Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.209
Peer-review started: January 31, 2015
First decision: April 27, 2015
Revised: May 15, 2015
Accepted: June 18, 2015
Article in press: June 19, 2015
Published online: August 26, 2015
Processing time: 209 Days and 4.4 Hours
Atherosclerosis is a chronic inflammatory disease associated with cardiovascular dysfunction including myocardial infarction, unstable angina, sudden cardiac death, stroke and peripheral thromboses. It has been predicted that atherosclerosis will be the primary cause of death in the world by 2020. Atherogenesis is initiated by endothelial injury due to oxidative stress associated with cardiovascular risk factors including diabetes mellitus, hypertension, cigarette smoking, dyslipidemia, obesity, and metabolic syndrome. The impairment of the endothelium associated with cardiovascular risk factors creates an imbalance between vasodilating and vasoconstricting factors, in particular, an increase in angiotensin II (Ang II) and a decrease in nitric oxide. The renin-angiotensin system (RAS), and its primary mediator Ang II, also have a direct influence on the progression of the atherosclerotic process via effects on endothelial function, inflammation, fibrinolytic balance, and plaque stability. Anti-inflammatory agents [statins, secretory phospholipase A2 inhibitor, lipoprotein-associated phospholipase A2 inhibitor, 5-lipoxygenase activating protein, chemokine motif ligand-2, C-C chemokine motif receptor 2 pathway inhibitors, methotrexate, IL-1 pathway inhibitor and RAS inhibitors (angiotensin-converting enzyme inhibitors)], Ang II receptor blockers and ranin inhibitors may slow inflammatory processes and disease progression. Several studies in human using anti-inflammatory agents and RAS inhibitors revealed vascular benefits and reduced progression of coronary atherosclerosis in patients with stable angina pectoris; decreased vascular inflammatory markers, improved common carotid intima-media thickness and plaque volume in patients with diagnosed atherosclerosis. Recent preclinical studies have demonstrated therapeutic efficacy of vitamin D analogs paricalcitol in ApoE-deficient atherosclerotic mice.
Core tip: There are several reviews in the literature contributed to the pathophysiology, therapeutic options and clinical trials for atherosclerosis. However this is a first review to report the latest cellular and molecular mechanisms of the pathways of atherosclerosis including inflammation, renin-angiotensin system, oxidants/antioxidants imbalance and the efficacy of several therapeutic strategies in improving cardiovascular outcomes, and recent clinical trials reducing the progression of the pathogenesis of atherosclerosis.
- Citation: Husain K, Hernandez W, Ansari RA, Ferder L. Inflammation, oxidative stress and renin angiotensin system in atherosclerosis. World J Biol Chem 2015; 6(3): 209-217
- URL: https://www.wjgnet.com/1949-8454/full/v6/i3/209.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v6.i3.209
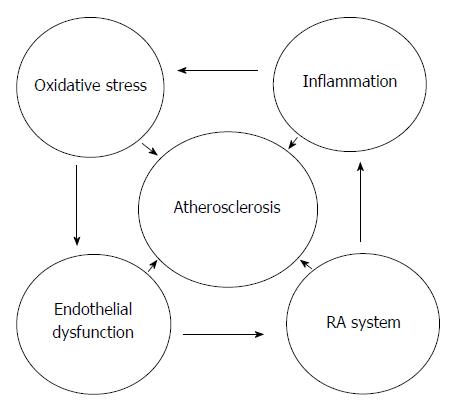
Atherosclerosis, a continuing chronic disease with inflammation manifesting in the vascular system of blood vessels is the primary origin of cardiovascular diseases (CVDs) in the developed countries of the globe. Its burden is higher in developing countries of Asia, Africa and South America (> 5000 per 100000) than in the developed countries of North America, Europe and Australia (< 3000 per 100000). The mortality due to this disease is anticipated to be at the first place in the globe by 2020[1]. It is represented by the development of vascular lesions or plaques in the blood vessels following inflammatory/oxidant response to endothelial damage[2,3]. The plaque mainly composed of blood cells, foam cells, lipids and proteins with calcium accumulation[3-5]. Finally it results to vascular expansion, vascular blockage, and inhibition of vascular blood flow leading to burst of the vascular wall[6,7]. In cardiovascular disease, blockage and rupture of atherosclerotic coronary arteries cause myocardial infarction, whereas blockage of carotid arteries cause stroke[2,6,8]. The impairment of the physiological functions of the endothelium is seen during initial phases of the atherosclerotic lesions due to oxidant damage. Endothelial damage is linked with heart and blood vessels risk factors such as diabetes, high blood pressure, nicotine, lipid disorders, obesity, and disorders of the metabolism[2,6,9,10]. The renin-angiotensin system (RAS) also plays an important role in the advancement of atherosclerosis by influencing on physiology of the endothelium, inflammatory reactions, thrombosis, and oxidant injury[9,10]. Ang II causes oxidant damage in vascular system by inducing oxidant species generation via activation of NADPH oxidase and these oxidant species oxidize cellular biomolecules including lipids, lipoproteins and DNA leading to endothelial impairment. The relationship between inflammation, oxidative stress, RAS system, endothelial dysfunction and atherosclerosis is depicted in Figure 1. This mini review presents precisely the mechanistic aspects of the events associated with atherosclerosis, implications of the inflammation, RAS and oxidative stress as well as the efficacy of several therapeutic strategies in improving cardiovascular system, physiology of the endothelium, and ameliorating the advancement of atherosclerotic events. Current clinical trials using anti-inflammatory, RAS blockers and antioxidants in attenuating the atherosclerotic lesions and preserving the pathophysiology of the endothelium is also reviewed.
