivered with VT termination (Figure 14 and 15). The final remap showed no more LPs, and programmed ventricular stimulation showed no VT inducibility. The patient had not reported any VT recurrences at the time of the 3-mo follow-up.
PN injury is a recognized complication of catheter ablation procedures, and has been reported following ablation of atrial, supraventricular, and VT and with multiple ablation modalities including RF, cryoballoon ablation, and LASER[1-7]. Particularly notable is the left PN that runs along the lateral LV wall before inserting in the diaphragm[12]. Due to the left PN course and the increasing number of epicardial VT ablations, it will not be uncommon to deal with the potential risk of PN injury in the future. In order to avoid PN complications, a common precaution is to establish the proximity of the ablation catheter tip in relation to the PN course. This is typically performed using high-output pacing, and includes 3D tags into the EAM in order to create a visual representation of the distance between the tip of the ablation catheter and the PN course. However, ablation in an area close to the PN is sometimes necessary, and different techniques have been described to avoid PN injury, such as the introduction of air and/or saline into the pericardial space, or introduction of a second deflectable sheath and different types of large balloons. Table 1 summarizes all cases reported in the literature of epicardial VT ablation, in which PN was displaced using various types of large balloons in the pericardial space. Buch et al[13] described the first case in 2007 in a 78-year-old patient with ischemic cardiomyopathy and drug-refractory VT originating from the epicardial posterosuperior aspect of the LV. They used an 18 mm × 40 mm dilatation catheter (Meditech, Boston Scientific) positioned close to the ablation tip obtaining PN displacement without complication[13]. Biase et al[10] reported a multicenter prospective comparison between methods for separating the PN from the epicardial surface. Interestingly, of the eight patients involved, the authors reported two cases of failure to prevent PN capture, and three cases of unsuccessful placement of the intrapericardial balloon. They found controlled “hydro-pneumopericardium” to be the best strategy for preventing PN injury during epicardial ablation[10]. Kumar and colleagues reported the largest series. Five patients with nonischemic cardiomyopathy underwent epicardial VT ablation and effective PN displacement by using the vascular or the gastrointestinal balloon. The authors reported two complications among these patients, including a case of pleuro-pericardial fistula and moderate pericarditis, which was resolved after colchicine administration[14]. One of the limitations of intrapericardial balloon placement is the inability to steer the balloon catheter, and the lack of support and stability. Fan et al[5] found that in order to overcome these limitations, the use of a steerable outer sheath to guide balloon placement was necessary for additional support and stability[5]. In this case series, the use of intrapericardial balloon placement was safely and successfully used to increase the distance between the PN and the ablation target area, thus eliminating PN capture with high-output pacing.
| Author | Number of cases | Procedure | Cardiomyopathy | Device used | Complications | Outcome |
| Buch E et al[13] | 1 | VT | ICM | 18 mm × 40 mm (Meditech, Boston Scientific) | None | Arrhythmia-free at 10 mo |
| Biase et al[10] | 2 | VT | 1 ICM – 1 NICM | 25 mm × 40 mm (NA) | None | No VT at 9 ± 3 mo |
| Kumar et al[14] | 5 | VT | 5 NICM | 18 mm × 20 mm (NMT, Boston Scientific); 18-20 mm esophageal balloon (Hercules 3, Cook) | 1 pleuro-pericardial fistula and pericarditis | No VT recurrence at median follow-up 13 mo |
| Fan et al[5] | 1 | VT | NICM | 18 mm × 60 mm (Braun Medical) | None | No acute VT inducibility |
PN injury can occur as one of the complications following epicardial VT ablation procedures, and may prevent successful ablation of these arrhythmias. Although few cases have been reported in the literature, PN displacement through the use of different types of large balloon catheters in the epicardial space to separate the PN from the ventricular epicardium during epicardial VT ablation seems to be feasible and reproducible, can help to avoid procedure-related morbidity, and can improve ablation success when performed in selected Centers and by experienced operators.