Atherosclerosis is a concurrent inflammatory disease which first starts in the endothelium of the arterial wall[3,4,11]. Impairment of the endothelium is the first physiological alteration in the pathophysiology of this disorder which is manifested by enhanced vascular constriction and depressed dilatation of the vascular endothelium as well as changes in the mediators of thrombosis. Endothelium-derived relaxing factor (EDRF) or nitric oxide (NO) plays an important role in preserving the endothelial vasodilatation and inhibiting the vasoconstriction triggered by angiotensin II and endothelin[3]. Inflammatory processes are manifested by enhanced biosynthesis of mediators of inflammation and thrombosis. The mediators and reactions include interleukin-6, monocyte chemoattractant protein-1 (MCP-1), intercellular adhesion molecule-1 (ICAM-1), endothelial-selectin, adhesion/infiltration of monocytes, oxidation of low density lipoprotein (LDL) and production of foam cells[12]. Foam cells are formed due to storage of excess cholesterol ester in the macrophages[12]. The transport of cholesterol regulated by ATP-binding cassette transporter A1 (ABCA1) and transport of oxidized LDL through CD36 regulate the excess of cholesterol ester in the macrophages[12]. Apart from excess foam cells, growth of smooth muscle/endothelial cells[3,13], collagens, matrix metalloproteinases (MMPs), fibronectin, and elastin are also responsible for plaque development[2,3,11]. Evidences suggest that cytokines and tissue factors also regulate pathophysiology of the endothelium due to inflammatory reactions. The influence of different cytokines and factors modulating the pathophysiology of the vessel wall is depicted in Table 1. Among the biomarkers of inflammation C-reactive protein (CRP) is important which is generated by hepatic cells and is also modulated by IL-6, IL-1 and TNF-α[14]. Evidences suggest that raised blood CRP level is one of the inflammatory biomarkers and predictors of cardiovascular diseases[15,16]. It is also implicated in the advancement of atherosclerotic lesions by regulating physiology of endothelium[3,17,18]. It enhances the production of VCAM-1, ICAM-1, selectins, and MCP-1 in the endothelium through induction of powerful constrictor of the vessels ET-1 and IL-6[3,17]. It ameliorates the synthesis of NO in the endothelium by depressing the transcription and translation of enzyme NO synthase[3,19]. It also plays a significant role in cooperating with the activities of other cytokines and factors. CRP induces the biochemical synthesis and physiological functions of PAI-1 in the endothelium[19]. PAI-1 is known to be actively involved in thrombosis during atherosclerosis process and inhibits destruction of the fibrin clot by suppressing plasminogen activation[20]. There is a good correlation between elevated blood PAI-1 concentration and death rate in patients with coronary heart diseases[20]. Apolipoprotein E as well as low-density lipoprotein (LDL)-receptor knock out animals display speedy atherosclerotic lesions[21,22]. These animals also have sizeable counts of macrophages/T cells in their plaques. Cross breading of apolipoprotein-E knock out with T-cell knock out and mice with deficient macrophages (osteoporotic op/op) revealed the influence of immune cells in the progression of atherosclerosis[23]. Inflammatory reactions are not only involved in progression of human vascular plaques generation but also have important role in the rupture of internal arterial plaques transforming chronic disorder into an acute thrombo-embolic disease. Several factors are implicated in the rupture of internal arterial plaques comprise of cytokines, cyclooxygenase-2, matrix metalloproteinases, and tissue factors[1,4,23]. Experimental evidences support crucial role for inflammatory reactions as a connection between risk factors for atherosclerotic disorder and pathophysiologic complexity of the disease[2]. Serum amyloid A (SAA) protein has also been implicated in the inflammatory reactions associated with atherosclerotic disorder and used as a biomarker for cardiac and vascular disorders as well as heart and vessels outcome[24]. TNF-α is one of the inflammatory cytokines involved in commencement as well as development of atherosclerosis. It induces transcription factor nuclear factor-κB (NF-κB), a key factor in the pathways of inflammation. In the process of atherosclerosis NF-κB induces the transcription of VCAM-1, ICAM-1, MCP-1, and E-selectin in smooth muscle/endothelial cells of the blood vessels[25]. TNF-α depletes NO levels in the endothelium causing decrease of endothelial dilatation leading to dysfunction of the endothelium[26,27]. TNF-α has been reported to cause apoptosis of the endothelial cells through dephosphorylation of protein kinase B (Akt) leading to endothelial damage[28,29]. Resistin exerts inflammatory reactions/vasoactive effects in cultured cells of the endothelium[8]. In atherosclerotic process resistin induces transcription of cellular factors such as VCAM-1 and MCP-1[30]. Cells from endothelium exposed to resistin deplete the levels of TNF receptor-associated factor (TRAF-3) which is a well known inhibitor of the endothelial activation[31]. It is suggested that augmented resistin concentration causes a significant dysfunction of the endothelium through activation of endothelial system. Furthermore, resistin exposure activates endothelial cells by increasing ET-1 release through induction of transcription of ET-1 indicating its role in the impairment of the endothelium[32]. Leptin up regulates ET-1 as well as NO synthase biosynthesis in the endothelial cells and augments generation of free radicals and oxidants[33,34], causing oxidative stress[35]. Leptin also increases the cellular growth as well as migration of cells of the endothelium[36] and cells of smooth muscle[37]. It induces the synthesis of MCP-1 in the cells of the aortic endothelium[38]. It enhances the aggregation of the platelets and vascular thrombus formation through leptin receptor pathways[33,34]. It directly augments concentrations of monocyte colony-stimulating factor (MCSF)[39], increases cholesterol levels in hyperglycemia[40], and promotes new blood vessel formation[41]. We have shown in our earlier studies elevated concentrations of factors involved in inflammatory pathway namely TNF-α, MCP-1, Cox-2, TGF-β1, iNOS, and Mn-SOD in ApoE-deficient atherosclerotic mice[42,43] proving the vascular inflammation as an integral process in the atherosclerotic pathophysiology.
| Cytokines/factors | Abbreviations |
| Tumor necrosis factor-alpha | TNF-α , TNF-a |
| Interleukine-6 | IL-6 |
| Interleukine-1beta | IL-1β, IL-1beta |
| Nuclear factor-kappa B | NF-κB |
| Monocyte chemoattractant protein-1 | MCP-1 |
| C-reactive protein | CRP |
| Intracellular adhesion molecule-1 | ICAM-1 |
| Vascular cell adhesion molecule-1 | VCAM-1 |
| Monocyte colony-stimulating factor | M-CSF, MCSF |
| Transforming growth factor beta1 | TGFβ1, TGF-β1 |
| Plasminogen activator inhibitor-1 | PAI-1 |
| Macrophage migration inhibitory factor | MIF |
| Cyclooxygenase-2 | COX-2 |
| Endothelin-1 | ET-1 |
| Angiotensin 2 | Ang II, ANG II |
| Endothelial-selectin | E-selectin |
| Platelet-selectin | P-selectin |
| Angiotensinogen 2 | ANGT 2 |
| Leptin | LEP |
| Inducible nitric oxide synthase | iNOS |
| Matrix metalloproteinase | MMP |
| Low density lipoprotein | LDL |
| Serum amyloid A | SAA |
| Apolipoprotein E | ApoE |
| Tumor necrosis factor receptor –associated factor | TRAF |
| Reactive oxygen species | ROS |
| Angiotensin type 1 receptor | ATR1 |
| Glutathione peroxidase 1 | GPx1 |
| Phospholipase A2 | PLA2 |
| Lipoprotein-associated phospholipase A2 | Lp-PLA2 |
| 5-Lipoxygenase-activating protein (FLAP) | FLAP |
| 5-Lipoxygenase | 5-LO |
| Chemokine motif ligand 2 | CCL2 |
| Chemokine motif receptor 2 (CCR2) | CCR2 |
| Toll like receptor 2 | TLR2 |
| Hear shock protein | HSP |
Oxidative stress is referred as imbalance of the cellular oxidants and antioxidants in the body. In atherosclerosis process Ang II causes oxidants/antioxidants imbalance in the vascular system by inducing oxidant species generation via activation of NADPH oxidase[44,45]. Earlier studies have shown that excess superoxide generation due to NADPH oxidase activation causes inflammation and further generation of inflammatory cytokines (TNF-alpha) through NF-κB activation[46-48]. The role of NF-κB activation is also demonstrated in atherosclerosis[47,49]. These reactive oxygen species (ROS) initiate vascular membrane lipid peroxidation leading to inflammation and production of TNF-αvia NF-κB induction[46,47] and other factors namely VCAM-1, MCP-1, TGF-β1, Matrix metalloproteinase 9 (MMP9), iNOS and Mn-SOD[50-52]. The ROS up regulate atherosclerotic events namely cell infiltration, migration, adhesion and platelet activation. These ROS oxidize cellular biomolecules including lipids, proteins and nucleic acids causing endothelial impairments[53]. However, cells are equipped with an intricate cellular defense system that includes antioxidant enzymes namely superoxide dismutase (SOD), catalase and glutathione peroxidase (GSHPx), tripeptide glutathione (GSH), antioxidant vitamins A, C, and E to scavenge oxidant species thereby attenuate oxidant injury[54]. Most importantly, depletion of a major cellular antioxidant such as GSH has been reported to cause vascular dysfunction[42,55-57]. The vascular system has also been shown to be equipped with antioxidant defense to combat oxidant damage[42,55,56]. However depletion of cellular antioxidants increases oxidant species build up leading to pathological and physiological impairments[42,43,55-57]. Several studies in ApoE knock out mouse model revealed down regulation of antioxidants in atherosclerosis[42,43,58] which suggests a relationship between declined antioxidants and enhanced pathological lesions. On the contrary, over expression of catalase which hydrolyses H2O2, suppressed tissue injury in ApoE knock out mouse model[21] indicating the implications of H2O2 degrading enzyme in atherosclerosis. Furthermore, additional studies suggest defensive capacity of glutathione peroxidase (GPx1) in atherosclerosis[58]. Down regulation of erythrocyte GPx1 level was linked with enhanced risk of heart and blood vessel injury and patients with atherosclerosis of carotid artery posses declined GPx1 levels[58]. Knock down of GPx1 in animal enhanced lipoprotein oxidation and depleted NO levels causing impairment of endothelium[58]. Diabetes-related atherosclerotic events are enhanced with deficit of GPx1 through increased inflammation and thrombosis in ApoE-deficient mouse model[58]. Several studies reported a lower level of tissue antioxidants in patients with renal and cardiovascular diseases[6,59-61].