Special thanks for support to Michela Grande, BEng, Abbott, Milan, Italy.
| 1. | Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, Chen PS, Chen SA, Chung MK, Nielsen JC, Curtis AB, Davies DW, Day JD, d'Avila A, de Groot NMSN, Di Biase L, Duytschaever M, Edgerton JR, Ellenbogen KA, Ellinor PT, Ernst S, Fenelon G, Gerstenfeld EP, Haines DE, Haissaguerre M, Helm RH, Hylek E, Jackman WM, Jalife J, Kalman JM, Kautzner J, Kottkamp H, Kuck KH, Kumagai K, Lee R, Lewalter T, Lindsay BD, Macle L, Mansour M, Marchlinski FE, Michaud GF, Nakagawa H, Natale A, Nattel S, Okumura K, Packer D, Pokushalov E, Reynolds MR, Sanders P, Scanavacca M, Schilling R, Tondo C, Tsao HM, Verma A, Wilber DJ, Yamane T. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275-e444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1684] [Cited by in RCA: 1625] [Article Influence: 180.6] [Reference Citation Analysis (0)] |
| 2. | Lee JC, Steven D, Roberts-Thomson KC, Raymond JM, Stevenson WG, Tedrow UB. Atrial tachycardias adjacent to the phrenic nerve: recognition, potential problems, and solutions. Heart Rhythm. 2009;6:1186–1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Durante-Mangoni E, Vecchio DD, Ruggiero G. Right diaphragm paralysis following cardiac radiofrequency catheter ablation for inappropriate sinus tachycardia. Pacing. Clin Electrophysiol. 2003;26:783-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Rumbak MJ, Chokshi SK, Abel N, Abel W, Kittusamy PK, McCormack J, Patel MM, Fontanet H. Left phrenic nerve paresis complicating catheter radiofrequency ablation for Wolff-Parkinson-White syndrome. Am Heart J. 1996;132:1281–1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Fan R, Cano O, Ho SY, Bala R, Callans DJ, Dixit S, Garcia F, Gerstenfeld EP, Hutchinson M, Lin D, Riley M, Marchlinski FE. Characterization of the phrenic nerve course within the epicardial substrate of patients with nonischemic cardiomyopathy and ventricular tachycardia. Heart Rhythm. 2009;6:59–64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Killu AM, Friedman PA, Mulpuru SK, Munger TM, Packer DL, Asirvatham SJ. Atypical complications encountered with epicardial electrophysiological procedures. Heart Rhythm. 2013;10:1613–21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Bai R, Patel D, Di Biase L, Fahmy TS, Kozeluhova M, Prasad S, Schweikert R, Cummings J, Saliba W, Andrews-Williams M, Themistoclakis S, Bonso A, Rossillo A, Raviele A, Schmitt C, Karch M, Uriarte JA, Tchou P, Arruda M, Natale A. Phrenic nerve injury after catheter ablation: should we worry about this complication? J Cardiovasc Electrophysiol. 2006;17:944–8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Della Bella P, Brugada J, Zeppenfeld K, Merino J, Neuzil P, Maury P, Maccabelli G, Vergara P, Baratto F, Berruezo A, Wijnmaalen AP. Epicardial ablation for ventricular tachycardia: a European multicenter study. Circ Arrhythm Electrophysiol. 2011;4:653-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 189] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 9. | Schmidt B, Chun KR, Ouyang F, Metzner A, Antz M, Kuck KH. Three-dimensional reconstruction of the anatomic course of the right phrenic nerve in humans by pace mapping. Heart Rhythm. 2008;5:1120-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Di Biase L, Burkhardt JD, Pelargonio G, Dello Russo A, Casella M, Santarelli P, Horton R, Sanchez J, Gallinghouse JG, Al-Ahmad A, Wang P, Cummings JE, Schweikert RA, Natale A. Prevention of phrenic nerve injury during epicardial ablation: comparison of methods for separating the phrenic nerve from the epicardial surface. Heart Rhythm. 2009;6:957-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Sosa E, Scanavacca M, D’Avila A, Pilleggi F. A New Technique to Perform Epicardial Mapping in the Electrophysiology Laboratory. J Cardiovasc Electrophysiol. 1996;7:531-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 667] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 12. | Sanchez-Quintana D, Cabrera JA, Climent V, Farre J, Weiglein A, Ho SY. How close are the phrenic nerves to cardiac structures? Implication for cardiac interventionalist. J Cardiovasc Electrophysiol. 2005;16:309-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 189] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Buch E, Vaseghi M, Cesario DA, Shivkumar K. A novel method for preventing phrenic nerve injury during catheter ablation. Heart Rhythm. 2007;4:95-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Kumar S, Barbhaiya CR, Baldinger SH, Koplan BA, Maytin M, Epstein LM, John RM, Michaud GF, Tedrow UB, Stevenson WG. Epicardial Phrenic Nerve Displacement During Catheter Ablation of Atrial and Ventricular Arrhythmias. Procedural Experience and Outcomes. Circ Arrhythm Electrophysiol. 2015;8:896-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Kharlamov AN S-Editor: Zhang L L-Editor: Filipodia E-Editor: Zhang YL