The renin-angiotensin system is thought to have an important implication in the pathophysiologic injury in atherosclerosis through induction of various cellular/molecular reactions[10,61,62]. Previous studies showed that angiotensin II (Ang II) produced by vascular tissues increases generation of ROS and induces synthesis of several factors via induction of AT II type 1 receptor (AT1R), leading to stockpile of inflammatory mediators and proliferation/migration of vascular cells[1,10,62]. These findings indicate that regional impact of AT1R activation in vascular wall plays a critical role in pathophysiology of concurrent inflammatory reactions through a direct action on local vascular cells. Several studies demonstrated an essential part of RAS in pathophysiology of heart and vascular system, including atherosclerotic disease[1,6,10,62,63]. Angiotensinogen (ANGT), a precursor of ANG II, is synthesized in fat cells[64]. Angiotensin II causes oxidative stress in cardiovascular systems by inducing ROS production via induction of NADPH oxidase[44,49]. These oxidant species begin oxidation reaction of lipids in the membranes of the blood vessels leading to inflammation and generation of inflammatory cytokines (TNF-α) through NF-κB activation[1,2,11,23,63,65]. These ROS oxidize cellular biomolecules namely lipids, proteins and nucleic acids causing oxidation of membrane phospholipids resulting in the impairments of the heart and blood vessels[1,2,11,23,57,63,65,66]. Vasoconstrictor Ang II instantly triggers the synthesis of ICAM-1, VCAM-1, MCP-1, and macrophage colony stimulating factor (M-CSF) in the walls of the blood vessels through induction of NF-κB-regulated genetic products[67]. Moreover Ang II stimulates production of oxidant species from NO leading to depletion of NO causing injury to blood vessels[3]. Enhanced ANGT 2 levels is associated with new blood vessels formation[68] and elevation of blood pressure[44,56,57]. These two disorders are closely linked with impairments of the endothelium. Additionally, CRP induces ATR1-receptor transcription and translation as well as enhanced AT1-receptor levels in blood vessel wall[69]. Importantly, activation of ATR1-receptors promote Ang II-induced ROS generation, migration/proliferation/remodeling of the cells of the blood vessels[70].
The influence of inflammatory reactions on the progression/maturation and rupture of thrombus in the vascular lumen of the blood vessels opens new therapeutic strategies for atherosclerotic disorder. One of the clinical trials (JUPITER) corroborates clinical advantage of the evaluation of the degree of inflammation in managing the therapeutic intervention to restrict the occurrence of the injury to the heart and vascular system. Academic and theoretical concepts of inflammatory reactions are being now utilized as a therapeutic tool in the clinics for the risk assessment as well as targeted therapeutics[2]. Current therapeutics effectiveness in preventing atherosclerosis such as HMG-CoA reductase inhibitors (statins), acetylsalicyclic acid (ASS), and RAS blockers deploy their influence through modulation of inflammation in the blood vessels[71]. Attentions are also focused to various therapeutic agents decreasing different modulators of inflammation process. Among these drugs are derivatives of thiazolidinedions (glitazones), HMG-CoA reductase inhibitors (statins), acetyl salicylic acid, ACE inhibitors, and ATR blockers. Glitazones are activators of nuclear peroxisome proliferator-activated receptor (PPAR)-γ. Glitazones are known to down regulate various factors involved in the process of inflammation and their activities therefore they can ameliorate the advancement of atherosclerotic events. Glitazones are also known to deplete TNF-α level in fat cells and abrogate TNF-α-induced synthesis of VCAM-1 and ICAM-1 in the endothelium of the blood vessels[8,17]. Rosiglitazone has been shown to reverse the progression of atherosclerotic activities of CRP in the endothelium of the blood vessels[8]. Glitazones also depress leptin-induced migration of the cells of the endothelium[33]. Glitazones inhibit resistin concentration in fat tissues[72,73]. Alternatively, inhibitors of matrix metalloproteinase (MMP) activity as well as vaccines are presently under developmental processes and clinical trials[23]. There are several anti-inflammatory agents have been used pre-clinically and clinically in the treatment of atherosclerotic cardiovascular diseases such as phospholipase A2 (PLA2) inhibitors[74], phospholipase A2 of lipoproteins (Lp-PLA2) inhibitors[75], 5-LO-activating protein (FLAP)[76], arachidonate 5-lipoxygenase (5-LO)[77], chemokine motif ligand 2 (CCL2), chemokine motif receptor 2 (CCR2) inhibitors[78], methotrexate[15,65], and IL-1 pathway inhibitor[79].
Antioxidant therapy has been shown to ameliorate cardiovascular oxidative stress by scavenging excess ROS and up regulating the antioxidant defense system[54,80]. The antioxidant N-acetylcysteine is reported to abrogate accelerated atherosclerotic events in ApoE knockout mouse models[80]. Our recent study demonstrated that vitamin D analog paricalcitol ameliorated the oxidative vascular injury by suppressing ROS-generating enzyme NADPH oxidase activity and inflammatory mediators and by up regulating the antioxidant defense system in ApoE-deficient mice[42,43]. There is a novel strategy against inflammation is being used by inhibiting the oxidation of lipoproteins to stop their entry into the cells of the blood vessels. The ARISE clinical trial investigated the influence of antioxidant succinobucol (AGI-1067) in patients who had myocardial infarction/ruptured atherosclerotic plaques had significant curative effects[81]. In preclinical investigation using antioxidant enzyme GPx1 afforded significant protection in a mouse model of atherosclerosis[58].
In preclinical and clinical investigations putative drugs used for high blood pressure have been reported to adequately suppress the events of atherosclerosis[6,62,63]. The inhibitors or blockers/antagonists of RAS such as ACE inhibitors and ATR blockers act either by depleting the generation of Ang II or by blocking the binding of Ang II to its receptors. RAS inhibitors or blockers/antagonists have both ancillary and concurring actions that enhance the NO concentration, decrease oxidants/antioxidants imbalance, inhibit RAS-induced inflammation, as depicted by actions of these drugs on several biomarkers of inflammation[6,10,17,23,62,63,82]. The above biochemical alterations achieve amelioration in the activities of the endothelium and pathophysiology of the vascular system in atherosclerosis. Additionally above biochemical alterations are also linked with advantage over lowering the blood pressure in patients with atherosclerosis and high blood pressure. These investigations have demonstrated that olmesartan medoxomil administration suppress the advancement of atherosclerotic process and significantly ameliorate the cardiovascular dysfunctions[10,63]. ATR1 blockers have been shown to decrease the new blood vessel formation in ApoE-deficient mouse model of atherosclerosis, through toll like receptor 2 and 4 (TLR2/TLR4)-arbitrated events of inflammation and activation of MMP, consequently inhibiting the proliferation of the vascular lesions as well as rupture of the plaques[83]. Our recent studies have shown that ACEI enalapril ameliorated the oxidative vascular injury by suppressing ROS-generating enzyme NADPH oxidase activity and inflammatory mediators and by up regulating the antioxidant defense system in ApoE-deficient mice[42,43].
Preclinical as well as clinical studies have clearly demonstrated and provided the evidence that vitamin D and its analogues suppresses the various factors and biomarkers associated with disorders of vascular system and permeate cells of the immune system against the activities of the inflammation[5,9,84-87]. The knock down of vitamin D receptor (VDR) and VDR-mediated signaling pathways in animals induces hypertension by elevating renin release from the kidneys and stimulates the process of atherosclerosis conceivably through regional induction of cellular RAS[9]. The insufficiency or depletion of vitamin D causes hypertension and enhances the progression of atherosclerotic lesions in animals[5]. Our recent study demonstrated that vitamin D analog paricalcitol ameliorated the oxidative vascular injury by suppressing ROS-generating enzyme NADPH oxidase activity and inflammatory mediators and by up regulating the antioxidant defense system in ApoE-deficient mice[42,43]. In animal model when vaccines derived against oxidized LDL and heat shock protein (HSP) administered have demonstrated inhibition of inflammation and progression of atherosclerotic lesions[88]. The prospect of clinical application of vaccines for prevention or treatment of atherosclerosis is presently under exploration[23]. The antibodies raised against the oxidized lipoproteins not only reveal activity of the disorder but also confer the prevention and treatment of atherosclerosis[7,63].
The investigations delineated in the present mini review furnish to our comprehending the role of inflammatory events, oxidants/antioxidants imbalance and RAS pathways in the pathophysiology of atherosclerosis as well as targeted therapeutic intervention. Preclinical as well as clinical investigations clearly demonstrate that inflammatory reactions operates all stages of atherosclerotic events, including commencement, advancement, and the complexity of the lesions. Inflammatory process is commonly associated with several risk factors for atherosclerotic plaque formation and modified pathophysiology of the blood vessels. Alteration of implicated risk factors has a clinical advantage through abrogating the inflammatory reactions and its consequences. Convincing verifications from clinical studies authenticates the application of the drugs inhibiting the inflammation process; RAS blockers and antioxidants as a therapeutic regimen that can prevent and treat the atherosclerotic lesions.
| 1. | Scott J. The pathogenesis of atherosclerosis and new opportunities for treatment and prevention. J Neural Transm Suppl. 2002;1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010;74:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 525] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 3. | Verma S, Wang CH, Li SH, Dumont AS, Fedak PW, Badiwala MV, Dhillon B, Weisel RD, Li RK, Mickle DA. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 747] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 4. | Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6017] [Cited by in RCA: 6107] [Article Influence: 254.5] [Reference Citation Analysis (0)] |
| 5. | Weng S, Sprague JE, Oh J, Riek AE, Chin K, Garcia M, Bernal-Mizrachi C. Vitamin D deficiency induces high blood pressure and accelerates atherosclerosis in mice. PLoS One. 2013;8:e54625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5059] [Cited by in RCA: 5174] [Article Influence: 207.0] [Reference Citation Analysis (0)] |
| 7. | Libby P. Lipid-lowering therapy stabilizes plaque, reduces events by limiting inflammation. Am J Manag Care. 2002;1 Suppl:4. [PubMed] |
| 8. | Verma S, Li SH, Wang CH, Fedak PW, Li RK, Weisel RD, Mickle DA. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation. 2003;108:736-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 476] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 9. | Ferder M, Inserra F, Manucha W, Ferder L. The world pandemic of vitamin D deficiency could possibly be explained by cellular inflammatory response activity induced by the renin-angiotensin system. Am J Physiol Cell Physiol. 2013;304:C1027-C1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Pacurari M, Kafoury R, Tchounwou PB, Ndebele K. The Renin-Angiotensin-aldosterone system in vascular inflammation and remodeling. Int J Inflam. 2014;2014:689360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 244] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 11. | Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15621] [Cited by in RCA: 15641] [Article Influence: 579.3] [Reference Citation Analysis (0)] |
| 12. | Allahverdian S, Pannu PS, Francis GA. Contribution of monocyte-derived macrophages and smooth muscle cells to arterial foam cell formation. Cardiovasc Res. 2012;95:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Obikane H, Abiko Y, Ueno H, Kusumi Y, Esumi M, Mitsumata M. Effect of endothelial cell proliferation on atherogenesis: a role of p21(Sdi/Cip/Waf1) in monocyte adhesion to endothelial cells. Atherosclerosis. 2010;212:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1662] [Cited by in RCA: 1646] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 15. | Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1669] [Cited by in RCA: 1641] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 16. | Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131-2135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1646] [Cited by in RCA: 1724] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 17. | Pasceri V, Wu HD, Willerson JT, Yeh ET. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-gamma activators. Circulation. 2000;101:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 401] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 18. | Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 1250] [Article Influence: 54.3] [Reference Citation Analysis (2)] |
| 19. | Devaraj S, Xu DY, Jialal I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation. 2003;107:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 461] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 20. | Kohler HP, Grant PJ. Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med. 2000;342:1792-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 545] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 21. | Yang H, Roberts LJ, Shi MJ, Zhou LC, Ballard BR, Richardson A, Guo ZM. Retardation of atherosclerosis by overexpression of catalase or both Cu/Zn-superoxide dismutase and catalase in mice lacking apolipoprotein E. Circ Res. 2004;95:1075-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1628] [Cited by in RCA: 1730] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 23. | Stoll G, Bendszus M. Inflammation and atherosclerosis: novel insights into plaque formation and destabilization. Stroke. 2006;37:1923-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 357] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 24. | Johnson BD, Kip KE, Marroquin OC, Ridker PM, Kelsey SF, Shaw LJ, Pepine CJ, Sharaf B, Bairey Merz CN, Sopko G. Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109:726-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 329] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 25. | Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1551] [Cited by in RCA: 1547] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 26. | Bhagat K, Vallance P. Inflammatory cytokines impair endothelium-dependent dilatation in human veins in vivo. Circulation. 1997;96:3042-3047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 278] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 27. | Wang P, Ba ZF, Chaudry IH. Administration of tumor necrosis factor-alpha in vivo depresses endothelium-dependent relaxation. Am J Physiol. 1994;266:H2535-H2541. [PubMed] |
| 28. | Choy JC, Granville DJ, Hunt DW, McManus BM. Endothelial cell apoptosis: biochemical characteristics and potential implications for atherosclerosis. J Mol Cell Cardiol. 2001;33:1673-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 351] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 29. | Hermann C, Assmus B, Urbich C, Zeiher AM, Dimmeler S. Insulin-mediated stimulation of protein kinase Akt: A potent survival signaling cascade for endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:402-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 166] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Steppan CM, Brown EJ, Wright CM, Bhat S, Banerjee RR, Dai CY, Enders GH, Silberg DG, Wen X, Wu GD. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci USA. 2001;98:502-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 479] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 31. | Calabro P, Samudio I, Willerson JT, Yeh ET. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation. 2004;110:3335-3340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 232] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 32. | Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288:H2031-H2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 583] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 33. | Cooke JP, Oka RK. Does leptin cause vascular disease? Circulation. 2002;106:1904-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Konstantinides S, Schafer K, Loskutoff DJ. The prothrombotic effects of leptin possible implications for the risk of cardiovascular disease in obesity. Ann N Y Acad Sci. 2001;947:134-141; discussion 141-142. [PubMed] |
| 35. | Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999;13:1231-1238. [PubMed] |
| 36. | Park HY, Kwon HM, Lim HJ, Hong BK, Lee JY, Park BE, Jang Y, Cho SY, Kim HS. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med. 2001;33:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 328] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 37. | Artwohl M, Roden M, Hölzenbein T, Freudenthaler A, Waldhäusl W, Baumgartner-Parzer SM. Modulation by leptin of proliferation and apoptosis in vascular endothelial cells. Int J Obes Relat Metab Disord. 2002;26:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzmán M, Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem. 2001;276:25096-25100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 443] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 39. | Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57-65. [PubMed] |
| 40. | O’Rourke L, Gronning LM, Yeaman SJ, Shepherd PR. Glucose-dependent regulation of cholesterol ester metabolism in macrophages by insulin and leptin. J Biol Chem. 2002;277:42557-42562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Sierra-Honigmann MR, Nath AK, Murakami C, García-Cardeña G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 983] [Cited by in RCA: 978] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 42. | Husain K, Suarez E, Isidro A, Ferder L. Effects of paricalcitol and enalapril on atherosclerotic injury in mouse aortas. Am J Nephrol. 2010;32:296-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Suarez-Martinez E, Husain K, Ferder L. Adiponectin expression and the cardioprotective role of the vitamin D receptor activator paricalcitol and the angiotensin converting enzyme inhibitor enalapril in ApoE-deficient mice. Ther Adv Cardiovasc Dis. 2014;8:224-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, Griendling KK. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 415] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 45. | Hattori Y, Akimoto K, Nishikimi T, Matsuoka H, Kasai K. Activation of AMP-activated protein kinase enhances angiotensin ii-induced proliferation in cardiac fibroblasts. Hypertension. 2006;47:265-270 [. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Ungvari Z, Csiszar A, Kaminski PM, Wolin MS, Koller A. Chronic high pressure-induced arterial oxidative stress: involvement of protein kinase C-dependent NAD(P)H oxidase and local renin-angiotensin system. Am J Pathol. 2004;165:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Zhang L, Ma Y, Zhang J, Cheng J, Du J. A new cellular signaling mechanism for angiotensin II activation of NF-kappaB: An IkappaB-independent, RSK-mediated phosphorylation of p65. Arterioscler Thromb Vasc Biol. 2005;25:1148-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 48. | Brasier AR. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 457] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 49. | Zhang X, Wu M, Jiang H, Hao J, Zhang Q, Zhu Q, Saren G, Zhang Y, Meng X, Yue X. Angiotensin II upregulates endothelial lipase expression via the NF-kappa B and MAPK signaling pathways. PLoS One. 2014;9:e107634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Jiang X, Zeng HS, Guo Y, Zhou ZB, Tang BS, Li FK. The expression of matrix metalloproteinases-9, transforming growth factor-beta1 and transforming growth factor-beta receptor I in human atherosclerotic plaque and their relationship with plaque stability. Chin Med J (Engl). 2004;117:1825-1829. [PubMed] |
| 51. | Gustafsson S, Lind L, Söderberg S, Zilmer M, Hulthe J, Ingelsson E. Oxidative stress and inflammatory markers in relation to circulating levels of adiponectin. Obesity (Silver Spring). 2013;21:1467-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Lu W, Jiang JP, Hu J, Wang J, Zheng MZ. Curcumin protects against lipopolysaccharide-induced vasoconstriction dysfunction via inhibition of thrombospondin-1 and transforming growth factor-β1. Exp Ther Med. 2015;9:377-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Hamza SM, Dyck JR. Systemic and renal oxidative stress in the pathogenesis of hypertension: modulation of long-term control of arterial blood pressure by resveratrol. Front Physiol. 2014;5:292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 54. | Rodrigues SD, França KC, Dallin FT, Fujihara CK, Nascimento AJ, Pecoits-Filho R, Nakao LS. N-acetylcysteine as a potential strategy to attenuate the oxidative stress induced by uremic serum in the vascular system. Life Sci. 2015;121:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Husain K, Ferder L, Mizobuchi M, Finch J, Slatopolsky E. Combination therapy with paricalcitol and enalapril ameliorates cardiac oxidative injury in uremic rats. Am J Nephrol. 2009;29:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 56. | Husain K, Vazquez M, Ansari RA, Malafa MP, Lalla J. Chronic alcohol-induced oxidative endothelial injury relates to angiotensin II levels in the rat. Mol Cell Biochem. 2008;307:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 57. | Vaziri ND, Wang XQ, Oveisi F, Rad B. Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension. 2000;36:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 263] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 58. | Lewis P, Stefanovic N, Pete J, Calkin AC, Giunti S, Thallas-Bonke V, Jandeleit-Dahm KA, Allen TJ, Kola I, Cooper ME. Lack of the antioxidant enzyme glutathione peroxidase-1 accelerates atherosclerosis in diabetic apolipoprotein E-deficient mice. Circulation. 2007;115:2178-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 59. | Wang L, Manson JE, Buring JE, Lee IM, Sesso HD. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension. 2008;51:1073-1079 [. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 243] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 60. | Agarwal R, Acharya M, Tian J, Hippensteel RL, Melnick JZ, Qiu P, Williams L, Batlle D. Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int. 2005;68:2823-2828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 254] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 61. | da Cunha V, Tham DM, Martin-McNulty B, Deng G, Ho JJ, Wilson DW, Rutledge JC, Vergona R, Sullivan ME, Wang YX. Enalapril attenuates angiotensin II-induced atherosclerosis and vascular inflammation. Atherosclerosis. 2005;178:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 62. | Wu H, Cheng XW, Hu L, Hao CN, Hayashi M, Takeshita K, Hamrah MS, Shi GP, Kuzuya M, Murohara T. Renin inhibition reduces atherosclerotic plaque neovessel formation and regresses advanced atherosclerotic plaques. Atherosclerosis. 2014;237:739-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Mason RP. Optimal therapeutic strategy for treating patients with hypertension and atherosclerosis: focus on olmesartan medoxomil. Vasc Health Risk Manag. 2011;7:405-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11:327-332. [PubMed] |
| 65. | Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT). J Thromb Haemost. 2009;7 Suppl 1:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 201] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 66. | Younes M, Siegers CP. Mechanistic aspects of enhanced lipid peroxidation following glutathione depletion in vivo. Chem Biol Interact. 1981;34:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 211] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 67. | Tham DM, Martin-McNulty B, Wang YX, Da Cunha V, Wilson DW, Athanassious CN, Powers AF, Sullivan ME, Rutledge JC. Angiotensin II injures the arterial wall causing increased aortic stiffening in apolipoprotein E-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1442-R1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Esposito K, Nicoletti G, Giugliano D. Obesity, cytokines and endothelial dysfunction: a link for the raised cardiovascular risk associated with visceral obesity. J Endocrinol Invest. 2002;25:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Wang CH, Li SH, Weisel RD, Fedak PW, Dumont AS, Szmitko P, Li RK, Mickle DA, Verma S. C-reactive protein upregulates angiotensin type 1 receptors in vascular smooth muscle. Circulation. 2003;107:1783-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 393] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 70. | Nickenig G, Harrison DG. The AT(1)-type angiotensin receptor in oxidative stress and atherogenesis: Part II: AT(1) receptor regulation. Circulation. 2002;105:530-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 259] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 71. | Sukhova GK, Williams JK, Libby P. Statins reduce inflammation in atheroma of nonhuman primates independent of effects on serum cholesterol. Arterioscler Thromb Vasc Biol. 2002;22:1452-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 170] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 72. | Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1280] [Cited by in RCA: 1256] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 73. | Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, Olefsky JM. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968-2974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 550] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 74. | Rosenson RS, Elliott M, Stasiv Y, Hislop C. Randomized trial of an inhibitor of secretory phospholipase A2 on atherogenic lipoprotein subclasses in statin-treated patients with coronary heart disease. Eur Heart J. 2011;32:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 75. | Wilensky RL, Shi Y, Mohler ER, Hamamdzic D, Burgert ME, Li J, Postle A, Fenning RS, Bollinger JG, Hoffman BE. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nat Med. 2008;14:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 302] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 76. | Hakonarson H, Thorvaldsson S, Helgadottir A, Gudbjartsson D, Zink F, Andresdottir M, Manolescu A, Arnar DO, Andersen K, Sigurdsson A. Effects of a 5-lipoxygenase-activating protein inhibitor on biomarkers associated with risk of myocardial infarction: a randomized trial. JAMA. 2005;293:2245-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 77. | Tardif JC, L’allier PL, Ibrahim R, Grégoire JC, Nozza A, Cossette M, Kouz S, Lavoie MA, Paquin J, Brotz TM. Treatment with 5-lipoxygenase inhibitor VIA-2291 (Atreleuton) in patients with recent acute coronary syndrome. Circ Cardiovasc Imaging. 2010;3:298-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 78. | Gilbert J, Lekstrom-Himes J, Donaldson D, Lee Y, Hu M, Xu J, Wyant T, Davidson M. Effect of CC chemokine receptor 2 CCR2 blockade on serum C-reactive protein in individuals at atherosclerotic risk and with a single nucleotide polymorphism of the monocyte chemoattractant protein-1 promoter region. Am J Cardiol. 2011;107:906-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 79. | Crossman DC, Morton AC, Gunn JP, Greenwood JP, Hall AS, Fox KA, Lucking AJ, Flather MD, Lees B, Foley CE. Investigation of the effect of Interleukin-1 receptor antagonist (IL-1ra) on markers of inflammation in non-ST elevation acute coronary syndromes (The MRC-ILA-HEART Study). Trials. 2008;9:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 80. | Ivanovski O, Szumilak D, Nguyen-Khoa T, Ruellan N, Phan O, Lacour B, Descamps-Latscha B, Drüeke TB, Massy ZA. The antioxidant N-acetylcysteine prevents accelerated atherosclerosis in uremic apolipoprotein E knockout mice. Kidney Int. 2005;67:2288-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 81. | Tardif JC, McMurray JJ, Klug E, Small R, Schumi J, Choi J, Cooper J, Scott R, Lewis EF, L’Allier PL. Effects of succinobucol (AGI-1067) after an acute coronary syndrome: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:1761-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 159] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 82. | Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6011] [Cited by in RCA: 5676] [Article Influence: 218.3] [Reference Citation Analysis (0)] |
| 83. | Cheng XW, Song H, Sasaki T, Hu L, Inoue A, Bando YK, Shi GP, Kuzuya M, Okumura K, Murohara T. Angiotensin type 1 receptor blocker reduces intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Hypertension. 2011;57:981-989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 84. | Artaza JN, Mehrotra R, Norris KC. Vitamin D and the cardiovascular system. Clin J Am Soc Nephrol. 2009;4:1515-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 85. | Gunta SS, Thadhani RI, Mak RH. The effect of vitamin D status on risk factors for cardiovascular disease. Nat Rev Nephrol. 2013;9:337-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 86. | Norman PE, Powell JT. Vitamin D and cardiovascular disease. Circ Res. 2014;114:379-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 365] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 87. | Panizo S, Barrio-Vázquez S, Naves-Díaz M, Carrillo-López N, Rodríguez I, Fernández-Vázquez A, Valdivielso JM, Thadhani R, Cannata-Andía JB. Vitamin D receptor activation, left ventricular hypertrophy and myocardial fibrosis. Nephrol Dial Transplant. 2013;28:2735-2744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 88. | Maron R, Sukhova G, Faria AM, Hoffmann E, Mach F, Libby P, Weiner HL. Mucosal administration of heat shock protein-65 decreases atherosclerosis and inflammation in aortic arch of low-density lipoprotein receptor-deficient mice. Circulation. 2002;106:1708-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 187] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Choe W, Ramana KV, Rholam M S- Editor: Tian YL L- Editor: A E- Editor: Wang CH